Abstract
Background: Sodium oxybate is used in the treatment of narcolepsy. Currently no published literature supports its safety during breastfeeding, although it has a favorable pharmacokinetic profile for minimizing exposure.
Materials and Methods: We report a case of a 27-year-old primigravida with narcolepsy who was taking sodium oxybate for symptom control and contacted our Lactation Study Center for advice. Based on our current pharmacokinetic knowledge, she was advised to avoid breastfeeding 4 hours after a dose.
Results: Follow-up phone interviews were done and the patient reported that the feeding schedule was manageable, and she was able to exclusively breastfeed for 6 months of her infant's life. Based on pediatric records, her infant's growth and development were excellent. There were no noted side effects of the medication for the infant.
Conclusions: This is the first report to our knowledge of breastfeeding during maternal therapy with sodium oxybate, which appears to be compatible with safe, exclusive breastfeeding when managed appropriately.
Introduction
Breastfeeding women are often faced with difficult decisions about the impact of their medication use on the breastfeeding process and the safety of their breastfed infants. This is especially true for breastfeeding women who present with uncommon conditions necessitating use of medications never formally tested during lactation, such as narcolepsy. In an effort to expand the available evidence for breastfeeding women with narcolepsy, we present a case study of a breastfeeding mother with narcolepsy using sodium oxybate (Xyrem®). We looked for possible effects of the drug on her infant for the first 6 months of life and documented her experience with a recommended feeding schedule.
Narcolepsy affects 0.05% of the general population, and its role in pregnancy and childbirth is poorly explored, although one small study reported no clinically relevant adverse effects on pregnancy, childbirth, or the newborn.1–3 Treatment options for hypersomnia and/or cataplexy include amphetamines, modafinil, and sodium oxybate.2 The 2011 guidelines from the European Academy of Neurology recommend sodium oxybate as first-line therapy for cataplexy.4 Specific recommendations in pregnant and breastfeeding women with narcolepsy are lacking, and physician surveys have indicated a wide range of practices.1 One case report on maternal use of mixed amphetamine salts while lactating has been published; there are no published reports on maternal use of sodium oxybate for narcolepsy during breastfeeding.5,6
Sodium oxybate is the sodium salt of gamma hydroxybutyrate (GHB), which has potent sedative, hypnotic, and amnestic properties and is used to treat insomnia, clinical depression, cataplexy, and narcolepsy.7 Although the mechanism of action for sodium oxybate in the treatment of narcolepsy is unknown, it enhances the GABAB receptor's inhibitory effects on noradrenergic, dopaminergic, and thalamocortical neurons. The manufacturer's prescribing information states that sodium oxybate is well-absorbed orally (88% bioavailability), is minimally protein bound, achieves peak serum concentration in 30–75 minutes, and is rapidly metabolized through the Kreb's cycle to carbon dioxide and water. It is eliminated primarily through exhalation with an elimination half-life of 30–60 minutes.8 Scharf et al. conducted a pharmacokinetic study in 1998 in patients with narcolepsy, and at 4 hours postadministration, 50% of patients had undetectable GHB concentrations, whereas the remaining patients had low concentrations at approximately twice the level of detection. The elimination half-life ranged from 36.9 to 71.4 minutes, consistent with the healthy volunteer studies conducted by the manufacturer.7
Materials and Methods
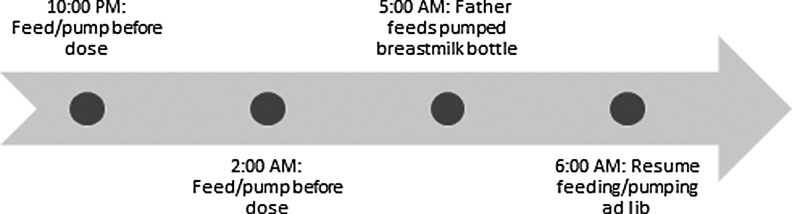
The case patient was identified through a telephone consultation to the Lactation Study Center at the University of Rochester because she was concerned about the safety of her medication regimen, which included sodium oxybate 4 g twice nightly (10:00 PM and 2:00 AM), fluoxetine 20 mg daily, and cetirizine 5 mg daily. This regimen was taken throughout pregnancy. The patient was a 27-year-old, primiparous female, with a medical history significant for narcolepsy (diagnosed 7 years before) and asthma. She had delivered a healthy, full-term, newborn male infant and initiated breastfeeding 2 days before her lactation consultant contacted the Lactation Study Center. Given the lack of data on the use of sodium oxybate during breastfeeding to guide decision making, the following pharmacodynamic and pharmacokinetic parameters were considered for analyzing potential risk: mechanism of action, possible adverse effects, oral bioavailability, protein binding, time to peak concentration, and elimination half-life. Unfavorable properties of the drug that might increase infant exposure include minimal protein binding, high oral bioavailability, and the potential for infant sedation and respiratory depression. Favorable properties include a short elimination half-life, rapid attainment of peak serum concentration, and metabolism independent of end organ maturity. Breastmilk concentrations do not always closely parallel maternal serum levels although this is a common assumption when evaluating medications for which there is no published data. Therefore, on the basis of the very brief half-life, it was deemed unlikely that the drug would significantly accumulate in breastmilk; however, the uncertainty of this assumption was explained to the mother.9 It also seemed unlikely the drug would undergo significant ion trapping in breastmilk given its low pKa.9,10 Based on this risk assessment, to ensure nearly complete elimination of the drug, a recommendation was made to breastfeed or pump before the 10:00 PM and 2:00 AM doses, and to use previously expressed breastmilk or formula for any feeding occurring within 4 hours after a dose. This recommendation was based on Scharf et al.'s pharmacokinetic study showing low rates of detection in maternal plasma after 4 hours.7 After 6:00 AM, breastfeeding or expression ad libitum was considered safe to resume.
