Abstract
We investigated the role of the calcitonin (Miacalcin) in the progression of osteoarthritis (OA) and in nociceptive behavior in an experimental rat model of OA and osteoporosis. OA was induced by anterior cruciate ligament transection (ACLT) of the right knee and by bilateral ovariectomy (OVX) in Wistar rats. Nociceptive behaviors (secondary mechanical allodynia and weight-bearing distribution of the hind paws) were analyzed prior to surgery and every week, beginning at 12 weeks after surgery, up to 20 weeks. At 20 weeks, histopathological studies were performed on the cartilage of the knee joints. Immunohistochemical analysis was performed to examine the effect of calcitonin on transforming growth factor (TGF)-β1 expression in articular cartilage chondrocytes. Rats subjected to ACLT + OVX surgery showed obvious OA changes in the joints. Animals subjected to ACLT + OVX and treated with calcitonin showed significantly less cartilage degeneration and improved nociceptive tests compared with animals subjected to ACLT + OVX surgeries alone. Moreover, calcitonin increased TGF-β1 expression in chondrocytes in ACLT + OVX-affected cartilage. Subcutaneous injection of calcitonin (1) attenuated the development of OA, (2) concomitantly reduced nociception, and (3) modulated chondrocyte metabolism, possibly by increasing cellular TGF-β1 expression.
Osteoarthritis (OA), is a complex disease characterized by bone remodeling, synovium inflammation, and cartilage loss. Although OA is classically defined as a progressive degenerative disease of the articular cartilage, inflammation plays a key role in its pathogenesis1. The pain associated with OA is primarily localized to the affected joint, but a number of OA patients also exhibit increased nociception in adjacent or even remote areas of the body2. Patients with ruptured anterior cruciate ligaments (ACL) develop post-traumatic OA of the knee3.
Although ovariectomy (OVX) is a classical technique to induce osteoporosis, it also successfully induces OA4. Studies using animal models have shown that OVX can successfully induce OA in the rat articular cartilage5. The pathological changes observed in OVX rats were similar in nature to those observed very early in human OA, such as mild erosion and loss of proteoglycans, as described previously6.
Calcitonin is a 32-amino acid hormone produced by thyroid gland parafollicular cells that, similar to 1,25-dihydroxyvitamin D, increases calcium and phosphate uptake to counteract the effects of parathyroid hormone7. Calcitonin is approved for the treatment of postmenopausal osteoporosis, malignancy-associated hypercalcemia, and Paget disease, all of which involve accelerated bone turnover8. Clinical trials involving patients with spinal fractures and knee OA suggest that calcitonin has anti-nociceptive effects, as evidenced by the reduced consumption of analgesic drugs9. Bagger et al. demonstrated significant inhibition of not only cartilage degradation but also bone resorption with an oral formulation of calcitonin in a 3-month intervention study of healthy elderly women10. In cultured isolated chondrocytes and in vitro explants, calcitonin may stimulate collagen type II and proteoglycan synthesis11, suggesting potential anabolic effects of the hormone on cartilage. Although several positive effects of calcitonin on chondrocytes have been demonstrated, the biological mechanism underlying its potential direct effects on nociception and OA development remain unclear.
Transforming growth factor-beta (TGF-β) is a multi-functional cytokine involved in crucial biological processes such as extracellular matrix synthesis, cell proliferation and differentiation, and tissue repair12. Intra-articular injection of TGF-β induces an increase in proteoglycan synthesis and articular cartilage content in naïve murine knee joints13. Loss of TGF-β signaling in cartilage induces chondrocyte hypertrophy, leading to cartilage degeneration14, and pharmacological activation of the TGF-β pathway has therefore been proposed to preserve articular cartilage integrity during OA15. The aim of the present study was to assess the effect of calcitonin on OA development in ACL transection (ACLT)- and OVX-induced OA rats and the anti-nociceptive effect of calcitonin in OA rats by measuring TGF-β1 expression in the articular cartilage by immunohistochemistry.
Methods
Animals
The use of rats conformed to the Guiding Principles in the Care and Use of Animals approved by the Council of the American Physiology Society and was approved by the National Sun Yat-Sen University Animal Care and Use Committee (approval number PTCH-2-300-005-2). Three-month-old male Wistar rats (body weight = 295–320 g) were maintained under climate-controlled conditions on a 12-h light-dark cycle at 22–24 °C with a relative humidity of 50–55%.
Surgical technique for induction of OA and Osteoporosis
OA was induced in rats by ACLT of the right knee; the left knee was not treated. Rats were anesthetized with 3% isoflurane in an oxygen/room air mixture (1:1). The surgical procedure was modified from the protocol described in previous studies16. Osteoporosis was induced in rats by bilateral OVX. The surgical procedure was modified from the protocol described in a previous study6. The animals were not immobilized after surgery and were allowed daily unrestricted cage activity. They were closely monitored for infections and other complications for 20 weeks after surgery.
Experimental design and calcitonin treatment
The animals were divided randomly into 5 groups. Group I (naïve group; n = 8) rats did not undergo surgery or treatment. Group II (calcitonin 15 U group; n = 6) animals underwent no surgery and received 15 U calcitonin (Miacalcin; calcitonin salmon synthetic, Novartis, Basel, Switzerland) via subcutaneous injection. In group III (ACLT + OVX group; n = 8), rats underwent both ACLT and OVX surgeries, and received 0.1 mL distilled water via subcutaneous injection. Group IV was referred to as the ACLT + OVX + 3 U calcitonin group (n = 6) and the rats underwent both ACLT and OVX surgeries and received 3 U calcitonin via subcutaneous injection two times per week for 9 consecutive weeks, beginning 12 weeks after surgery until 20 weeks. Group V was referred to as ACLT + OVX + 15 U calcitonin group (n = 7) and the rats received the same treatment as group IV except the calcitonin dose was 15 U. Measurements of secondary mechanical allodynia and hind paw weight distribution were performed as nociceptive behavioral tests prior to and 12 through 20 weeks after surgery. Changes in knee joint width were measured on the same schedule. At 20 weeks after surgery, gross morphology and histopathological examinations were performed on the cartilage of the femoral condyle and tibial plateau of the knee joint. Immunohistochemical analysis was performed to examine the effect of calcitonin on TGF-β1 expression in articular cartilage chondrocytes.
Assessment of nociception
Secondary mechanical allodynia
Allodynia was assessed with von Frey Filaments (North Coast Medical, Inc. Morgan Hill, CA, USA). The filament diameters corresponded to a logarithmic scale of the force exerted, and thus the perceived intensity was measured on linear and interval scales. The withdrawal threshold was determined by Chaplan’s up-down method involving the use of alternate large and small fibers to determine the 50% withdrawal threshold17. Each von Frey filament was applied to the plantar surface of the paw for 5 s. Briefly, when the rat lifted its paw in response to pressure, the filament size was recorded, and a weaker filament was then tested. The von Frey filament was applied to each paw for 5 trials at approximately 3-min intervals.
