Abstract
Circulating endothelial progenitor cells (EPCs) play a role in the regeneration of damaged brain tissue. However, the relationship between circulating EPC levels and functional recovery in intracerebral hemorrhage (ICH) has not yet been tested. Therefore, our aim was to study the influence of circulating EPCs on the outcome of ICH. Forty-six patients with primary ICH (males, 71.7%; age, 72.7 ± 10.8 years) were prospectively included in the study within 12 hours of symptom onset. The main outcome variable was good functional outcome at 12 months (modified Rankin scale ≤2), considering residual volume at 6 months as a secondary variable. Circulating EPC (CD34+/CD133+/KDR+) levels were measured by flow cytometry from blood samples obtained at admission, 72 hours and day 7. Our results indicate that patients with good outcome show higher EPC numbers at 72 hours and day 7 (all p < 0.001). However, only EPC levels at day 7 were independently associated with good functional outcome at 12 months (OR, 1.15; CI95%, 1.01–1.35) after adjustment by age, baseline stroke severity and ICH volume. Moreover, EPC levels at day 7 were negatively correlated to residual volume (r = −0.525; p = 0.005). In conclusion, these findings suggest that EPCs may play a role in the functional recovery of ICH patients.
Intracerebral hemorrhage (ICH) represents 10–15% of all strokes1. Early mortality ranges between 32% and 52% within the first 30 days, only 10% of patients will live independently after 1 month, and only one in five patients will be autonomous at 6 months2. Despite being the most severe cerebral vascular disorder, there is no specific pharmacological treatment. Early surgery, even if does not increase the disability or death rate at 6 months, might have only a small clinically relevant survival advantage for patients with spontaneous superficial ICH without intraventricular hemorrhage3. Therefore, it is imperative to search for new therapeutic options. In this regard, the beneficial effects of bone marrow-derived progenitor cells (BMPCs) have been demonstrated in animal models of ICH, as evidenced by reduced tissue loss, immature neuron formation, synaptogenesis, neuronal migration, and neurological improvement4,5,6,7. In clinical studies regarding patients after acute ischemic stroke, the increase of circulating endothelial progenitor cells (EPCs), a subtype of BMPCs, has been associated with good neurological and functional outcome, reduced infarct growth and neurological improvement8,9,10,11,12,13. Likewise, high blood levels of circulating CD34+ progenitor cells have been associated with improved functional outcome at three months and reduced brain injury in patients with primary ICH14. Furtheremore, a recent study involving 16 patients with ICH has shown increased levels of circulating EPCs15. However, the role of circulating EPCs in the recovery of ICH patients is largely unknown. Our aim was therefore to study the influence of circulating EPCs in the outcome of ICH.
Results
We prospectively studied 46 patients with primary ICH (71.7% male, mean age 72.7 ± 10.8 years). Median [quartiles] NIHSS score at admission was 11 [5,16], and mean time from stroke onset was 4.7 ± 4.6 hours.
Primary Outcome
Table 1 shows the main characteristics of patients classified by outcome groups. Nineteen (41.3%) patients showed good functional outcome at 12 months. Patients with good functional outcome were younger and showed milder stroke severity, lower rate of ventricular extension and leukoaraiosis, and smaller ICH and edema volumes at admission. Likewise, patients with good outcome (n = 19) showed higher EPC numbers at 72 hours (18.2 ± 5.5 vs. 11.2 ± 7.3; p = 0.003) and at day 7 (22.7 ± 5.3 vs. 13.5 ± 9.1; p = 0.001), but not at admission (14.2 ± 5.1 vs. 10.9 ± 6.1; p = 0.108) (Fig. 1). However, only EPC levels at day 7 were independently associated with good functional outcome at 12 months (OR, 1.15; CI95%, 1.01 to 1.35, p = 0.039) after adjustment by age, baseline stroke severity, and ICH volume (Table 2).
