Abstract
AIM: To compare the efficacy and safety of yttrium-90 radioembolization (Y90RE) and transarterial chemoembolization (TACE) in hepatocellular carcinoma patients.
METHODS: Bibliographic research was conducted on main scientific databases. When there was no statistically significant heterogeneity, pooled effects were calculated using a fixed-effects model by means of Mantel-Haenszel test, otherwise, a random-effects model was used with DerSimonian and Laird test. Summary estimates were expressed in terms of odds ratios (ORs) and 95%CI. The probability of publication bias was assessed using funnel plots and with Begg and Mazumdar’s test. Sensitivity analysis was finally conducted using the method of excluding extreme data.
RESULTS: A total of 10 studies were analyzed, of which 2 randomized controlled trials. Survival rate (SR) assessed at 1 year showed an absolute similarity between the two treatment groups (OR = 1.01, 95%CI: 0.78-1.31, P = 0.93). As long as time elapsed since the treatment, ORs for survival rate tended to significantly increase, thus meaning better long-term outcomes in patients who underwent Y90RE (2-year SR: OR = 1.43, 1.08-1.89, P = 0.01; 3-year SR: OR = 1.48, 1.03-2.13, P = 0.04). Meta-analysis of plotted hazard ratios (HRs) determined a non-significant overall estimate in favor of Y90RE (HR = 0.91, 0.80-1.04, P = 0.16). Y90RE showed a statistically significant benefit as compared to TACE in terms of higher progression-free survival rate assessed at 1 year (OR = 1.67; 95%CI: 1.10-2.55; P = 0.02). Pooled analyses do not revealed a statistically significant increase in OR for tumor objective responses after Y90RE with respect to TACE (OR = 1.22, 95%CI: 0.69-2.16, P = 0.50). A non-significant trend in favor of Y90RE was observed according to adverse event rate (OR = 0.70, 0.38-1.30, P = 0.26).
CONCLUSION: Our meta-analysis reveals that Y90RE and TACE show similar effects in terms of survival, response rate and safety profile, although tumor progression is delayed after radioembolization.
Keywords: Yttrium-90 radioembolization, Transarterial chemoembolization, Hepatocellular carcinoma, Survival, Prognosis, Recurrence
Core tip: A clear evidence in support of the superiority of yttrium-90 radioembolization (Y90RE) over chemoembolization (TACE) in hepatocellular carcinoma patients is still lacking. Results of our meta-analysis reveal that Y90RE and TACE show similar effects in terms of survival, response rate and safety profile, although tumor progression is delayed after radioembolization. Similar results were found as for objective response rate and safety profile. The sole statistical difference was with regard to 1-year progression-free survival, which resulted significantly in favor of Y90RE (OR = 1.67, P = 0.02).
INTRODUCTION
Hepatocellular carcinoma (HCC) is a global health problem, representing the third most common cause of cancer-related death and the leading cause of mortality among patients with cirrhosis[1,2]. Thanks to the recent improvements in surveillance protocols, diagnostic tools and therapeutic armamentaria, nowadays early HCC diagnosis is feasible in 30%-60% of cases in developed countries[3]. However, a substantial proportion of patients develop tumoral portal vein thrombosis (PVT) or a multifocal pattern as a result of HCC recurrence or progression, leading to an advanced disease stage not amenable to curative treatments.
Transarterial chemoembolization (TACE) is the most widely used primary treatment for unresectable HCC and the recommended first line-therapy for patients in intermediate stage[2,4,5]. The rationale for TACE is that intra-arterial infusion of a cytotoxic agent followed by embolization of the tumor-feeding blood vessels will result in a strong cytotoxic and ischemic effect[4,5].
A novel technique in the field of loco-regional treatments for HCC is called transarterial radioembolization with yttrium-90 (Y90RE), which induces tumor necrosis by means of injection of glass or resin microsphere loaded with yttrium-90[6,7]. Y90RE, which is in fact a novel form of liver-directed brachytherapy, has already demonstrated its efficacy in HCC patients leading to delayed time to progression (TTP) and prolonged overall survival (OS)[8,9]. Commonly adopted Y90-loaded microspheres present usually a small size (< 40 μm), therefore due to their microembolic effect they can be used even in patients with portal vein occlusion. Furthermore, because of absence of flow obstruction, in the case of Y90RE there is no hypoxia-initiated cascade and therefore typical post-TACE sequelae as post-embolization syndrome are less common[6,7].
