Abstract
Objective
Appetitive responses to weight loss are mediated by a nutrient-sensing neural network comprised of melanocortin neurons. The role of neural melanocortin-3 receptors (MC3R) in mediating these responses is enigmatic. Mc3r knockout mice exhibit a paradoxical phenotype of obesity and reduced feeding-related behaviors in situations of nutrient scarcity. Here we examined whether MC3Rs expressed in mesolimbic neurons regulate feeding-related motivational responses.
Methods
Interactions between Mc3r genotype, cognitive function and energy balance on food self-administration were assessed using operant conditioning with fixed- and progressive ratio (FR1/PR1) settings. Inhibition of Mc3r transcription by a loxP-flanked transcriptional blocker (TB) in C57BL/6JN mice (Mc3rTB/TB) was reversed in mesolimbic neurons using DAT-Cre (DAT-MC3R).
Results
Caloric restriction (CR) caused 10–15% weight loss and increased motivation to acquire food rewards during training sessions. c-Fos-expression in the nucleus accumbens was increased 1 h following food presentation. While exhibiting weight loss, total food self-administration, enhanced motivation to self-administer food rewards in training sessions held during CR and c-Fos-activation in the nucleus accumbens following re-feeding were all markedly attenuated in Mc3rTB/TB mice. In contrast, cognitive abilities were normal in Mc3rTB/TB mice. Total food self-administration during FR1 sessions was not rescued in DAT-MC3R mice, however enhanced motivational responses to self-administer food rewards in PR1 conditions were restored. The nutrient-partitioning phenotype observed with Mc3r-deficiency was not rescued in DAT-MC3R mice.
Conclusions
Mesolimbic MC3Rs mediate enhanced motivational responses during CR. However, they are insufficient to restore normal caloric loading when food is presented during CR and do not affect metabolic conditions altering nutrient partitioning.
Keywords: Melanocortins, Melanocortin-3 receptors, Dopamine, Motivation, Appetite, Caloric restriction
Graphical abstract
Highlights
-
•
Food-related motivational responses in mice increase with caloric restriction (CR).
-
•
Melanocortin-3 receptors (MC3R) are required for food-related motivational responses.
-
•
MC3Rs role in food-related motivational responses depends on metabolic condition.
-
•
Mesolimbic MC3Rs increase food-related motivational responses during CR.
1. Introduction
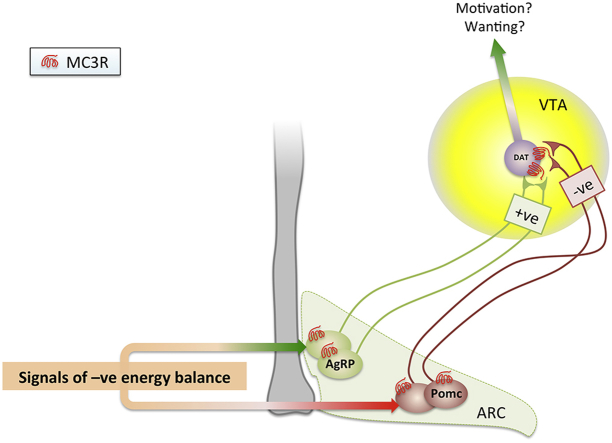
The central nervous melanocortin system forms a focal point for the nutrient-sensing neural networks coordinating appetitive and metabolic responses to internal cues of metabolic state [1]. The core system is comprised of two populations of neurons in the arcuate nucleus of the hypothalamus (ARC) expressing the endogenous melanocortin ligands. Activation of neurons expressing proopiomelanocortin (POMC), a precursor polypeptide processed to produce α− and γ−melanocortin stimulating hormones (α−/γ−MSH), inhibits feeding behaviors and increases metabolic rate. Neurons co-expressing agouti-related peptide (AgRP), neuropeptide Y (Npy) and GABA act oppositely, promoting expression of feeding-related behaviors while activating metabolic programs conserving and storing energy.
The acute feeding and metabolic responses to melanocortin analogs requires melanocortin-4 receptors (MC4Rs), a 7-transmembrane G protein-coupled receptor expressed throughout the brain [2], [3], [4]. Clinical relevance is indicated by observation of hyperphagic obesity syndromes in humans inheriting nonsense mutations in the POMC or MC4R genes [1], [5]. Another member of the melanocortin receptor family, the melanocortin-3 receptor (MC3R), is expressed in hypothalamic and limbic structures controlling autonomic function and complex ingestive behaviors [6], [7], [8]. Deletion of the Mc3r gene results in altered nutrient partitioning that favors accrual of fat mass (FM) in mice [8], [9], [10], [11], [12], [13]. MC3R mutations may also cause obesity in humans [14]. While MC4Rs have been linked to circuits governing appetite and energy expenditure [15], the exact physiological roles of neural MC3Rs related to weight control have remained enigmatic.
Experiments using Mc3r and Mc4r knockout (−/−) mice treated with MSH-analogs indicate MC3Rs are neither necessary nor sufficient for inhibiting feeding behaviors [10], [16], [17], [18], [19] or stimulating energy expenditure [17], [19]. Both hyper- and hypophagia have sometimes been observed in Mc3r-deficient mice, suggesting sensitivity to genetic background and housing conditions [10], [13], [14]. The obese phenotype of Mc3r−/− mice has therefore been widely considered to be metabolic, with altered partitioning of nutrients between lean tissues and lipid reserves. Factors contributing to this metabolic condition have been proposed to include hypercorticosteronemia [20], attenuated fasting-induced lipolysis in white adipose tissue [20], failure to suppress hepatic de novo lipogenesis during situations of caloric insufficiency [21], [22], and increased differentiation of mesenchymal stem cells to adipocytes [14].
Mc3r-deficiency in mice is associated with a paradoxical phenotype in which obesity is associated with reduced feeding-related anticipatory responses [8], [23], [24], [25], [26]. MC3Rs are necessary for adaptive feeding responses during fasting [20] and hypocaloric restricted feeding paradigms [8], [23], [24], [26]. MC3Rs may modulate responses to internal cues of metabolic state inducing food anticipatory activity (FAA), a progressive increase in locomotor activity anticipating food presentation when mice are fed a single hypocaloric meal at daily intervals [27]. FAA involves a coordinated phase shift in rhythms of appetitive behaviors and wakefulness to coincide with nutrient consumption [28], [29]. Attenuated FAA in Mc3r−/− mice may at least partially result from insensitivity of AgRP/NPY neurons to internal cues of metabolic state [20], [25]. As observed for MC4Rs [15], MC3R-dependent pathways affecting feeding behavior, peripheral metabolism and protection from obesity may be distributed between discrete populations of cells in the central nervous system and periphery.
The studies presented herein had two objectives. We first assessed whether deficits in food anticipatory behaviors observed in Mc3r-deficient mice manifest as reduced feeding-related motivational responses, using operant conditioning to measure self-administration of food rewards in situations of nutrient sufficiency (ad libitum feeding) and caloric restriction (CR). We report that MC3Rs are required for the full expression of feeding-related motivational responses, but only in situations of caloric insufficiency. Second, we assessed whether MC3Rs expressed in the mesolimbic dopaminergic system receiving inputs from AgRP and POMC neurons [6], [7], [30], [31], regulate feeding-related motivational responses. Injection of melanocortin agonists into the ventral tegmental area (VTA) alters reward-related behaviors towards food and addictive substances, and affects changes in dopamine transmission to the ventral striatum/nucleus accumbens (NAc) [31], [32]. We report that rescuing MC3Rs in the mesolimbic dopaminergic system enhances some feeding-related motivational responses during situations of caloric insufficiency, but has no impact on the obese phenotype.
2. Materials and methods
2.1. Animals
All studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Scripps Research Institute in Jupiter, FL. Animals were maintained in a 12 h light/dark cycle, at standard room temperature (23 °C) and were singly housed during the experimental procedure. All studies were performed in male mice during the light phase of the cycle. Training sessions and feeding times were kept consistent every day in each group of animals.
The role of MC3Rs in regulating motivational responses was assessed using Mc3rtm1Butl (Mc3rTB/TB) mice. The expression of Mc3r gene in this strain is suppressed by insertion of a loxP-flanked transcription blocker (TB) into the genes 5′ UTR [8]. The initial studies of food self-administration used male Mc3rTB/TB and littermate controls produced from Mc3rTB/+ breeding pairs on a mixed C57BL/6J;C57BL/6N background. While the LoxTBMc3r strain was developed using Bruce 4 embryonic stem cells derived from C57BL/6J mice [8], contamination with C57BL/6N is presumed to have occurred at some stage during colony maintenance. The ratio of genes derived from the 6J to 6N substrains in the mice used for these experiments is therefore unclear. The role of MC3Rs and MC4Rs in regulating motivational responses to melanocortin agonists administered icv was further assessed using B6;129S4-Mc4rtm1Lowl/J mice (Mc4rTB/TB) purchased from the Jackson Laboratory. In this strain, Mc4r expression is suppressed by insertion of the loxP-flanked TB into the genes 5′ UTR [33].