Table 1. Baseline clinical characteristics, vascular risk factors, stroke subtype, biochemical parameters and neuroimaging findings in patients with good or poor outcome at 12 months.
| Good Outcome n = 19 | Poor Outcome n = 27 | p | |
|---|---|---|---|
| Age (years) | 67.7 ± 10.7 | 76.1 ± 9.8 | 0.010 |
| Male, (%) | 73.7 | 70.4 | 0.806 |
| Time from stroke onset, h | 5.1 ± 4.5 | 4.4 ± 4.8 | 0.562 |
| Vascular risk factors | |||
| History of hypertension, (%) | 63.2 | 63.0 | 0.989 |
| History of diabetes, (%) | 10.5 | 11.1 | 0.950 |
| History of atrial fibrillation, (%) | 10.5 | 25.9 | 0.195 |
| History of hyperlipidemia, (%) | 36.8 | 28.5 | 0.363 |
| Smoking habit, (%) | 10.5 | 3.7 | 0.356 |
| Alcohol consumption, (%) | 21.1 | 14.8 | 0.583 |
| Biochemistry and vital signs at admission | |||
| Body temperature (°C) | 36.4 ± 0.8 | 36.1 ± 0.5 | 0.110 |
| Systolic blood pressure (mm Hg) | 165.9 ± 29.6 | 162.2 ± 27.9 | 0.665 |
| Diastolic blood pressure (mm Hg) | 88.2 ± 21.0 | 86.4 ± 21.2 | 0.781 |
| Glucose levels (mg/dL) | 123.0 ± 34.2 | 140.2 ± 37.8 | 0.121 |
| Fibrinogen (mg/dL) | 407.7 ± 98.4 | 451.5 ± 108.0 | 0.174 |
| Leucocytes (×103/mL) | 9.3 ± 2.2 | 10.2 ± 3.7 | 0.344 |
| Platelets (×103/mL) | 232.9 ± 44.1 | 232.1 ± 68.1 | 0.965 |
| INR | 1.2 ± 0.5 | 1.6 ± 1.2 | 0.077 |
| Neuroimaging findings | |||
| ICH volume at admission (cc) | 15.4 ± 12.6 | 42.3 ± 36.6 | <0.0001 |
| Edema volume at admission (cc) | 24.7 ± 14.3 | 51.4 ± 40.5 | 0.007 |
| Ventricular extension, (%) | 15.8 | 55.6 | <0.0001 |
| Leukoaraiosis, (%) | 5.3 | 51.9 | <0.0001 |
| Clinical characteristics | |||
| NIHSS at admission | 7 [5, 10] | 13 [9, 16] | <0.0001 |
| Diagnosis | |||
| Topographic: | 0.847 | ||
| – Deep, (%) | 63.2 | 66.7 | |
| – Lobar, (%) | 36.8 | 33.3 | |
| Etiologic: | 0.271 | ||
| – Hypertensive, (%) | 63.1 | 44.0 | |
| – Amyloid, (%) | 31.6 | 32.0 | |
| – Anticoagulants, (%) | 0 | 16.0 | |
| – Undetermined, (%) | 5.3 | 8.0 | |
| Number of EPCs (CD34+/CD133+/KDR+) | |||
| EPCs at admission | 14.2 ± 5.1 | 10.9 ± 6.1 | 0.108 |
| EPCs at 72 hours | 18.2 ± 5.5 | 11.2 ± 7.3 | 0.003 |
| EPCs at day 7 | 22.7 ± 5.3 | 13.5 ± 9.1 | <0.0001 |
INR: International Normalized Ratio; ICH: Intracerebral hemorrhage; NIHSS: National Institute of Health Stroke Scale.
Figure 1. Temporal profile of mean circulating EPC numbers in ICH patients with good or poor outcome at 12 months.
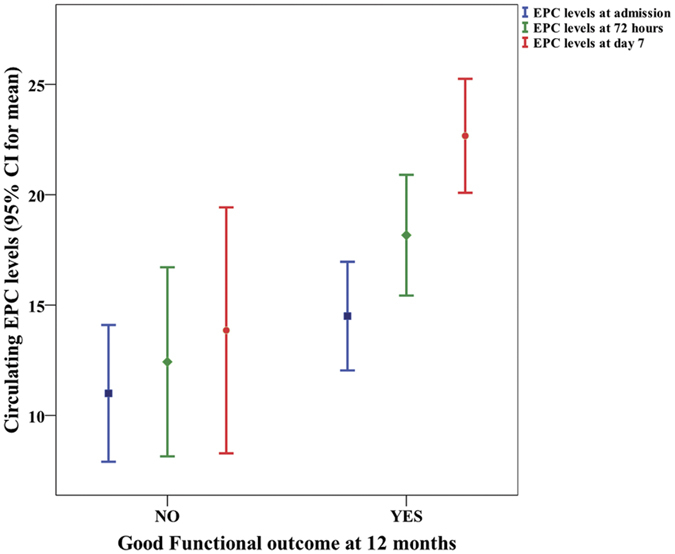
Table 2. Crude and adjusted OR of good outcome at 12 months for EPCs numbers at 72 hours and day 7.