Although several studies comparing the two loco-regional techniques have been recently published, whether there is a clear superiority of one treatment over the other is still debated.
In this study, we performed a meta-analysis to compare the efficacy of Y90RE and TACE in treating patients with unresectable HCC considering as main endpoints survival rate (SR), progression-free survival (PFS) and adverse events rate. We think that the comparison of these two procedures could help to better define the treatment strategy in intermediate/advanced HCC patients.
MATERIALS AND METHODS
Inclusion and exclusion criteria
This meta-analysis only included studies meeting the following criteria: (1) studies comparing Y90RE and TACE in HCC patients; (2) studies published in English; and (3) articles reporting at least one of the following data: TTP, survival and adverse events.
Search strategy
Figure 1 reports the search strategy followed in the meta-analysis.
Figure 1.
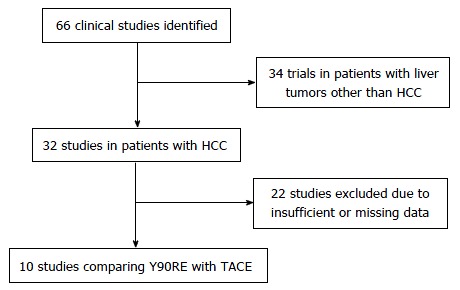
Flow chart summarizing study selection. HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; Y90RE: Yttrium-90 radioembolization.
Bibliographic research was conducted on PubMed, EMBASE, Cochrane Library and Embase including all studies fulfilling inclusion criteria published until January 2016. Keywords used included ‘‘transcatheter arterial chemoembolization”, “TACE”, “transcatheter arterial radioembolization”, “TARE”, “liver cancer”, “hepatocellular carcinoma” and “HCC”. Relevant reviews and meta-analyses of loco-regional treatments in unresectable HCC were examined for potential suitable studies. Authors of included studies were contacted to obtain full text or further information when needed.
Data extraction and management
Data extraction was conducted by two reviewers (Facciorusso A and Muscatiello N) using a standardized approach (PRISMA Statement)[10]. Data on publication details (year of publication, name of first author and country), study characteristics (patients’ age and sex, study design, sample size, Child-Pugh stage, interventions, follow-up duration), OS, TTP, and 1-year SR were gathered. Case reports and abstracts or studies with insufficient data were excluded. In case of repetitive publications from the same population, only most recent and complete articles were included.
The quality of the included studies was assessed by two authors independently (Facciorusso A and Muscatiello N) according to the currently accepted criteria described elsewhere[11,12].
Disagreements were resolved by discussion and following a third opinion (Serviddio G).
Statistical analysis
χ2 and I2 tests were used for across studies comparison of the percentage of variability attributable to heterogeneity beyond chance. P < 0.10 for χ2 test and I2 < 25% were interpreted as low-level heterogeneity.
When there was no statistically significant heterogeneity, pooled effects were calculated using a fixed-effects model by means of Mantel-Haenszel test; otherwise, a random-effects model was used with DerSimonian and Laird test. Summary estimates were expressed in terms of odds ratios (ORs) and 95%CI.
Probability of publication bias was assessed using funnel plots and with Begg and Mazumdar’s test. To explore eventual sources of heterogeneity, we compared summary results obtained from subsets of studies grouped according to their design or quality. Sensitivity analysis was finally conducted using the method of excluding extreme data (the maximum or the minimum).
All statistical analyses were conducted using RevMan version 5 from the Cochrane collaboration. For all calculations a two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
Selection of studies
After initial screening, 66 potentially relevant articles were identified; 34 were excluded because dealing with non-HCC patients and 22 due to missing or incomplete data. Clinical data of 1557 patients from 10 studies were finally pooled to compare Y90RE and TACE (Figure 1).
Characteristics of included articles
A total of 10 studies published from 2005 to 2015 were analyzed, which included 461 HCC patients treated with Y90RE and 1096 who underwent TACE[13-22] (Table 1).
