ABSTRACT
Background: Chlorhexidine gluconate is a widely used antimicrobial agent. Adding chlorhexidine and quaternary ammonium compounds to filling materials, such as composite resins, acrylic resins, and glass ionomer cements increases the antibacterial property of restorative materials. This study includes antibacterial property of glass ionomer restorative cements with chlorhexidine gluconate.
Aim: The primary objective of our study was to compare the antimicrobial properties of two commercially available glass ionomer cements with and without chlorhexidine gluconate on strains of mutans streptococci.
Materials and methods: Two glass ionomers (Fuji II Conventional and Fuji IX) were used. Chlorhexidine gluconate was mixed with glass ionomer cements, and antimicrobial properties against mutans streptococci were assessed by agar diffusion. The tested bacterial strain was inhibited and the antimicrobial properties decreased with time.
Results: The highest amount of antimicrobial activity with mean inhibitory zone was found in Fuji II with chlorhexidine gluconate followed by Fuji IX with chlorhexidine gluconate, Fuji II without chlorhexidine gluconate, and Fuji IX without chlorhexidine gluconate.
Conclusion: The results of the study confirmed that the addition of 5% chlorhexidine gluconate to Fuji II and Fuji IX glass ionomer cements resulted in a restorative material that had increased antimicrobial properties over the conventional glass ionomer cements alone for Streptococcus mutans.
How to cite this article: Yadiki JV, Jampanapalli SR , Konda S, Inguva HC, Chimata VK. Comparative Evaluation of the Antimicrobial Properties of Glass Ionomer Cements with and without Chlorhexidine Gluconate. Int J Clin Pediatr Dent 2016;9(2):99-103.
Keywords: Antimicrobial properties, Chlorhexidine gluconate, Glass ionomer cements.
INTRODUCTION
Caries disease still remains a major public health problem despite the widespread use of fluoride and the decline in caries prevalence observed in the majority of highly industrialized countries.1 Because of low socioeconomic status, caries epidemiology still remains an indispensible part of dental public health leading to an increase in caries prevalence.
Seppa et al2 reported that glass ionomers have antibacterial properties in vitro. Also, the growth of Streptococcus mutans has been reported to be inhibited in vivo around conventional and silver glass ionomers, which has generally been attributed to fluoride released by the materials.3
There may be direct correlation between fluoride release and antimicrobial effects of glass ionomer cements.
Recently, researchers modified filling materials, such as composite resins, acrylic resins, and glass ionomer cements by adding chlorhexidine and quaternary ammonium compounds.4 Moreover, bacteriostatic and bactericidal agents have the potential to be used in combination with glass ionomer cements to obtain an antibacterial restorative material.
Chlorhexidine gluconate is a widely used antimicrobial agent as it is both inhibitory and lethal to vegetative Gram-positive and Gram-negative bacteria at relatively high dilutions.5 Major characteristics of chlorhexidine that contribute to its success as an antiplaque agent include its substantivity and broad spectrum of antibacterial activity.6 It creates bacteriostatic environment by binding to oral surfaces and subsequent release of the compound over a long period of time. Our study was aimed to evaluate the antimicrobial properties of glass ionomer materials (Fuji II and Fuji IX) with and without chlohexidine gluconate against S. mutans.
MATERIALS AND METHODS
Materials used were Fuji II conventional (Type II GIC), Fuji IX (Type IX GIC; G.C. Corporation, Tokyo, Japan), and 5% chlorhexidine gluconate solution (Basic Pharma Life Sciences Pvt. Ltd, Ankleshwar, Gujarat).
Method of Preparation of Material Disks
Fuji II with chlorhexidine gluconate, Fuji IX with chlorhexidine gluconate, Fuji II without chlorhexidine gluconate, and Fuji IX without chlorhexidine gluconate were indicated with groups A to D respectively. A total of 10 specimens were prepared from each material.