Weight-bearing distribution test
The effect of joint damage on weight distribution through the right (OA) and left (contralateral) knees was measured using a dual channel weight averager (Singa Technology Corporation, Taipei, Taiwan), which independently measures weight bearing by each hind paw. The change in hind paw weight distribution was used as an index of joint discomfort and was determined as described previously18,19. Briefly, rats were placed in an angled plexiglass chamber positioned so that each hind paw rested on a separate force plate. The force exerted by each hind limb (measured in grams) was averaged over a 5-s period. Each data point is the mean of three 5-s readings. The hind paw weight distribution is expressed as the difference in weight bearing between the contralateral and ipsilateral limbs.
Knee joint width, gross morphology, and histopathological findings of joints
Changes in knee joint width were measured bilaterally using calipers (AA847R, Aesculap, AG, Tuttlingen, Germany) prior to surgery and every week, beginning at 12 weeks after surgery, up to 20 weeks. The width of the knee joint was measured from the medial to the lateral aspect of the knee joint (at approximately the level of the medial and lateral joint lines). Immediately after sacrifice, each knee was examined for gross morphological changes, such as cartilage lesions, of the femoral condyle and tibial plateau as previously described20. Briefly, cartilage erosion was graded on a scale of 0–4, with 0 = normal surface appearance; 1 = minimal fibrillation or a slight yellowish discoloration of the surface; 2 = erosion extending to the superficial or middle layers; 3 = erosion extending to the deep layer; and 4 = erosion extending to the subchondral bone. The joints were sectioned 0.5 cm above and below the joint line, fixed in 10% neutral-buffered formalin for 3 days, and then decalcified for 2 weeks in buffered 12.5% ethylenediaminetetraacetic acid and formalin solution. The joints were then sectioned mid-sagittally, washed with tap water, placed in embedding cassettes for dehydration, clearing, and infiltration by an automatic tissue processor (Tissue-Tek, Sakura Finetek Japan Co., Ltd., Japan), embedded in paraffin blocks using a tissue embedding center (EC780-1; EC780-2, USA), and cut into 2-μm sections using a rotary microtome (HM340E, Microm, Biotechnical Services, Inc., San Diego, CA, USA) from the central weight-bearing surfaces of the femoral condyles and tibial plateaus of both knees. The sections were stained with hematoxylin and eosin and Safranin-O/fast green to assess the general morphology and matrix proteoglycan of the cartilage. Articular cartilage was graded under microscopic examination according to the Osteoarthritis Research Society International (OARSI) grading system21. This system comprises 6 histological grades and 4 histological stages. The total score (score = grade × stage) ranges from 1 point (normal articular cartilage) to 24 points (no repair).
Immunohistochemical staining for TGF-β1
Cartilage specimens were processed for immunohistochemical analysis as previously described22,23. Briefly, sections (2 μm) of the paraffin-embedded specimens were placed on slides, deparaffinized with xylene, and dehydrated in a graded series of ethanol solutions, and then endogenous peroxidase activity was quenched by incubation in 0.3% hydrogen peroxide for 30 min. The antigen was retrieved by enzymatic digestion with proteinase K (20 mM; Sigma, St. Louis, MO, USA) in phosphate-buffered saline (PBS) for 20 min. After 2 10-min washes with PBS, the sections were incubated with PBS containing 5% normal goat serum for 30 min to block non-specific binding. The slides were incubated with primary antibodies against anti-TGF-β1 (1:200 dilution, cat. ab92486; Abcam, Cambridge, UK; polyclonal rabbit antibody). The sections were then incubated for 90 min with biotinylated anti-rabbit IgG (Vector Labs, Burlingame, CA, USA) diluted 1:200 with 1% BSA in PBS. Thereafter, the sections were treated with the avidin-biotin complex technique using an ABC kit (Vectastain ABC kit; Vector Labs), followed by 3,3′-diaminobenzidine tetrahydrochloride (Vectastain ABC kit; Vector Laboratories) for 5 min. The different antigens present in each cartilage specimen were quantified by determining the number of positively stained chondrocytes in the entire thickness of cartilage, as previously described23. The cartilage was divided into 6 microscopic fields (3 each in the superficial and deep zones) (magnification, 400×), and the results were averaged. Prior to evaluating each OA specimen, an intact cartilage surface was identified for use as a marker to validate the results of morphometric analysis. The final results were expressed as the percentage of chondrocytes stained positive for each antigen (cell score), with a maximum score of 100% for each cartilage specimen. Each slide was reviewed by 2 independent readers who were blinded to the treatment groups.
In contrast, for double-immunofluorescent staining of TGF-β1 and articular chondrocyte markers (doublecortin)24, the cartilage sections were incubated with a mixture of anti-TGF-β1 (1:200 dilution, cat. ab64715; Abcam; monoclonal mouse antibody) and anti-doublecortin (ab18723; Abcam; polyclonal rabbit antibody) antibodies overnight at 4 °C, and then followed by a mixture of Alexa Fluor 488-conjugated chicken anti-mouse IgG antibody (1:400 dilution; cat. A-21200; Molecular Probes, Eugene, OR, USA; green fluorescence) and DyLight 549-conjugated donkey anti-rabbit IgG antibody (1:400 dilution, cat. 711-506-152; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA; red fluorescence) for 40 min at room temperature. We then acquired the double-immunostaining images using Leica TCS SP5 II confocal microscope (Leica Instruments).
Data and statistical analysis
All continuous data were presented as the mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to test for significant differences among the means of various scores of experimental groups. To compare the mean differences between the treatment and naïve groups, the Student-Newman-Keuls method post-hoc test was used. The trends for the changes in nociceptive behavior and knee joint width were tested using repeated-measures ANOVA. Differences resulting in P-values of less than 0.05 were considered significant.
Results
All rats in our study survived to sacrifice without infection.
Nociceptive behavior (secondary mechanical allodynia and weight-bearing distribution)
Figure 1 shows the effects of calcitonin on secondary mechanical allodynia. The paw withdrawal latency was significantly increased in the ACLT + OVX + 3 U calcitonin group compared to the ACLT + OVX group at 13, 14, 16, and 24 weeks after surgery (0.83 ± 0.15 vs. 0.45 ± 0.03 g; 0.71 ± 0.18 vs 0.31 ± 0.04 g; 0.83 ± 0.18 vs 0.43 ± 0.03 g and 1.33 ± 0.15 vs 0.77 ± 0.12 g respectively; P < 0.05; Fig. 1). This effect was the same as that observed in the ACLT + OVX + 15 U calcitonin group than in the ACLT + OVX group at 13–20 weeks after surgery (1.29 ± 0.12 vs 0.45 ± 0.03 g; 1.25 ± 0.16 vs 0.31 ± 0.04 g; 1.26 ± 0.12 vs 0.45 ± 0.03 g; 1.27 ± 0.06 vs 0.43 ± 0.03 g; 1.28 ± 0.15 vs 0.52 ± 0.14 g; 1.71 ± 0.14 vs 0.7 ± 0.09 g; 1.53 ± 0.28 vs 0.85 ± 0.17 g and 1.59 ± 0.19 vs 0.77 ± 0.12 g, respectively; P < 0.05; Fig. 1).
