Abstract
Water activated by non-thermal plasma creates an acidified solution containing reactive oxygen and nitrogen species, known as plasma-activated water (PAW). The objective of this study was to investigate the effects of different storage temperatures (25 °C, 4 °C, −20 °C, −80 °C) on bactericidal activities against S. aureus and physicochemical properties of PAW up to 30 days. Interestingly, PAW stored at −80 °C yielded the best antibacterial activity against Staphylococcus aureus, 3~4 log reduction over a 30-day period after PAW generation; meanwhile, PAW stored at 25 °C, 4 °C, and −20 °C, respectively, yielded 0.2~2 log decrease in cell viability after the same exposure and storage time. These results were verified by scanning electron microscope (SEM). The physicochemical properties of PAW stored at different temperatures were evaluated, including pH, oxidation reduction potential (ORP), and hydrogen peroxide, nitrate, nitrite anion and NO radical levels. These findings suggested that bacterial activity of PAW stored at 25 °C, 4 °C, −20 °C decreased over time, and depended on three germicidal factors, specifically ORP, H2O2, and NO3−. Moreover, PAW stored at −80 °C retained bactericidal activity, with NO2− contributing to bactericidal ability in association with H2O2. Our findings provide a basis for PAW storage and practical applications in disinfection and food preservation.
Plasma-activated water (PAW), prepared by non-thermal plasma treatment of distilled water, has gained increasing attention as an aqueous disinfectant. Various plasma sources are used to activate water, including direct current (DC), low-frequency discharge, radio frequency discharge, pulsed coronas, dielectric barrier discharge, atmospheric pressure plasma jets, and microwave discharge1,2,3,4. PAW can efficiently inhibit a wide range of microorganisms such as Hafnia alvei, Escherichia coli, Saccharomyces cerevisiae, Staphylococcus aureus, and Candida albicans5,6,7,8,9. The main advantages of using PAW for bacterial inhibition include less adverse impact on the environment, and no need for transportation and storage of potentially hazardous chemicals; indeed, chemical sanitizers, especially chlorine-based products, have raised increasing public health concerns about the risk of carcinogenic chlorinated organic compounds10. As a green disinfection product, PAW is a promising alternative to traditional sanitizers applied in agriculture (sterilization of fruits and vegetables) and food industry (disinfection of poultry products). R. Ma11 reported the potential of PAW for inhibiting S. aureus inoculated to strawberries. In addition, Y. Xu12 pointed out that PAW soaking is a promising method for fresh-keeping of postharvest A. bisporus.
Based on discharge type, working gas, and the chemical composition of the surrounding environment, various chemical reactions induced by plasma can be initiated, with a number of resulting primary and secondary species penetrating or dissolving into the liquid. Among the chemical species in PAW, OH· radical, atomic oxygen, ozone, and hydrogen peroxide are considered the main reactive oxygen species (ROS), playing a dominant role in the inactivation process13,14. The contribution of nitrogen-based reactive species also has to be considered: nitric oxide and its derivatives formed with water, including nitrites, nitrates and peroxynitrites15,16,17,18,19. Synergistic effects of reactive species are possible in plasma-treated liquid for microbial inhibition. Indeed, the antimicrobial properties of PAW were tentatively attributed to the synergetic effects of acidic pH and nitrite/nitrate remaining in noticeable concentrations in the solution6,8,19. Reactive-nitrogen- and -oxygen-based species play an important role in the toxic effect of PAW, and have DNA, RNA, proteins, and lipids as principal targets20,21. Moreover, both non-thermal plasma and PAW could catalyze the generation of intracellular ROS, consequently causing oxidative stress in bacterial cells22,23,24.
However, active species in PAW are difficult to assess due to short life spans and fast disproportionation in the plasma-liquid systems. Studies evaluating the effect of atomic oxygen on bacterial killing in water are scarce, since excited atomic oxygen only has a life time of ~30 ns25. It is also worth noting that trace amounts of ozone (a few ppm) were detected in air26. In water, ozone can live for 1 000 s at room temperature27. OH, with extreme reactivity, can be easily scavenged by virtually any organic molecule in its close vicinity, which accounts for its short life-span of about 10−9s28. Exploring PAW stability, Matthew J Traylor8 pointed out the complexity of PAW solutions, where multiple chemical components exert varying biological effects in differing time scales, after measuring physicochemical properties and bacterial inhibition over a 7-day period.
Storage conditions are important factors affecting the physicochemical properties and bactericidal activity of PAW. However, little information is available concerning the effects of storage conditions such as temperature, lighting, and sealing. The aim of this study was to investigate the physicochemical properties and bactericidal activities of PAW stored at different temperatures. The effects of storage temperatures on pH, ORP, hydrogen peroxide, nitrate and nitrite anion levels and the stability of bactericidal efficiency of PAW were assessed for up to 30 days after generation. PAW’s antimicrobial efficacy against S. aureus was examined by colony forming unit (CFU) count, and further verified by SEM. OES was employed to detect the main excited reactive species in PAW. Moreover, PAW’s physicochemical characteristics, including pH, ORP, and hydrogen peroxide, nitrate and nitrite anion levels, were recorded. Finally, ESR spectroscopy was used to detect NO radicals in PAW. Understanding the physicochemical properties and bactericidal activity of PAW before and after storage is important to the practical application in inactivating harmful microorganisms in food industries and agriculture.
Experimental Section
Plasma Microjet Device and PAW Generation
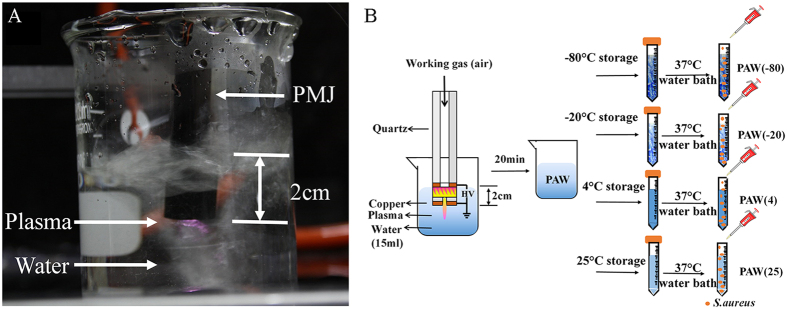
The air plasma generator is schematically illustrated in Fig. 1(A), and was designed based on the HEDBS structure29. The system, mainly consisting of copper electrodes and quartz dielectric, was set at the end of a quartz tube (inlet diameter, 1.5 mm). Air with 260 L/h gas flow rate was injected into the quartz tube, and the high voltage electrode connected to a power source (20 kHz). A homogeneous plasma was generated in the discharge gap of 0.5 mm and a plasma jet reaching 7 mm long ejected through the end outlet of 0.5 mm. All device parts were fixed to each other to prevent accidental displacement.