The patient consented to be interviewed by telephone at 3 and 6 months postpartum and to have her child's pediatrician share the results of the 2, 4, and 6 month Ages and Stages Questionnaires®, the infant's growth charts, and clinical impressions regarding the infant's growth and development. She continued the medication regimen already described throughout the observation period.
Results
The recommended feeding and medication schedule was reportedly easy to follow; Figure 1 details the schedule followed by the family, with a bottle of expressed breastmilk fed by the father after the second dose of sodium oxybate at 5:00 AM. The mother reported breastfeeding on demand during the day, about eight times per day. The mother was able to pump 3–5 fluid ounces on each side, and was pumping two to three times daily at 1 month of age, once daily at 3 months of age, and once weekly by 6 months of age. The infant was exclusively breastfed or breastmilk fed for 6 months, at which point solid foods were started.
FIG. 1.
Dosing/feeding schedule followed by the parents.
At 3 months postpartum, the mother reported having more daytime sleepiness than during pregnancy, which she attributed to the demands of a newborn infant. At the 6 month interview, the mother reported that her narcolepsy symptoms were at her baseline before delivery. When asked about any special safety precautions taken because of her narcolepsy, the mother reported placing the baby in a bassinet on his back to sleep. She breastfed with supervision for the first month of life to ensure that she did not fall asleep while nursing, and also breastfed the baby in a rocker to prevent falling asleep. She had no episodes of narcolepsy or cataplexy while breastfeeding.
For the first 6 months of life, the infant was stable, showed no apparent side effects of the medication, and grew and developed appropriately. The infant's growth charts showed 50th percentiles for height, weight, and weight/height. Head circumference was at the 50th percentile at birth and the 75th percentile at 2 months of age and thereafter. Ages and Stages Questionnaires were administered at 2, 4, and 6 months of age and the infant scored high normal for all categories of development. There were no other clinical concerns.
Discussion and Conclusion
Sodium oxybate for the treatment of narcolepsy was associated with an excellent clinical outcome for the infant in our case of an exclusively breastfeeding woman with narcolepsy. The reassuring pharmacokinetic profile of the drug appeared to have been a reliable indicator for its clinical safety. This is the first report to our knowledge on the use of sodium oxybate during breastfeeding. More cases are needed to build a body of evidence so that we may appropriately counsel pregnant and breastfeeding women with narcolepsy about the use of this drug.
Acknowledgments
The authors would like to acknowledge the contributions of Constance Baldwin and Norma Barton to this case report.
Disclosure Statement
No competing financial interests exist.
References
- 1.Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med 2014;35:571–587 [DOI] [PubMed] [Google Scholar]
- 2.Wise MS, Arand DL, Auger RR, et al. Treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007;30:1712–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurovich-Horvat E, Tormášiová M, Slonková J, et al. Assessment of pregnancy outcomes in Czech and Slovak women with narcolepsy. Med Sci Monit 2010;16:SR35–SR40 [PubMed] [Google Scholar]
- 4.Billiard M, Dauvilliers Y, Dolenc-Groselj L, et al. Management of narcolepsy in adults. In: European Handbook of Neurological Management, 2nd ed., Gilhus NE, Barnes MP, Brainin M, eds., Vol. 1 Oxford, United Kingdom: Wiley-Blackwell, 2011, pp. 513–528 [Google Scholar]
- 5.Hoque R, Chesson A. Conception, pregnancy, delivery, and breastfeeding in a narcoleptic patient with cataplexy. J Clin Sleep Med 2008;4:601–603 [PMC free article] [PubMed] [Google Scholar]
- 6.Öhman I, Norstedt Wikner B, Beck O, et al. Narcolepsy treated with racemic amphetamine during pregnancy and breastfeeding. J Hum Lact 2015;31:374–376 [DOI] [PubMed] [Google Scholar]
- 7.Scharf MB, Lai AA, Branigan B, et al. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep 1998;21:507–514 [DOI] [PubMed] [Google Scholar]
- 8.Xyrem® [package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2015 [Google Scholar]
- 9.Lawrence RA, Lawrence RM. Chapter 12: Medications, Herbal Preparations, and Natural Products in Breast Milk. In: Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession. 8th ed. Philadelphia, PA: Elsevier, 2016, pp. 365–373 [Google Scholar]
- 10.National Center for Biotechnology Information. PubChem Compound Database; CID = 3037032. https://pubchem.ncbi.nlm.nih.gov/compound/3037032 (accessed March9, 2016)