To assess the role of Mc3rs expressed by dopaminergic neurons in regulating feeding-related behaviors, we used Cre-mediated recombination to selectively restore transcription in Mc3rTB/TB mice. B6.SJL-Slc6a3tm1.1(cre)Bkmn/J mice (DAT-Cre) were used to excise the TB in mesolimbic neurons in the ventral tegmental area (VTA) [34], generating DAT-cre;Mc3rTB/TB mice (hereafter referred to as DAT-MC3R). DAT-cre(-ve);Mc3rTB/+ females were mated with DAT-cre(+ve);Mc3rTB/+ males. DAT-cre and ‘true’ wild type controls (WT: negative for both the Cre and TB-modified Mc3r alleles), Mc3rTB/TB and DAT-MC3R were derived from the same litters.
To visualize Mc3r-expressing neurons, we used Tg(Mc3r-EGFP)BX153Gsat mice (hereafter referred to as Mc3r-eGFP) purchased from the Mutant Mouse Regional Resource Center [7], [35]. This strain carries a bacterial artificial chromosome containing a modified Mc3r gene; an enhanced green fluorescent protein (eGFP) followed by polyadenylation signal were inserted into the ATG transcription initiation codon. Cre activity in Mc3r-positive neurons in the VTA was verified using a reporter strain expressing red fluorescent protein variant (tdTomato) (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) [36] crossed with the DAT-Cre and Mc3r-eGFP strains.
2.2. Behavioral testing
Self-administration experiments were performed in operant chambers housed in sound-attenuating cubicles (Coulbourn Instruments). Each chamber was equipped with two levers, a food pellet hopper and a house light. One lever was predetermined to be the “active” lever (lever pressing resulted in a positive outcome/food reward), while the other lever was “inactive” (lever pressing had no outcome). Total active lever pressings (not only the pressings that lead to reward, but also pressings during the time-out period after pellet acquisition) are presented. An initial evaluation indicated similar results when only the pressings that lead to reward were analyzed (data not shown). Inactive lever pressings were assessed. Active and inactive levers were counterbalanced between animals.
Adult mice aged 3.5–4 months were initially subjected to caloric restriction (CR) for 14 d before the start of behavioral testing. During this period, mice were provided 2.7 g of standard rodent chow (Teklad Global 19.1% Protein Rodent Diet, Harlan Laboratories) at the time coinciding with the completion of self-administration sessions later in the study. This feeding protocol resulted in a 10–15% reduction of body weight, and is compatible with previously described protocols used to study anticipatory behaviors in Mc3r-deficient mice [37], [38]. During training, chow was provided once per day after the training session (2.5 g/d). The first two days of habituation involved mice undergoing a “magazine schedule” in the chambers, a 30 min session during which a 20 mg chocolate flavored food pellet (sucrose; Dustless Precision Pellets 20 mg, Bioserv) was released from the food hopper every minute coinciding with activation of a light cue over the active lever (total of 30 pellets). The mice were then trained for 7 d in a Fixed Ratio 1 (FR1) schedule; pressing the active lever resulted in presentation of a sucrose pellet and activation of a light cue. Pressing the inactive lever had no outcome. Prior to shifting to the more demanding progressive ratio (PR) schedule, mice were shifted to 2 d training each at FR2 or FR5 (2 or 5 lever pressings required to obtain a food pellet) (data not shown). The mice were then subjected to 10 d of training in a PR1 schedule during which acquisition of each additional food reward required an additional lever press (n+1: 5, 6, 7 etc. lever pressings). Break point was determined as the effort (number of pressings) the animal did in order to obtain the last pellet.
Two lines of experiments were performed. The first line of experiments compared food self-administration in wild type (WT) and Mc3rTB/TB littermates. These experiments involved two cohorts of mice subjected to either 30 min training sessions with a 20 s time out period following each reward (shown as experiment 1), or 60 min training sessions with a 10 s time out period during the PR1 (data not shown). The longer session was used for analyzing food self-administration of DAT-MC3R mice (experiment 2). After 10 d training in PR1, mice were returned to ad libitum feeding conditions and the PR1 schedule maintained. Furthermore, we replaced sucrose with standard chow pellets (20 mg) and animals were tested in ad libitum feeding conditions in order to assess any potential genotype difference in preference for sucrose versus standard chow. For drug treatment a 2 h session was performed with animals that were trained in the PR1 schedule using sucrose pellets as reward.
Cognitive flexibility was assessed in a separate experiment using mice trained in a 60 min PR1 schedule session and mice maintained in a state of negative energy balance. During that session the inactive lever was switched to serve as the active (pressing of that lever resulted in a food reward) while the previously active lever served as an inactive (no outcome). The time taken for mice to adapt to inversion of the active and inactive levers was then used to assess cognitive performance.
2.3. Intracerebroventricular administration of MSH
Mice were anesthetized using isoflurane inhalation. A stainless steel cannula (Plastics One) was implanted into the right lateral ventricle and the mechanism was fixed to the skull using four stainless steel screws and dental cement. Stereotaxic coordinates were obtained in accordance to Paxinos and Watson mouse brain atlas (anteriorposterior; −0.2 mm; midlateral; +1 mm and dorsoventral; −2 mm). Injectors extending 0.5 mm beyond the cannula were used. D-trp8-γ-MSH (Tocris Bioscience) was dissolved in artificial cerebrospinal fluid (ACSF, Tocris Bioscience) (0.5 nm/μl). 1 nM of D-trp8-γ-MSH was injected at a rate of 0.5 μl/min into the lateral ventricle, with ACSF (2 μl at a rate of 0.5 μl/min) used as control. At the end of the experiment, ink was injected via the cannula, animals were sacrificed and brains were extracted and inspected for ink distribution.
2.4. Morris water maze
Spatial learning and memory was assessed using the Morris water maze/navigation task as described previously [39], [40]. Briefly, the mice had to learn to use distal cues to locate a platform hidden in a circular tank (48-in. diameter, water temperature 23 °C). Before starting the training, a visual platform test (platform elevated above water level and no distal cues) was performed to assess visual ability of the mice. The animals were then trained for 8 consecutive days in 4 trials per day (intertrial interval, approx. 30 min). The start position varied randomly for each trial. The platform was placed in the NW compartment of the tank. The mice had 60 s to locate the platform or they were guided to it. Once the platform was reached, the mice were left 15 s on the platform. The % of success and latency to find the platform were averaged per day, and used as acquisition parameters. In order to assess reference memory and memory consolidation, two probe trials (each, 60 s), during which the platform was removed, were performed 24 h and 10 days following the acquisition. Time spent in each compartment (NW, NE, SW, SE) of the tank was recorded. Performance was tracked using EthoVision XT (Noldus Information Technology, Inc).
2.5. Quantitative real time PCR
Mice were decapitated, brains snap-frozen on dry-ice/isopentane slurry and stored at −80 °C. The VTA was punched from frozen brains and total RNA extracted using Trizol reagent. Total RNA was treated with DNA-free kit (Ambion, Life Technologies) to remove genomic DNA contamination. 200 ng RNA was used for cDNA synthesis using a Superscript III Reverse transcription kit (Invitrogen) according to manufacturer's instructions. Quantitative RT-PCR was performed in a 384-well plate using TaqMan gene expression assays for Mc3r and Dat (Mm00434876_s1 and Mm00438388_m1; Applied Biosystems) using an Applied Biosystems 7900 HT Detection System. RPL30 (Mm01611464_g1; Applied Biosystems) was used as a reference gene. Expression data were analyzed according to the ΔΔCt method.
2.6. Immunohistochemistry
To assess feeding-related changes in neural activity during CR, we examined how feeding affects c-Fos immunoreactivity. Adult naïve WT and Mc3rTB/TB mice were each divided into two groups (minimum sample size of 3 mice/group): CR without food at time of sacrifice and CR with 1 h of food access using the same protocol applied in the self-administration studies. CR was applied for 14 d, on the day of sacrifice half received a regular meal (ad libitum access) while half did not. The food was provided mid-light cycle, every day at the same time.
Animals were initially perfused transcardially with 10 ml 0.01 M PBS followed by 50 ml of 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were postfixed in 4% PFA overnight at 4 °C, and then cryoprotected for 24–48 h in 30% sucrose at 4 °C. Following the sucrose cryoprotection procedure, brains were snap frozen in isopentane (−40 °C) and 20 μm coronal sections were cut on a cryostat (Leica) and mounted on Superfrost Plus Microscope Slides (Fisher Scientific). Sections were initially incubated for 1 h at room temperature with a blocking solution (5% normal donkey serum and 0.5% Triton in 0.01 M PBS). Immunohistochemistry was performed using an anti-c-Fos rabbit antiserum synthesized against amino acids 4–17 of the human protein (1:1000, Ab-5 ref#PC38, Calbiochem). Expression of eGFP was assessed using and anti-GFP rabbit antibody (for the reporter mice; 1:200, Cat# A-11122, ThermoFisher Scientific) in 5% normal donkey serum and 0.5% Triton in 0.01 M PBS.