Figure 1.
(A) Image of PAW generation, and (B) schematic diagram of plasma jet and experimental arrangement, including PAW generation and storage at different temperatures.
As shown in Fig. 1(B), PAW was produced by placing the plasma jet beneath the water surface. The distance between the PMJ end and liquid surface was 20 mm. Every 10 ml sterile distilled water was activated by plasma for 20 min to obtain PAW, which was stored in centrifuge tubes. Four different storage temperatures were adopted, including 25 °C, 4 °C, −20 °C, and −80 °C for 1, 3, 7, 15, and 30 days, respectively. For simplified description of the different PAW states, PAW(25), PAW(4), PAW(−20), and PAW(−80) represented PAW stored at 25 °C, 4 °C, −20 °C, and −80 °C after plasma activation, respectively. Meanwhile, PAW-0 referred to water without plasma activation.
Bacterial strains, growth conditions, and PAW treatment
S. aureus (China General Microbiological Culture Collection Center, CGMCC number 1.2465), was used as a model organism in inhibitory tests. Bacteria were grown at 37 °C in Luria-Bertani (LB) medium to logarithmic phase (absorbance of 0.15 at 600 nm on SPECTROstar Omega plate reader, BMG, Germany), about 108/mL bacteria. Afterwards, 1 ml of the bacterial suspension was centrifuged at 5000 r/min for 10 min, and resuspended in 1 ml distilled water. Then, 100 μl of the resulting S. aureus suspension was added into 10 ml PAW equilibrated at 37 °C (5 min), for 20 min incubation. S. aureus suspension treated with PAW-0 was setup as the negative control. All experiments were repeated three times for statistical analysis.
Sterilization Ability of PAW
Colony count assay was used to assess kinetic killing curves and antimicrobial effects of a given compound or device. In brief, after 20 min PAW treatment, tenfold serial dilutions of 100 μl treated suspension were plated on LB agar culture medium, and incubated at 37 °C for about 21 h before CFU count. The sterilization ability of PAW was evaluated by the Log Reduction, calculated as follows:
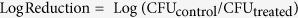 |
Scanning electron microscope (SEM, Quanta 200FEG) was used to evaluate cell morphology before and after PAW treatment. For SEM sample preparation, cells were pelleted by centrifugation (5000 rpm), and dehydrated by alcohol gradient (30, 50, 70, 80, 90, and 100%) until desiccation. The samples were then gilded and observed under an electron microscope.
OES Detection of the Main Excited ROS in PAW
To identify the main excited reactive species in plasma activated water, OES was employed in the 200–1000 nm range along the axial direction of the PMJ with the AvaSpec-2048-8 Fiber Optic. Plasma discharge formed beneath the water surface and one end of the fiber optics cable was used to acquire light signals at the bottom of the water container (quartz tube), approximately 5 mm away from the exit nozzle30.
Evaluation of PAW’s Physical and Chemical Properties
After PAW storage at different temperatures for various times, pH, ORP, and H2O2 contents were immediately detected to evaluate its physical and chemical properties. ORP, an indicator of the ability of a solution to oxidize and related to oxidizer concentration, activity, or strength31, was employed to evaluate global reactive oxygen species (ROS) levels in PAW. ORP in each sample was measured with a redox sensitive electrode (Mettler-Toledo LE501). H2O2 concentrations in PAW samples were quantified by Hydrogen Peroxide Assay Kit (Beyotime, Jiangsu, China); pH was monitored by a microprocessor pH-meter (Mettler-Toledo LE438).
Detection of nitrate and nitrite in PAW
The concentrations of nitrate anions (NO3−) and nitrite anions (NO2−) in PAW samples were measured by spectrophotometry32,33,34. After PAW storage at different temperature, 10 ml PAW were added 200 μl of 1 mol/L hydrochloric acid and 20 μl of 0.8% sulfamic acid. NO3− levels in PAW samples were determined by ultraviolet absorption spectrometry (NanoDrop 8000) at a single wavelength of 220 nm. As for NO2−, sulphanilamide was used as the diazotizing reagent, and N-(1-naphthy1)-ethylenediamine hydrochloride as the coupling reagent. After plasma activation, 200 μl of 10 g/L sulphanilamide was added into 10 ml PAW, and incubated at room temperature for 2 min, subsequently adding 200 μl of 1.0 g/L N-(1-naphthy1)-ethylenediamine hydrochloride for 20 min at room temperature. After incubation, NO2− levels were measured at 540 nm.
Measurement of NO radicals
N-methyl-D-glucamine dithiocarbamate (MGD) (99%, J&K Scientific Ltd. China) and FeSO4·7H2O (AR, National Medicine Group Chemical Reagent Co. Ltd) were used to trap NO free radicals produced in PAW by ESR spectroscopy, which result in the stable paramagnetic complex NO-Fe2+(MGD)2 with a characteristic ESR feature. After PAW melting, 250 μl MGD (1 mol/L) and 250 μl Fe2+ (0.3 mol/L) were added into 0.5 ml samples immediately. The reaction was initiated by addition of iron. The final product was imbibed by a capillary and detected in the resonator cavity of an ESR spectrometer (ER-200D-SRC/E-500, Bruker Ltd, German) operated at room temperature. The nitric oxide donor sodium nitroprusside (SNP, Beyotime, Jiangsu, China) was detected by ESR spectroscopy as standard for assessing NO radical concentrations in PAW. ESR experiments were carried out under following conditions: central magnetic field, 3369.850 Gauss; sweep width, 100.0 Gauss; frequency, 9.628 GHz; modulation frequency, 100 kHz; power, 7.971 mW.
