Abstract
In socially monogamous prairie voles (Microtus ochrogaster), mating induces three primary types of behavior; namely, partner preference, selective aggression toward conspecific strangers, and bi-parental care, making this rodent an ideal model system to study sociality and underlying neurochemical mechanisms associated with monogamous mating strategies. Here, we highlight species differences in neurochemical receptor distributions associated with mating experience leading to the establishment of stable pair-bonds. Specifically, we illustrate the role of nucleus accumbens dopamine in programming the formation and maintenance of monogamous bonds and describe the role of anterior hypothalamic vasopressin in the regulation of selective aggression. We conclude by discussing recent molecular work in voles and emphasize the importance of this rodent for future research in the behavioral neurobiology field.
Keywords: Vasopressin, oxytocin, dopamine, corticotrophin-releasing hormone, nucleus accumbens, lateral septum, anterior hypothalamus, aggression, affiliation, parental care
Introduction
Attraction and sex are hard-wired, universal, behaviors programmed in single cell organisms as well as in complex nervous systems important for sociality, competition, and reproductive success. How the brain changes after sexual experience to control social behavior is of great interest to scientists across different disciplines and to the public and society in general. Copulation comes with both benefits and costs to species survival and evolution. In the socially monogamous prairie vole (Microtus ochrogaster), for example, mating induces partner preference, aggression, and bi-parental care [1] - behaviors that promote fitness yet threaten survival. So, why would evolution program a socially monogamous brain when the majority of animals mate indiscriminately? Over the past few decades, a constellation of molecules, neurotransmitters, hormones, and genes have been identified that begin to unravel the complexity of prairie vole mating strategies in the context of species reproductive fitness [2]. In this review, we focus on recent data illustrating the critical role(s) of the neuropeptides arginine vasopressin (AVP), oxytocin (OT), corticotrophin-releasing hormone (CRH) and the neurotransmitter dopamine (DA) in the regulation of mating-induced social behavior in voles. We end by discussing recent and future work that holds great promise in elucidating the molecular neurobiology and functional significance of attachment.
The socially monogamous prairie vole
The prairie vole is a microtine rodent species that displays unique patterns of social behavior associated with a monogamous mating strategy. Sexually naïve prairie voles are gregarious and highly affiliative. After mating, they display three types of social behavior: partner preference between mates, selective aggression toward conspecific strangers but not their partner, and bi-parental care of offspring (Figure 1). Extensive research has been conducted to examine the neurochemicals and neural circuitry underlying these innate behaviors [1,2]. There are other microtine species, such as meadow (Microtus pennsylvanicus) and montane (Microtus montanus) voles, that are taxonomically quite similar to prairie voles but socially promiscuous, do not develop mating-induced partner preference or selective aggression, and only display maternal care [3]. Together, these vole species provide an excellent comparative model system to study mating-induced development and/or changes in social behaviors associated with different life strategies and allow for investigation of the underlying neurobiological mechanisms.
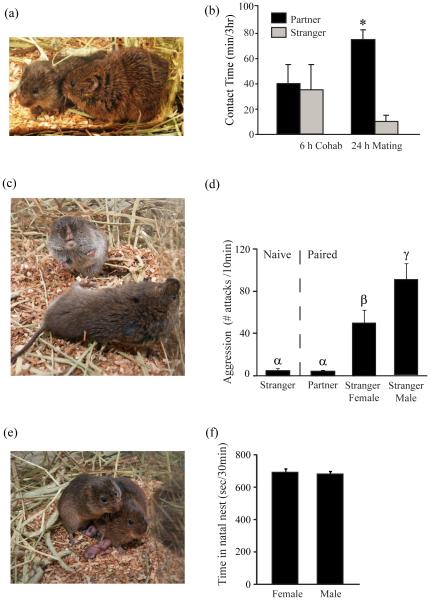
Figure 1. Laboratory characterization of mating-induced pair-bonding behavior.
(a) Photo depicts a pair-bonded male and female prairie vole displaying side-by-side (cuddling) contact (Photo by C. Badland & A. Smith). (b) In male and female prairie voles, 6 hrs of social cohabitation, without mating, is not sufficient to induce partner preference, as voles spend approximately an equal amount of contact time with their partner or with a stranger. Conversely, 24 hrs of cohabitation with successful copulation promotes partner preference formation, as voles spend significantly more time in side-by-side contact with their partner than with an unfamiliar stranger during a 3 hr partner preference assay. (c) Photo shows a pair-bonded male prairie vole (top) preparing to attack an unfamiliar stranger male prairie vole (bottom; Photo by C. Badland & A. Smith). (d) Sexually inexperienced (Naïve) male prairie voles do not display aggressive behavior toward a stranger, although successful mating and two weeks of social cohabitation engenders escalated selective aggression toward stranger male and female conspecifics but not toward familiar female partners. (e) Photo illustrates a pair-bonded male and female prairie vole huddling over and protecting their newly born pups (Photo by C. Badland & A. Smith). (f) Male and female prairie vole parents spend equivalent time in their natal nest huddling, contacting, and licking/grooming their offspring. Bars indicate means ± standard error of the mean. Bars with different Greek letters differ significantly from each other. *: p < 0.05. Adapted from [2,32•,40,61].