Table 1.
Characteristics of the included studies
| Ref. | Arm | Sample size | Recruitment period | Study design | Region | CP (A/B/C) | BCLC (A/B/C/D) | Quality |
| Ahmad et al[13] | Y90RE | 24 | 1990-2003 | R | United States | NA | NA | M |
| TACE | 52 | |||||||
| Kooby et al[14] | Y90RE | 27 | 1996-2006 | R | United States | 13/14/0 | NA | H |
| TACE | 44 | 22/22/0 | ||||||
| Carr et al[15] | Y90RE | 99 | 1992-2005 | R | United States | NA | NA | M |
| TACE | 691 | |||||||
| Salem et al[16] | Y90RE | 123 | 1999-2008 | R | United States | 67/54/2 | 43/65/13/2 | H |
| TACE | 122 | 67/53/2 | 47/61/12/2 | |||||
| Lance et al[17] | Y90RE | 38 | 2008-2010 | R | United States | 31/7/0 | NA | H |
| TACE | 35 | 24/11/0 | ||||||
| Moreno-Luna et al[18] | Y90RE | 61 | 1998-2008 | R | United States | 53/8/0 | 12/35/14/0 | H |
| TACE | 55 | 44/11/0 | 23/13/19/0 | |||||
| Pitton et al[19] | Y90RE | 12 | 2010-2012 | RCT | Germany | 10/2/0 | 0/12/0/0 | H |
| TACE | 12 | 9/3/0 | 1/11/0/0 | |||||
| El Fouly et al[20] | Y90RE | 44 | 2009-2011 | R | Egipt/Germany | 37/7/0 | NA | M |
| TACE | 42 | 33/9/0 | ||||||
| Kolligs et al[21] | Y90RE | 13 | 2009-2012 | RCT | Germany/Spain | 9/3/1 | 5/5/3/0 | M |
| TACE | 15 | 9/4/2 | 4/8/3/0 | |||||
| Akinwande et al[22] | Y90RE | 20 | 2007-2013 | R | United States | 7/11/2 | 0/0/20/0 | M |
| TACE | 28 | 14/13/1 | 0/0/28/0 |
CP: Child-Pugh; BCLC: Barcelona Clinic Liver Cancer; Y90RE: Yttrium-90 radioembolization; TACE: Transarterial chemoembolization; R: Retrospective; RCT: Randomized controlled trial; NA: Not available; H: High; M: Moderate.
Among these articles, 8 were retrospective studies[13-18,20,22] and 2 were randomized controlled trials (RCTs)[19,21]. Overall, 5 studies (of which 1 RCT) were judged high quality[14,16-19] and 5 (of which 1 RCT) moderate quality[13,15,20-22] (Table 2). In all the studies, the two treatment cohorts were well-balanced in terms of either clinical parameters and tumoral stage (Table 2).
Table 2.
Risk of bias assessment and quality of included studies
| Selection | Comparability | Outcome | Overall quality | |||||
| Observational studies1 | ||||||||
| Ahmad et al[13] | ++ | + | ++ | 5 | ||||
| Kooby et al[14] | +++ | ++ | ++ | 7 | ||||
| Carr et al[15] | ++ | ++ | ++ | 6 | ||||
| Salem et al[16] | ++++ | ++ | +++ | 9 | ||||
| Lance et al[17] | +++ | ++ | ++ | 7 | ||||
| Moreno-Luna et al[18] | ++++ | ++ | +++ | 9 | ||||
| El Fouly et al[20] | ++ | + | +++ | 6 | ||||
| Akinwande et al[22] | ++ | + | ++ | 5 | ||||
| Randomized controlled trials2 | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Pitton et al[19] | L | L | L | U | L | L | L | H |
| Kolligs et al[21] | L | H | U | U | H | L | L | M |
Study quality assessment performed by means of Newcastle/Ottawa scale (each asterisk represents if the respective criterion within the subsection was satisfied);
Cochrane Collaboration’s tool for assessing the risk of bias across 7 domains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. L: Low; H: High; U: Unclear; M: Moderate.