Statistical Analysis
Data were obtained from at least three independent experiments (n ≥ 3). Values are mean ± standard deviation (SD). Statistical analysis was performed using SPSS statistical package 17.0 (SPSS Inc., USA). Analysis of variance (ANOVA) was used to compare different treatments during PAW storage; significant differences were identified by the Student-Newman-Keuls multiple range test, with a confidence level at P ≤ 0.05. In addition, paired-samples t-test was employed to compare the effects of different treatment conditions on NO radicals in PAW after 30 days of storage. Significant differences were represented by *P < 0.05, **P < 0.01, and ***P < 0.001.
Results and Discussion
Bactericidal efficiency of PAW samples under different storage temperatures
S. aureus inhibition
The colony count assay was employed to assess the bactericidal effects of PAW samples stored at different temperatures for various days. Bactericidal ability of PAW increased with decreasing temperature, with −80 > −20 > 4 > 25 (°C) obtained in descending order for bacterial inhibition. Before storage, fresh PAW resulted in a log reduction of approximately 5 for S. aureus. As shown in Fig. 2, bacterial growth declined to 3.7 and 1.8 at −80 °C and 25 °C, respectively, after 1 day of storage. PAW stored at −80 °C retained higher antibacterial activity compared to the other temperatures, leading to a log reduction of 3~4 for S. aureus; at other temperatures, PAW reduced total bacterial growth by 1.8, 2.2, 2.9 logs compared with untreated control, which decreased to about 1 log after 30 days of storage. Generally speaking, significant differences in bacterial killing were obtained between PAW(−80) and PAWs stored at other temperatures. These findings indicated that loss of bactericidal activity in PAW(−80) was reduced compared with that of other temperatures, indicating PAW(−80) retained efficient content, and consequently bactericidal activity. The differences for various storage temperatures might be explained by great variations in the physicochemical properties of PAW in these conditions. These results were consistent with a previous research assessing acidic electrolyzed water (AEW), and revealing that storage at −18 °C may be a better method to retain the available chlorine concentration as well as bactericidal activity compared to storage at 25 °C35.
Figure 2. Inactivation rate of S. aureus treated with PAW stored at different temperatures for 30 days.
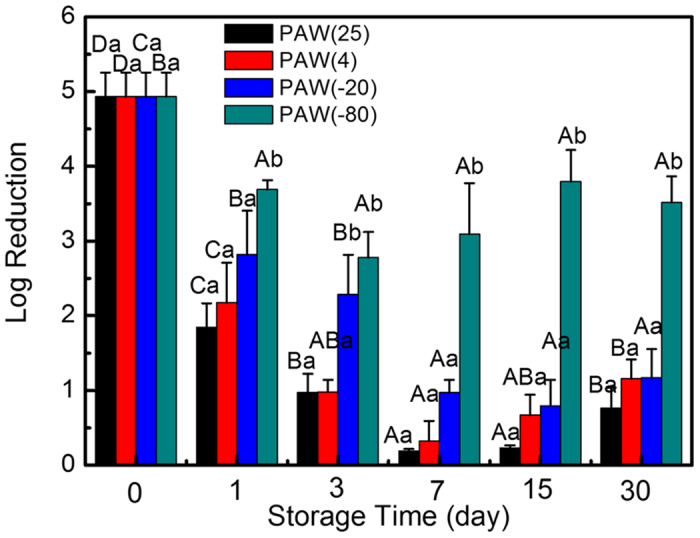
Different lowercase letters for the same storage time indicate significant differences (p < 0.05). Different capital letters for the same storage temperature but different times indicate significant differences (p < 0.05).
SEM assessment of S. aureus
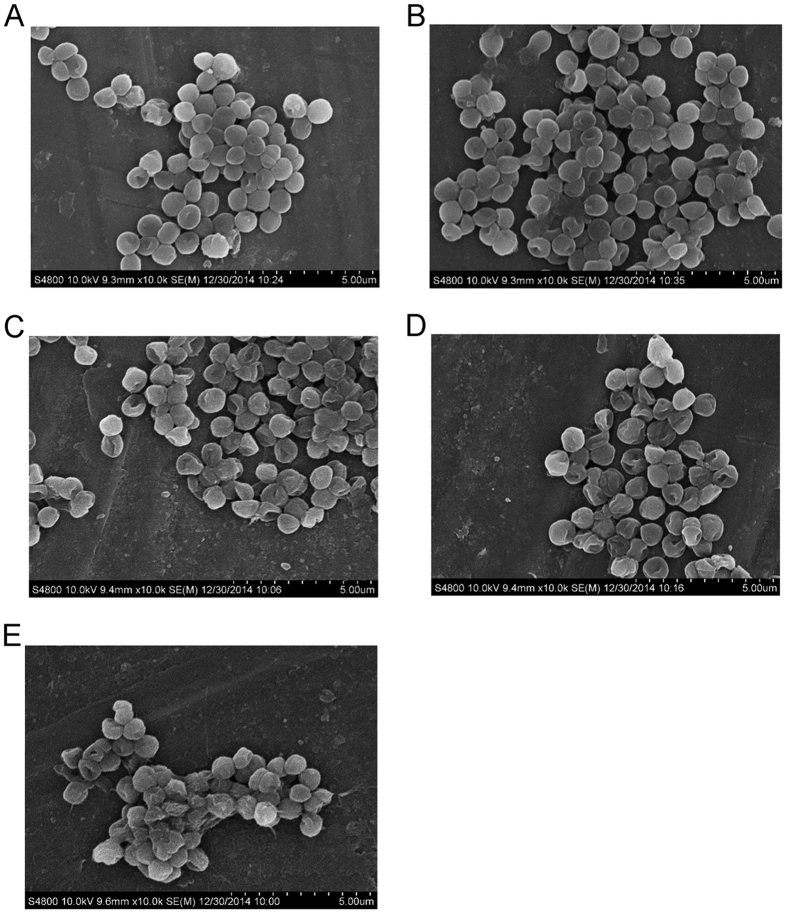
Scanning electron microscope (SEM) study further verified the stronger bactericidal ability of PAW(−80) compared to that of other temperatures. Typical SEM images of S. aureus before and after 20 min of PAW treatment following 30-day storage are shown in Fig. 3. Interestingly, the bacteria underwent a transition from initially smooth surfaces to surfaces with distortion, shrinkage and rupture of the outer layer after PAW treatment. The level of damage depended on storage temperature, and was more severe for −80 °C (Fig. 3(E)) compared to 25 °C (Fig. 3(B)), 4 °C (Fig. 3(C)), and −20 °C (Fig. 3(D)). The bacteria underwent a transition from smooth (Fig. 3(A)) to severely deformed (Fig. 3(E)) surfaces. These results were consistent with bacterial number reduction (Fig. 2), indicating the superiority of PAW stored at −80 °C over samples kept at other temperatures.