Specimens of four groups of cements were prepared by using a brass mold containing four holes measuring 11 × 5 mm diameter. 0.5 gm of chlorhexidine gluconate was added to 9.5 gm of liquid glass ionomer cements to obtain 5% formulation. Recommended powder/liquid ratio for restorative purposes by the manufacturers was adopted, i.e., 1 scoop of powder to 1 drop of liquid.
Materials were mixed and loaded into the specific holes in brass mold. Prepared specimens were inserted in the wells within 1 minute with sterile dental instruments. Surface was covered with glass slide and materials were allowed to set. After setting, the disk-shaped specimens were removed from the mold.
Agar Diffusion Assay
Standard strains of S. mutans (MTCC 497) were used to test the antimicrobial efficacy of two different restorative materials with and without chlorhexidine gluco-nate. Brain heart infusion broth was used for culture. Ten agar plates were used. Using a sterile swab, the surface of each agar plate was swabbed three times to ensure even distribution of the inoculum. After drying of the agar plates, four wells of 11 × 5 mm diameter were made in each agar plate using sterile agar punchers and the set disk-shaped specimens were inserted into the wells. The plates wee incubated aerobically for 48 hours.
The experiment was repeated ten times for each material and the zones of inhibition were measured independently. Mean zone of inhibition of each restorative material was calculated and subjected to statistical analysis.
RESULTS
Antimicrobial activity was determined by measuring the size of inhibition zones produced around the specimens (specimen + inhibition zone, in mm) with a digital caliper at three different points, and then the mean was recorded after 24 hours to obtain day 1 values (Fig. 1). This procedure was continued to obtain day 7 (Fig. 2) and day 14 (Fig. 3) values.
Fig. 1.
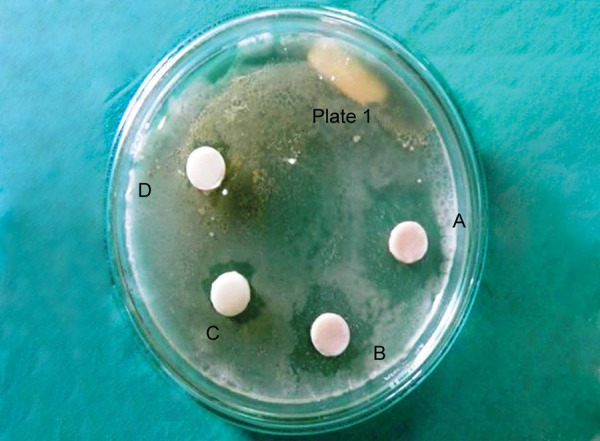
Zones of inhibition around restorative materials against Streptococcus mutans on day 1
Fig. 2.
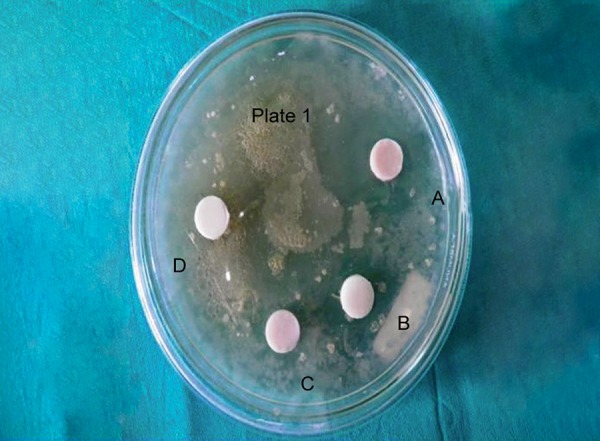
Zones of inhibition around restorative materials against Streptococcus mutans on day 7
Fig. 3.