Figure 1. Time course of the anti-allodynic effect of calcitonin in ACLT-induced OA/OVX-induced osteoporosis nociception pain model.

Data are the mean ± SEM of the ipsilateral hind paw of each group. *P < 0.05 vs the ACLT + OVX group.
The weight-distribution differential between the OA and contralateral hind paws in the ACLT + OVX + 3 U calcitonin group was lower than that of the ACLT + OVX group at 14–20 weeks after surgery (22.62 ± 3.28 g vs 42.33 ± 4.64 g; 19.22 ± 3.60 g vs. 41.11 ± 3.01 g; 28.33 ± 3.18 g vs 4.3.59 ± 5.64 g; 22.77 ± 4.26 g vs 39.2 ± 3.51 g; 28.57 ± 4.00 g vs 44.61 ± 5.98 g; 25.03 ± 5.13 g vs 45.89 ± 3.27 g and 20.85 ± 4.57 g vs 41.09 ± 5.58 g respectively; P < 0.05; Fig. 2). The same trend was observed in the comparison between the ACLT + OVX + 15 U calcitonin and ACLT + OVX groups at 13–20 weeks after surgery (28.00 ± 3.75 g vs 43.46 ± 3.97 g; 12.91 ± 1.64 g vs 42.33 ± 4.64 g; 12.00 ± 2.27 g vs 41.11 ± 3.01 g; 19.00 ± 2.83 g vs 43.59 ± 5.64 g; 15.47 ± 2.24 g vs. 39.20 ± 3.51 g; 23.93 ± 5.47 g vs. 44.61 ± 5.98 g; 20.33 ± 3.81 g vs. 45.89 ± 3.27 g and 17.30 ± 4.95 g vs. 41.09 ± 5.58 g respectively; P < 0.05; Fig. 2). Moreover, no significant difference in nociception in the contralateral limbs was observed among the experimental groups (data not shown).
Figure 2. Time course of the effect of calcitonin on weight bearing of the contralateral (left) and ipsilateral (right) ACLT hind paws for 20 weeks after ACLT/OVX surgery.
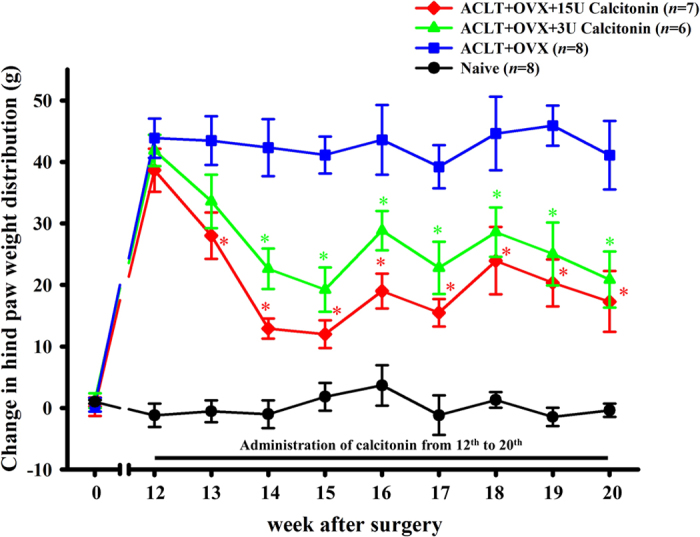
The baseline was set as the weight-bearing differential prior to surgery. Data are the mean ± SEM of each group. *P < 0.05 vs the ACLT + OVX group.
Change in knee joint width
There was a significant decrease in the width of the hind limb knee joint in the ACLT + OVX + 3 U calcitonin group compared with the ACLT + OVX group at 14–20 weeks after surgery (0.55 ± 0.1 vs 0.87 ± 0.05 mm; 0.63 ± 0.08 vs 0.81 ± 0.05 mm; 0.54 ± 0.05 vs 0.83 ± 0.08 mm; 0.55 ± 0.05 vs 0.78 ± 0.07 mm; 0.59 ± 0.08 vs 0.79 ± 0.08 mm; 0.59 ± 0.05 vs 0.79 ± 0.06 mm and 0.62 ± 0.07 vs 0.82 ± 0.07 mm, respectively; P < 0.05; Fig. 3). The same trend was observed for the comparison between the ACLT + OVX + 15 U calcitonin and the ACLT + OVX group at 13–20 weeks after surgery (0.56 ± 0.06 vs 0.88 ± 0.08 mm; 0.56 ± 0.05 vs 0.87 ± 0.05 mm; 0.47 ± 0.05 vs 0.81 ± 0.05 mm; 0.54 ± 0.03 vs 0.83 ± 0.08 mm; 0.57 ± 0.02 vs 0.78 ± 0.07 mm; 0.59 ± 0.03 vs 0.79 ± 0.08 mm; 0.56 ± 0.06 vs 0.79 ± 0.06 mm and 0.62 ± 0.08 vs 0.82 ± 0.07 mm respectively; P < 0.05; Fig. 3).
Figure 3. Time course of the effects of calcitonin on bilateral hind knee joint widths after ACLT/OVX.
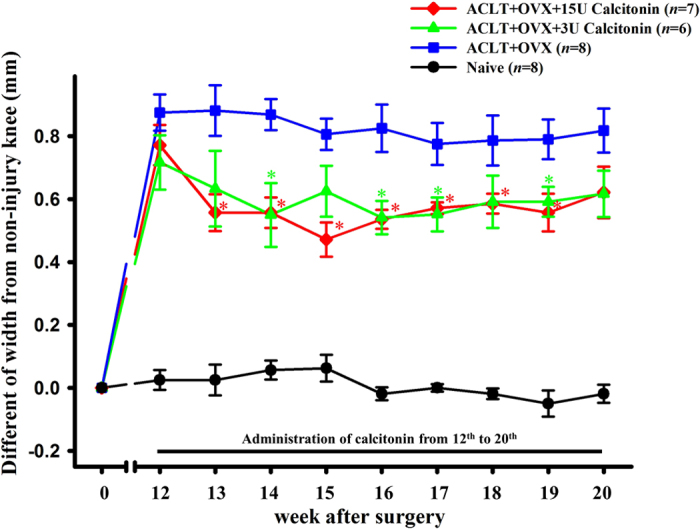
The data are the mean ± SEM of knee width, with baseline set as the widths prior to surgery. *P < 0.05 vs the ACLT + OVX group.
