Abstract
AIM: To facilitate translational research on cholelithiasis, we have developed a rat model of human gallstones by exploiting the unique biliopancreatic features of this species.
METHODS: Under anesthesia, 16 adult rats of equal genders underwent two times of abdominal surgery. First, their common bile duct (CBD) was ligated to cause cholestasis by total biliary obstruction (TBO). On day 0, 1, 3, 7, 14, 21 and 28 after TBO, magnetic resonance imaging (MRI) was conducted to monitor the dilatation of the CBD, and blood was sampled to analyze total serum bilirubin (TSB). Secondly, on day 30, the abdomen was re-opened and gallstone(s) collected from human patients were implanted in the dilated CBD as a virtual gallbladder (VGB), which was closed by suture ligation. This rat cholelithiasis model was examined by MRI, clinical observation, microcholangiography and histology.
RESULTS: All rats survived two laparotomies. After ligation, the CBD was dilated to a stable size of 4 to 30 mm in diameter on day 21-28, which became a VGB. The rats initially showed signs of jaundice that diminished over time, which paralleled with the evolving TSB levels from 0.6 ± 0.3 mg/dL before ligation, through a peak of 10.9 ± 1.9 mg/dL on day 14, until a nearly normalized value after day 28. The dilated CBD with thickened wall allowed an incision for implantation of human gallstones of 1-10 mm in diameter. The rat cholelithiasis was proven by in vivo MRI and postmortem microcholangiography and histomorphology.
CONCLUSION: A rat model cholelithiasis with human gallstones has been established, which proves feasible, safe, reliable, nontoxic and cost-effective. Given the gallstones of human origin, applications of this model may be of help in translational research such as optical detection and lysis of gallstones by systemic drug administration.
Keywords: Cholelithiasis, Rat, Gallbladder, Common bile duct, Cholestasis, Bilirubin, Gallstones
Core tip: The mouse and rat are common experimental animals. Unlike the mouse that has a gallbladder but difficulty in imaging studies, the rat does not have a gallbladder, which has hampered studies on imaging gallstones. To tackle this problem, we first induced a virtual gallbladder (VGB) in rats by ligation of the common bile duct (CBD) accompanying with gradual increase and normalization of serum bilirubin. Then we implanted gallstone(s) collected from human patients into the dilated CBD or VGB to create a cholelithiatic model in rats, which has been validated by in vivo magnetic resonance imaging, microcholangiography and histology. This rat model is deemed useful for translational research such as fluorescent visualization of gallstones for laparoscopic detection and differential diagnosis of cholelithiasis.
INTRODUCTION
Cholelithiasis refers to the presence of gallstones that are concreted from bile components in the biliary tract, usually in the gallbladder. Cholelithiasis is a clinical syndrome[1-3] with a global incidence in up to 20% of the adult population[2,4,5]. The risk factors for developing gallstones include gender, age, geographic location, genes, ethnicity, metabolic conditions, obesity, pregnancy, diet and alcohol or drug consumption[6,7]. Cholelithiasis can trigger acute cholecystitis or gallbladder inflammation leading to severe abdominal pain, jaundice and secondary infections by intestinal microorganisms[8,9]. More life-threatening complications include acute cholangitis or pancreatitis[10,11]. Stones larger than 3 cm or gallbladders packed with stones might also increase the risk of gallbladder cancer[6].
Over the past decades, research on diagnosis, prevention and treatment of cholelithiasis has been a popular focus of medical community. By using different methods including gallbladder infection, induced biliary stasis, and modified diet[12], animal models of cholelithiasis have been created to resemble human pathophysiology. However, these models are often cost-ineffective and unreliable with a low rate of gallstone formation or marked hepatotoxicity[12,13]. In addition, their gallstone compositions may differ from that in humans, making it difficult to extrapolate experimental findings to clinic applications.
Rodents including mice and rats are inexpensive and widely applied experimental animals. Rats are 10-fold bigger in dimension than mice, which eases many of the research procedures including noninvasive imaging diagnosis using clinical scanners[14]. But, comparing to mice, rats lack a gallbladder by nature[12], which otherwise would make it impossible to research cholelithiasis in this species. However, based on its unique biliopancreatic features[15], we hypothesized that it could be feasible in the rat first to create a hollow biliary organ as a virtual gallbladder (VGB), and then to implant human gallstones in it without compromising normal bile flow at 22.5 mL/d[16]. To test this hypothesis, we conducted two phases of abdominal surgery to ligate and dilate the common bile duct (CBD) first and, one month later, to implant gallstones in order to create a rat model of cholelithiasis. We further validated this model by in vivo magnetic resonance imaging (MRI), serial blood bilirubin tests, postmortem microcholangiography, and histomorphological examinations. Potential implications of this rat model of human cholelithiasis for translational research include fluorescent visualization of gallstones for laparoscopic detection and differential diagnosis of gallstones after systemic administration of small molecular drugs that undergo hepatobiliary excretion as being proven preliminarily[17]. Similarly, new litholytic chemicals can be tested this way for therapy.