Figure 3. Scanning electron microscope (SEM) images of S. aureus after PAW treatment for 20 min.
(A) Untreated; (B) 25 °C; (C) 4 °C; (D) −20 °C; (E) −80 °C for 30 days, respectively (magnification x10000).
Physicochemical properties of PAW during storage
OES Detection of Main Excited ROS in PAW
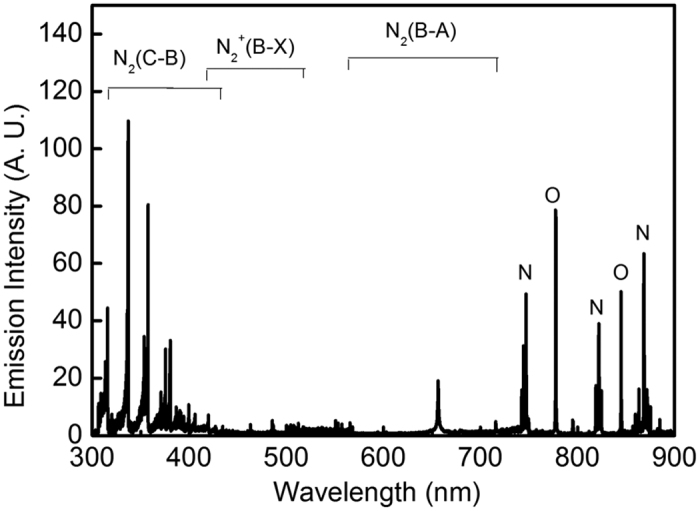
OES was employed to investigate the main excited active species generated in PAW. In the end-on spectra of the PAW, PMJ operated beneath the water surface at an operating current of 35 mA and voltage of 100 V (Fig. 4(A)). OES results are dominated by the second positive system of 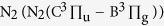 , first negative system of
, first negative system of 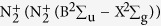 , and first positive system of
, and first positive system of 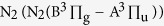 at 300–420 nm, 400–520 nm, and 560–730 nm, respectively, as well as N with O emission lines at 730–900 nm. O and N generated from discharge are mainly formed through dissociation of O2 and N2, respectively, which are excited from the ground state by electron impact (1) and (2)29.
at 300–420 nm, 400–520 nm, and 560–730 nm, respectively, as well as N with O emission lines at 730–900 nm. O and N generated from discharge are mainly formed through dissociation of O2 and N2, respectively, which are excited from the ground state by electron impact (1) and (2)29.
Figure 4. End-on optical emission spectra of PMJ ranging from 300 to 900 nm operated in water.
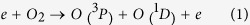 |
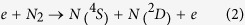 |
OES data indicated that excited atomic oxygen and nitrogen were produced in water by the plasma. Atomic oxygen is a chemically reactive species which can cause damage in biological molecules36. Atomic oxygen is readily converted into other reactive oxygen species, such as O3, ·OH and H2O2, due to high activity through chemical reactions37,38,39. Nitrites, nitrates, S-nitrosothiols and nitrosamines are metabolites of NO and mediators of the related cytotoxic effects, namely inhibition of mitochondrial respiration DNA damage leading to gene mutation, protein alteration and loss of function, necrosis, and apoptosis40.
PH and ORP in PAW samples
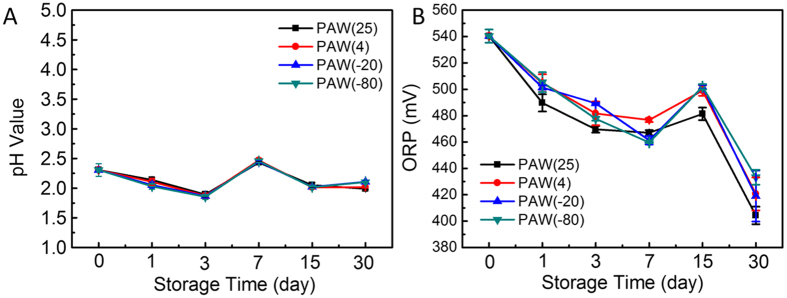
Physicochemical parameters of PAW showed significant differences at all storage temperatures compared with the control group, PAW-0. Solution pH and ORP were obtained during the storage process (Fig. 5). After plasma activation for 20 min, PAW’s pH decreased to 2.3 from 6.8, while ORP reached about 540 mV from the initial 250 mV. On the other hand, there was no significant change among the four different storage temperatures for the same time. As shown in Fig. 5, pH values of all samples only slightly changed with increasing storage time, and remained essentially stable during the 30-day storage in all four conditions; the pH of PAW remained around 2.0 regardless of storage temperature.
Figure 5.
Changes of (A) pH and (B) ORP of PAW stored at different temperatures over storage time.
ORP is regarded as an important factor influencing microbial inhibition. A high ORP can damage the outer and inner membranes41. Thus, it is of great significance to explore changes in PAW ORP for monitoring bactericidal efficacy. Figure 5(B) shows ORP for PAW samples stored under four different conditions for 30 days, respectively. As for different temperatures, changes followed a similar trend; they decreased slightly during the initial seven storage days by 15.6%, 13.3%, 17.1%, and 17.5%, respectively, and were reduced by 15.6%, 13.3%, 10.0%, and 6.2%, respectively, from days 7 to 30.