Gross morphology
Gross characteristics of cartilage degeneration, such as fibrillation, erosion and ulcer formation, and osteophyte formation, were observed in the femoral condyles and tibial plateau in the ACLT + OVX groups. Markedly less severe cartilage damage was observed in both groups treated with calcitonin. In the naïve and 15 U calcitonin groups, the cartilage was macroscopically normal, with a glistening, smooth surface, and no cartilage defects or osteophytes were observed. A significant difference (P < 0.05) in the gross morphology score was observed between the ACLT + OVX and the ACLT + OVX + 3 U calcitonin and ACLT + OVX + 15 U calcitonin groups (Table 1). The grades of cartilage damage in both calcitonin-treated groups were significantly lower than in the ACLT + OVX groups (Table 1). Naïve rats treated with 15 U calcitonin were normal in gross appearance (data not shown).
Table 1. Macroscopic and histological evaluation of articular cartilage of the femoral condyle and tibial plateau at 20 weeks after surgery.
| Score Group | Macroscopic score | Osteoarthritis score (OARSI score) |
|---|---|---|
| ACLT + OVX (n = 8) | 2.5 (1.4, 2.7)* | 15.2 (7.6, 18.2)* |
| ACLT + OVX + 3U calcitonin (n = 6) | 1.2 (0.4, 1.3)*† | 5.6 (3.7, 7.2)*† |
| ACLT + OVX + 15U calcitonin (n = 7) | 0.9 (0.5, 1.1)*† | 4.4 (2.8, 6.1)*† |
| Naïve (n = 8) | 0.5 (0.2, 0.6) | 1.8 (1.2, 2.8) |
| Calcitonin 15U (n = 6) | 0.6 (0.1, 0.4) | 2.2 (1.5, 3.2) |
Values are given as the mean ± SEM. The groups received surgeries and treatments as described in the Methods; OARSI score: Osteoarthritis Research Society International (OARSI) grading system. *P < 0.05 vs the naïve group; †P < 0.05 vs the ACLT + OVX groups.
Microscopic findings
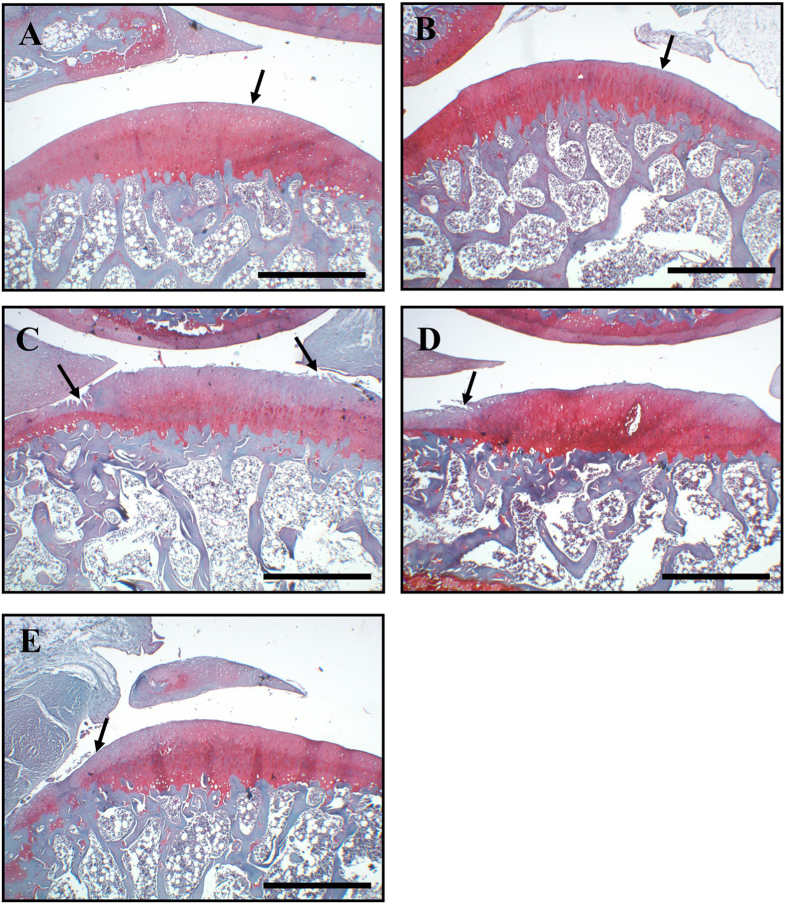
Histopathological examination with hematoxylin and eosin or Safranin-O/fast green staining revealed that the cartilage in the ACLT + OVX, ACLT + OVX + 3 U calcitonin, and ACLT + OVX + 15 U calcitonin groups exhibited various degrees of pathological changes (Fig. 4). In the naïve (Fig. 4A) and 15 U calcitonin (Fig. 4B) groups, the cartilage appeared histologically normal. A thin, glistening, smooth lamina filled with flattened chondrocytes was observed, and no loss of proteoglycan was present in the matrix based on Safranin-O/fasting green staining. Specimens from the ACLT + OVX (Fig. 4C) groups exhibited significantly higher incidence rates and greater severity of surface erosions than those from the naïve group. The ACLT + OVX + 3 U calcitonin (Fig. 4D) and ACLT + OVX + 15 U calcitonin (Fig. 4E) groups showed marked reductions in the severity of cartilage lesions, but fibrillation and fissures extending into the superficial layer of cartilage were observed. The OARSI scores of the calcitonin-treated groups were significantly lower than in the ACLT + OVX groups (Table 1). In addition, calcitonin alone at 15 U showed no detrimental effects on the cartilage of naïve rats (Fig. 4B and Table 1). None of the experimental groups in the present study showed changes in the gross or histological appearance of the hip or ankle joints (n = 3) at 20 weeks after surgery (data not shown).
Figure 4. Histopathological evaluation of articular cartilage of the knee joint.
In both the naïve (A) and calcitonin 15 U (B) groups, the surface of the superficial cartilaginous layer was smooth (arrow) and the cartilage matrix was prominently stained with H/E. Specimens from the ACLT + OVX group (C) showed decreased cartilage thickness, disappearance of the surface layer cells (arrow), a fissure extending into the transitional and radial zones, and chondrocyte hypocellularity in the transitional and radial zones. The specimens from the ACLT + OVX + 3 U calcitonin group (D) and ACLT + OVX + 15 U calcitonin groups (E) showed mild irregularity of the surface layer, fibrillation of and fissures within the superficial cartilaginous layer (arrow), and slight diffuse hypercellularity in the transitional and radial zones. Stain, H/E; original magnification, ×40. Scale bar = 200 μm.