Even though studying pair-bonding behavior in voles doesn’t fully model all aspects of human attachment, this experimental approach provides a vehicle to deliver neural mechanisms programming human attraction leading to the formation of intimate partnerships. There is significant conservation between both behavioral and neurochemical mechanisms controlling prairie vole and human mating drives and interpersonal attachment behavior. Thus, the vole represents an ideal model system for translational and basic research investigating the neurobiology underlying social behavior(s) associated with various mental health deficits that are characterized by problems with attachment such as those that suffer from autism spectrum disorders.
Neurochemical regulation of pair-bonding behavior
Research in voles has revealed a variety of neuromodulators in the regulation of mating-induced pair-bonding and comparable work in humans has implicated several of these same neurochemical systems. For example, the nine amino-acid (nonapeptide) AVP has been implicated in human aggressive behavior, as higher levels of AVP assayed from cerebrospinal fluid taken from aggressive patients has been shown to be associated with a history of violent behavior in both men and women [4]. Furthermore, a structurally similar nonapeptide, OT, administered intra-nasally in human couples, significantly enhanced several dimensions of positive communication between one-another such as agreeableness, positive regard for the self and partner, consolation, and increased eye-contact during a simulated couple’s quarrel as well as significantly reduced salivary cortisol levels after a semi-naturalistic conflict [5]. Another important element of prosocial behavior, prerequisite for social affiliation, is trust. Trust is necessary to social, economic, and/or political success and without it these faculties quickly disintegrate. However, at the dawn of the new millennium, next to nothing was known about the biological origins of trust in humans. Thus, in a similar double-blind placebo-controlled set of experiments as outlined above, participants were intra-nasally administered OT and reported remarkable increases in their level of perceived trust during social interactions which wasn’t a general effect of OT enhancing participants’ readiness to bear risks. Conversely, OT specifically affected an individual’s willingness to accept social risks during interpersonal interactions [6••]. Together, these data are in line with vole research substantiating OT as a neurobiological hallmark of prosocial approach behavior in gregarious animals and trust among humans – a necessary prerequisite to social affiliation. Finally, recent real-time functional magnetic resonance imaging research found enhanced neurotransmission in dopamine-rich brain regions, in the right ventral tegmental area (VTA) and right caudate nucleus, when participants view photographs of their life-long deeply-loving partners but not when they saw other familiar people from their life [7]. Furthermore, even at a relatively early stage of a pair-bond, activation in the left VTA positively correlated with facial attractiveness while activation in the right anteromedial caudate nucleus was associated with the intensity of romantic passion in individuals recently falling “in love” [8]. These persistent and enduring brain activation patterns suggest that DA-ergic reward circuits may encode the arousal component of the intense “high” when people are motivated to develop and maintain a life-long pair-bond, rather than representing an ephemeral emotional state of being, fleeting rationale decision, or general sex drive, per se. Taken together, these findings in humans suggest the possibility that similar neurochemicals and psychological facets of attachment and romance that resemble elements of pair-bonding behavior in voles may extend to people as well.
Partner Preference Formation
Evidence describing the neurobiology of human mating preferences is mixed, primarily due to the complex nature of attraction in people and the experimental limitations of cognitive neuroscience research. Therefore, investigating pair-bonding behavior in voles represents a valuable animal model system to reveal underlying neurochemical mechanisms programming social decision-making. To date, neurobiological research examining voles has implicated the neuromodulators AVP, OT, DA, and CRH in the regulation of partner preference formation.