Sections were incubated for 24 h with the first antibodies at 4 °C followed by 3 washes in 0.01 M PBS. Subsequently, the slices were incubated for 2 h with the following secondary antibody (1:400) anti-rabbit Alexa 488 (ThermoFisher Scientific) and washed 3 times in 0.01 M PBS. FluorSave Reagent (Calbiochem) was used for mounting sections. For analysis of Cre activation of tdTomato transcription, no staining against the tdTomato fluorophore was required.
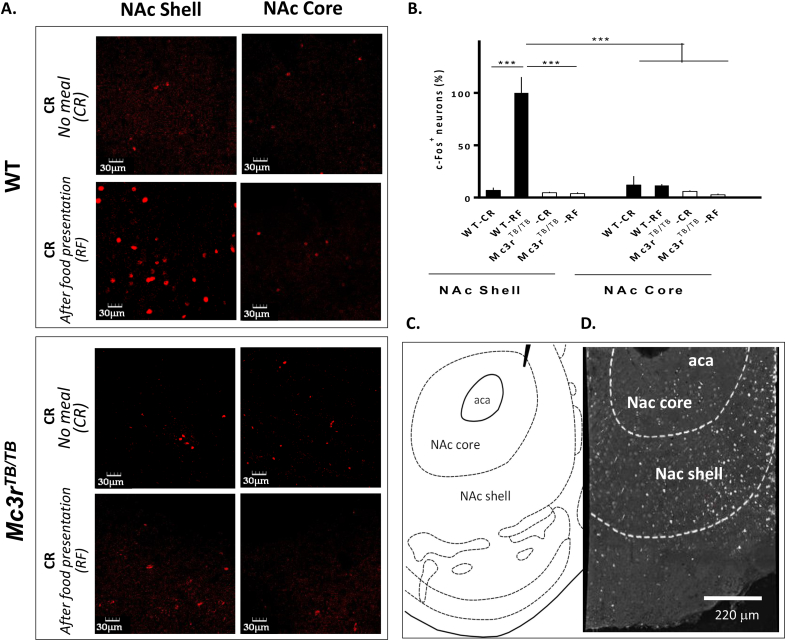
Immunofluorescence was visualized using an Olympus FluoView 1000 laser-scanning confocal microscope using Olympus 40× oil objective for the c-Fos study. For c-Fos labeling, identical settings were used to image all sections within a different group of mice WT– Mc3rTB/TB pairs (nucleus accumbens; NAc at the level of +0.9–1.2 mm from bregma). For each brain two images per section (one for each hemisphere) were acquired. Quantitative image analysis was performed on raw data using ImageJ software. c-Fos positive cell staining was defined as particles with an area of at least 50 pixels. The data are expressed as percentage of WT group subjected to CR with food (i.e., “baseline” conditions for mice subjected to CR for 14 d, with tissue collected without food on the final day). For the reporter animals, images from single layer sections were obtained in order to reveal co-localization. For the image illustrating spatial compartmentalization of the NAc shown in Figure 3, we constructed a mosaic using 25 images obtained with 40× oil objective.
Figure 3.
Increased c-Fos expression in the nucleus accumbens (NAc) shell of WT but not Mc3rTB/TBmice after a meal during CR. (A) C-Fos expression in the NAc shell and core of a representative WT and Mc3rTB/TB mouse. (B) Quantification of c-Fos positive neurons in the NAc shell and core; RC refers to mice subjected to CR but not fed on the day of tissue collection, while RF refers to mice subjected to CR and given food 1 h prior to tissue collection. (C) Imaging showing compartmentalization of NAc core and shell according to Paxinos and Watson atlas (1.18 mm from bregma). (D) Montage of photomicrographs taken from a WT animal after re-feeding, showing the area of c-Fos staining induced with feeding. CR: caloric restriction; RF: tissue harvested after re-feeding. ***p < 0.001.
2.7. Assessment of body composition
Body composition was determined by using nuclear magnetic resonance (NMR, Bruker Minispec) to measure fat mass (FM) and fat-free mass (FFM) before the beginning of CR (3.5–4 months old).
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 and IBM SPSS Statistics vers. 23. Data were initially analyzed using one or two-way Analysis of Variance (ANOVA) with or without repeated measures according to experimental design. Further analysis was performed using Bonferonni's posthoc test for multiple comparisons when appropriate. Comparisons between two groups were analyzed using t-test. Body composition data obtained using NMR were analyzed by ANCOVA using body weight as a covariate [41], [42]. P values of <0.05 were considered significant.
3. Results
3.1. Mc3rs are required for increased motivation to self-administer food during CR
Mc3r-deficient mice exhibit reduced re-feeding during situations of fasting and hypocaloric feeding schedules [20], [24]. To determine whether this phenotype reflects reduced feeding-related motivational responses, we compared self-administration of food rewards in a 30 min training session in mice subjected to CR to induce weight loss. The rationale for using the 30 min session was based on earlier studies in which food access was restricted temporally to a 4 h period using a mechanical barrier [24]. Following food access, mice exhibit a nutrient-loading (‘gorging’) phase, consuming 60–70% of total caloric intake in 30–40 min, followed by 200–210 min of low intensity feeding (nibbling). Gorging is markedly attenuated in Mc3rTB/TB mice with no compensation during the nibbling phase, suggesting premature satiation or a lack of re-feeding responses. We therefore examined food-self administration in mice in which access was limited to a period of time corresponding to the nutrient-loading phase.
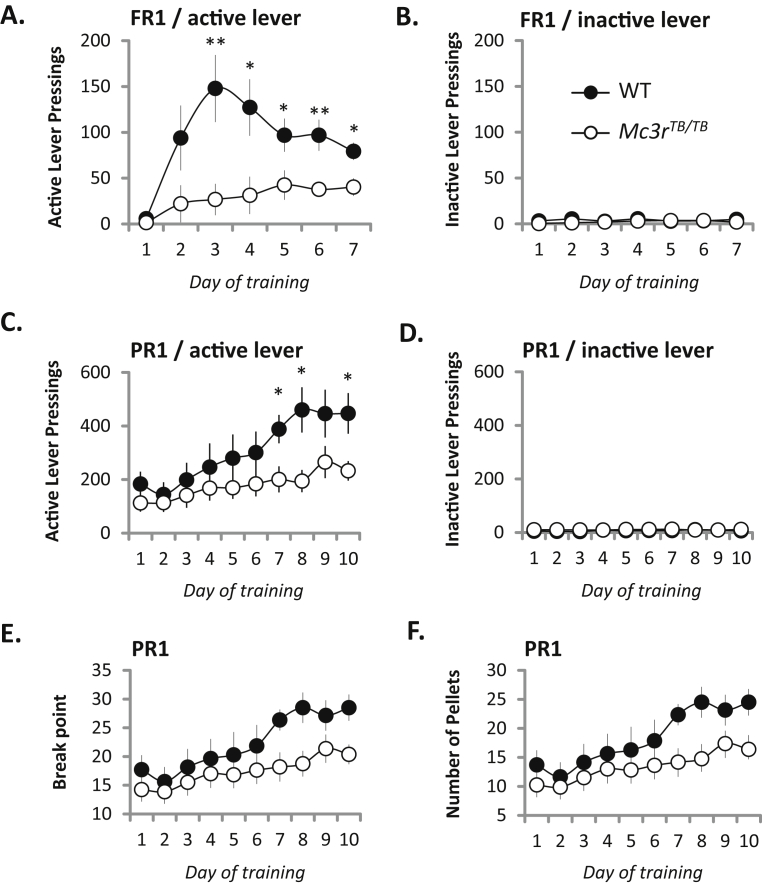
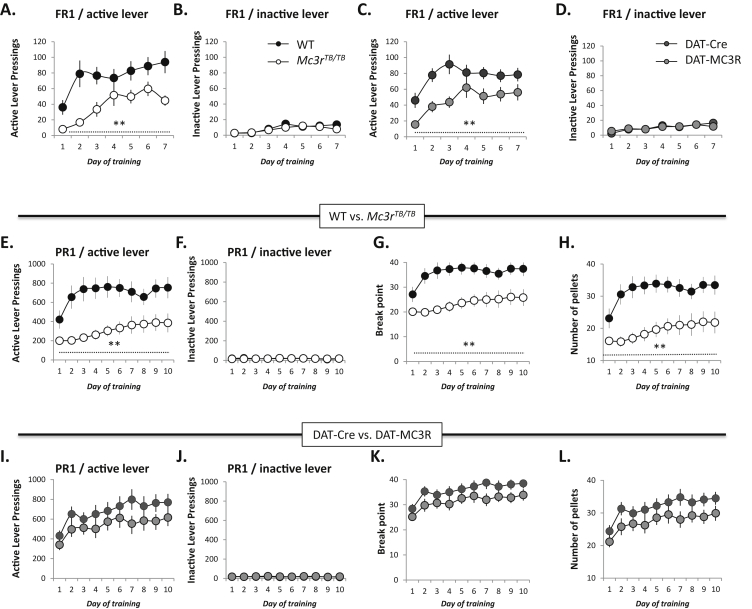
We anticipated that motivation to self-administer food rewards during CR would be increased when compared to ad libitum feeding conditions. This condition also enables rapid acquisition of the self-administration behavior. Analysis of self-administration during the 7 d FR1 arm of the 30 min training session when 1 lever press results in acquisition of 1 sucrose pellet indicated reduced feeding responses to CR in Mc3rTB/TB mice (Figure 1A). For the 30 min training session, there was a significant interaction between genotype and time in total active lever pressings [F(6,78) = 3.357, p < 0.01], with the genotype effect attaining statistical significance on days 3–7 (Figure 1A). Inactive lever pressing activity was negligible, and not affected by genotype (Figure 1B).