Immunohistochemical detection of TGF-β1, confocal double-immunofluorescent staining of TGF-β1 and articular chondrocyte marker doublecortin in the articular cartilage
The immunolocalization of phosphorylated TGF-β1 proteins in the cartilage specimens is shown in Fig. 5, and fewer TGF-β1-positive cells were observed in the naïve group (Fig. 5A) than in the ACLT + OVX group (Fig. 5B). In the ACLT + OVX, ACLT + OVX + 3 U calcitonin (Fig. 5C), ACLT + OVX + 15 U calcitonin (Fig. 5D), and 15 U calcitonin alone groups, 20 weeks after surgery, the numbers of TGF-β1-immunoreactive cells increased in the superficial and transitional cartilaginous zones. Calcitonin treatment increased ACLT + OVX-rats upregulation of TGF-β1 expression in cartilage chondrocytes (Fig. 5C,D). Quantification of the number of TGF-β1-immunoreactive cells revealed a significant increase in ACLT + OVX-induced upregulation of TGF-β1-positive cells in the calcitonin-treated ACLT + OVX and calcitonin alone groups (Fig. 5F, P < 0.05). To further confirm which cells were affected by calcitonin after ACLT + OVX, we used confocal microscopy to examine the chondrocytes in cartilage tissues. Localization of TGF-β1 (red color; Fig. 5G) and the chondrocyte marker protein, doublecortin (green color; Fig. 5H), in the articular cartilage of the ACLT + OVX + 15 U calcitonin group was determined using the double-labeling immunofluorescent staining method. The merged images of Fig. 5I indicated that TGF-β1 was colocalized with doublecortin (yellow; white arrow). The major cell type expressing TGF-β1 was chondrocytes in the articular cartilage tissues.
Figure 5. Distribution of TGF-β1 protein immunoreactivity and confocal double-immunofluorescent staining of TGF-β1 and articular chondrocyte marker doublecortin in the cartilage.
Positive immunoreactivity of the TGF-β1 protein is indicated by the red-brown color (arrows). The panels show the distribution of anti-TGF-β1 immunoreactivity in the cartilage of the (A) naïve, (B) calcitonin 15 U, (C) ACLT + OVX, (D) ACLT + OVX + 3 U calcitonin, and (E) ACLT + OVX + 15 U calcitonin groups. All samples were stained with antibodies against TGF-β1 protein. (F) Quantitative analysis showed that calcitonin significantly enhanced the number of TGF-β1-positive cells in the cartilage of ACLT + OVX knees. Scale bar = 100 μm. *P < 0.05 compared with the naïve group, #P < 0.05 compared with the ACLT + OVX group. Representative confocal immunofluorescence microscopy images showing the localization of TGF-β1 (G; green in color) and doublecortin (H; red in color) of articular cartilage in the ACLT + OVX + 15 U calcitonin group. Colocalization is indicated by yellow (I) and arrows. The confocal results showed that TGF-β1 was primarily co-localized with articular chondrocytes. Scale bars are 50 μm for all images.
Discussion
This is the first report showing that subcutaneous administration of calcitonin attenuated the development of OA and concomitant nociceptive behavior (secondary mechanical allodynia and weight-bearing distribution) and decreased inflammation in the knee joint in an experimental OA model in rats. Interestingly, calcitonin enhanced TGF-β1 expression in articular cartilage chondrocytes in this experimental model and in naïve rats.
The putative effect of calcitonin on the progression of OA has been investigated clinically, focusing on the positive effects on cartilage degradation10. Calcitonin was identified more than 40 years ago25, possesses potent anti-resorptive effects, and has been shown to be mediated by directly binding calcitonin to the calcitonin receptor on osteoclasts26. Calcitonin may directly target chondrocytes in the articular cartilage because human cartilage cells express the calcitonin receptor27. Signaling of calcitonin through the calcitonin receptor and binding to G-coupled receptors to activate adenylylcyclase can increase cAMP levels28, inhibit matrix metalloproteinase (MMP) activity11, and have chondroprotective potential11,29. Calcitonin also acts on osteoblasts to increase their proliferation and alkaline phosphatase activity in vitro30, which is associated with the increased synthesis and deposition of bone matrix collagen30. In in vitro culture of chondrocytes from human OA hips and knees, calcitonin appears to decrease collagenolytic activity and markedly stimulate the attachment of chondrocytes to fibronectin31. Badurski et al. were the first to report that salmon calcitonin reduced cartilage erosion and protected against cartilage glycosaminoglycan loss in experimental OA32. Manicourt et al. reported that the urinary level of deoxypyridinoline (a specific marker of bone resorption) significantly diminished in surgical-induced OA models by 3 weeks after subcutaneous injection with calcitonin33. Cartilage degradation may be assessed by measurements of the C-terminal telopeptide of type II collagen (CTX-II). Use of CTX-II as a marker for the progression of cartilage lesions and its direct relationship with radiological grades and clinical scores for OA have been demonstrated previously. Circulating high levels of CTX-II have been shown to be associated with OA and progression of the disease34. In in vitro studies of the culture of articular cartilage explants, direct chondroprotective effects were observed for calcitonin, by inhibiting matrix metalloproteinase activity and causing a dose-dependent decrease in CTX-II levels35 (ref). Studies by Karsdal et al. also demonstrated that oral salmon calcitonin given twice daily resulted in reductions in the markers of bone resorption and cartilage degradation (urine CTX-II)36. Thus, it is believed that early identification of CTX-II levels would be a useful tool for achieving early diagnosis of OA development and taking preventive action.