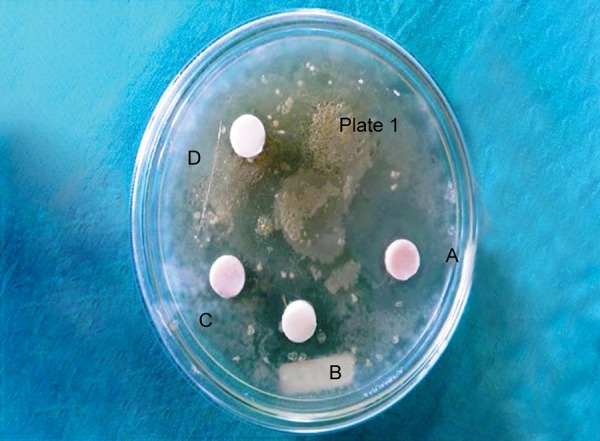
Zones of inhibition around restorative materials against Streptococcus mutans on day 14
All four groups of restorative materials showed antimicrobial properties. Streptococcus mutans was inhibited and the antimicrobial properties decreased with time.
The mean inhibition zone of Fuji II with chlorhexidine gluconate vs Fuji II without chlorhexidine gluconate (Graph 1) and mean inhibition zone of Fuji IX with chlorhexidine gluconate vs Fuji IX without chlorhexidine gluconate (Graph 2) were compared.
Graph 1.
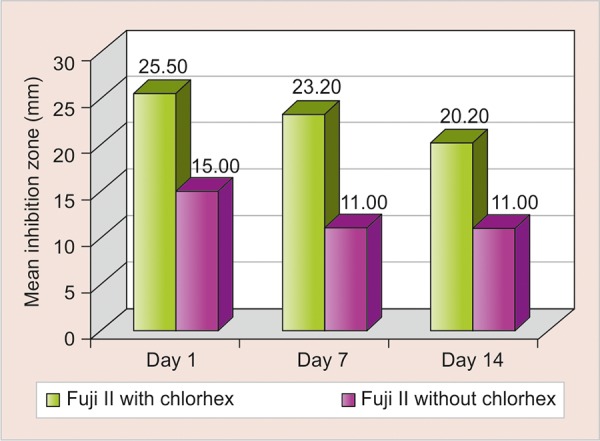
Comparison of mean inhibition zone of Fuji II with chlorhexidine gluconate vs Fuji II without chlorhexidine gluconate
Graph 2.
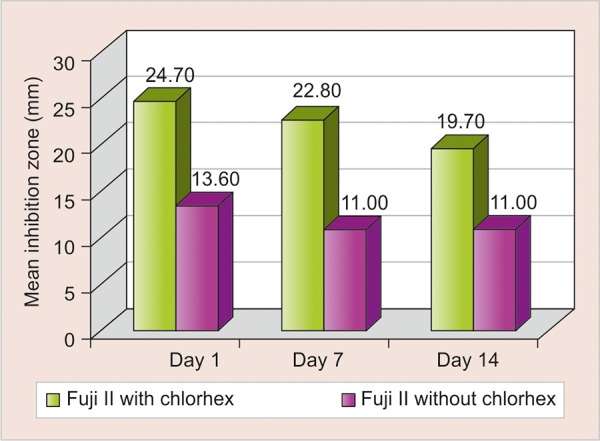
Comparison of mean inhibition zone of Fuji IX with chlorhexidine gluconate vs Fuji IX without chlorhexidine gluconate
The highest amount of antimicrobial activity with mean inhibitory zone was found in Fuji II with chlorhexidine gluconate (25.50 ± 1.269) followed by Fuji IX with chlorhexidine gluconate (24.70 ± 0.949), Fuji II without chlorhexidine gluconate (15.00 ± 0.667), and Fuji IX without chlorhexidine gluconate (13.60 ± 0.516) on day 1 (Table 1).