Figure 1.
Self-administration of food rewards and relevant motivation is reduced in Mc3rTB/TBmice during situations of caloric insufficiency. All mice were subjected to 14 d of CR (2.7 g of chow/day) prior to training; during the trials mice were provided 2.5 g of chow/day in addition to the amount of food (20 mg sucrose pellets) acquired during the trials. Total active (A, C) and inactive lever pressings (B, D) in a 30 min session in a fixed ratio 1 (FR1) (A, B) or progressive ratio 1 (PR1) schedule (C, D). Break point during the PR1 schedule (E). Number of pellets acquired during the PR1 schedule (F). The mice were subjected to 30 min training sessions and maintained in a CR state during the course of the trials. * p < 0.05, **p < 0.01 compared to WT.
The effort required to obtain the food reward in the FR1 schedule (one lever press) is minimal; these results are therefore consistent with attenuated re-feeding responses in Mc3rTB/TB mice during situations of negative energy balance [20], [24]. The failure of Mc3rTB/TB mice to increase food intake during the course of several days of repetitive 30 min training sessions could indicate a role in optimizing instrument performance. Alternatively, Mc3rTB/TB mice may be less motivated to adapt to a shortened time interval.
We next assessed motivation to self administer food rewards using a progressive ratio protocol (Figure 1C–F). In the PR1 arm, Mc3rTB/TB mice also exhibited a time-dependent significant reduction in active lever pressings [genotype × time interaction F(1, 117) = 1.97, p < 0.05], with statistical significance attained on days 7, 8 and 10 (p < 0.05) of training relative to controls (Figure 1C). Although the same trend was observed in the break point and number of pellets, these effects did not reach significance in the 30 min session. As observed during the FR1 training session, pressing of the inactive leaver was minimal and not affected by genotype (Figure 1D).
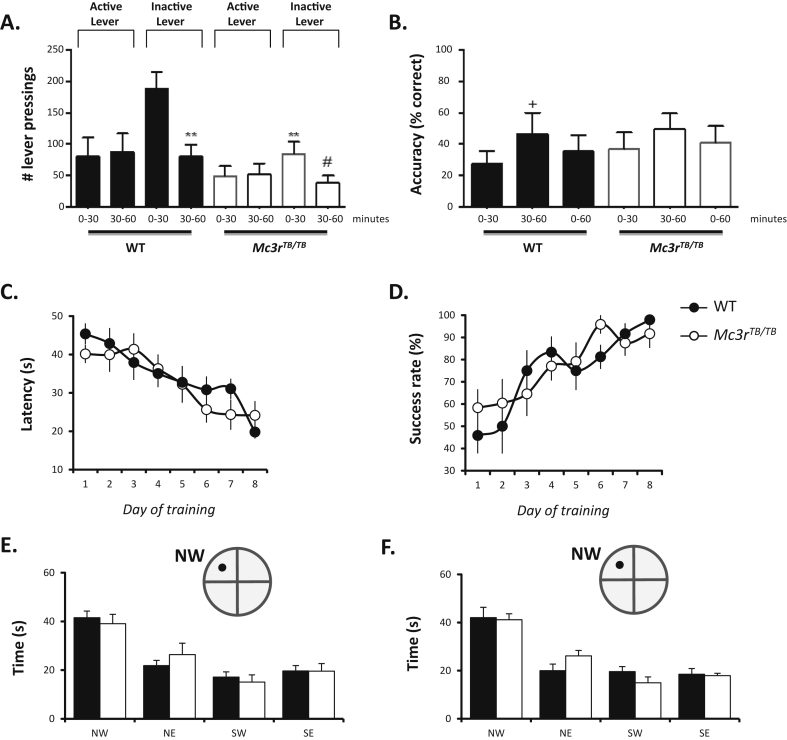
3.2. Reduced lever pressing is not the result of cognitive deficits
To determine whether impaired cognitive function explains reduced food self-administration, we used an active/inactive lever shift task. Mc3rTB/TB mice exhibited a normal adaptation to the new setting, rapidly shifting focus to the new active lever (Figure 2A,B). A Morris water maze task was used to compare spatial learning and memory in a separate batch of WT and Mc3rTB/TB mice (Figure 2C–F). There was no significant effect of genotype during the 8 d of acquisition, measured by latency and % of success to find the platform (Figure 2C,D); there was also no deficit in long-term memory as measured in time spent in each quadrant on probe days 1 and 2 (Figure 2E,F).
Figure 2.
Cognitive function of Mc3rTB/TBmice is normal. Cognitive abilities of Mc3rTB/TB mice (white bars/circles) were compared to control WT littermates (black bars/circles) by testing their ability to adapt during a lever-switching test (A, B), and by testing spatial memory in a Morris water maze (C–F). WT and Mc3rTB/TB mice demonstrated increased inactive lever pressings in the first 30 min of the session but they adapted in the second part of the session (30–60 min) (A, B). Acquisition of water maze task (C, D), probe 1 (E) and probe 2 (F). **p < 0.01 compared to WT 0–30 min inactive; #p < 0.05 compared to Mc3rTB/TB 0–30 inactive; +p < 0.05 compared to 0–30 min WT.
3.3. MC3Rs are required for activation of mesolimbic neurons by feeding during CR
MC3Rs expressed in mesolimbic dopamine neurons have been suggested to regulate reward-related behaviors, affecting dopamine turnover and altering sucrose preference in female mice [7]. Activation of dopaminergic neurons in the VTA has been shown to induce preferential dopamine release in the shell of the NAc [43]; an area that influences motivation to obtain food primarily by determining its reward value [44], [45]. Previous studies indicated that re-feeding but not fasting increases c-Fos activation in the NAc [44]. In the current study, re-feeding (1 h after) induced c-Fos activation in the NAc Shell, but not core, of WT but not Mc3rTB/TB mice (Figure 3A,B).
3.4. Rescuing limbic Mc3r transcription using DAT-Cre
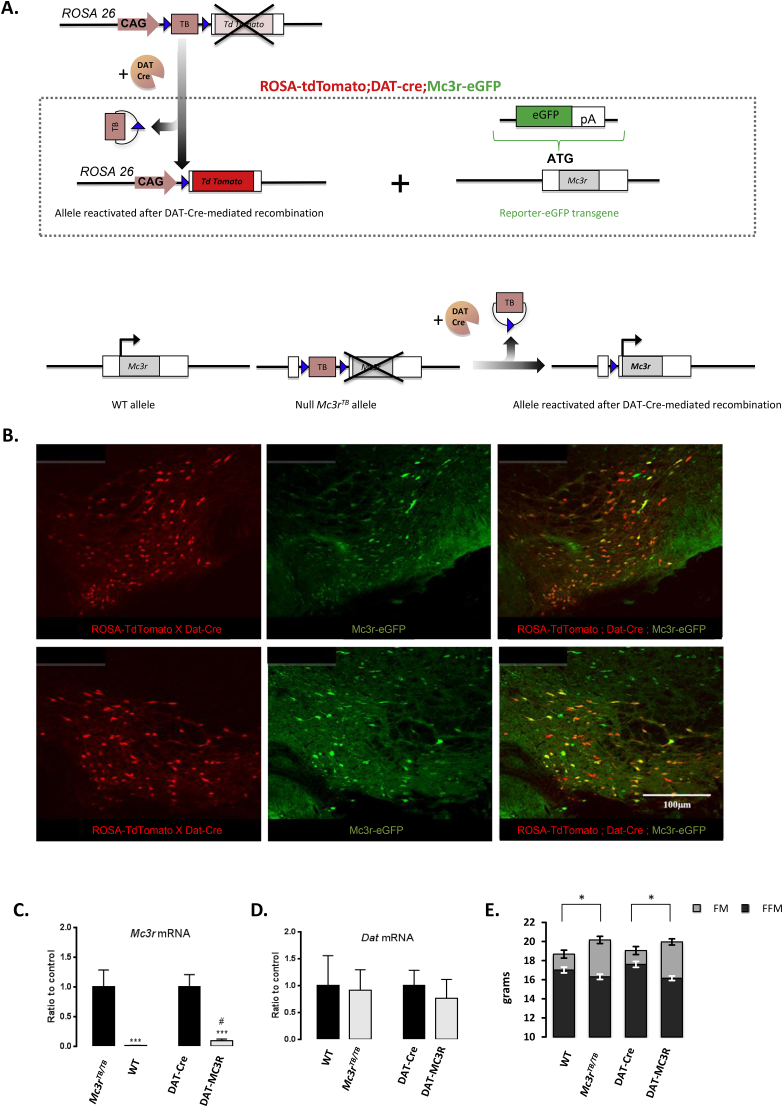
The role of MC3Rs expressed in the limbic system was investigated using DAT-Cre to rescue Mc3r transcription by removal of the loxTB sequence (Figure 4A). We first confirmed co-localization of Cre activity in Mc3r (+ve) neurons in the VTA by crossing DAT-Cre;tdTomato reporter mice with MC3R-eGFP mice (Figure 4B). DAT-MC3R mice exhibited a partial rescue when Mc3r mRNA expression was measured quantitatively in the VTA using qRT-PCR (Figure 4C). Expression of Dat mRNA was normal in all four lines (WT, DAT-Cre, DAT-MC3R and Mc3rTB/TB) (Figure 4D).