Prior research has demonstrated that 18-24 hrs of mating, but not 6 hrs of cohabitation, reliably induces partner preference in both male and female prairie voles (Figure 1a&b; [3,9••]). Such mating also leads to changes in the activity of several neuromodulators, listed above, which have been implicated in social behavior in prairie voles. Because genetically-related vole species were originally shown to differ in their social life mating strategies, a comparative approach was initially used to investigate intra-species differences in social behavior and underlying neurobiology. Monogamous and promiscuous voles differ in the pattern of AVP-V1a receptor (V1aR) distribution in the brain, specifically in reward-related regions such as the ventral pallidum (VP; Figure 2), indicating species differences in response to centrally released AVP (Figure 2; [10,11]). Intracerebroventricular (icv) infusion of a V1aR antagonist inhibits mating-induced partner preference while infusions of AVP induce this behavior in both male and female prairie voles without mating [9••,12]. In male prairie voles, mating enhances AVP gene expression in the bed nucleus of the stria terminalis (BNST) yet decreases AVP-immunoreactive (ir) fiber density in its projection area – the lateral septum (LS), suggesting increased AVP release in this area [13,14]. Indeed, intra-LS administration of AVP induces partner preference whereas a V1aR antagonist inhibits mating-induced partner preference. In the VP, V1aR antagonism impairs mating-induced partner preference whereas over-expression of V1aR by adeno-associated viral vector-mediated gene transfer (AAV-V1aR), in sexually naïve males, facilitates partner preference. Furthermore, AAV-V1aR over-expression in the VP enhances partner preference in promiscuous male meadow voles [15••] and facilitates social recognition in mice [16•].
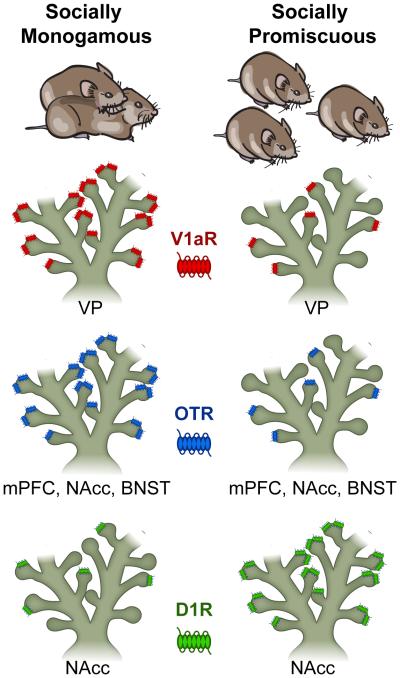
Figure 2. Microtine species differences in neurotransmitter receptor distribution and social behavior.
Prairie voles display a socially monogamous life strategy after mating and pair-bonding (Left Image) while Meadow and Montane voles’ exhibit socially promiscuous behavior (Right Image). Vasopressin V1a-type receptor (V1aR) expression in the ventral pallidum (VP) and oxytocin receptor (OTR) density in the medial prefrontal cortex (mPFC), nucleus accumbens (NAcc), and bed nucleus of the stria terminalis (BNST) are both higher in sexually naïve prairie voles (Left) while promiscuous voles have fewer endogenous V1a/OT receptors available in these brain regions (Right). Species differences in neuropeptide receptor density have been shown to explain why these systems may be involved in the evolution of divergent mating strategies in across rodent species. Conversely, dopamine D1 receptor (D1R) expression is higher in the NAcc of non-monogamous voles (Right) and lower in prairie voles (Left). Species differences in dopamine neurotransmitter receptor density have been shown to explain why prairie voles display mating-induced selective aggression while meadow voles exhibit general levels of aggressive behavior toward conspecific animals. (Illustration by C. Badland). Adapted from [19,21•,22,26,28••,29•,62-64].
Similarly, species differences are found in the patterns of OT receptor (OTR) distribution in the vole brain – in regions critical in the regulation of social behavior and motivation like the medial prefrontal cortex (mPFC), nucleus accumbens (NAcc), and BNST (Figure 2; [17•]). ICV OT injection induces partner preference in both male and female prairie voles, and this effect is blocked by infusion of an OTR antagonist [12]. In female prairie voles, OT release is increased in the NAcc during mating [18•], pharmacological blockade of OTRs in the NAcc (or the prelimbic cortex (PLC)) prevents mating-induced partner preference, and intra-NAcc OT infusion induces this behavior without mating [19,20]. Furthermore, AAV-OTR over-expression in the NAcc facilitates partner preference formation in sexually naïve female prairie voles, but not in female meadow voles (Figure 3; [21•]). Finally, blocking OTRs in the LS of male prairie voles also prevents mating-induced partner preference [22].