Various clinical and animal studies have reported different results for the effect of calcitonin on OA. Owing to the limited literature on calcitonin dosage, there is still no optimal calcitonin concentration, volume, or injection schedule that can reliably yield desired results. The subcutaneous injection was started 12 weeks after surgery because quantitative computed tomography (QCT)37 and dual x-ray absorptionmetry (DXA)38 have previously detected a reduction in bone mineral density in both femoral condyles and tibial plateaus at 12 weeks after ACLT, with a marked reduction in the internal compartment of the OA joint. Further studies are required to establish the optimal duration of calcitonin administration for OA. It is not sufficient to analyze the effects of calcitonin on only cartilage histopathology to determine its effectiveness on OA pathology. It is important to monitor changes in the subchondral bone because they are related to the changes in the cartilage. Joint unloading and early and persistent synovitis might cause a rapid loss of the subchondral trabecular bone. A 30% reduction in load bearing by an osteoarthritic knee has been shown to persist for at least 45 months after ACLT39. Calcitonin has been shown to improve the bone mineral density and volume fraction, as assessed by μCT in an ACLT dog model40. In the present study, the calcitonin dose used in rats was the equivalent dosage used in humans. Several animal models of OA are available, and each model mimics different mechanisms through which OA initiates and develops. There is an apparent need for an OA model that directly mimics a human form of the disease while simultaneously providing a convenient methodological tool for preclinical investigations. OVX interferes with estrogen production and increases bone and cartilage turnover. OVX alone has been shown to produce signs of OA in rats4 and to increase OA pathology in traumatic OA models in the rabbit41 and rats42. The prevalence of OA is higher in post-menopausal women than in men43. In the present study, establishment of the OVX + ACLT model resulted in joint instability, which could effectively mimic the pathological changes detected in human OA. Thus, this model is considered to be suitable for evaluating therapeutic agents for OA. The main limitation of the animal model used in our study is that we did not detect the pathology of osteoporosis but could only analyze the OA pathogenesis. In the present study, both the macroscopic and OARSI scores were significantly lower in the ACLT + OVX surgery plus calcitonin-treated animals compared to in those that received surgery alone. Subcutaneous calcitonin administration significantly reduced the severity of cartilage degradation in the ACLT + OVX knee. Our results indicate that calcitonin treatment had cartilage-protective effects in rat knee joints. Although previous studies have indicated that the effects of calcitonin include bone and both cartilage anti-catabolic and cartilage anabolic, the curative effects should be evaluated in long-term clinical trials. Several reports have implicated the involvement of the serotonergic44 and catecholaminergic systems45 in calcitonin-induced anti-nociception. In humans, the similarities between calcitonin and morphine-induced analgesia, as well as reports of calcitonin-induced elevation of plasma β-endorphine levels, suggest that the endogenous opiate system mediates the analgesic action of calcitonin46. In behavioral studies, repeated systemic injections of calcitonin inhibited formalin-induced hyperalgesia and OVX-induced hyperalgesia in rats47, while single injections had no effects48. Studies using rat and mouse models have indicated that OA pain is associated with the release of a sensory neuropeptide, calcitonin gene-related peptide (CGRP)49, and an increased CGRP release50, by joint afferents”. Bullock et al.51 suggested that the role of CGRP receptor systems might be an important target to modulate pain during OA51. Castro et al. used a quantitative method to associate joint pain with weight bearing in the ACLT rat model52. Our previous study showed that intra-articular injection of magnesium sulfate attenuated secondary mechanical allodynia and thermal hyperalgesia, in a rat model with collagenase-induced OA23. In the present study, ACLT + OVX caused a painful response in the injured right hind-paw in rats, causing the animal to redistribute its body weight in favor of the non-injured left limb. This nociceptive behavior was also characterized by decreases in the secondary mechanical allodynia threshold and significant decreases in weight distribution in the ACLT + OVX knee. Changes in joint width were measured to determine tissue swelling and as an index of inflammation18. The addition of calcitonin treatment reduced the knee joint width compared with the groups that received ACLT + OVX surgery alone. Since salmon calcitonin is a polypeptide, it may have some side effects on human body, such as decreased food appetite, nausea, and vomiting, among others. It may induce allergic response53. In the present study, we found that calcitonin may decrease nociceptive pain. The above data indicate that calcitonin decreases nociception and inflammation. However, the exact mechanism of the anti-nociceptive and anti-inflammatory activities of calcitonin requires further investigation. We apologize for the lack of comparison of the results for the contralateral left (non-injury) leg, including OA score, histopathological evaluation, and TGF-β level. In the original study design, there were 5 experimental groups, including the naïve, calcitonin 15 U, ACLT + OVX, ACLT + OVX + 3U calcitonin, and ACLT + OVX + 15U calcitonin groups. The results of OA score and the histopathological and immunohistochemical data of TGF-β were only compared amongst groups for the right (injury) legs. In our previous study42, the OA scores and histopathological findings of the contralateral non-injury legs showed normal findings.
The role of TGF-β in the pathogenesis of OA has gained attention in recent years. TGF-β1 has also been shown to down-regulate cartilage-degrading enzymes and counteract the catabolic cytokine interleukin (IL)-1, both in vivo and in vitro54. The ability of TGF-β to counteract IL-1 is also lost with age55. TGF-β1 generally enhances the chondrogenic differentiation of bone-marrow derived mesenchymal stem cells56. Therefore, TGF-β1 appears to be a good candidate for cartilage repair. The main TGF-β signaling route is through specific membrane receptors (activin receptor-like kinase receptors, ALKs) and its intracellular effectors, the Smad proteins57. Interruption of the TGF-β/Smad3 signaling pathway leads to chondrocyte hypertrophic differentiation and cartilage degeneration14. TGF-β induced nerve growth factor mRNA in bovine and human chondrocytes in an ALK5/Smad2/3-dependent manner58, and add a new layer to the complex pathogenic role of TGF-β in OA pathogenesis. TGF-β1 is considered essential for cartilage integrity and is present at high levels in normal cartilage but shows reduced expression in cartilage in OA59. Li et al. suggested that vitamin D supplementation showed protective effects in OVX-induced OA, partly through the TGF-β1 pathway60. Asporin inhibits TGF-β-mediated expression of cartilage matrix genes such as collagen type II and aggrecan; it also inhibits accumulation of proteoglycans61. Serra et al.62 demonstrated that overexpressing a dominant negative TGF-β receptor resulted in terminal chondrocyte differentiation and subsequent OA62. Taken together, these results elucidate the importance of endogenous TGF-β in maintaining cartilage integrity in OA. In the present study, we observed increase TGF-β1 expression in the calcitonin-treated ACLT + OVX groups, and treatment with calcitonin alone may increase TGF-β1 expression in naïve cartilage chondrocytes. This is the first demonstration that calcitonin treatment increases TGF-β1 expression in both ACLT/OVX-affected and naïve rat chondrocytes. Whether the TGF-β1 pathway is a crucial and multifaceted signaling pathway in OA pathogenesis requires further analysis. We hypothesize that TGF-β1 is at least partially responsible for OA development and nociception. These data, as well as the analgesic effects of calcitonin on the model, support that calcitonin is a new option for the treatment of OA.
Additional Information
How to cite this article: Wen, Z.-H. et al. Calcitonin attenuates cartilage degeneration and nociception in an experimental rat model of osteoarthritis: role of TGF-β in chondrocytes. Sci. Rep. 6, 28862; doi: 10.1038/srep28862 (2016).
Acknowledgments
The study was supported by Pingtung Christian Hospital, Taiwan (PS100008) and Ministry of Science and Technology, Taiwan (101-2314-B-475-001-MY3).
Footnotes
Author Contributions Dr. Y.-H.J. had full access to all of the study data and assumes responsibility for its integrity and for the accuracy of the data analysis. Study design: C.-C.T., Y.-C.C. and Z.-H.W. Data acquisition: Y.-C.C. and W.-F.C. Data analysis and interpretation: Y.-C.C., C.-C.T., Y.-Y.L., S.-P.H. and S.-C.L. Manuscript preparation: S.-Y.H. and H.-P.L. Statistical analysis: C.-C.T. and Y.-C.C.