Table 1: Mean values of inhibition zones (mm) of four groups of restorative materials against Streptococcus mutans
|
Mean values of inhibition zones |
Standard deviation |
Analysis of variance |
|||||||||||||
| Materials | Day 1 | Day 7 | Day 14 | Day 1 | Day 7 | Day 14 | (Sig) | ||||||||
| Group A | 25.50 | 23.20 | 20.20 | 1.269 | 1.229 | 1.317 | <0.001* | ||||||||
| Group B | 24.70 | 22.80 | 19.70 | 0.949 | 0.632 | 1.252 | |||||||||
| Group C | 15.00 | 11.00 | 11.00 | 0.667 | 0.000 | 0.000 | |||||||||
| Group D | 13.60 | 11.00 | 11.00 | 0.516 | 0.000 | 0.000 | |||||||||
*Highly significant (p < 0.001)
Tukey HSD post hoc analysis showed that the mean inhibition in both Fuji II and Fuji IX with chlorhexidine gluconate was significantly greater than that of Fuji II and Fuji IX without chlorhexidine gluconate up to 14 days. One-way analysis of variance showed that the difference in mean inhibition zone was statistically highly significantly between four groups (p < 0.001) up to 14 days (Table 2 and Graph 3).
Table 2: Comparison of intragroup change in inhibition zone from day 1 to 7 and then at day 14
| Materials | Comparison |
Mean difference |
“t”-value | p-value | |||||
| Group A | Day 1 vs day 7 | 2.30 ± 0.68 | 10.776 | <0.001* | |||||
| Day 1 vs day 14 | 5.30 ± 1.06 | 15.821 | <0.001* | ||||||
| Day 7 vs day 14 | 3.00 ± 0.67 | 14.230 | <0.001* | ||||||
| Group B | Day 1vs day 7 | 1.90 ± 0.74 | 8.143 | <0.001* | |||||
| Day 1 vs day 14 | 5.00 ± 1.25 | 12.677 | <0.001* | ||||||
| Day 7 vs day 14 | 3.10 ± 0.99 | 9.858 | <0.001* | ||||||
| Group C | Day 1 vs day 7 | 4.00 ± 0.67 | 18.974 | <0.001* | |||||
| Day 1 vs day 14 | 4.00 ± 0.67 | 18.974 | <0.001* | ||||||
| Day 7 vs day 14 | – | – | – | ||||||
| Group D | Day 1 vs day 7 | 2.60 ± 0.52 | 15.922 | <0.001* | |||||
| Day 1 vs day 14 | 2.60 ± 0.52 | 15.922 | <0.001* | ||||||
| Day 7 vs day 14 | – | – | – |
*Highly significant (p < 0.001)
Graph 3.
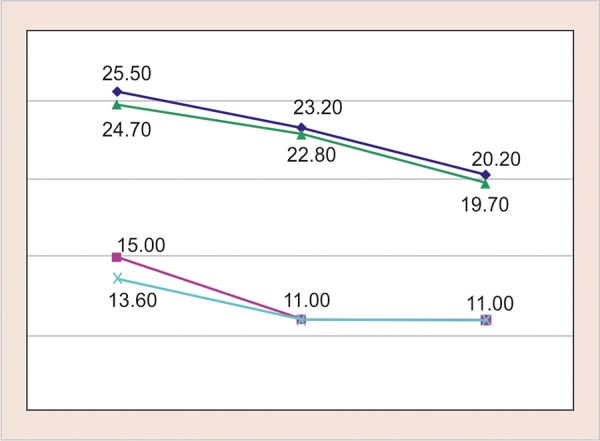
Mean inhibition zone in four groups on days 1, 7, and 14
Group A―Fuji II with chlorhexidine gluconate
Group B―Fuji IX with chlorhexidine gluconate
Group C―Fuji II without chlorhexidine gluconate
Group D―Fuji IX without chlorhexidine gluconate
DISCUSSION
Our study demonstrated that the addition of chlorhexidine gluconate to Fuji II and Fuji IX glass ionomer cements resulted in a restorative material that had increased antimicrobial properties over the conventional glass ionomer alone for S. mutans. There was significant difference in antimicrobial activity of four groups of materials. This antimicrobial activity of the cements may be due to fluoride release.
The mutans group streptococci are the most cari-ogenic microorganisms because of their metabolic characteristics and activity. The risk of developing caries in patients is determined by the level of microorganisms in saliva.