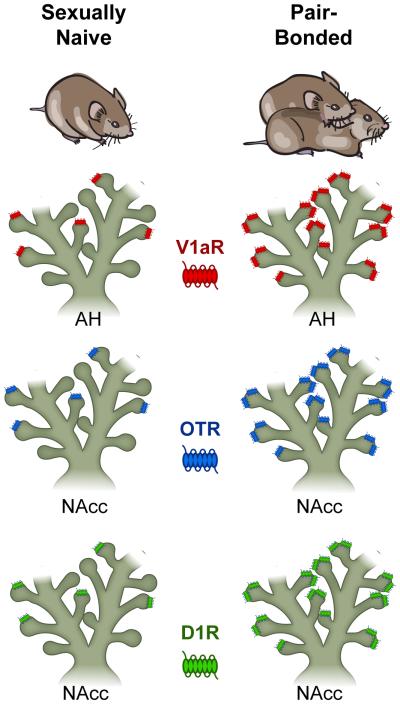
Figure 3. Pair-bonding-induced neuromodulator receptor plasticity and sociality in the prairie vole.
Sexually naïve male prairie voles (Left) and pair-bonded male prairie voles (Right) exhibit experience-dependent changes in neurotransmitter receptor density in select brain regions including the anterior hypothalamus (AH) and nucleus accumbens (NAcc). Specifically, successful mating and pair-bonding site-specifically enhances the density of vasopressin V1a-type receptor (V1aR) expression in the AH and oxytocin receptor (OTR) and dopamine D1-type receptor (D1R) density in the NAcc. These mating-induced neuroplastic changes in receptor densities explain the behavioral switch from general patterns of social affiliation and aggression to robust social memory and selective aggression in pair-bonded male prairie voles. (Illustration by C. Badland). Adapted from [28••,32•,34••,64].
The mesocorticolimbic DA circuit underlies emotional valence to control intrinsically-motivated behavioral drives like hunger and mating [23,24]. Not surprisingly, this system has also been identified as being important in the regulation of social memory encoding partner preference and attachment. The first series of experiments that examined the central role of DA and partner preference formation employed a behavioral pharmacological approach. Peripheral (intraperitoneal (ip)) treatment of a non-selective DA receptor (DAR) antagonist was sufficient to block mating-induced partner preference whereas treatment of a DAR agonist facilitated partner preference without mating [25]. Similar effects were found by treating prairie voles with a D2R-, but not D1R-, specific antagonist/agonist, indicating that D2R activation is critical for partner preference formation [25]. In the NAcc of both male and female prairie voles, mating was found to induce DA release [26,27] and administration of a non-selective DAR or D2R-specific antagonist impaired mating-induced partner preference whereas treatment of a non-selective DAR or D2R-specific agonist facilitated partner preference formation in the absence of mating experience [27,28••]. Conversely, intra-NAcc D1R activation prevented partner preference induced by mating or by D2R activation [28••]. This receptor-specific DA-ergic regulation of partner preference was found to be due to the opposing effects of D1R/D2R, via the activity of the intra-cellular cyclic adenosine monophosphate (cAMP) signaling cascade and its conjugated G-proteins, site-specifically, within the rostral NAcc shell [29•].
In addition to AVP, OT, and DA, the stress neuropeptide, CRH, also plays a significant role in the regulation of pair-bonding behavior. Male prairie voles treated with exogenous CRH display partner preference, without mating, which can be blocked with co-administration of a CRH receptor antagonist. Site-specific micro-infusion of CRH in the NAcc facilitates, while CRH receptor antagonist treatment inhibits, partner preference formation in male prairie voles. Pair-bonding with a female also significantly increases CRH mRNA in the BNST of males.
CRH is secreted from the paraventricular nucleus of the anterior hypothalamus (PVN) and binds to CRH receptors in the anterior pituitary which synthesizes adrenocorticotrophic hormone (ACTH). ACTH is then released into the bloodstream and acts on receptors expressed on the adrenal cortex which produces glucocorticoids, like corticosterone (CORT), that then bind to glucocorticoid receptors (GRs) in the brain during stress. The prairie vole is glucocorticoid resistant and has approximately 5-to 10-times greater basal plasma CORT, 3-times higher basal levels of ACTH, and 10-times lower affinity for the GR-type-1 receptor relative to non-monogamous rodents. In female prairie voles, cohabitation with a male significantly reduces serum CORT levels. Furthermore, adrenalectomy or GR antagonist treatment in females is sufficient to facilitate while CORT injections, or exposure to swim stress, prevents partner preference formation. In short, these results suggest that decreases in hypothalamic pituitary adrenal (HPA) axis activity facilitate the formation of partner preference in female prairie voles. In males, the story is completely the opposite. Adrenalectomy inhibits partner preference formation and this effect can be reversed with CORT replacement. Furthermore, males experiencing the loss of a bonded partner exhibit significantly higher levels of circulating CORT and adrenal gland weight, implicating the HPA axis in partner separation.