References
- Pelletier J. P., Martel-Pelletier J. & Abramson S. B. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 44, 1237–1247, doi: 10.1002/1529-0131(200106) (2001). [DOI] [PubMed] [Google Scholar]
- Bajaj P., Baja P., Graven-Nielsen T. & Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain 93, 107–114, doi: 10.1016/S0304-3959(01)00300-1 (2001). [DOI] [PubMed] [Google Scholar]
- Lohmander L. S. et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomized, double blind, placebo controlled multicentre trial. Ann Rheum Dis 55, 424–431 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høegh-Andersen P. et al. Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res Ther 6, R169–180, doi: 10.1186/ar1152 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard B. C., Ostergaard S., Christiansen C. & Karsdal M. A. The effect of oral calcitonin on cartilage turnover and surface erosion in the ovariectomized rat model. Arthritis Rheum 56, 2674–2678, doi: 10.1002/art.22797 (2007). [DOI] [PubMed] [Google Scholar]
- Walter H. et al. Immunohistochemical analysis of several proteolytic enzymes as parameters of cartilage degradation. Pathol Res Pract 194, 73–81, doi: 10.1016/S0344-0338(98)80073-3 (1998). [DOI] [PubMed] [Google Scholar]
- Zaidi M. et al. Forty years of calcitonin—where are we now? A tribute to the work of Iain Macintyre, FRS. Bone 30, 655–663, doi: 10.1016/S8756-3282(02)00688-9 (2002). [DOI] [PubMed] [Google Scholar]
- Tanko L. B. et al. Safety and efficacy of a novel salmon calcitonin (sCT) technology-based oral formulation in healthy postmenopausal women: acute and 3-month effects on biomarkers of bone turnover. J Bone Miner Res 19, 1531–8, doi: 10.1359/JBMR.040715 (2004). [DOI] [PubMed] [Google Scholar]
- Badurski J., Jeziernicka E., Naruszewicz K. & Racewicz A. Comparative analysis of three treatment regimens for treating gonarthritis with calcitonin, naproxen and flavonoids based on EULAR criteria and visual analogue scale (VAS). Pol Tyg Lek 50, 37–40 (1995). [PubMed] [Google Scholar]
- Bagger Y. Z. et al. Oral salmon calcitonin induced suppression of urinary collagen type II degradation in postmenopausal women: a new potential treatment of osteoarthritis. Bone 37, 425–430, doi: 10.1016/j.bone.2005.04.032 (2005) [DOI] [PubMed] [Google Scholar]
- Sondergaard B. C. et al. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthritis Cartilage 14, 759–768, doi: 10.1016/j.joca.2006.01.014 (2006). [DOI] [PubMed] [Google Scholar]
- Pujol J. P. et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connective Tissue Research 49, 293–7, doi: 10.1080/03008200802148355 (2008). [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J. & van der Berg W. B. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest 71, 279–290 (1994). [PubMed] [Google Scholar]
- Yang X. et al. TGF-b/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol 153, 35–46, http://www.jcb.org/cgi/content/full/153/1/35 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson E. N., van der Kraan P. M. & van den Berg W. B. TGF-beta and osteoarthritis. Osteoarthritis Cartilage 15, 597–604, doi: 10.1016/j.joca.2007.02.005 (2007). [DOI] [PubMed] [Google Scholar]
- Jean Y. H. et al. Intra-articular injection of cyclooxygenase-2 inhibitor parecoxib attenuates osteoarthritis progression in anterior cruciate ligament-transection knee in rats: role of excitatory amino acids. Osteoarthritis Cartilage 1, 638–645, doi: 10.1016/j.joca.2006.11.008 (2007). [DOI] [PubMed] [Google Scholar]
- Chaplan S. R. et al. Quantitative assessment of tactile allodynia in the rat paw. 1 J Neuro Meth 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
- Fernihough J. et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain 112, 83–93, doi: 10.1016/j.pain.2004.08.004 (2004). [DOI] [PubMed] [Google Scholar]
- Wen Z. H. et al. Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthritis Cartilage 18, 1192–1202, doi: 10.1016/j.joca.2010.05.012 (2010). [DOI] [PubMed] [Google Scholar]
- Fernandes J. C. et al. Effects of tenidap on canine experimental osteoarthritis. I. Morphologic and metalloprotease analysis. Arthritis Rheum 38, 1290–1303, doi: 10.1002/art.1780380918 (1995). [DOI] [PubMed] [Google Scholar]
- Pritzker K. P. et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 214, 13–29, doi: 10.1016/j.joca.2005.07.014 (2006). [DOI] [PubMed] [Google Scholar]
- Jean Y. H. et al. Increase in excitatory amino acid concentration and transporters expression in osteoarthritic knees of anterior cruciate ligament transected rabbits. Osteoarthritis Cartilage 16, 1442–1449, doi: 10.1016/j.joca.2008.04.008 (2008). [DOI] [PubMed] [Google Scholar]
- Lee C. H. et al. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: association with attenuation of N-methyl-D-aspartate (NMDA) receptor subunit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthritis Cartilage 17, 1485–93, doi: 10.1016/j.joca.2009.05.006 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Doublecortin is expressed in articular chondrocytes. 2 Biochem Biophys Res Commun 363, 694–700, doi: 10.1016/j.bbrc.2007.09.030 (2007). [DOI] [PubMed] [Google Scholar]
- Kumar M. A., Foster G. V. & MacIntyre I. Further evidence for calcitonin. A rapid-acting hormone which lowers plasmacalcium. Lancet 35, 480–2 (1963). [DOI] [PubMed] [Google Scholar]
- Chambers T. J. & Moore A. The sensitivity of isolated osteoclasts to morphological transformation by calcitonin. J Clin Endocrinol Metab 57, 819–824 (1983). [DOI] [PubMed] [Google Scholar]
- Segovia-Silvestre T. et al. Identification of the calcitonin receptor in osteoarthritic chondrocytes. BMC Res Notes 13, 407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuestner R. E. et al. Cloning and characterization of an abundant subtype of the human calcitonin receptor. Mol Pharmacol 46, 246–55 (1994). [PubMed] [Google Scholar]
- Karsdal M. A. et al. Calcitonin is involved in cartilage homeostasis: is calcitonin a treatment for OA? Osteoarthritis Cartilage 14, 617–624, doi: 10.1016/j.joca.2006.03.014 (2006). [DOI] [PubMed] [Google Scholar]
- Farley J. R. et al. The anti-bone-resorptive agent calcitonin also acts in vitro to directly increase bone formation and bone cell proliferation. Endocrinology 123, 159–167, doi: http://dx.doi.org/10.1210/endo-123-1-159 (1988). [DOI] [PubMed] [Google Scholar]
- Hellio M. P. et al. Calcitonin inhibits phospholipase A2 and collagenase activity of human osteoarthritic chondrocytes. Osteoarthritis Cartilage 5, 121–128, doi: 10.1016/S1063-4584(97)80005-2 (1997). [DOI] [PubMed] [Google Scholar]