Figure 4.
Rescuing Mc3r expression in the VTA has no effect on body composition. (A) Schematic of the strategy used to visualize colocalization of Cre activity in Mc3r positive neurons of transgenic ROSA-tdTomato;DAT-Cre;Mc3r-eGFP mice (upper panel) and for restoring Mc3r transcription in DAT-MC3R mice (lower panel). (B) Photomicrographs showing Cre-mediated recombination in MC3R expressing neurons in the VTA of ROSA-tdTomato;DAT-cre;Mc3r-eGFP mouse. The upper and lower panels are representative of more anterior (upper) or posterior (lower) section. (C) Analysis of Mc3r expression in WT (n = 5), Mc3rTB/TB mice (n = 9); DAT-Cre (n = 9) and DAT-MC3R mice (n = 15) mice by qRT-PCR. (D) Dat expression is not affected by genotype. *p < 0.05, ***p < 0.001 compared to there corresponding control; #p < 0.05 compared to Mc3rTB/TB mice. (E) Assessment of fat mass (FM) and fat-free mass (FFM) adjusted for body weight. ***p < 0.001 compared to there corresponding control for FM and FFM.
3.5. Limbic MC3Rs regulate feeding-related motivational responses but not adiposity
The nutrient partitioning phenotype classically associated with Mc3r-deficiency, increased FM and reduced FFM [8], [9], [10], was not rescued in DAT-MC3R mice (Figure 4E). For the DAT-MC3R study, FM and FFM data were analyzed using ANCOVA with genotype as an independent variable and body weight (BW) as a covariate. There were significant effects of Mc3r genotype on FM (increased) and FFM (reduced) (both p < 0.001), but no significant effect of DAT-Cre genotype and no interaction.
We next assessed whether limbic MC3Rs are involved in regulate feeding-related motivational responses (Figure 5). For this analysis, data are presented comparing Mc3rTB/TB and DAT-MC3R with the appropriate controls (WT, DAT-Cre) (Figure 5A–D for FR1 training sessions; E-L for PR1 training sessions). During the FR1 training session, Mc3rTB/TB [Mc3r effect; F(1, 19) = 12.244, p < 0.01] and DAT-MC3R [Mc3r effect; F(1, 26) = 10.279, p < 0.01] mice both exhibited reduced active lever pressings/food self-administration compared to their corresponding controls (Figure 5A,C). As before, the level of inactive lever pressing was minimal and not affected by genotype (Figure 5B,D). There was no significant difference between the WT (Cre negative) and DAT-Cre controls for any of these parameters.
Figure 5.
Effect of rescuing VTA Mc3r expression on self-administration of food rewards during CR. Data shown are from mice subjected to FR1 (A–D) and PR1 (E–L) training sessions after 14 d of CR to induce weight loss. As observed before (Figure 1A,B), Mc3rTB/TB mice exhibited decreased active (A) but not inactive (B) lever pressings compared to WT littermates in the FR1 schedule. A similar phenotype was evident in DAT-MC3R mice compared to their controls (DAT-Cre mice) (C, D). However, during the PR1 training sessions there was evidence for an improved performance of DAT-MC3R mice. As observed previously (Figure 1C–F), Mc3rTB/TB mice exhibited reduced active lever pressings (E), lower break point (G) and reduced pellet acquisition (H) compared to WT littermates. The decreased motivation to self-administer food rewards under PR1 conditions was at least partially restored in the DAT-MC3R mice, which relative to their controls showed no significant difference in active lever pressing (I), break point (K) or pellet acquisition (L). Pressing of the inactive lever was minimal during the PR1 training sessions, irrespective of genotype (F, J). Note also that the number of pellets obtained during PR1 was lower compared to FR1 (<40 vs. 80 pellets; for FR1 the number of pellets acquired is equal to the number of lever pressings). **p < 0.01 compared to corresponding WT.
During the PR1 session, Mc3rTB/TB mice again showed reduced motivation [F(1, 19) = 13.364, p < 0.01 for the active lever pressings; F(1, 19) = 13.770, p < 0.001 for the break point and F(1, 19) = 13.770, p < 0.001 for the rewards compared to their respective control groups (Figure 5E–H)]. However, we now observed evidence for a rescue, with DAT-MC3R mice exhibiting self-administration levels closer to their controls (DAT-Cre mice, p > 0.05) (Figure 5I–L).
3.6. Restoring ad libitum food access results in loss of phenotype
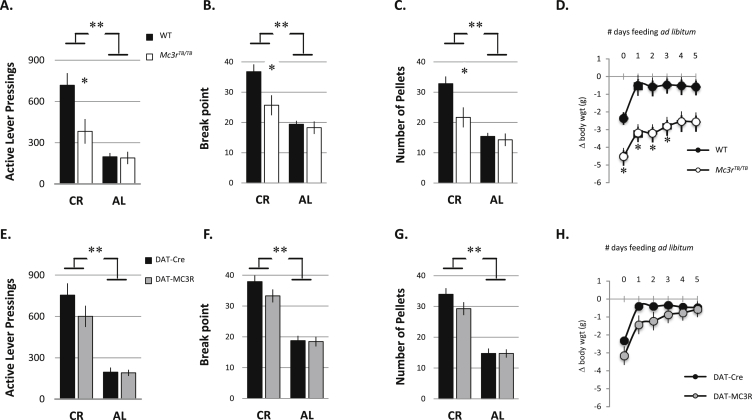
Up to this time, self-administration had been tested in mice maintained at a body weight 10–15% below normal. Releasing mice from CR in the PR1 condition by restoring ad libitum access to chow resulted in an immediate reduction in self-administration as assessed using lever presses, break point and pellets obtained during the training session (p < 0.01) (Figure 6A–C). Reduced motivation associated with loss of MC3R signaling is thus dependent on metabolic state. DAT-MC3R mice exhibited no difference in self-administration in the CR state during the final trainings sessions, and exhibited a normal response to AL feeding (Figure 6E–G).
Figure 6.
Comparison of food self-administration during CR and ad libitum feeding conditions. Active lever pressings (A, E), break point (B, F), number of pellets acquired (C, G) and body weight rebound (D, H) are shown. The data during CR are the mean of final three days of PR1 training (days 8–10); in panels D and H the change in body weight from baseline condition (i.e., prior to CR-induced weight loss) are shown as Δ body weight in grams. *p < 0.05 compared to WT; **p < 0.01 compared to the corresponding CR condition; CR: caloric restriction; AL: ad Libitum; wgt, body weight.
Results from assessing changes in body weight from baseline at the end of the PR1 training sessions, when the animals were returned to ad libitum feeding, were consistent with pellet data during the PR1 training sessions. Weight loss at the end of training was more pronounced in Mc3rTB/TB mice relative to controls (Figure 6D), although this was due to WT mice regaining some of the weight that had been lost with the initial 14 d of CR during the FR and PR trial sessions. This difference might therefore be attributed to reduced food self-administration by Mc3rTB/TB mice. There was no difference between DAT-Cre and DAT-MC3R mice (Figure 6H), consistent with improved performance during PR training. WT, DAT-Cre and DAT-MC3R mice demonstrated an immediate body weight (BW) rebound following returning back to ad libitum feeding conditions (p < 0.001); this response was delayed in Mc3rTB/TB mice.