Selective Aggression
Among the neurochemicals implicated in maladaptive forms of escalated aggression and violence in humans and laboratory animals [30], AVP and DA have been shown to be important in the regulation of adaptive forms of agonistic behavior such as selective aggression in prairie voles. Following mating and extended cohabitation, males that are pair-bonded with a female exhibit aggression toward conspecific male and female strangers but not toward their familiar female partner (selective aggression; Figure 1c&d), and this behavior is important in maintaining established pair-bonds [31]. Selective aggression is associated with increased neuronal activation, measured by Fos-ir labeling, in several brain areas including the anterior hypothalamus (AH), LS, medial preoptic area (MPOA), BNST, and posterior dorsal medial amygdala (MeAPD; [32•,33]). In the AH, selective aggression is accompanied by activation of AVP expressing neurons and increased AVP release [32•,34••]. Administration of the V1aR antagonist, icv or site-specifically into the AH, diminishes selective aggression [9••,34••]. Conversely, administration of AVP enhances selective aggression in sexually naïve males, and this AVP-facilitated aggression can be blocked by concurrent administration of a V1aR antagonist [9••,34••]. Furthermore, pair-bonded males exhibit an increased density in V1aR binding in the AH compared to their sexually naïve counterparts (Figure 3), and over-expression of V1aR in the AH, by AAV-V1aR, facilitates selective aggression in sexually naïve males [34••]. These data demonstrate that AH-AVP is involved in the regulation of selective aggression in male prairie voles. AH-AVP has also been shown to regulate aggression in Syrian hamsters [35]. In human clinical studies, CSF levels of AVP are associated with a lifetime history of physical violence and assault in individuals with borderline personality disorder [4] and may control the perception of social cues conveying anger in research participants [36].
The frequency and intensity of physical aggression is typically observed more in males than in females across many species. Because of these sex differences, previous research examined the potential role of steroid hormones, like androgens, in the development of aggressive behavior. However, castration in male prairie voles and male rats has no effect on aggression. Therefore, circulating testosterone cannot be the sole contributor of aggressive behavior. For example, AVP infused directly into the MeAPD facilitates territorial aggression in castrated male rats. Because the act of aggression relies heavily on motivation and emotional valence, these affective states are encoded via central DA.
The mesocorticolimbic DA system has been implicated in prairie vole aggression [28••]. Two weeks of pair-bonding lead to enhanced expression of D1Rs, but not D2Rs, in the rostral NAcc shell in the male prairie vole brain (Figure 3). Furthermore, male meadow voles exhibit significantly more D1-like DA receptors in the NAcc than do male prairie voles (Figure 2), providing evidence to potentially explain why non-monogamous animals display general levels of aggression while socially monogamous species exhibit “jealousy”-like behavior including patterns of mating-induced selective aggression directed toward other conspecifics except toward their familiar partner (Figure 1c&d). Pharmacological blockade of D1Rs in the NAcc reduces selective aggression in pair-bonded males. In parallel drug experiments, repeated treatment of the commonly abused psychostimulant, amphetamine (d-AMPH), enhanced aggression toward conspecific females as well as toward a female partner which prevented partner preference formation [34••,37••]. Pharmacologically, these drug-addled pair-bonding deficits can be reversed via micro-infusion of OT in the mPFC [38•]. Finally, these alterations in social behavior overlap with an increase in NAcc-D1R and AH-V1aR, indicating that drugs of abuse can hijack neuroplasticity evolved to retain social fidelity [34••,37••].
Bi-parental Care
Although the neurobiology of maternal behavior has been extensively studied, we know virtually nothing about the neurobiology of paternal behavior, mainly due to the lack of appropriate animal models that display bi-parental care. Thus, prairie voles provide a unique opportunity to study the neurochemical regulation of paternal behavior. Both female as well as male prairie voles spend equal amounts of time in their natal nest providing parental care for their offspring (Figure 1e&f). Pup exposure induces an increase in Fos-ir in the accessory olfactory bulb (AOB), MPOA, BNST, MeAPD, and LS in male prairie, but not meadow, voles, suggesting increased, regional neuronal activation associated with the display of paternal behavior in prairie voles [39,40]. When this socioemotional circuit is impaired via bulbectomy or MeAPD lesioning, paternal behavior is dramatically decreased in male prairie voles [41,42]. After pairing with a female for two weeks or becoming fathers, male prairie voles display higher levels of paternal behavior associated with altered AVP activity in the brain including increased AVP mRNA expression in the BNST and PVN [13,14] and decreased AVP-ir fiber density in the LS [43,44]. Infusion of AVP or OT in the brain (icv) enhances paternal behavior in sexually naïve male prairie voles whereas V1aR or OTR blockade decreases pup retrieval and huddling and increases pup attacks [45]. The LS has also been identified as a target brain area in which infusions of AVP enhance and a V1aR antagonist impairs paternal behavior in male prairie voles [46]. In other laboratory rodents, AVP in the LV increases maternal behavior in female rats [47] and in new father marmosets, whom also display paternal behavior, the density of V1aRs and dendritic spines in the mPFC is significantly increased, compared to non-fathers [48].