Survival
Data on overall survival were available for 1481 patients enrolled in 9 studies[14-22], which estimated this outcome by means of Kaplan-Meier curves and compared the two groups using log-rank test. Table 3 describes summary estimates for SR at three consecutive time-points, specifically at 1, 2 and 3 years. SR at 1 year was reported in all the aforementioned nine studies and showed an absolute similarity between the two treatment groups (OR = 1.01, 95%CI: 0.78-1.31, P = 0.93). As long as time elapsed since the treatment, ORs for survival rate tended to significantly increase, thus meaning better long-term outcomes in patients who underwent Y90RE (2-year SR: OR = 1.43, 1.08-1.89, P = 0.01; 3-year SR: OR = 1.48, 1.03-2.13, P = 0.04). Notably, the number of studies reporting long-term outcomes tended to decrease with 7 studies assessing 2-year SR[15-20,22] and only 5 reporting 3-year SR[15,16,18-20]. No evidence of heterogeneity was found at any time points (Table 3).
Table 3.
Odds ratios and heterogeneity of 1-year, 2-year and 3-year survival rate
| Survival estimate | No. of studies | No. of patients | OR (95%CI) | P-value |
Heterogeneity |
|
| I2 | P | |||||
| 1-yr SR | 9 | 1481 | 1.01 (0.78-1.31) | 0.93 | 0% | 0.71 |
| 2-yr SR | 7 | 1382 | 1.43 (1.08-1.89) | 0.01 | 0% | 0.93 |
| 3-yr SR | 5 | 1261 | 1.48 (1.03-2.13) | 0.04 | 0% | 0.44 |
SR: Survival rate; OR: Odds ratio.
In order to obtain a more robust and reliable estimate of patient survival, we performed a meta-analysis of plotted hazard ratios (HRs) from 7 studies which provided data to calculate this parameter[14-20], obtaining as result a non-significant overall estimate in favor of Y90RE (HR = 0.91, 0.80-1.04, P = 0.16; Figure 2). There was only a low level of heterogeneity among studies [χ2 = 7.32, df = 6 (P = 0.29), I2 = 18%)] and no publication bias was detected by using funnel plot (Figure 3) and performing Begg and Mazumdar’s test (P = 0.51). Subgroup analysis retrieving separately results of the only RCT and observational studies did not alter the final findings of our meta-analysis (P = 0.77 and 0.16, respectively). Sensitivity analysis was also performed by restricting analysis to high-quality studies and by excluding each article once per time and it showed that the outcome effect was coherent (data not shown).
Figure 2.
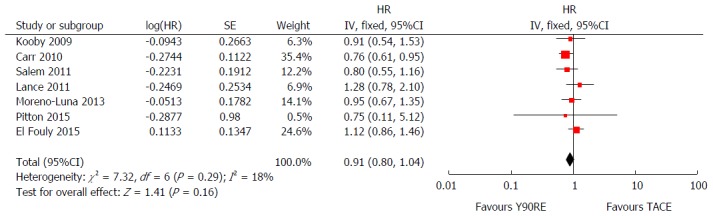
Forest plot of hazard ratios for overall survival after yttrium-90 radioembolization and transarterial chemoembolization. Overall estimate was non-significantly in favor of Y90RE (HR = 0.91, 0.80-1.04, P = 0.16). There was only a low level of heterogeneity among studies [χ2 = 7.32, df = 6 (P = 0.29), I2 = 18%)]. Y90RE: Yttrium-90 radioembolization; TACE: Transarterial chemoembolization; HR: Hazard ratio.
Figure 3.
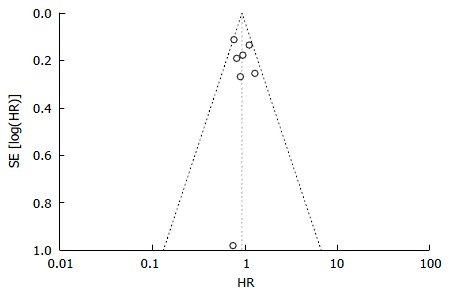
Funnel plot for detection of publication bias with regard to overall survival. No evidence of publication bias was detected. HR: Hazard ratio.