The synergetic effects of acidic pH and ROS always provide a warranty of relatively good germicidal efficacy6. In addition, high ORP indicates a solution with high oxidative strength14. There was no obvious change between different temperatures, demonstrating that they are not pivotal factors in sterilizing against S. aureus among different storage conditions.
H2O2 Concentrations in PAW samples
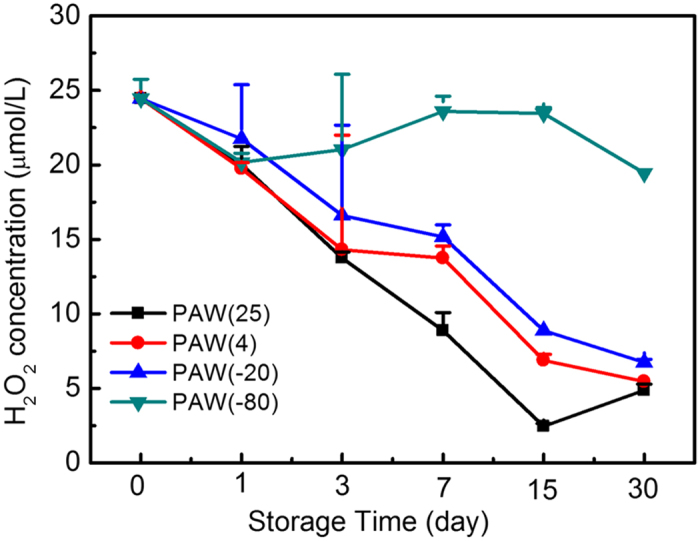
H2O2 is thought to be involved in the antimicrobial properties of PAW, and previous investigations have assessed the antimicrobial contributions of H2O2 in PAW. R. Burlica42 observed that approximately 3 mM H2O2 contributes a 2-log reduction in bacterial colony formation, while M. Naitaliet6 noted that 10 μM acidified H2O2 yields a 0.4-log reduction. We measured the H2O2 concentration of PAW immediately, approximately 24.4 μM (Fig. 6). H2O2 concentrations in PAW stored at −80 °C were approximately 20 μM and did not vary significantly over 30 days. With regard to PAW samples stored at other temperatures, H2O2 levels decreased to 6.0 μM after 30 days of storage from approximately 24.4 μM, indicating that H2O2 in PAW has reacted with various substances under such conditions. H2O2 is considered a strong oxidizer, especially in acidic environments43; thus loss of H2O2 might play an important role in different disinfection efficiencies observed.
Figure 6. H2O2 concentration in PAW samples stored at different temperatures and times.
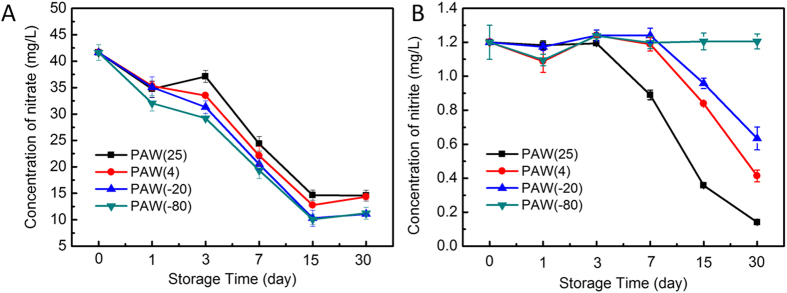
Nitrate and Nitrite Concentrations in PAW
In addition to ROS, reactive nitrogen species (RNS) such as nitrites, nitrates and peroxynitrites have also attracted attention from scientists for their sterilization mechanisms in PAW20,44,45,46,47. Formation of nitrogen transient species was evidenced mainly indirectly through detection of nitrogen products NO2− and NO3− in plasma treated liquid. Thus, NO3− and NO2− levels in PAW were also monitored by ultraviolet spectrophotometry. As shown in Fig. 7(A), NO3− concentrations had a similar trend in PAW samples for all conditions, decreasing with storage time. As for storage temperature, no significant differences in NO3− concentrations among various storage conditions were obtained. With respect to NO2−, concentrations in PAW(−80) were effectively constant over 30 days, about 1.2 μM. However, nitrite levels in PAW varied, decreasing from 1.2 μM to 0.1 μM, 1.2 μM to 0.4 μM, and 1.2 μM to 0.6 μM at 25 °C, 4 °C, and −20 °C, respectively, within 30 days. Meanwhile, nitrite levels were highly related to storage temperature, decreasing along with increasing temperature. Furthermore, significant differences in NO2− concentrations between PAW(25) and PAW(−80) were obtained (p < 0.05). In addition, the post-discharge reaction between hydrogen peroxide and nitrite ions occurring in water after treatment with plasma determined the formation of peroxynitrite: NO2− +H2O2+H+→ONOOH + H2O48. Peroxynitrite chemistry was shown to significantly participate in the antibacterial properties of PAW6,8,18,19. NO3− amounts in PAW samples significantly decreased after storage, while NO2− and H2O2 amounts dropped faster after storage at the other three temperatures compared with −80 °C, indicating that PAW(−80) is more favorable to keep NO2−, H2O2, and consequently, bactericidal activity. For solutions treated by liquid-phase plasmas, the antimicrobial properties of PAW were tentatively attributed to the synergetic effects of H2O2 and nitrite remaining in noticeable concentrations. This would be good news for users in practical applications, because PAW(−80) can avoid active ingredient loss during storage.
Figure 7.
Concentration of (A) nitrate and (B) nitrite in PAW stored at different temperatures and times.