Because good parenting represents a critical social behavior necessary for healthy development of offspring and species survival, it’s not surprising to also learn that central DA plays a critical role in the regulation of parental behavior. Like its role in maternal behavior [49], central DA has also been implicated in paternal behavior. In male prairie voles, neurons that express tyrosine hydroxylase (TH; rate limiting enzyme for DA biosynthesis) in the BNST and MeAPD are activated by pup interaction [50]. Peripheral administration (ip) of a DAR antagonist reduces pup licking and contact yet increases pup huddling without affecting locomotor activity in both male and female prairie voles [51•]. DA is released in the NAcc of female rats exposed to pup stimuli [52] and released DA regulates maternal behavior in a DAR-specific manner [53].
The HPA axis has also been described in parental behavior of voles. For example, swim stress experience significantly enhances time spent huddling over and licking and grooming, pups but isn’t observed in unstressed male father controls. Importantly, these effects on paternal behavior are not observed in female prairie voles which suggest that, like the sexually dimorphic effects of CRH on partner preference formation, CRH may exert sex-specific effects on parental behavior as well. Finally, icv micro-infusion of urocortin-II, a closely related member of the CRH neuropeptide family, significantly increased passive parental behavior in both male and female prairie voles, without affecting locomotor or anxiety-like behaviors.
Conclusions and future directions
In humans, AVP, OT, DA, and CRH underlie many social behaviors including bonding, aggression, and parental care. These systems work in concert with one another to control levels of sociality. Mating in the socially monogamous prairie vole induces long-lasting neuroplasticity in circuits that regulate an enduring suite of pair-bonding, mate guarding, and parental behavior. In the prairie vole, the same neuromodulators interact to produce these robust behavioral patterns after copulation. Emerging research using the prairie vole has begun to investigate the neurobiology of pair-bond functions such as the role of OT on partner’s stress-buffering [54-56•] and consolation [57••]. Furthermore, exciting molecular work demonstrates epigenetic regulation of AVP and OT neuropeptidergic systems underlying mating-induced pair-bonding [58••]. Novel semi-naturalistic field studies in prairie voles has found that DNA variation in the V1aR gene includes polymorphisms that predict the epigenetic status and neuronal expression of V1aR in a spatial memory circuit and this genomic diversity in V1aR is favored by selection [59••]. Finally, our most recent data reveal neurochemical interactions between AVP, serotonin (5-HT), and CRH, in a neuronal microcircuit, encoding a male prairie vole’s decision to affiliate or fight his female partner or a stranger female [60••]. Together with previous studies, the prairie vole field is ripe for incorporating contemporary molecular genetic tools like genomic tract-tracing, opto/chemo-genetic approaches, and gene editing via CRISPR technology. By adding these techniques to the vole toolbox, the field will be able to molecularly target and genetically manipulate neurotransmitter systems underlying the neuroplasticity accompanied by sexual experience and the resulting innate social drives that ensue.
Highlights.
Mating induces partner preference, aggression, and bi-parental care in prairie voles.
Species differences in mating strategies are explained by neurogenetic variation.
Vasopressin, oxytocin, dopamine, and stress peptide signaling facilitate attachment.
Nucleus accumbens dopamine programs formation and maintenance of pair-bonds.
Anterior hypothalamic vasopressin regulates selective aggression.
ACKNOWLEGEMENTS
The authors would like to thank Dirson João Stein and the editor’s for providing very helpful feedback on our review. The work referenced in this manuscript was supported by National Institutes of Health grants F31-MH79600 & F32-GM096591 to KLG, MHR01-58616, DAR01-19627, & DAK02-23048 to ZXW, and NIH Program Training Grant T32 NS-07437.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
The authors declare no biomedical, financial, or potential conflicts of interest.