PFS
Data on tumor progression was available in 4 studies[16,19-21]. Y90RE showed a statistically significant benefit in terms of higher PFS rate assessed at 1 year (OR = 1.67; 95%CI: 1.10-2.55; P = 0.02) (Figure 4). There was no evidence of heterogeneity among individual studies (P = 0.66; I2 = 0%), hence a fixed model was used. Furthermore, there was no publication bias detected using funnel plot and Begg and Mazumdar’s
Figure 4.
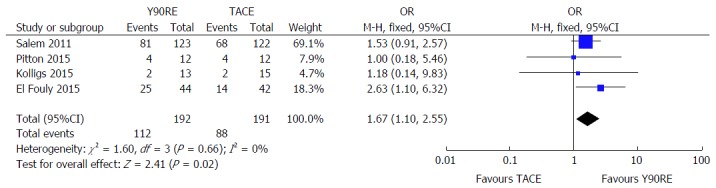
Forest plot for 1-year progression-free survival after yttrium-90 radioembolization and transarterial chemoembolization. Y90RE showed a statistically significant benefit in terms of higher progression-free survival rate assessed at 1 year (OR = 1.67, 1.10-2.55, P = 0.02). There was no evidence of heterogeneity among individual studies (P = 0.66; I2 = 0%). Y90RE: Yttrium-90 radioembolization; TACE: Transarterial chemoembolization; OR: Odds ratio.
test was not significant (P = 0.304 and P = 0.412, respectively). A low sensitivity to individual studies resulted after performing sensitivity analysis.
Objective response rate
There were eight studies containing information about objective response rate[13-16,18,20-22] enrolling 407 and 1041 patients treated with Y90RE and TACE, respectively. Pooled analyses did not reveal a statistically significant increase in OR for tumor objective responses after Y90RE with respect to TACE (OR = 1.22, 95%CI: 0.69-2.16, P = 0.50) (Figure 5). There was, however, evidence of heterogeneity across these studies (P = 0.004; I2 = 67%), therefore we performed a subgroup analysis in order to explore the cause of this heterogeneity, which was mainly due to some outlier studies[13,15,20,21]. In fact, tumor response assessment is dependent on a number of variables, such as radiologic criteria adopted, local expertise, imaging technique used (whether computed tomography-scan or magnetic resonance imaging) and time of response evaluation. Unfortunately, conducting a meta-regression analysis taking into account all these variables was not possible due to the small number of studies. No evidence of publication bias was detected.
Figure 5.
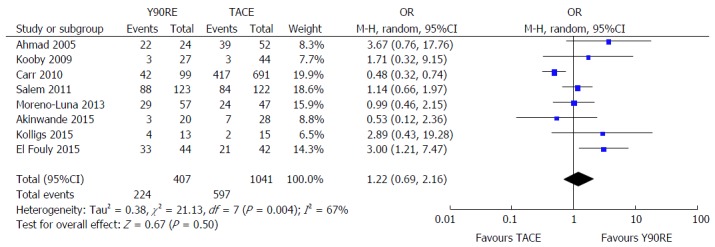
Forest plot for objective response rate after yttrium-90 radioembolization and transarterial chemoembolization. Pooled analyses do not revealed a statistically significant increase in odds ratio for tumor objective responses after Y90RE with respect to TACE (OR = 1.22, 95%CI: 0.69-2.16, P = 0.50). There was, however, evidence of heterogeneity across these studies (P = 0.004; I² = 67%), mainly due to some outlier studies. Y90RE: Yttrium-90 radioembolization; TACE: Transarterial chemoembolization; OR: Odds ratio.
Toxicity
Seven studies reported toxicity data of their treated patients[13,14,16-18,21,22]. A non-significant trend in favor of Y90RE was observed (OR = 0.70, 0.38-1.30, P = 0.26), but with high evidence of heterogeneity (I2 = 52%, P = 0.05) (Figure 6). Major responsible of heterogeneity was the study by Salem et al[16], since I2 dropped to 13% after exclusion of this article. No evidence of publication bias was detected.
Figure 6.