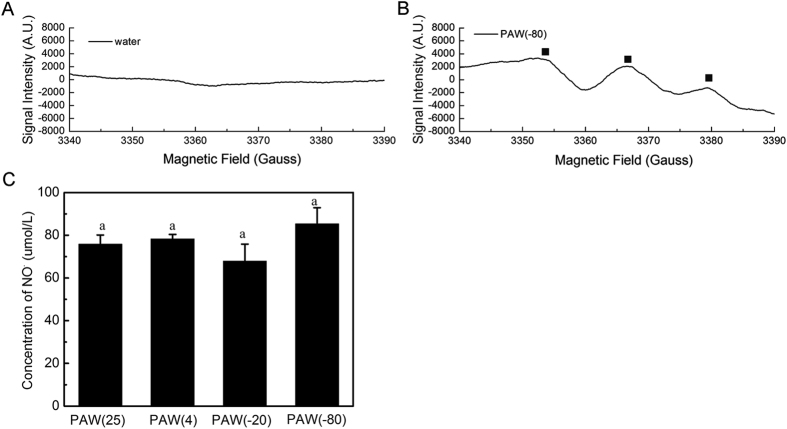
NO radical amounts in PAW samples
The direct spin trapping reaction between Fe2+(MGD)2 and NO produces the spin adduct NO-Fe2+(MGD)2 that is characterized by a tripling ESR spectrum, with a peak intensity ratio of 1:1:1. As shown in Fig. 8(A,B), NO was detected in PAW but virtually absent in the PAW-0 control. NO-Fe2+(MGD)2 signals detected in PAW demonstrated that NO radical is generated in solutions activated by plasma. After 30-day storage, relative NO-Fe2+(MGD)2 signal intensities for the same experimental conditions in PAW(25), PAW(4), PAW(−20) and PAW(−80) achieved 75.84, 78.34, 67.91, 85.28 μmol/L, respectively, according to the standard concentration of NO donor. There were no significant differences among treatment groups, indicating that NO radical is not a key factor affecting sterilization ability of PAWs stored at different temperatures. However, potential antimicrobial effects of NO have also been reported. T. Lai49 observed that exogenous NO can induce generation of ROS and cause oxidative damage to proteins during the germination process, resulting in growth inhibition of Penicillium expansum. After PAW storage, reactive nitrogen oxide species (RNOS) cause oxidative and nitrosative damage by altering DNA, inhibiting enzyme function, and inducing lipid peroxidation, which account for the majority of NO’s antimicrobial properties. The results demonstrated that NO radicals play a pivotal role in S. aureus inhibition, nearly independently of temperature.
Figure 8. Electron spin resonance (ESR) spectrum of MGD-NO signal in PAW(−80) and NO concentrations in PAW stored at different temperature for 30 days.
Same lowercase letters for the 30th indicate insignificant differences (p > 0.05).
Pearson correlation analysis of ORP, H2O2, and NO3 −, and S. aureus inhibition along with storage time
The results demonstrated that bactericidal ability of PAW(−80) remained stable during storage, while that of PAW(−20), PAW(4), PAW(25) displayed a decreasing trend with increasing storage time. The Pearson’s correlations between physicochemical parameters and S. aureus growth reduction induced by PAW(25), PAW(4), PAW(−20) during 30 days of storage were evaluated, respectively. As shown in Table 1, Pearson correlation coefficients in PAW(25), PAW(4), and PAW(−20) in association with ORP were 0.729, 0.661, and 0.723, respectively; those between the above treatment groups and H2O2 were 0.851, 0.783, and 0.861, respectively. With regard to NO3−, Pearson correlation coefficients were 0.727, 0.742, and 0.905, respectively. Significantly positive correlation was obtained during the whole storage period relative to pH and NO2−. In general, PAW’s inhibitory activity changed with storage time, and depended on three germicidal factors, specifically ORP, H2O2, and NO3−. ORP is considered an important factor affecting microbial killing as it can damage outer and inner membranes of E. coli O157:H7, subsequently leading to inhibition41. H2O2 is considered an important constituent in sterilization. Nitrate, as a long-lived secondary product, is likely responsible for the extended biological effects of plasma-activated water after plasma treatment8. ORP, H2O2 and their combination with nitrates have been proposed to be the dominant factors in PAW(25), PAW(4), and PAW(−20) affecting microbial killing over storage time.
Table 1. Pearson correlation coefficients between ORP, H2O2, and NO3 − +, and inactivation of S. aureus for PAW(25), PAW(4), and PAW(−20) treatment during 30 days of storage.
| Treatments | Pearson correlation coefficient |
||||
|---|---|---|---|---|---|
| pH | ORP | H2O2 | NO3− | NO2− | |
| PAW(25) | 0.307 | 0.729 | 0.851 | 0.727 | 0.530 |
| PAW(4) | 0.259 | 0.661 | 0.783 | 0.742 | 0.274 |
| PAW(−20) | 0.134 | 0.723 | 0.861 | 0.905 | 0.439 |
Comparisons of physicochemical properties of PAW stored at different temperatures for 30 days
To the best of our knowledge, whether storage temperature significantly affects bactericidal activity of PAW remains unclear. In this study, sterilization efficiency of PAW(−80) was better compared to values obtained for other treatment groups, with PAW(−80) > PAW(−20) > PAW(4) > PAW(25). These differences might be explained by distinct physicochemical properties of PAW after storage at various temperatures.
As shown in Table 2, ORP in different PAWs remained almost unchanged after 30 days of storage, with no significant differences among storage temperatures (p > 0.05). However, pH and nitrate of PAW(−80) and PAW(−20) significantly differed from values obtained for PAW(4) and PAW(25) (<0.05); the differences between PAW(−80) and PAW(−20) were insignificant (p > 0.05). Interestingly, PAW(−80) showed significant differences compared with PAW samples stored at other temperatures (25, 4 or −20 °C), specifically H2O2 and NO2−. Both H2O2 and NO2− levels in PAW(−80) remained stable, while the other treatment groups showed decreased levels as shown in Table 2. H2O2 amounts in PAW(−80) were 3.2, 2.9, an 1.8 times, respectively, higher compared with PAW(25), PAW(4) and PAW(−20) values at the end of storage. Similarly, nitrite ion concentrations in PAW(−80) were significantly different from those of other treatment groups (<0.05). It is possible that the reaction between acidified nitrites and 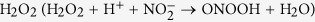 is affected by temperature. Nitrite production may thus be transiently encountered and biologically active in PAW.
is affected by temperature. Nitrite production may thus be transiently encountered and biologically active in PAW.