| OR (CI95%), p | OR (CI95%), adjusted p | |
|---|---|---|
| EPCs at 72 hours | 1.18 (1.04 to 1.34), p = 0.009 | 1.10 (0.95 to 1.29), p = 0.189 |
| EPCs at day 7 | 1.31 (1.09 to 1.58), p = 0.002 | 1.15 (1.01 to 1.35), p = 0.039 |
| NIHSS at admission | 0.94 (0.81 to 1.09), p = 0.405 | |
| ICH volume at admission | 0.98 (0.93 to 1.03), p = 0.383 | |
| Age | 0.95 (0.86 to 1.04), p = 0.295 |
NIHSS: National Institute of Health Stroke Scale; ICH: Intracerebral hemorrhage.
Secondary Outcomes
Figure 2 shows the correlation of circulating EPCs at admission, 72 hours and day 7 with ICH residual volume at 6 months. No correlation was found between circulating EPC levels at admission and residual ICH volume at 6 months (Pearson correlation coefficient, r = −0.158; p = 0.412). However, an exponential negative correlation was found between residual ICH volume and EPC levels at 72 hours (r = −0.421; p = 0.032), and at day 7 (r = −0.525; p = 0.005). In the multivariate analysis, both circulating levels of EPCs at 72 hours (B, −1.01; CI95%, −1.79 to −0.21; p = 0.015) and especially at day 7 (B, −0.86; CI95%, −0.1.44 to −0.28; p = 0.005) were independently associated with residual ICH volume at 6 months. However, only circulating EPC levels at day 7 (B, −0.54; CI95%, −0.93 to −0.15; p = 0.009) were independenly associated with residual ICH volume at 6 months after adjusting by baseline stroke severity, as well as ICH and edema volumes at admission.
Figure 2. Scatterplot showing the correlation of circulating EPCs at admission, 72 hours and day 7 with ICH residual volume at 6 months.
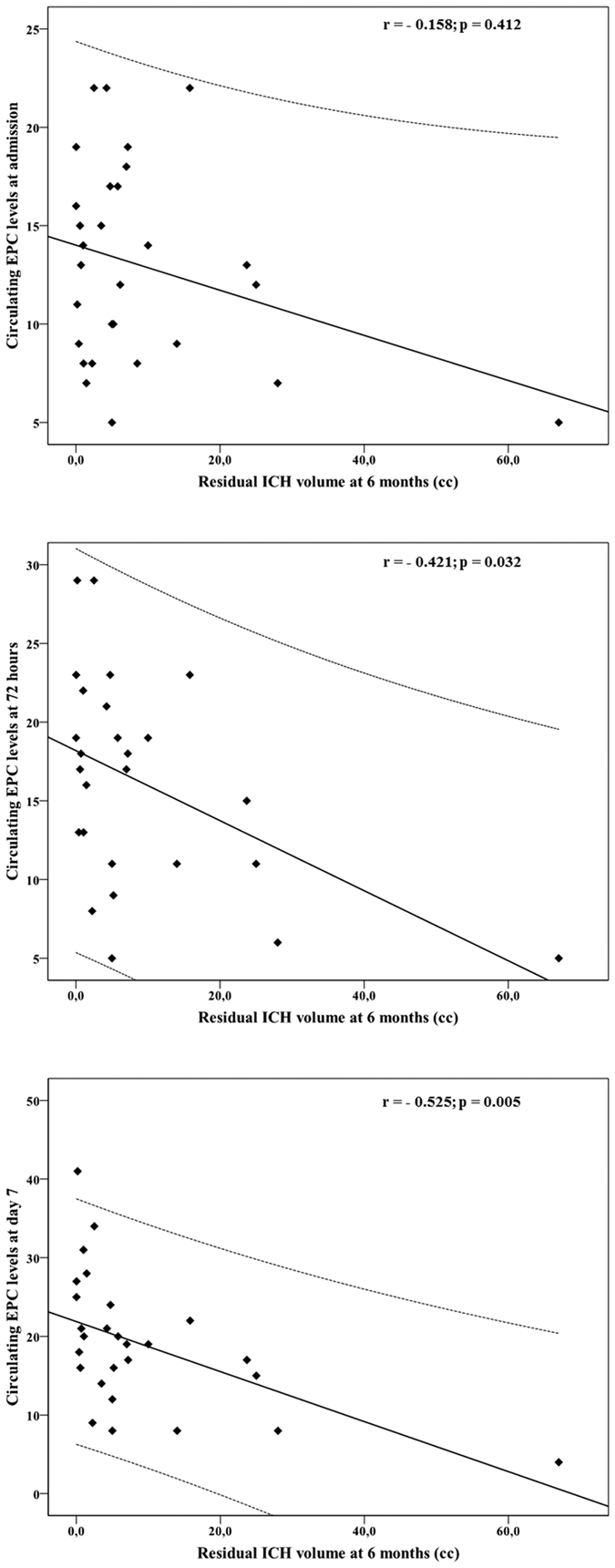
No correlation was found between circulating EPC levels at admission and ICH residual volume at 6 months (Pearson correlation coefficient, r = −0.158; p = 0.412). However, an exponential relation was found between ICH residual volume and EPC levels at 72 hours (r = −0.421; p = 0.032), and at day 7 (r = −0.525; p = 0.005).