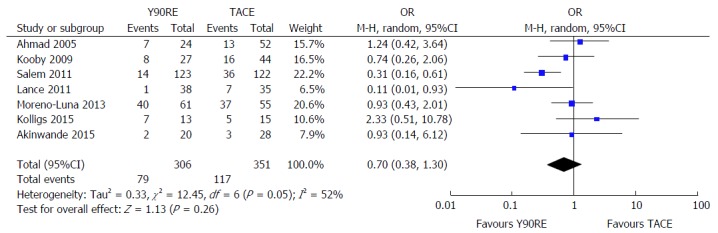
Forest plot for serious adverse event rate after yttrium-90 radioembolization and transarterial chemoembolization. A non-significant trend in favor of Y90RE was observed (OR = 0.70, 0.38-1.30, P = 0.26), but with a high evidence of heterogeneity (I2 = 52%; P = 0.05). Y90RE: Yttrium-90 radioembolization; TACE: Transarterial chemoembolization; OR: Odds ratio.
DISCUSSION
TACE is actually the recommended first-line therapy for patients with unresectable intermediate HCC[2-4]. In this setting where curative surgical or ablative treatments are not feasible, palliation with TACE has been found to improve survival as compared to best supportive care[23,24]. The pressing need for novel therapeutic regimens able to improve response rates and survival while reducing treatment-related complications led to the development of new drug-delivery systems, such as drug-eluting beads (DEBs)[4,25]. Although whether there is a clear superiority of DEB-TACE over conventional TACE using lipiodol is still matter of debate[12,26,27], increasing interest has been recently raised on smaller DEBs which seem able to induce wider necrosis of the target lesion since they achieve a more distal embolization, thus also obstructing collateral channels[28]. As a consequence, survival estimates after TACE has considerably improved in the last years, reaching more than 40 mo of median OS in two recent studies[29,30].
On the other hand, Y90 transarterial radioembolization is a form of brachytherapy in which intra-arterially injected 90Y-loaded microspheres serve as sources for internal radiation purposes, with no significant vessel occlusion thus rendering this treatment feasible even in patients with PVT, which is a well-known contraindication to TACE[6,7].
In particular radioembolization produces average disease control rates above 80% and is usually very well tolerated. Main complications do not result from the microembolic effect, even in patients with portal vein occlusion, but rather from excessive irradiation of non-target liver tissue[6,7,9].
Although several studies comparing the two therapies have been published so far, a clear evidence in support of the superiority of one technique over the other in terms of OS is still lacking. Therefore, given the similarity in survival outcomes between the two procedures, post-hoc analyses indicated that a randomized study with > 1000 patients would be required to establish equivalence of survival times between patients treated with Y90RE and TACE[16].
Aim of our meta-analysis was hence to compare these two trans-arterial treatments in terms of overall survival, progression-free survival, objective response rate and safety profile in unresectable HCC patients.
A total of 10 studies were identified and statistically analyzed, which included 461 HCC patients treated with Y90RE and 1096 treated with chemoembolization. Of note, all the included studies were from the West and in particular 70% from United States. Average quality was moderate-high.
Survival rate assessed at 1 year was similar between the two therapeutic groups (OR = 1.01, P = 0.93), but as long as time elapsed since the treatment ORs for survival rate tended to significantly increase, thus meaning better long-term outcomes in patients who underwent Y90RE (P = 0.01 and 0.04 at 2 and 3 years, respectively). Notably, the number of studies reporting long-term outcomes tended to decrease from 10 (all the included studies) which reported 1-year SR to only 5 reporting 3-year SR[15,16,18-20] (Table 3).
In order to obtain a more robust and reliable estimate of patient survival, we performed a meta-analysis of plotted HRs, obtaining as result a non-significant overall estimate in favor of Y90RE (HR = 0.91, 0.80-1.04, P = 0.16; Figure 2).
Therefore, our analysis seem to confirm the non-superiority of one treatment over the other as found in previous papers[16,18].
Unfortunately, subgroup analysis performed on the basis of baseline tumor stage or other clinical prognostic factors known to influence HCC patients’ survival, such as ferritin level[31] or drug therapy used[32], was not feasible due to the low number of available studies and the absence of outcomes stratification in most of them.