Table 2. Physicochemical properties, including ORP, H2O2, and NO3 − for PAW(25), PAW(4), PAW(−20) and PAW(−80) for 30 days.
| Treatments | Physicochemical properties |
||||
|---|---|---|---|---|---|
| pH | ORP | H2O2 | NO3− | NO2− | |
| PAW(25) | 1.99 ± 0.01a | 404 ± 7a | 4.60 ± 0.57a | 14.57 ± 1.03b | 0.14 ± 0.01a |
| PAW(4) | 2.02 ± 0.02a | 421 ± 13a | 4.98 ± 0.44a | 14.35 ± 0.55b | 0.41 ± 0.03b |
| PAW(−20) | 2.11 ± 0.02b | 419 ± 19a | 6.98 ± 0.44b | 11.09 ± 0.13a | 0.63 ± 0.07c |
| PAW(−80) | 2.10 ± 0.02b | 433 ± 6a | 19.46 ± 0.29c | 11.24 ± 1.13a | 1.21 ± 0.04d |
Different lowercase letters indicate significant differences (p < 0.05).
Our results indicated that bactericidal activity could be preserved at −80 °C during PAW storage. Consequently, −80 °C has the potential to keep freshness and product sanitization in melted PAW. Compared with PAW samples stored at other temperatures, physicochemical parameters of PAW(−80) had significant changes (Table 2). This is good news for PAW users, since in practice PAW can be prepared and stored at −80 °C until needed, avoiding loss of constituents during storage. Furthermore, melted PAW can be treated as common water because of reduced adverse impact on the human body as well as the environment.
Conclusion
We demonstrated that PAW(−80) is more efficient than PAWs stored at other temperatures (25 °C, 4 °C, and −20 °C) for sterilization. With regard to pH, ORP, and NO3−, minimal changes of PAW were observed. H2O2 and nitrite concentrations presented the same trend, decreasing with increasing storage temperature. Evaluation of NO radicals by ESR showed that short-lived species slightly contribute to the differences in sterilization efficiency of PAW stored at different temperatures. This suggested that PAW(−80) can preserve bactericidal activity, and H2O2 and NO2− levels, leading to S. aureus inhibition.
This work unravels the importance of storage temperature on physicochemical properties and bactericidal efficiency of PAW, providing a basis for practical application of PAW in inactivating harmful microorganisms in medicine and foodstuff. In the future, other storage conditions should be assessed, e.g. light, agitation, and packaging.
Statement of Novelty
Plasma-activated water (PAW) as a novel disinfectant can be used to inhibit harmful microorganisms in food industry and medical settings. However, the short life of active species in PAW is an important limitation for practical applications. Here, physicochemical properties and bactericidal activities of PAW stored at 25, 4, −20, −80 °C, respectively, were assessed. Results showed PAW stored at −80 °C retained efficient inhibitory activity against S. aureus. Moreover, NO2− contributed to the antimicrobial property, in combination with H2O2. These findings provide a basis for PAW storage and practical applications in food and medical industry.
Highlights
PAW stored at −80 °C retains bactericidal activity.
NO2− contributes to the antibacterial property of PAW(−80) in combination with H2O2.
Bactericidal ability of PAW stored at 25, 4, and −20 °C decreased over time.
Disinfection properties depended on ORP, H2O2, and NO3−.
Additional Information
How to cite this article: Shen, J. et al. Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci. Rep. 6, 28505; doi: 10.1038/srep28505 (2016).
Acknowledgments
This research was sponsored by Peking University Biomed-X Foundation.
Footnotes
Author Contributions J.Z. and J.F. designed this study. J.S. and Y.T. were involved in the experimentation. Y.L. analyzed data and R.M. prepared the figures. Y.T. wrote the manuscript with assistance from J.S. and Q.Z. All authors reviewed the manuscript.
References
- Fridman A., Chirokov A. & Gutsol A. Non-thermal atmospheric pressure discharges. J. Phys D: Appl Phys. 38, R1–R24 (2005). [Google Scholar]
- Laroussi M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: review, analysis, and prospects. IEEE Trans. Plasma Sci. 30, 1409–1415 (2002). [Google Scholar]
- Moreau M., Orange N. & Feuilloley M. G. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 26, 610–617 (2008). [DOI] [PubMed] [Google Scholar]
- Park G. Y. et al. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci. Technol. 21, 4 (2012). [Google Scholar]
- Kamgang-Youbi G. et al. Impact on disinfection efficiency of cell load and of planktonic/adherent/detached state: case of Hafnia alvei inactivation by plasma activated water. Appl. Microbiol. Biotechnol. 81, 449–457 (2008). [DOI] [PubMed] [Google Scholar]
- Naitali M., Kamgang-Youbi G., Herry J. M., Bellon-Fontaine M. N. & Brisset J. L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 76, 7662–7664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamgang-Youbi G. et al. Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett. Appl. Microbiol. 48, 13–18 (2009). [DOI] [PubMed] [Google Scholar]
- Traylor M. J. et al. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D: Appl. Phys. 44, 472001 (2011). [Google Scholar]
- Oehmigen K., Hahnel M., Brandenburg R., Wilke C. & Weltmann K.-D. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process. Polym. 7, 250–257 (2010). [Google Scholar]
- Ölmez H. & Kretzschmar U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT-Food Sci Technol. 42, 686–693 (2009). [Google Scholar]
- Ma R. et al. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 300, 643–651 (2015). [DOI] [PubMed] [Google Scholar]
- Xu Y., Tian Y., Ma R., Liu Q. & Zhang J. Effect of plasma activated water on the postharvest quality of button mushrooms. Agaricus bisporus, Food Chem. 197, 436–444 (2016). [DOI] [PubMed] [Google Scholar]
- Pavlovich M. J., Chang H., Sakiyama Y., Clark D. S. & Graves D. B. Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J. Phys. D: Appl. Phys. 46, 145202 (2013). [Google Scholar]
- Zhang Q., Liang Y., Feng H., Ma R., Tian Y., Zhang J. & Fang J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 102, 203701 (2013). [Google Scholar]