On the other hand, no correlation was found between circulating EPC levels at day 7 and NIHSS at admission (r = −0.237; p = 0.178). However, a significant negative correlation was observed between circulating EPC levels at day 7 and NIHSS at discharge (r = −0.607; p < 0.0001), at 3 months (r = −0.570; p < 0.0001) and at 12 months (r = −0.591; p < 0.0001).
Discussion
Main results and clinical relevance
To the best of our knowledge, this study is the first prospective analysis that evaluates the relationship between circulating levels of EPCs (defined as CD34+/CD133+/KDR+ cells) and brain injury in patients with ICH. Remarkably, circulating EPC levels at day 7 were independently associated with good functional outcome at 12 months. This favourable effect on the primary endpoint was supported by residual ICH volume reduction at 6 months and milder neurological deficits. Overall, these findings support cellular therapy with EPCs as a new therapeutic approach for patients with ICH, a severe vascular disorder with currently no specific pharmacological treatment.
Possible mechanisms underlying EPC effects on recovery and ICH residual volume
We have demonstrated that circulating EPCS increase at day 7 in response to ICH, and that the greater the magnitude of this increases the better the clinical outcome at 12 months. These findings are in line with experimental studies demonstrating the beneficial effects of BMPCs in animal ICH models as evidenced by reduced tissue loss, immature neuron formation, synaptogenesis, neuronal migration and neurological improvement4,5,6,7. The fact that patients with good outcome showed higher EPC levels at day 7, but not at admission, supports the hypothesis that EPCs can mediate processes of chronic vessel repair and neurorepair. Furthermore, supporting this hypothesis, we found a negative correlation between increased levels of EPCs and smaller ICH residual volume at 6 months as well as milder neurological deficits. However, the mechanisms by which EPCs are associated with better functional outcome and with smaller residual ICH volume need further investigation. We hypothesize that this may occur through a triple mechanism: (a) the repairing of the damaged vessels through re-endotelization as previously shown in ventricular assist devices in humans16,17 (b) the developing of new vessels through neovascularization18,19,20 and (c) the paracrine action of EPCs promoting angiogenesis21,22,23,24. A possible fourth mechanism may add up in the acute phase of ICH, where EPCs may play an early role in protecting the blood-brain barrier (BBB), as some studies have indeed suggested in ischemic stroke25. This is consistent with other data from this study showing that reduced flow mediated dilation (which is positively correlated with EPCs; r = 0.602; p < 0.0001) holds a negative association with ICH and edema growth at 48–72 hours (data not shown).
On the other hand, previous studies by our group have observed that higher levels of growth factors at 72 hours after ICH are independently associated with a good functional outcome at 3 months, lesion volume reduction and relative neurological improvement ≥50% at day 9026. Likewise, we have previously demonstrated that higher levels of growth factors are associated with higher levels of circulating EPCs in human ischemic stroke27. Therefore, it is tempting to postulate that the beneficial effects of growth factors in ICH may be mediated by EPCs.