In the above cited study by Salem et al[16], time to progression was significantly longer after Y90RE (13.3 mo vs 8.4 mo, P = 0.023), but it did not translate directly into improved survival. Such a finding was confirmed in our meta-analysis where OR for 1-year PFS resulted significantly in favor of Y90RE (OR = 1.67, P = 0.02) (Figure 4).
No significant difference according to the other two analyzed outcomes (response rate and major adverse event rate) was found.
The apparent discrepancy between the significant benefit of Y90RE in terms of progression-free survival and the non-significant trends found with regard to the other outcomes are likely due to the complex multistep pathogenesis of HCC and the different course of underlying liver cirrhosis[33-35]. In this regard, a post-progression survival analysis would be interesting and represents in our opinion one of the most important targets of incoming clinical research in hepato-oncology[36,37].
There are some limitations to our study. First, we included both RCTs and observational studies, thus resulting in greater heterogeneity (as seen with regard to response rate analysis) and higher risk of selection and reporting bias. This, in addition to the aforementioned lack of data stratification and subgroup analysis, calls for a carefully interpretation of our findings. Moreover, technical details varied widely throughout the included studies either in TACE cohorts (for instance some studies adopted conventional TACE whereas others DEB-TACE) and in Y90RE arms (for instance as for dosimetry protocol)[38].
In conclusion, our meta-analysis reveals that Y90RE and TACE show similar effects in unresectable HCC patients in terms of OS, response rate and safety profile, although tumor progression is delayed after radioembolization. Further properly sized RCTs are warranted in order to confirm these results.
COMMENTS
Background
Transarterial chemoembolization (TACE) is the most widely used primary treatment for unresectable hepatocellular carcinoma (HCC) and the recommended first line-therapy for patients in intermediate stage. A novel technique in the field of loco-regional treatments for HCC is called transarterial radioembolization with yttrium-90 (Y90RE), which induces tumor necrosis by means of injection of glass or resin microsphere loaded with yttrium-90. Although several studies comparing the two loco-regional techniques have been recently published, whether there is a clear superiority of one treatment over the other is still debated.
Research frontiers
This meta-analysis reveals that Y90RE and TACE show similar effects in unresectable HCC patients in terms of overall survival, response rate and safety profile, although tumor progression is delayed after radioembolization. Further properly sized randomized controlled trials are warranted in order to confirm these results.
Innovations and breakthroughs
The authors’ findings stand for a similarity in treatment effects between Y90RE and TACE in HCC patients. Their meta-analysis constitutes the most up-to-date overview of studies comparing the two techniques.
Applications
The present report allows understanding the role of two transarterial treatments in HCC patients.
Terminology
TACE: Transarterial treatment whose rationale is that the intra-arterial infusion of a cytotoxic agent followed by embolization of the tumor-feeding blood vessels will result in a strong cytotoxic and ischemic effect; Y90RE: Novel form of liver-directed brachytherapy which induces tumor necrosis by means of injection of glass or resin microsphere loaded with yttrium-90.
Peer-review
This meta-analysis aims to compare the efficacy and safety of Y90RE and TACE in HCC. It is a nicely written manuscript. The analysis is well performed.