- Hayami T. et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum 50, 1193–1206, doi: 10.1002/art.20124 (2004). [DOI] [PubMed] [Google Scholar]
- Manicourt D. H. et al. Treatment with calcitonin suppresses the responses of bone, cartilage, and synovium in the early stages of canine experimental osteoarthritis and significantly reduces the severity of the cartilage lesions. Arthritis Rheum 42, 1159–1167, doi: 10.1002/1529-0131(199906) (1999). [DOI] [PubMed] [Google Scholar]
- Meulenbelt I. et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage 15, 379–385, doi: http://dx.doi.org/10.1016/j.joca.2006.09.007 (2007). [DOI] [PubMed] [Google Scholar]
- Sondergaard B. C. et al. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthritis Cartilage 14, 759–768, doi: http://dx.doi.org/10.1016/j.joca.2006.01.014 (2006). [DOI] [PubMed] [Google Scholar]
- Karsdal M. A. et al. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study. Osteoarthritis Cartilage 18, 150–159, doi: 10.1016/j.joca.2009.08.004 (2010). [DOI] [PubMed] [Google Scholar]
- Boyd S. K. et al. Early regional adaptation of periarticular bone mineral density after anterior cruciate ligament injury. J Appl Physiol 89, 2359–2364 (2000). [DOI] [PubMed] [Google Scholar]
- Wohl G. R. et al. Periarticular cancellous bone changes following anterior cruciate ligament injury. J Appl Physiol 91, 336–342 (2001). [DOI] [PubMed] [Google Scholar]
- O’Connor B. L. et al. Gait alterations in dogs after transection of the anterior cruciate ligament. Arthritis Rheum 32, 1142–1147, doi: 10.1002/anr.1780320913 (1989). [DOI] [PubMed] [Google Scholar]
- Behets C. et al. Effects of calcitonin on subchondral trabecular bone changes and on osteoarthritic cartilage lesions after acute anterior cruciate ligament deficiency. J Bone Miner Res 19, 1821–1826, doi: 10.1359/JBMR.040609 (2004). [DOI] [PubMed] [Google Scholar]
- Calvo E. et al. Osteoporosis increases the severity of cartilage damage in an experimental model of osteoarthritis in rabbits. Osteoarthritis Cartilage 15, 69–77, doi: http://dx.doi.org/10.1016/j.joca.2006.06.006 (2007). [DOI] [PubMed] [Google Scholar]
- Yang P. Y. et al. Effects of tibolone on osteoarthritis in ovariectomized rats: association with nociceptive pain behaviour. Eur J Pain 18, 680–690. doi: 10.1002/j.1532-2149.2013.00406.x. Epub 2013 Oct 2 (2014). [DOI] [PubMed] [Google Scholar]
- Felson D. T. et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 38, 1500–1505, doi: 10.1002/art.1780381017 (1995). [DOI] [PubMed] [Google Scholar]
- Colado M. I. et al. Involvement of central serotonergic pathways in analgesia elicited by salmon calcitonin in the mouse. Eur J Pharmacol 252, 291–297 (1994). [DOI] [PubMed] [Google Scholar]
- Guidobono F. et al. Role of catecholamines in calcitonin-induced analgesia. Pharmacology 31, 342–348 (1985). [DOI] [PubMed] [Google Scholar]
- Lyritis G. P. & Trovas G. Analgesic effects of calcitonin. Bone 30, 71S–4S (2002). [DOI] [PubMed] [Google Scholar]
- Shibata K. et al. Ovariectomy-induced hyperalgesia and antinociceptive effect of elcatonin, a synthetic eel calcitonin. Pharmacol Biochem Behav 60, 371–376, doi: 10.1016/S0091-3057(98)00015-X (1998). [DOI] [PubMed] [Google Scholar]
- Elliott K. et al. Dextromethorphan suppresses both formalin-induced nociceptive behavior and the formalin-induced increase in spinal cord c-fos mRNA. Pain 61, 401–409, doi: 10.1016/0304-3959(94)00214-Y (1995). [DOI] [PubMed] [Google Scholar]
- Fernihough J., Gentry C., Bevan S. & Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Let 11, 388(2), 75–80, doi: 10.1016/j.neulet.2005.06.044 (2005). [DOI] [PubMed] [Google Scholar]
- Ogbonna A. C. et al. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur J Pain 17, 514–526, doi: 10.1002/j.1532-2149.2012.00223 (2013). [DOI] [PubMed] [Google Scholar]
- Bullock C. M. et al. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol 66, 2188–2200, doi: 10.1002/art.38656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R. R., Cunha F. Q., Silva F. S. & Rocha F.A.C. A quantitative approach to measure joint pain in experimental osteoarthritis-evidence of a role of nitric oxide. Osteoarthritis Cartilage 14, 769–776, doi: 10.1016/j.joca.2006.01.013 (2006). [DOI] [PubMed] [Google Scholar]
- Nieves J. W., Komar L., Cosman F. & Lindsay R. Calcium potentitates the effects of estrogen and calcitonin on bone mass: review and analysis. Am J Clin Nutr 67, 18–24 (1998). [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J. & van der Berg W. B. Protection from interleukin 1 induced destruction of articular cartilage by transforming growth factor beta: studies in anatomically intact cartilage in vitro and in vivo. Ann Rheum Dis 52, 185–191 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J. & van der Berg W. B. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis 53, 593–600 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worster A. A., Nixon A. J., Brower-Toland B. D. & Williams J. Effect of transforming growth factor beta1 on chondrogenic differentiation of cultured equine mesenchymal stem cells. Am J Vet Res 61, 1003–1010 (2000). [DOI] [PubMed] [Google Scholar]
- van der Kraan P. M., Blaney Davidson E. N., Blom A. & van den Berg W. B. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage 17, 1539–1545, doi: 10.1016/j.joca.2009.06.008 (2009). [DOI] [PubMed] [Google Scholar]
- Blaney Davidson E. N. et al. TGF-beta is a potent inducer of nerve growth factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthritis Cartilage 23, 478–486, doi: 10.1016/j.joca.2014.12.005 (2015). [DOI] [PubMed] [Google Scholar]
- Blaney Davidson E. N., Vitters E. L., van der Kraan P. M. & van den Berg W. B. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instabilityinduced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis 65, 1414–1421, doi: 10.1136/ard.2005.045971 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. et al. Vitamin D prevents articular cartilage erosion by regulating collagen II turnover through TGF-β1 in ovariectomized rats. Osteoarthritis Cartilage 24, 345–353, doi: 10.1016/j.joca.2015.08.013 (2016). [DOI] [PubMed] [Google Scholar]
- Kizawa H. et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet 37, 138–144, doi: 10.1038/ng1496 (2005). [DOI] [PubMed] [Google Scholar]
- Serra R. et al. Expression of a truncated, kinase defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol 139, 541–552 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]