The most attractive advantage of the glass ionomers is their ability to release fluoride in the immediate vicinity of the cement. Fluoride release seems to be the most probable reason for the inhibitory effect on acid production. Fluoride availability from glass ionomer is pH controlled, the rate-controlling factors being salivary phosphate and proteins. Shashibhushan et al7 reported that there is a direct correlation between the amount of fluoride release and the antibacterial activity.
Vermeersch8 reported that the low pH of glass ionomer cements while setting may contribute more to their antimicrobial properties than their fluoride-leaching capabilities.
Emilson9 reported that no antimicrobial compound with the exception of fluoride has been shown to be more effective in the prevention of dental caries than chlorhexidine. It has the property to inhibit the action of glucosyltransferase enzyme responsible for accumulation of bacteria on the tooth surface. Sugar transport and acid production in oral bacteria is also affected by chlorhexidine. In humans, the clinical efficiency of the combination of chlorhexidine and fluoride was tested by Luoma et al10 in children.
The results obtained display a very significant fall in the mutans streptococci count on days 1, 7, and 14 (Graph 3).
Türkün et al11 reported that antimicrobial activity was dependent upon the concentration of disinfectant added to glass ionomer cements and others indicated no dose-response effects.12,13 In our study, 5% chlorhexidine gluconate solution was added to glass ionomer liquid. The bacterial strain S. mutans was inhibited and the antimicrobial properties decreased with time may be because of decrease in available chlorhexidine.
The high concentrations of chlorhexidine in the mixture increases the time of antimicrobial effect on S. mutans but decreases the physical properties of the material.
The decrease in the physical properties of the gluconate form of chlorhexidine is related to the fact that it is a liquid and leaches out more rapidly than the powder or diacetate form of chlorhexidine.13 Furthermore, the stability of chlorhexidine solution is adversely affected by exposure to higher temperatures of light, which may happen during storage of glass ionomer liquid. On the contrary, the amount of chlorhexidine should be kept as low as possible, as the chlorhexidine does not contribute to the formation of the glass ionomer network, and therefore, high amounts of chlorhexidine would weaken the scaffold and compromise the physical properties of the antibacterial glass ionomer cements.14 In our study, 5% chlorhexidine gluconate solution was added to glass ionomer liquid 1 minute before preparing the specimens.15
In our study, the highest amount of antimicrobial activity with mean inhibitory zone was found in Fuji II with chlorhexidine gluconate group followed by Fuji IX with chlorhexidine gluconate, Fuji II without chlorhexidine gluconate and Fuji IX without chlorhexidine gluconate. These results coincided with findings reported by Frencken et al16 and Shashibhushan et al.7 Fuji IX contains high amounts of glass, immediately after mixing, it becomes highly viscous and in wet environment it shows increased surface hardness.
It is considered that release of limited amounts of chlorhexidine is due to viscosity and hardness of Fuji IX irrespective of its contents and solubility. Therefore, it can be concluded that chlorhexidine-containing glass ionomer cements showed a superior effect in inhibiting growth of microorganisms compared with conventional glass ionomer cements alone. Research is going on in developing glass ionomer cements with antibacterial effects by the addition of bactericides such as chlorhexidine.
CONCLUSION
All four groups of restorative materials showed antimicrobial properties against S. mutans and the properties decreased with time.
The highest amount of antimicrobial activity with mean inhibitory zone was found in Fuji II with chlorhexidine gluconate followed by Fuji IX with chlorhexidine gluconate, Fuji II without chlo-rhexidine gluconate and Fuji IX without chlorhexidine gluconate.