Study limitations
This study has some limitations. First, we did not include a control group. Nevertheless, a previous study had already shown increased levels of circulating EPCs in ICH patients compared with a control group15. Another limitation is that we did not determine EPC levels at longer intervals after the acute phase of ICH, so we cannot conclude if good recovery was associated with an acute and transient increase in EPC levels or if it was the result of a sustained increase of these progenitor cells during the follow-up. However, the favourable effect of the EPC level increase at day 7 was independent of other important prognostic variables at baseline. Lack of consensus over optimal definition for EPCs and the best measurement method may justify many contradictory results concerning EPCs up to date. We have used CD34, CD133 and KDR markers, as well as flow cytometry analysis because both techniques are amply used and recommended for EPC characterization and counting28. We have not directly measured functionality of EPCs. Nevertheless, the most accepted method for EPC characterization is flow cytometry, and several studies in ischemic stroke patients have virtually come to the same results using this technique and cell culture for CFU-EC counts (which best expresses functionality of EPCs) suggesting a parallelism relationship between EPC numbers and functionality8,10,11.
Conclusions.
In conclusion, a higher increase in circulating EPC levels at day 7 is associated with a better outcome and reduced ICH residual volume in ICH patients. However, whether if EPCs are able to incorporate into brain hemorrhagic areas and promote neurorepair in humans remains to be clarified. Finally, the role of EPCs as a therapeutic tool able to promote chronic neurorepair of brain tissue damaged by an ICH needs to be further explored.
Methods
Study population and patient characteristics
Between October 2012 and February 2014, 59 consecutive patients with a first-ever primary non-traumatic ICH of less than 12 hours from clinical onset and previously independent for their daily living activities were prospectively included in the study. Patients with previously altered functional capacity (modified Rankin Scale (mRS) ≥ 1)29 (n = 3), chronic inflammatory diseases (n = 2), severe hepatic (n = 1) or renal (n = 1) diseases, cancer (n = 2) or infectious disease in the 15 days prior to inclusion (n = 1) were excluded. Furthermore, 2 patients did not accept their participation in the study, so a total of 46 patients were finally included.
This research was carried out in accordance with the Declaration of Helsinki of the World Medical Association (2008) and approved by the Ethics Committee of the Servizo Galego de Saúde. Informed consent was obtained from each patient or their relatives after full explanation of the procedures.
Clinical variables
All patients were admitted to an acute stroke unit and treated according to the guidelines of the Cerebrovascular Diseases Study Group of the Spanish Society of Neurology30. Etiological diagnosis was made according the Guidelines for the management of spontaneous intracerebral hemorrhage in adults from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group31. Medical history recording potential vascular risk factors, blood and coagulation tests, 12-lead ECG, and chest radiography was obtained at admission.
To evaluate neurologic deficit, the National Institute of Health Stroke Scale (NIHSS)32 was performed at admission, 24 and 72 hours, at discharge, and at 3 and 12 months. Functional outcome was evaluated at discharge and at 3 and 12 months by using the mRS29. Both NIHSS and mRS were evaluated by internationally certified neurologists.
Antihypertensive treatment with intravenous labetalol or urapidil was administered in case of systolic blood pressure >185 mmHg or diastolic blood pressure >105 mmHg. Low-dose subcutaneous heparin was used for the prevention of deep vein thrombosis and pulmonary thromboembolism.
Neuroimaging studies
A CT study was performed at admission, at 72 hours, between days 4th–7th, and at 6 months. ICH and perihematomal edema volumes were calculated by a standard planimetric method using a semiautomated process. The perimeter of appropriate high and low-attenuation zones was traced, calculating lesion areas for each slice which were multiplied by slice thickness to yield lesion volumes. The residual cavity volume of ICH at 6 months was determined using the same volumetric method described; each volume calculation was done three times, the mean value being taken as definitive.
ICH topography was classified as lobar when it predominantly affected the cortical or subcortical white matter of the cerebral lobes, or as deep when it was limited to the internal capsule, the basal ganglia or the thalamus. The presence of intraventricular extension of the hematoma was also recorded.
All neuroimaging evaluations were made by the same neuroradiologist, who had no knowledge of the patients’ clinical or laboratory results.