- Lukes P., Brisset J. L., Locke B. R. et al. Biological effects of electrical discharge plasma in water and in gas–liquid environments Plasma Chemistry and Catalysis in Gases and Liquids Edited by Parvulescu (Wiley Press, 2012). [Google Scholar]
- Laroussi M. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process. Polym. 2, 391–400 (2005). [Google Scholar]
- Dobrynin D., Fridman G., Friedman G. & Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 11, 115020 (2009). [Google Scholar]
- Van Gils C. A. J., Hofmann S., Boekema B. K. H. L., Brandenburg R. & Bruggeman P. J. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J. Phys. D: Appl. Phys. 46, 175203 (2013). [Google Scholar]
- Oehmigen K. et al. Estimation of possible mechanisms of Escherichia coli inactivation by plasma treated sodium chloride solution. Plasma Process. Polym. 8, 904–913 (2011). [Google Scholar]
- Cabiscol E., Tamarit J. & Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 3, 3–8 (2000). [PubMed] [Google Scholar]
- Fang F. C. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2, 820–832 (2004). [DOI] [PubMed] [Google Scholar]
- Lunov O. et al. Cell death induced by ozone and various non-thermal plasmas: therapeutic perspectives and limitations. Sci Rep. 4, 7129–7129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunov O. et al. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials, 82, 71–83 (2016). [DOI] [PubMed] [Google Scholar]
- Tian Y. et al. Assessment of the Physicochemical Properties and Biological Effects of Water Activated by Non‐thermal Plasma Above and Beneath the Water Surface. Plasma Process. Polym. 12, 439–449 (2015). [Google Scholar]
- Reuter S., Niemi K., Schulz-von der Gathen V. & Dobele H. F. Generation of atomic oxygen in the effluent of an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 18, 015006 (2009). [Google Scholar]
- Sun P. et al. Inactivation of Bacillus subtilis spores in water by a direct-current, cold atmospheric-pressure air plasma microjet. Plasma Process. Polym. 9, 157–164 (2012). [Google Scholar]
- Glaze W. H., Kang J. & Chapin D. H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone: Sci. Eng. 9, 335–352 (1987). [Google Scholar]
- Aikens J. & Dix T. A. Perhydroxyl radical (HOO.) initiated lipid peroxidation. J. Biol. Chem. 266, 15091–15098 (1991). [PubMed] [Google Scholar]
- Yu S. et al. Dielectric barrier structure with hollow electrodes and its recoil effect. Appl. Phys. Lett. 106, 244101 (2015). [Google Scholar]
- Ma R. et al. An evaluation of anti-oxidative protection for cells against atmospheric pressure cold plasma treatment. Appl. Phys. Lett. 100, 123701 (2012). [Google Scholar]
- McPherson L. L. Understanding ORP’s role in the disinfection process. Water Eng. Manage, 140, 29–31 (1993). [Google Scholar]
- Collos Y. et al. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J Appl. Phycol. 11, 179–184 (1999). [Google Scholar]
- Barnes H. & Folkard A. R. The determination of nitrites. Analyst 76, 599–603 (1951). [Google Scholar]
- Shinn M. B. Colorimetric method for determination of nitrate. Ind.Eng.Chem.Anal.Ed. 1, 33–35 (1941). [Google Scholar]
- Xie J., Sun X. H., Pan Y. J. & Zhao Y. Physicochemical properties and bactericidal activities of acidic electrolyzed water used or stored at different temperatures on shrimp. Food Res. Int. 47, 331–336 (2012). [Google Scholar]
- Park G., Lee H., Kim G. & Lee J. K. Global model of He/O2 and Ar/O2 atmospheric pressure glow discharges. Plasma Process. Polym. 5, 569–576 (2008). [Google Scholar]
- Mukkavilli S., Lee C. K., Varghese K. & Tavlarides L. L. Modeling of the electrostatic corona discharge reactor. IEEE Trans. Plasma Sci. 16, 652–660 (1988). [Google Scholar]
- Teodoru S., Kusano Y. & Bogaerts A. The effect of O2 in a humid O2/N2/NOx gas mixture on NOx and N2O remediation by an atmospheric pressure dielectric barrier discharge. Plasma Process. Polym. 9, 652–689 (2012). [Google Scholar]
- Tatarova E. et al. Microwave air plasma source at atmospheric pressure: Experiment and theory. J. Appl. Phys. 108, 123305 (2010). [Google Scholar]
- Fukumura D., Kashiwagi S. & Jain R. K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 6, 521–534 (2006). [DOI] [PubMed] [Google Scholar]
- Liao L. B., Chen W. M. & Xiao X. M. The generation and inactivation mechanism of oxidation–reduction potential of electrolyzed oxidizing water. J. Food Eng. 78, 1326–1332 (2007). [Google Scholar]
- Burlica R., Grim R., Shih K., Balkwill D. & Locke B. Bacteria inactivation using low power pulsed gliding arc discharges with water spray. Plasma Process. Polym. 7, 640–649 (2010). [Google Scholar]
- Jones C. W. Applications of Hydrogen Peroxide and Derivatives (Royal Society of Chemistry, Cambridge, 1999). [Google Scholar]
- Lee K., Paek K. H., Ju W. T. & Lee Y. Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygen, Journal of microbiology (Seoul, Korea) 44, 269–275 (2006). [PubMed] [Google Scholar]
- Barraud N. et al. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa, J Bacteriol. 188, 7344–7353 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel U., Andrasch M., Weltmann K.-D. & Ehlbeck J. Inactivation of Vegetative Microorganisms and Bacillus atrophaeus Endospores by Reactive Nitrogen Species (RNS). Plasma Process. Polym. 11, 110–116 (2014). [Google Scholar]
- Hao X. et al. Nitric oxide generation with an air operated non-thermal plasma jet and associated microbial inactivation mechanisms. Plasma Process. Polym. 11, 1044–1056 (2014). [Google Scholar]
- Lukes P., Dolezalova E., Sisrova I. & Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 23, 015019 (2014). [Google Scholar]
- Lai T., Li B., Qin G. & Tian S. Oxidative damage involves in the inhibitory effect of nitric oxide on spore germination of Penicillium expansum. Curr. Microbiol. 62, 229–234 (2011). [DOI] [PubMed] [Google Scholar]