This study concludes that the addition of chlorhexidine gluconate to Fuji II and Fuji IX glass ionomer cements resulted in a restorative material that had increased antimicrobial properties over the conventional glass ionomer cements alone for S. mutans.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1. Marthaler TM. Changes in dental caries. Caries Res. 2004 May-Jun;38(3):173–181. doi: 10.1159/000077752. [DOI] [PubMed] [Google Scholar]
- 2. Seppa L, Toppa-Saarinen E, Luoma H. Effect of different glass ionomers on the acid production and electrolyte metabolism of streptococcus mutans Ingbritt. Caries Res. 1992;26(6):434–438. doi: 10.1159/000261483. [DOI] [PubMed] [Google Scholar]
- 3. Forss H, Jokinen J, Spets-Happonen S, Seppa L, Luoma H. Fluoride and mutans streptococci in plaque grown on glass ionomer and composite. Caries Res. 1991 Feb;25(6):454–458. doi: 10.1159/000261410. [DOI] [PubMed] [Google Scholar]
- 4. Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003 Sep;19(6):449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 5. Davies GE, Francis J, Martin AR, Rose FL, Swain G. 1:6-di-4’-chlorophenyl-diguanidohexane (Hibitane). Laboratory investigation of a new antibacterial agent of high potency. Br J Pharmacol Chemother. 1954 Jun;9(2):192–196. doi: 10.1111/j.1476-5381.1954.tb00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gjermo P. Chlorhexidine and related compounds. J Dent Res. 1989;68(special issue):1602–1608. [Google Scholar]
- 7. Shashibhushan KK, Basappa N, Subba Reddy VV. Comparison of antibacterial activity of three fluorides and zinc releasing commercial glass ionomer cements on strains of mutans streptococci: an in vitro study. J Indian Soc Pedod Prev Dent. 2008;26(Suppl 2):S56–S61. [PubMed] [Google Scholar]
- 8. Vermeersch G, Leloup G, Delmee M, Vreven J. Antibacterial activity of glass-ionomers, compomers and resin composites: relationship between acidity and material setting phase. J Oral Rehabil. 2005 May;32(5):368–374. doi: 10.1111/j.1365-2842.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- 9. Emilson CG. Potential efficacy of chlorhexidine against mutans streptococci and dental caries. J Dental Res. 1994 Mar;73(3):682–691. doi: 10.1177/00220345940730031401. [DOI] [PubMed] [Google Scholar]
- 10. Luoma H, Murtomaa H, Nuuja T, Nyman A, Nummikoski P, Ainamo J, Luoma AR. A simultaneous reduction of caries and gingivitis in a group of school children receiving chlorhexidine-fluoride applications. Results after 2 years. Caries Res. 1978;12(5):290–298. doi: 10.1159/000260347. [DOI] [PubMed] [Google Scholar]
- 11.Türkün LS, Türkün M, Erturul F, Ate M, Brugger S. Long-term antibacterial effects and physical properties of a chlorhexidine-containing glass ionomer cement. J Esthet Restor Dent. 2008 Feb;20(1):29–44. doi: 10.1111/j.1708-8240.2008.00146.x. [DOI] [PubMed] [Google Scholar]
- 12. Jedrychowski JR, Caputo AA, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidine. J Oral Rehabil. 1983;10:373–381. doi: 10.1111/j.1365-2842.1983.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi Y, Imazato S, Kaneshiro AV, Ebisu S, Frencken JE, Tay FR. Antibacterial effects and physical properties of glass-ionomers cements containing chlorhexidine for the ART approach. Dent Mater. 2006 Jul;22(7):647–652. doi: 10.1016/j.dental.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 14. Sanders BJ, Gregory RL, Moore K, Avery DR. Antibacterial and physical properties of resin-modified glass-ionomers combined with chlorhexidine. J Oral Rehabil. 2002 Jun;29(6):553–558. doi: 10.1046/j.1365-2842.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- 15. Botelho MG. Inhibitory effects on selected oral bacteria of antibacterial agents incorporated in a glass ionomer cement. Caries Res. 2003 Mar-Apr;37(2):108–114. doi: 10.1159/000069019. [DOI] [PubMed] [Google Scholar]
- 16. Frencken JE, Imazato S, Toi C, Mulder J, Mickenautsch S, Takahashi Y, Ebisu S. Antibacterial effect of chlorhexidine-containing glass ionomer cement in vivo: a pilot study. Caries Res. 2007 Feb;41(2):102–107. doi: 10.1159/000098042. [DOI] [PubMed] [Google Scholar]