EPC quantification in peripheral blood by Flow Cytometry
Circulating EPC leves were measured by flow cytometry according to methods described elsewhere33 in blood samples obtained at admission, 72 hours and at day 7. Blood samples were processed within 1–2 hours after collection by a single researcher who had no knowledge of the patients’ clinical or radiological results. In brief, circulating EPCs were analyzed for the expression of specific surface antigens using direct flow cytometry (BD FACSAria IIu, BD, Franklin Lakes, NJ, USA). Cells were labelled with FITC-conjugated anti-CD34 (BD, Franklin Lakes, NJ, USA), PE-conjugated anti-KDR (R&D Systems, Minneapolis, MN, USA) and APC-conjugated anti-CD133 (clone AC133 from Miltenyi Biotec, Bergisch Gladbach, Germany) monoclonal antibodies. We considered EPCs as triple CD133+/CD34+/KDR+ staining cells in the mononuclear cell fraction. In all analyses, 5 × 105 events were acquired, scored using a FACSAria IIu analyzer (BD, Franklin Lakes, NJ, USA), and processed using the PC FACSDiva software program (BD, Franklin Lakes, NJ, USA). Cell count was always expressed per 106 events.
Outcome Variables
The primary endpoint was good functional outcome (mRS ≤ 2) at 12 months. ICH Residual volume at 6 months and the evolution of NIHSS score during the first 12 months were evaluated as secondary outcome variables.
Statistical Analysis
The statistical analysis was conducted in SPSS 18.0 (SPSS, Chicago, IL, USA) for Mac.
Sample size calculation
Sample size was calculated using the statistical EPIDAT software (http://www.sergas.es/MostrarContidos_N3_T01.aspx?IdPaxina=62714), based on an EPC levels increase >200% in patients with acute ischemic stroke who showed good functional outcome according to previous studies8. The minimum sample size calculated to detect this effect was made accepting an alpha level of 5% and an 80% power.
Statistical tests for univariate analysis
Results were expressed as percentages for categorical variables and as mean (SD) or median and range (25th and 75th percentiles) for continuous variables depending on whether their distribution was normal or not. The Kolmogorov-Smirnov test was used to assess normality. Proportions were compared using the chi-square or Fisher test, while the continuous variables between groups were compared with the Student’s t or the Mann-Whitney tests depending on whether their distribution was normal or not. Bivariate correlations were performed using Pearson’s coefficient (normally distributed variables) or Spearman coefficient (variables without normal distribution).
Statistical tests for multivariate analysis
The association of EPCs and good functional outcome (mRS ≤ 2 at 12 months) was assessed using logistic regression analysis; the influence on residual ICH volume was assessed by multiple linear regression models. Both logistic regression analysis and multivariable linear regression models were adjusted for those variables with a proven biological relevance for each endpoint in order to avoid spurious associations. Residual plots were examined to detect potential non-linear relationships between the outcome variable and continuous independent variables. Results were expressed as adjusted odds ratios (ORs) or Beta estimate with the corresponding 95% confidence intervals (95% CI).
Additional Information
How to cite this article: Pías-Peleteiro, J. et al. Increased Endothelial Progenitor Cell Levels are Associated with Good Outcome in Intracerebral Hemorrhage. Sci. Rep. 6, 28724; doi: 10.1038/srep28724 (2016).
Acknowledgments
This study has been partially supported by grants from Instituto de Salud Carlos III (PI14/01879), Spanish Research Network on Cerebrovascular Diseases RETICS-INVICTUS (RD12/0014), Xunta de Galicia (Consellería Educación GRC2014/027) and the European Union program FEDER. Furthermore, F. Campos (CP14/00154) and T. Sobrino (CP12/03121) are recipients of a research contract from Miguel Servet Program of Instituto de Salud Carlos III. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions Conception and design of the study (T.S. and J.C.). Molecular data acquisition and analysis (M.P.-M., E.L.-A., F.C. and T.S.). Clinical and radiological data adquisition and analysis (J.P.-P., M.R.-Y. and M.B.). Handled funding and supervision (T.S. and J.C.). Statistical analysis (J.C.). Manuscript drafting (J.P.-P. and T.S.). Critical revision for important intellectual content (F.C., M.B. and M.R.-Y). Supervision (M.P.-M., E.L.-A., F.C., M.R.-Y. and M.B). All authors reviewed the manuscript.