COMPETING FINANCIAL INTERESTS
The authors declare no biomedical, financial, or potential conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 2.Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- 4.Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- 5.Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 6••.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. This was the first series of laboratory experiments to demonstrate that intranasal administration of oxytocin enhanced trust among humans during social interactions, the results of which concur with animal research demonstrating a critical role of oxytocin as a biological endophenotype encoding prosocial approach behavior. [DOI] [PubMed] [Google Scholar]
- 7.Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. J Comp Neurol. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- 8.Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- 9••.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. This seminal report identified central vasopressin as being necessary and sufficient to regulate affiliation and aggression in pair bonded male prairie voles. [DOI] [PubMed] [Google Scholar]
- 10.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Young LJ, Liu Y, Insel TR. Species differences in vasopressin receptor binding are evident early in development: comparative anatomic studies in prairie and montane voles. J Comp Neurol. 1997;378:535–546. [PubMed] [Google Scholar]
- 12.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZX, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J Neuroendocrinol. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 15••.Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. AAV-V1aR over-expression in the ventral pallidum of promiscuous male meadow voles enhanced partner preference formation after mating. [DOI] [PubMed] [Google Scholar]
- 16••.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. AAV-V1aR expression in the lateral septum (LS) of V1aR knockout mice (V1aRKO) rescues social recognition, while AAV-V1aR over-expression in the LS of wild type mice ehances sociality. [DOI] [PubMed] [Google Scholar]
- 17•.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. This study was the first to report species differences in the distribution of oxytocin receptor (OTR) binding using receptor autoradiography. Prairie voles exhibit higher levels of OTR expression in the prelimbic cortex, bed nucleus of the stria terminalis, and nucleus accumbens compared to nonmonogamous montane voles. These species differences in OTR distribution were also neurotransmitter specific, as no differences were observed in the distribution of benzodiazepine or mu opioid receptor expression in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 20.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 21•.Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. AAV-OTR over-expression in the nucleus accumbens (NAcc) of sexually naive female prairie voles accelerated partner preference after cohabitation with a male. However, the same manipulation in nonmonogamous meadow voles did not facilitate partner preference formation. These data were the first to demonstrate a direct relationship between OTR density in the NAcc and variation in social attachment behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 23.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 24.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- 26.Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav Neurosci. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 28••.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. NAcc DA D1-like receptor (D1R) activation prevented pair bond formation, whereas D2-like receptor (D2R) activation facilitated it in male prairie voles. Pair-bonded male prairie voles exhibited a significant upregulation of NAcc D1R, but not D2R, and pharmacological blockade of D1R abolished mating-induced selective aggression. [DOI] [PubMed] [Google Scholar]
- 29•.Aragona BJ, Wang Z. Opposing regulation of pair bond formation by cAMP signaling within the nucleus accumbens shell. J Neurosci. 2007;27:13352–13356. doi: 10.1523/JNEUROSCI.3216-07.2007. Similar to the effects of activating D2-like receptors, decreasing cAMP signaling, by blocking cAMP binding sites on protein kinase A (PKA), facilitated partner preference formation in male prairie voles. Conversely, increasing cAMP signaling, by preventing the activation of inhibitory G-proteins by activating stimulatory G-proteins, or stimulating PKA, prevented the formation of mating-induced partner preferences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miczek KA, DeBold JF, Gobrogge KL, Newman E, de Almeida RM. The Role of Neurotransmitters in Violence and Aggression. In: Sturmey P, editor. Handbook of Violence and Aggression. Wiley; 2016. [Google Scholar]
- 31.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 32•.Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. This was the first study to identify activity in a specific brain nucleus, the anterior hypothalamus (AH), being site-specifically associated with the display of selective aggression in pair bonded male prairie voles. Furthermore, these Fos positive neurons , in aggressive males, co-expressed vasopressin (AVP) or tyrosine hydroylase, suggesting a relationship between AVP and dopamine signalling in the AH and heightened levels of aggression. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- 34••.Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. Vasopressin (AVP) signalling in the anterior hypothalamus (AH) is both necessary and sufficient to regulate selective aggression in pair-bonded male prairie voles. AH-AVP also mediates amphetamine-induced aggression toward stranger females and familiar female partners. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferris CF, Melloni RH, Jr., Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 37••.Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci U S A. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. Sexually naïve male prairie voles develop conditioned place preference to repreated amphetamine (AMPH) exposure, which impairs mating-induced partner preference. Pharmacological blockade of DA D1R in the NAcc rescues mating-induced partner preference in AMPH-treated males. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Young KA, Liu Y, Gobrogge KL, Wang H, Wang Z. Oxytocin reverses amphetamine-induced deficits in social bonding: evidence for an interaction with nucleus accumbens dopamine. J Neurosci. 2014;34:8499–8506. doi: 10.1523/JNEUROSCI.4275-13.2014. Repeated exposure to AMPH inhibits partner preference formation and decreases OTR-ir in the NAcc and DA D2R-ir in the NAcc of female prairie voles. Intra-mPFC infusion of oxytocin restores partner preferences altered by AMPH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZXI TR. Parental behavior in voles. Advanced Study of Behavior. 1996;25:361–384. [Google Scholar]