3.7. Increased motivation to obtain sucrose versus chow rewards in ad libitum fed mice is not dependent on MC3Rs
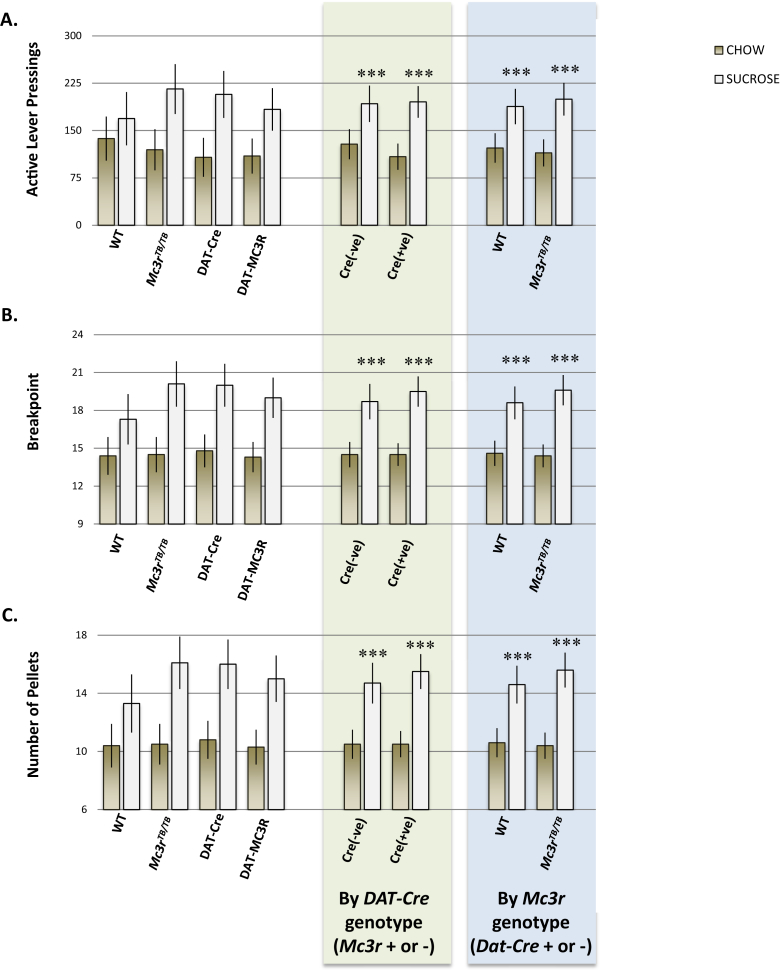
We next examined whether preference for sucrose consumption is affected by genotype, comparing self-administration of sucrose pellets or standard chow pellets in AL feeding condition. Self-administration of sucrose pellets was significantly higher compared to chow pellets [diet effect; F(1,31) = 38.652, p < 0.001], indicating increased motivation to work for sucrose versus standard chow food rewards, irrespective of Mc3r or DAT-Cre genotype and without evidence of interaction (Figure 7). Increased preference for sucrose was also observed in other experiments in which male WT and Mc3rTB/TB mice were provided the choice of sweetened or non-sweetened purified diets (data not shown).
Figure 7.
Comparison of sucrose and standard chow self-administration in ad libitum feeding conditions. The data shown are after 6 d of exposure to chow pellets in ad libitum feeding condition; the mice had been exposed to sucrose pellets throughout the experiment with the data shown here being recorded after 7 d of ad libitum feeding. (A) Active lever pressings; (B) break point; (C) number of pellets. ***p < 0.001 compared to corresponding chow.
3.8. MC3Rs are insufficient for inhibition of food self-administration by MSH
Finding that MC3Rs promote feeding-related motivational behaviors contradicts previous results using Mc4r−/− mice in which the inhibitory effects of MSH on food intake were shown to be MC4R-dependent and MC3R-independent [10], [16], [17], [18]. However, a recent study did observe that γ−MSH increases self-administration of sucrose rewards when administered into the VTA of rats [31]. We therefore compared the effect of D-Trp8-γ−MSH, a high affinity melanocortin receptor agonist exhibiting MC3R specificity when assayed using human receptors [46], administered icv on food self-administration in PR1 schedule of reinforcement, investigating MC3R-specific effects using LoxTBMc4r (Mc4rTB/TB) mice [33].
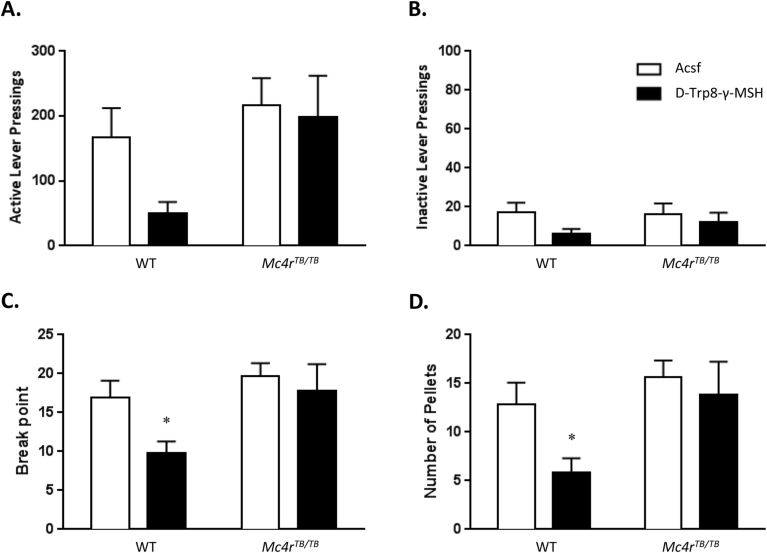
While the number of active lever pressings was lower in WT mice treated with D-Trp8-γ−MSH compared to vehicle treated controls (Figure 8A), the overall effect assessed using 2-way ANOVA (genotype, treatment) was not significant. Significant treatment effects were observed for inactive lever pressings [F(1,14) = 8.909, p < 0.01] (Figure 8B), break point [F(1,14) = 5.833, p < 0.05] (Figure 8C), and the total number of pellets [F(1,14) = 5.832, p < 0.05] (Figure 8D). However, no significant genotype effect or interaction (genotype × treatment) was observed for any of these parameters (p > 0.05, Figure 8). An analysis focused on the WT group indicated the anticipated inhibitory effect of treatment on break point [t(7) = 2.703, p < 0.05] and rewards [t(7) = 2.703, p < 0.05], but not on pressing of the inactive lever. No effect reached significance in the Mc4rTB/TB mice. These results are consistent with previous results in showing that activation of MC3Rs in the central nervous system by MSH, in absence of MC4Rs, does not produce an inhibition of food intake, or of feeding-related motivational behaviors.
Figure 8.
Reduced motivation to self-administer food rewards associated with D-Trp8-γ-MSH requires functional MC4Rs. D-Trp8-γ-MSH significantly reduced motivation for food in WT mice but the effect did not reach significance in the Mc4rTB/TB group. (A) Active and (B) inactive lever pressings, (C) break point; (D) total number of pellets. *p < 0.05 compared to WT Acsf control group.
4. Discussion
The present study had two objectives. First we determined whether MC3Rs are required for the regulation of feeding-related motivational behaviors. The experimental paradigm was designed to identify interactions between Mc3r genotype and changes in metabolic state due to caloric restriction. The rationale for comparing motivation to self-administer food rewards in different feeding conditions was based on previous studies that indicated deficits in neuroendocrine and locomotor responses to restricted feeding paradigms [8], [20], [21], [23], [24], [26]. Mc3r-deficiency has sometimes been associated with hypophagia when chow is freely available [10], suggesting to us that motivation to self-administer food rewards might also be reduced in situations of caloric sufficiency. However, our results suggest that the motivational phenotype of Mc3rTB/TB mice is context-specific, with self-administration of food rewards reduced in situations of negative energy balance induced by CR but normal in situations of caloric sufficiency.
We also observed that activation of neurons in the NAc by feeding signals is markedly attenuated in Mc3rTB/TB mice. The NAc is involved in motivation, reinforcement, goal-directed behavior, pleasure and reward, and is an area important for regulating food related processes [47], [48]. Acute re-feeding following a fast has been shown to increase c-Fos immunoreactivity in the NAc [44]. This finding adds further significance in indicating a critical role for MC3Rs in the expression of appetitive responses to weight loss.
Having identified a motivational phenotype, the second objective was to assess the involvement of limbic MC3Rs in the expression of feeding-related motivational responses. We combined genetic and pharmacological tools with the self-administration paradigm to assess the role of limbic MC3Rs. Reduced motivation to work for food rewards in Mc3rTB/TB mice in situations of negative energy is at least partially restored by selective re-expression of the endogenous MC3Rs in the VTA. When food is available with minimal effort (FR1), Mc3rTB/TB and DAT-MC3R mice exhibited similar reductions in the self-administration of food. However, in PR1 training sessions, a schedule that can be consider as a more clear indication of motivation since the animals have to work more to obtain a food reward, DAT-MC3R mice exhibited improved performance. This suggests that MC3Rs expressed in the VTA regulate motivational responses to CR and may have an important role in the defense of body weight during situations of nutrient scarcity. However, actions of VTA MC3Rs are not sufficient to restore normal caloric loading during situations of negative energy balance.