References
- Qureshi A. I. et al. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 344, 1450–1460 (2001). [DOI] [PubMed] [Google Scholar]
- Adeoye O. & Broderick J. P. Advances in the management of intracerebral hemorrhage. Nat. Rev. Neurol. 6, 593–601 (2010). [DOI] [PubMed] [Google Scholar]
- Mendelow A. D. et al. STICH II Investigators.Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 382, 397–408 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried D. et al. Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J. Neurosurg. 104, 313–318 (2006). [DOI] [PubMed] [Google Scholar]
- Seyfried D. M. et al. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 1224, 12–19 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Huang Z., Xu Y. & Zhang S. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol. Res. 28, 104–112 (2006). [DOI] [PubMed] [Google Scholar]
- Li B. et al.The effect of CXCL12 on endothelial progenitor cells: potential target for angiogenesis in intracerebral hemorrhage. J. Interferon Cytokine Res. 35, 23–31 (2015). [DOI] [PubMed] [Google Scholar]
- Sobrino T. et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 38, 2759–2764 (2007). [DOI] [PubMed] [Google Scholar]
- Sobrino T., Blanco M., Pérez-Mato M., Rodríguez-Yáñez M. & Castillo J. Increased levels of circulating endothelial progenitor cells in patients with ischaemic stroke treated with statins during acute phase. Eur. J. Neurol. 19, 1539–1546 (2012). [DOI] [PubMed] [Google Scholar]
- Chu K. et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 39, 1441–1447 (2008). [DOI] [PubMed] [Google Scholar]
- Yip H. K. et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke 39, 69–74 (2008). [DOI] [PubMed] [Google Scholar]
- Cesari F. et al. Bone marrow-derived progenitor cells in the early phase of ischemic stroke: Relation with stroke severity and discharge outcome. J. Cereb. Blood Flow Metab. 29, 1983–1990 (2009). [DOI] [PubMed] [Google Scholar]
- Bogoslovsky T. et al. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology 75, 2059–2062 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino T. et al. Cd34+ progenitor cells likely are involved in the good functional recovery after intracerebral hemorrhage in humans. J. Neurosci. Res. 89, 979–985 (2011). [DOI] [PubMed] [Google Scholar]
- Paczkowska E. et al. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: the role of endothelin-1. J. Neurol. Sci. 325, 90–99 (2013). [DOI] [PubMed] [Google Scholar]
- Shi Q. et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 92, 362–367 (1998). [PubMed] [Google Scholar]
- Peichev M. et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95, 952–958 (2000). [PubMed] [Google Scholar]
- Kaneko Y. et al. Cell therapy for stroke: Emphasis on optimizing safety and efficacy profile of endothelial progenitor cells. Curr. Pharm. Des. 18, 3731–3734 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. & Folkman J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2, 727–739 (2002). [DOI] [PubMed] [Google Scholar]
- Asahara T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997). [DOI] [PubMed] [Google Scholar]
- Murohara T. et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Invest. 105, 1527–1536 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihata H. et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler. Thromb. Vasc. Biol. 22, 1804–1810 (2002). [DOI] [PubMed] [Google Scholar]
- Asahara T. et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 85, 221–228 (1999). [DOI] [PubMed] [Google Scholar]
- Grant M. B. et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat. Med. 8, 607–612 (2002). [DOI] [PubMed] [Google Scholar]
- Marti-Fabregas J. et al. Endothelial progenitor cells in acute ischemic stroke. Brain. Behav. 3, 649–655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino T. et al. High serum levels of growth factors are associated with good outcome in intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 29, 1968–1974 (2009). [DOI] [PubMed] [Google Scholar]
- Sobrino T. et al. Temporal profile of molecular signatures associated with circulating endothelial progenitor cells in human ischemic stroke. J. Neurosci. Res. 90, 1788–1793 (2012). [DOI] [PubMed] [Google Scholar]
- Yoder M. C. Human Endothelial Progenitor Cells. Cold Spring Harb. Perspect. Med. 2, a006692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P. D. & Hantson L. Assessment scales for the evaluation of stroke patients. J. Stroke Cerebrovasc. Dis. 7, 113–127 (1998). [DOI] [PubMed] [Google Scholar]
- Rodríguez-Yáñez M. et al. Clinical practice guidelines in intracerebral haemorrhage. Neurologia 28, 236–249 (2013). [DOI] [PubMed] [Google Scholar]
- Broderick J. et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 38, 2001–2023 (2007). [DOI] [PubMed] [Google Scholar]
- Brott T. et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20, 864–870 (1989). [DOI] [PubMed] [Google Scholar]
- Fadini G. P. et al. Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis 207, 213–219 (2009). [DOI] [PubMed] [Google Scholar]