- 41.Kirkpatrick B, Williams JR, Slotnick BM, Carter CS. Olfactory bulbectomy decreases social behavior in male prairie voles (M. ochrogaster) Physiol Behav. 1994;55:885–889. doi: 10.1016/0031-9384(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 42.Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- 43.Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 44.Bamshad M, Novak MA, de Vries GJ. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physiol Behav. 1994;56:751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 45.Bales KL, Kim AJ, Lewis-Reese AD, Sue Carter C. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 48.Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- 49.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Northcutt KV, Wang Z, Lonstein JS. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol. 2007;500:103–115. doi: 10.1002/cne.21148. [DOI] [PubMed] [Google Scholar]
- 21•.Lonstein JS. Effects of dopamine receptor antagonism with haloperidol on nurturing behavior in the biparental prairie vole. Pharmacol Biochem Behav. 2002;74:11–19. doi: 10.1016/s0091-3057(02)00952-8. This study was the first to report the role of DA underlying biparental care in prairie voles. Peripheral administration (ip) of a DAR antagonist in both male and female prairie voles reduced pup licking and contact yet increased pup huddling without affecting locomotor activity. [DOI] [PubMed] [Google Scholar]
- 52.Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 53.Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm Behav. 2009;56:11–23. doi: 10.1016/j.yhbeh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Linton L, Altshuler R, Liu Y, Wang Z. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gobrogge K, Wang Z. Neuropeptidergic Regulation of Pair-bonding and Stress Buffering: Lessons from Voles. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. Restraint stress increases anxiety-like behavior and corticosterone (CORT) in female prairie voles recovering alone but not in females recovering with their male partner. This social buffering is accompanied by oxytocin (OT) release in the paraventricular nucleus (PVN) of the hypothalamus, while OT infusion directly into the PVN reduces restraint stress-induced anxiety-like behavior and CORT levels - which is blocked via PVN-OTR antagonism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. Prairie voles display enhanced partner-directed grooming toward familiar conspecifics (but not strangers) that have experienced an observed stressor, providing social buffering through consolation. Furthermore, prairie voles also mirror the fear response, anxiety-like behavior, and corticosterone increase of the stressed cagemate, suggesting a putative empathic response. Exposure to a stressed cagemate activates the anterior cingulate cortex, and OTR blockade in this brain region abolishes this partner-directed empathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Wang H, Duclot F, Liu Y, Wang Z, Kabbaj M. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat Neurosci. 2013;16:919–924. doi: 10.1038/nn.3420. Histone deacetylase inhibition facilitated partner preference formation in sexually naïve female prairie voles, which was associated with upregulation of V1aR and OTR in the NAcc via increases in histone acetylation at their respective promoters. Mating-induced partner preference triggers the same epigenetic regulation of V1aR and OTR gene promoters as the histone deacetylase inhibitor trichostatin A (TSA), demonstrating the first direct evidence for epigenetic regulation of pair-bonding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science. 2015;350:1371–1374. doi: 10.1126/science.aac5791. Results from semi-naturalistic field studies in prairie voles determined that DNA variation in the V1aR gene includes polymorphisms that predict the epigenetic status and neuronal expression of V1aR in a spatial memory circuit: hippocampus, laterodorsal thalamus, and retrosplenial cortex, and this genomic diversity in V1aR appears to be favored by selection. [DOI] [PubMed] [Google Scholar]
- 60••.Gobrogge K, Jia X, Liu Y, Wang Z. Neurochemical Mediation of Affiliation and Aggression Associated with Pair Bonding. Biological Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.02.013. This study was the first to report a novel neurochemical node in the male prairie vole brain programming the decision to affiliate or fight. CRH projections from the MeAPD to the AH, and CRH neurons in the AH synapsing in the LS, were activated during aggression but silent when males were affiliating. Conversely, 5-HT neurons projecting from the dorsal raphe (DR) to the AH were recruited when males were affiliating but quiet when males were fighting. Subsequent in-vivo behavioral pharmacology and real-time reverse microdialysis experiments, in the AH, coroborate the notion that a behavioral “switch” microcircuit exists in the pair bonded male prairie vole brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young KA, Gobrogge KL, Wang Z. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci Biobehav Rev. 2011;35:498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards S, Self DW. Monogamy: dopamine ties the knot. Nat Neurosci. 2006;9:7–8. doi: 10.1038/nn0106-7. [DOI] [PubMed] [Google Scholar]