In self-administration paradigms, break point is the number of operant responses at which the subject ceases engaging in self-administration [49]. As expected, CR increased the break point for food reward in the WT mice, with motivation decreasing when mice returned to ad libitum feeding. WT mice also demonstrated an immediate weight rebound when returned to ad libitum feeding conditions. This response was delayed in Mc3rTB/TB mice, further supporting a role for Mc3rs in responding to signals of negative energy balance during nutrient scarcity.
Previous data suggest no deficits in sucrose intake and preference in male Mc3r deficient mice in ad libitum feeding conditions [7]. The current study finds Mc3rTB/TB mice are willing to work more for sucrose pellets compared to chow pellets in situations of caloric sufficiency, as observed for WT mice (Figure 7). Considering that feeding behavior is influenced by nutritional and hedonic aspects [50], the current results suggest a role for MC3Rs in primarily mediating responses to foods caloric value. Preference for palatable foods is not affected by loss of MC3Rs; however, during situations of caloric insufficiency, MC3Rs are required for adapting motivational responses to internal cues of metabolic state, a response involving mesolimbic MC3Rs. Note, however, that the current results only apply to males, as further experiments applying our protocols are needed to examine the sex-specific differences in the effect of Mc3r-deficiency on sucrose preference reported previously [7].
In order to ensure that the observed phenotype was not a cognitive impairment, mice were tested in a session during which the active and inactive levers were inverted to assess cognitive flexibility. Mc3rTB/TB mice did not demonstrate any cognitive deficit in the lever-switch task. In addition, results from the water maze task indicated that learning and memory capabilities of Mc3rTB/TB mice are normal.
Midbrain dopaminergic neurons demonstrate strong responses to rewards and play a critical role in motivation [51]. Animals will also self-administer drugs that activate VTA [52]. Previous research suggests that only 26–36% of TH-expressing neurons express Mc3rs, while 43–72% of the Mc3r-expressing neurons in the VTA are dopaminergic (depending on the anterior-posterior position) [7]. Considering that a significant number of VTA neurons expressing MC3Rs are not dopaminergic neurons, along with limitations in the efficiency of Cre-mediated recombination, finding that Mc3r-expression in DAT-MC3R mice was 10% of normal may thus not come as a surprise. However, this level of activity was clearly sufficient to improve deficits in motivation for food self-administration in DAT-MC3R mice. We also observed rostral-caudal differences in the % co-localization of Mc3r- and DAT-expressing neurons in the VTA, with co-localization being more common in the anterior VTA (Figure 4B upper panel).
It is also worth noting the response of “first-order” melanocortin neurons releasing AgRP and α−MSH is attenuated, but likely not completely lost, in Mc3r-deficient mice during situations of caloric insufficiency [20], [25]. When combined with the low level of Cre-mediated recombination, the behavioral phenotype observed in DAT-MC3R mice may significantly underestimate the relative importance of limbic MC3Rs in the expression of feeding-related motivational behaviors.
The results from the current study are also significant in separating the altered feeding-related motivational response and obese phenotypes. MC3Rs expressed by dopaminergic neurons are not critical for regulating body composition (fat vs. fat-free mass). However, beyond appetite control a mechanism linking activity of mesolimbic dopamine neurons to metabolic control is not clear at this time. The role of MC3Rs in regulating feeding behavior in ad libitum feeding conditions is also controversial, as is the contribution of food intake to the obese phenotype [9], [10], [11], [13], [14]. Moreover, defining the contribution of deficits in the control of feeding behavior and metabolism to subtle obese phenotypes in mice is technically challenging [53], [54]. Reduced physical activity was initially postulated as contributing to the obese phenotype of the original Mc3r−/− mice on mixed backgrounds (129;B6) [9], [10], [55]. Reduced movement was also observed in Mc3rTB/TB mice on different genetic backgrounds [8], [24]. Given that DAT-MC3R exhibited the same obese phenotype, we did not examine metabolic rate or home cage activity. Even if the activity phenotype associated with loss of MC3Rs were to be altered in DAT-MC3R mice, the significance would be unclear. The contribution of physical activity to obese phenotypes of mice housed at room temperature has been suggested to be minor, only becoming significant when housed at thermoneutrality [56].
Our data using DAT-MC3R mice appear partially contradictory to the literature indicating that MC3Rs are not sufficient to mediate the anorexigenic or orexigenic responses to melanocortin analogs [10], [16], [17], [18], [57]. A recent study demonstrated that intra-VTA infusion of γ-MSH increased the number of sucrose rewards obtained in a progressive ratio schedule of self-administration paradigm [31]. However, the study differed in using rats and did not report break point data, making the significance of the outcome difficult to interpret.
Administration of MSH or AgRP directly into the VTA is known to produce acute behavioral responses, increasing dopamine transmission and inducing grooming and rearing behavior [32]. However, the lack of effect of D-Trp8-γ−MSH on food self-administration in Mc4rTB/TB mice is consistent with DAT-MC3Rs failing to rescue food intake in FR1 conditions (Figure 5A–D). A caveat to this conclusion is that D-Trp8-γ−MSH administered icv will affect other brain sites, and not only affect neurons in the VTA.
Several explanations for the discrepancy between acute responses to MSH and DAT-MC3R phenotype are possible. First, DAT-MC3Rs are simply unable to compensate for the attenuated response of AgRP neurons to signals of negative energy balance [20], [25]. Our data suggest that MC3Rs in the VTA can contribute to restoring motivation. However, they cannot compensate for the attenuated response of AgRP neurons to signal negative energy balance. A second theory, although less likely, is that MC3Rs affect signal processing that result in changes over time (plasticity), as compared to the well-known acute and immediate changes in feeding behavior associated with activation of MC4Rs. Another possibility is adaptations of the melanocortin system and reward circuits to a non-physiological condition (selective expression on DAT neurons only) occur during development. This could involve effects on synaptic plasticity and the strength of signaling between neuronal populations involved in regulating appetitive responses. Thus, acute inhibition of stimulation of MC3Rs may not result in acute feeding-related behavioral changes but, if repetitive, over time could result in altered signaling and motivational responses (i.e., amplification or suppression) that anticipate food rewards in response to internal cues of metabolic state. Investigating this hypothesis would however be difficult to technically challenging using current model, as it would require differentiating between the effects of losing signal from AgRP/Npy neurons [20], [25] and altered MC3R signaling. Finally, the CR condition itself could affect the outcome, as it has been shown previously that learned feeding schedules can attenuate the actions of melanocortin analogs in rats [58].
5. Conclusions
The specific roles of MC3Rs expressed in the central nervous system have not been well studied. Our previous results suggest MC3Rs in the ventromedial hypothalamus may be involved in metabolic control [8], while MC3Rs expressed on melanocortin neurons in the arcuate nucleus may have an autoreceptor function [59], [60]. The current results suggest MC3Rs expressed by dopaminergic neurons in the VTA are involved in regulating the expression of motivational responses during situations of negative energy balance.
Acknowledgments
The research was supported by grants from the NIH (DK073189 to AB; DA034116 and R03 DA033499 to CAM). AB and CAM also received support from the Scripps Florida Fund. We thank Dr Melissa Kazantzis for assistance with measurement of body composition, Dr Erica Young and Dr In-Jee You for advice on food self-administration studies, Dr Cristina Grande and Dr Roy G Smith for comments on data analysis, and Dr Mark Southern for assistance with the analysis of data from the operant conditioning chambers. We also thank Luise Angelini, technician veterinarian, for providing assistance with the animal healthcare.