Footnotes
Conflict-of-interest statement: None of the authors have received fees for serving as a speaker or are consultant/advisory board member for any organizations; None of the authors have received research funding from any organizations; None of the authors are employees of any organizations; None of the authors own stocks and/or share in any organizations; None of the authors own patents.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 30, 2016
First decision: April 15, 2016
Article in press: June 2, 2016
P- Reviewer: Al-Gayyar MMH, Eskens FALM, Wong GLH S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: Evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009–2019. doi: 10.4254/wjh.v7.i16.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 6.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58:2188–2197. doi: 10.1002/hep.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [PMC free article] [PubMed] [Google Scholar]
- 11.Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344. doi: 10.3109/02656736.2015.1127434. [DOI] [PubMed] [Google Scholar]
- 12.Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. 2016;48:571–577. doi: 10.1016/j.dld.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad J, Rhee J, Carr BI. The effects of hepatic artery chemotherapy on viral hepatitis in patients with hepatocellular carcinoma. Dig Dis Sci. 2005;50:331–335. doi: 10.1007/s10620-005-1606-0. [DOI] [PubMed] [Google Scholar]
- 14.Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, Staley CA, Kim HS. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305–1314. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, Spain J, Srinivas S, Shrikanthan S, Aucejo FN, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1697–1705. doi: 10.1016/j.jvir.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714–723. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, Weinmann A, Schreckenberger M, Galle PR, Otto G, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015;38:352–360. doi: 10.1007/s00270-014-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, Mueller S, Barakat E, Lauenstein T, Bockisch A, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627–635. doi: 10.1111/liv.12637. [DOI] [PubMed] [Google Scholar]
- 21.Kolligs FT, Bilbao JI, Jakobs T, Iñarrairaegui M, Nagel JM, Rodriguez M, Haug A, D’Avola D, op den Winkel M, Martinez-Cuesta A, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35:1715–1721. doi: 10.1111/liv.12750. [DOI] [PubMed] [Google Scholar]
- 22.Akinwande O, Kim D, Edwards J, Brown R, Philips P, Scoggins C, Martin RC. Is radioembolization ((90)Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis. Surg Oncol. 2015;24:270–275. doi: 10.1016/j.suronc.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 25.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, Cascella T, Marchianò A, Camerini T, Bhoori S, et al. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:645–653. doi: 10.1111/jgh.13147. [DOI] [PubMed] [Google Scholar]
- 28.Spreafico C, Cascella T, Facciorusso A, Sposito C, Rodolfo L, Morosi C, Civelli EM, Vaiani M, Bhoori S, Pellegrinelli A, et al. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Intervent Radiol. 2015;38:129–134. doi: 10.1007/s00270-014-0907-0. [DOI] [PubMed] [Google Scholar]
- 29.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, Marinis A, Kelekis A, Alexopoulou E, Chatziioannou A, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119–1128. doi: 10.1007/s00270-012-0394-0. [DOI] [PubMed] [Google Scholar]
- 31.Facciorusso A, Del Prete V, Antonino M, Neve V, Crucinio N, Di Leo A, Carr BI, Barone M. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29:1905–1910. doi: 10.1111/jgh.12618. [DOI] [PubMed] [Google Scholar]
- 32.Facciorusso A, Del Prete V, Crucinio N, Muscatiello N, Carr BI, Di Leo A, Barone M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J Gastroenterol Hepatol. 2015;30:1643–1650. doi: 10.1111/jgh.12988. [DOI] [PubMed] [Google Scholar]
- 33.Facciorusso A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: recent findings and new perspectives. Curr Diabetes Rev. 2013;9:382–386. doi: 10.2174/15733998113099990068. [DOI] [PubMed] [Google Scholar]
- 34.Facciorusso A, Antonino M, Del Prete V, Neve V, Scavo MP, Barone M. Are hematopoietic stem cells involved in hepatocarcinogenesis? Hepatobiliary Surg Nutr. 2014;3:199–206. doi: 10.3978/j.issn.2304-3881.2014.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facciorusso A, Licinio R, Carr BI, Di Leo A, Barone M. MEK 1/2 inhibitors in the treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2015;9:993–1003. doi: 10.1586/17474124.2015.1040763. [DOI] [PubMed] [Google Scholar]
- 36.Reig M, Rimola J, Torres F, Darnell A, Rodriguez-Lope C, Forner A, Llarch N, Ríos J, Ayuso C, Bruix J. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013;58:2023–2031. doi: 10.1002/hep.26586. [DOI] [PubMed] [Google Scholar]
- 37.Facciorusso A, Del Prete V, Antonino M, Crucinio N, Neve V, Di Leo A, Carr BI, Barone M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig Liver Dis. 2014;46:1014–1019. doi: 10.1016/j.dld.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Chiesa C, Mira M, Maccauro M, Spreafico C, Romito R, Morosi C, Camerini T, Carrara M, Pellizzari S, Negri A, et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: development of an individualized treatment planning strategy based on dosimetry and radiobiology. Eur J Nucl Med Mol Imaging. 2015;42:1718–1738. doi: 10.1007/s00259-015-3068-8. [DOI] [PubMed] [Google Scholar]