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Girardet C., Butler A.A. Neural melanocortin receptors in obesity and related metabolic disorders. Biochimica et Biophysica Acta. 2014;1842(3):482–494. doi: 10.1016/j.bbadis.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mountjoy K.G., Mortrud M.T., Low M.J., Simerly R.B., Cone R.D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology. 1994;8(10):1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 3.Kishi T., Aschkenasi C.J., Lee C.E., Mountjoy K.G., Saper C.B., Elmquist J.K. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. The Journal of Comparative Neurology. 2003;457(3):213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 4.Liu H., Kishi T., Roseberry A.G., Cai X., Lee C.E., Montez J.M. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(18):7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo G.S., Heisler L.K. Unraveling the brain regulation of appetite: lessons from genetics. Nature Neuroscience. 2012;15(10):1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 6.Roselli-Rehfuss L., Mountjoy K.G., Robbins L.S., Mortrud M.T., Low M.J., Tatro J.B. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(19):8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippert R.N., Ellacott K.L., Cone R.D. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology. 2014;155(5):1718–1727. doi: 10.1210/en.2013-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begriche K., Levasseur P.R., Zhang J., Rossi J., Skorupa D., Solt L.A. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane G-protein-coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. The Journal of Biological Chemistry. 2011;286(47):40771–40781. doi: 10.1074/jbc.M111.278374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler A.A., Kesterson R.A., Khong K., Cullen M.J., Pelleymounter M.A., Dekoning J. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 10.Chen A.S., Marsh D.J., Trumbauer M.E., Frazier E.G., Guan X.M., Yu H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 11.Sutton G.M., Trevaskis J.L., Hulver M.W., McMillan R.P., Markward N.J., Babin M.J. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147(5):2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellacott K.L., Murphy J.G., Marks D.L., Cone R.D. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148(12):6186–6194. doi: 10.1210/en.2007-0699. [DOI] [PubMed] [Google Scholar]
- 13.Butler A.A. The melanocortin system and energy balance. Peptides. 2006;27(2):281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee B., Koo J., Yun Jun J., Gavrilova O., Lee Y., Seo A.Y. A mouse model for a partially inactive obesity-associated human MC3R variant. Nature Communications. 2016;7:10522. doi: 10.1038/ncomms10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krashes M.J., Lowell B.B., Garfield A.S. Melanocortin-4 receptor-regulated energy homeostasis. Nature Neuroscience. 2016;19(2):206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh D.J., Hollopeter G., Huszar D., Laufer R., Yagaloff K.A., Fisher S.L. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nature Genetics. 1999;21(1):119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 17.Chen A.S., Metzger J.M., Trumbauer M.E., Guan X.M., Yu H., Frazier E.G. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Research. 2000;9(2):145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 18.Kumar K.G., Sutton G.M., Dong J.Z., Roubert P., Plas P., Halem H.A. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides. 2009;30(10):1892–1900. doi: 10.1016/j.peptides.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lute B., Jou W., Lateef D.M., Goldgof M., Xiao C., Pinol R.A. Biphasic effect of melanocortin agonists on metabolic rate and body temperature. Cell Metabolism. 2014;20(2):333–345. doi: 10.1016/j.cmet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renquist B.J., Murphy J.G., Larson E.A., Olsen D., Klein R.F., Ellacott K.L. Melanocortin-3 receptor regulates the normal fasting response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(23):E1489–E1498. doi: 10.1073/pnas.1201994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton G.M., Begriche K., Kumar K.G., Gimble J.M., Perez-Tilve D., Nogueiras R. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2010;24(3):862–872. doi: 10.1096/fj.09-142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girardet C., Begriche K., Ptitsyn A., Koza R.A., Butler A.A. Unravelling the mysterious roles of melanocortin-3 receptors in metabolic homeostasis and obesity using mouse genetics. International Journal of Obesity (London) Supplement. 2014;4:S37–S44. doi: 10.1038/ijosup.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton G.M., Perez-Tilve D., Nogueiras R., Fang J., Kim J.K., Cone R.D. The melanocortin-3 receptor is required for entrainment to meal intake. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(48):12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begriche K., Marston O.J., Rossi J., Burke L.K., McDonald P., Heisler L.K. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes, Brain, and Behavior. 2012;11(3):291–302. doi: 10.1111/j.1601-183X.2012.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girardet C., Mavrikaki M., Southern M.R., Smith R.G., Butler A.A. Assessing interactions between Ghsr and Mc3r reveals a role for AgRP in the expression of food anticipatory activity in male mice. Endocrinology. 2014;155(12):4843–4855. doi: 10.1210/en.2014-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begriche K., Sutton G.M., Butler A.A. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiology & Behavior. 2011;104(4):546–554. doi: 10.1016/j.physbeh.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mistlberger R.E. Neurobiology of food anticipatory circadian rhythms. Physiology & Behavior. 2011;104(4):535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Patton D.F., Mistlberger R.E. Circadian adaptations to meal timing: neuroendocrine mechanisms. Frontiers in Neuroscience. 2013;7:185. doi: 10.3389/fnins.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saper C.B. The central circadian timing system. Current Opinion in Neurobiology. 2013;23(5):747–751. doi: 10.1016/j.conb.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietrich M.O., Bober J., Ferreira J.G., Tellez L.A., Mineur Y.S., Souza D.O. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nature Neuroscience. 2012;15(8):1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandit R., Omrani A., Luijendijk M.C., de Vrind V.A., Van Rozen A.J., Ophuis R.J. Melanocortin 3 receptor signaling in midbrain dopamine neurons increases the motivation for food reward. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roseberry A.G., Stuhrman K., Dunigan A.I. Regulation of the mesocorticolimbic and mesostriatal dopamine systems by alpha-melanocyte stimulating hormone and agouti-related protein. Neuroscience and Biobehavioral Reviews. 2015;56:15–25. doi: 10.1016/j.neubiorev.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Backman C.M., Malik N., Zhang Y., Shan L., Grinberg A., Hoffer B.J. Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis. 2006;44(8):383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- 35.Gong S., Zheng C., Doughty M.L., Losos K., Didkovsky N., Schambra U.B. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 36.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGregor R., Wu M.F., Barber G., Ramanathan L., Siegel J.M. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(43):15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollander J.A., Pham D., Fowler C.D., Kenny P.J. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Frontiers in Behavioral Neuroscience. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenk G.L. Assessment of spatial memory using the radial arm maze and Morris water maze. Current Protocols in Neuroscience. 2004 doi: 10.1002/0471142301.ns0805as26. Chapter 8 (Unit 8 5A) [DOI] [PubMed] [Google Scholar]
- 40.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allison D.B., Paultre F., Goran M.I., Poehlman E.T., Heymsfield S.B. Statistical considerations regarding the use of ratios to adjust data. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1995;19(9):644–652. [PubMed] [Google Scholar]
- 42.Packard G.C., Boardman T.J. The misuse of ratios, indices, and percentages in ecophysiological research. Physiological Zoology. 1988;61(1):1–9. [Google Scholar]
- 43.Lecca D., Cacciapaglia F., Valentini V., Gronli J., Spiga S., Di Chiara G. Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology. 2006;184(3–4):435–446. doi: 10.1007/s00213-005-0280-4. [DOI] [PubMed] [Google Scholar]
- 44.Wu Q., Lemus M.B., Stark R., Bayliss J.A., Reichenbach A., Lockie S.H. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and re-feeding in male mice. Endocrinology. 2014;155(3):840–853. doi: 10.1210/en.2013-1831. [DOI] [PubMed] [Google Scholar]
- 45.Berridge K.C. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiology & Behavior. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grieco P., Balse P.M., Weinberg D., MacNeil T., Hruby V.J. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. Journal of Medicinal Chemistry. 2000;43(26):4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 47.Roitman M.F., Wheeler R.A., Wightman R.M., Carelli R.M. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nature Neuroscience. 2008;11(12):1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verhagen L.A., Luijendijk M.C., Korte-Bouws G.A., Korte S.M., Adan R.A. Dopamine and serotonin release in the nucleus accumbens during starvation-induced hyperactivity. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2009;19(5):309–316. doi: 10.1016/j.euroneuro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Richardson N.R., Roberts D.C. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 50.Vucetic Z., Reyes T.M. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdisciplinary Reviews Systems Biology and Medicine. 2010;2(5):577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- 51.Bromberg-Martin E.S., Matsumoto M., Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fields H.L., Hjelmstad G.O., Margolis E.B., Nicola S.M. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annual Review of Neuroscience. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 53.Butler A.A., Kozak L.P. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59(2):323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tschop M.H., Speakman J.R., Arch J.R., Auwerx J., Bruning J.C., Chan L. A guide to analysis of mouse energy metabolism. Nature Methods. 2012;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marks D., Cone R.D. The role of the melanocortin-3 receptor in cachexia. Annals of the New York Academy of Sciences. 2003;994:258–266. doi: 10.1111/j.1749-6632.2003.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 56.Virtue S., Even P., Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metabolism. 2012;16(5):665–671. doi: 10.1016/j.cmet.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutton G.M., Josephine Babin M., Gu X., Hruby V.J., Butler A.A. A derivative of the melanocortin receptor antagonist SHU9119 (PG932) increases food intake when administered peripherally. Peptides. 2008;29(1):104–111. doi: 10.1016/j.peptides.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benoit S.C., Clegg D.J., Barrera J.G., Seeley R.J., Woods S.C. Learned meal initiation attenuates the anorexic effects of the melanocortin agonist MTII. Diabetes. 2003;52(11):2684–2688. doi: 10.2337/diabetes.52.11.2684. [DOI] [PubMed] [Google Scholar]
- 59.Bagnol D., Lu X.Y., Kaelin C.B., Day H.E., Ollmann M., Gantz I. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19(18):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowley M.A., Smart J.L., Rubinstein M., Cerdan M.G., Diano S., Horvath T.L. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]