MATERIALS AND METHODS
Animals
Twenty adult Wistar rats of equal genders weighing 300-350 g were purchased from Charles River Laboratories, Inc. (St. Aubain les Elbeuf, France) and housed in environmentally controlled conditions including temperature at 23 °C ± 2 °C and relative humidity at 60% ± 10% on a 12:12 h light-dark cycle. Standard rat chow (Ssniff Spezialdiäten GmbH, Soest, Germany) and water were available ad libitum. All experimental procedures were approved by the institutional Animal Ethics Committee and complied with the European Ethics Committee guidelines (decree 86/609/EEC). As illustrated in Figure 1, eventually 16 rats were included after pilot tests in the first 4 rats.
Figure 1.
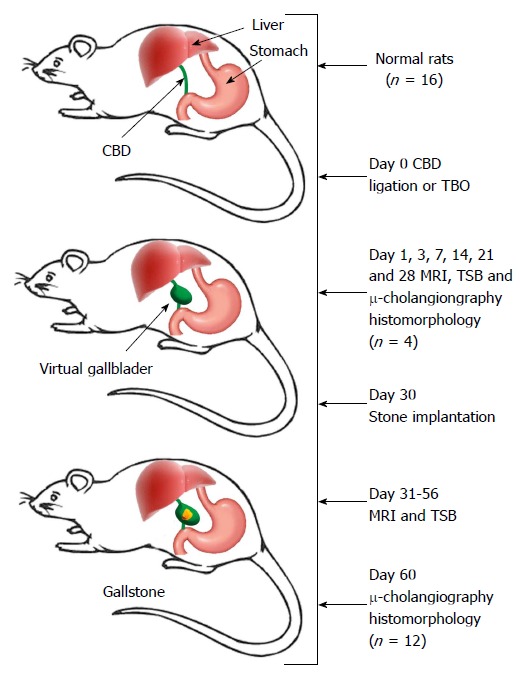
Flow chart of the experimental procedures. CBD: Common bile duct; MRI: Magnetic resonance imaging; TBO: Total biliary obstruction; TSB: Total serum bilirubin; μ-cholangiography: Microcholangiography.
Creation of a rat model of cholelithiasis
Ligation of CBD to create a VGB: Under anesthesia with intraperitoneal injection of sodium pentobarbital (Nembutal; Sanofi Sante Animal, Brussels, Belgium) at 40 mg per kilogram body weight and after shaving and sterilizing the skin, a midline abdominal incision of 4-5 cm was made through the skin, muscle and peritoneum (Figure 2A). The stomach and duodenal loop were exposed, and the CBD was identified underneath the hepatic hilum. The CBD was ligated adjacent to the duodenum[15,18] using a 5-0 silk suture (Ethicon, New Jersey, United States). The abdominal wall was closed with two layers of consecutive sutures and animals were allowed for recovery from anesthesia and surgery. The rat model of VGB was thus initiated (Figure 2A-2D).
Figure 2.
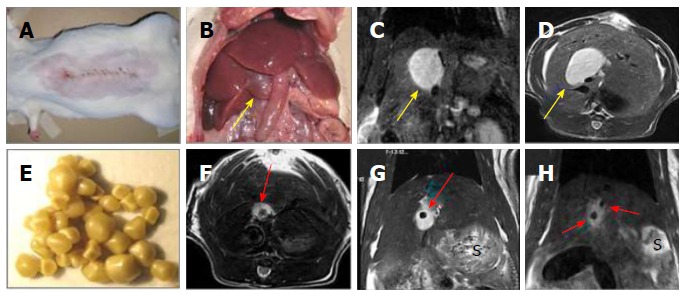
Creation of a rat model of cholelithiasis. A: Midline abdominal incision for CBD ligation; B: Laparotomy to expose the cholestasis-induced VGB (arrow); C: Coronal plane of T2-w MRI displaying an oval-shaped hyperintense dilated CBD or a VGB (arrow); D: Axial plane of T2-w MRI displaying the same oval-shaped hyperintense VGB (arrow); E: Gallstones from patients of cholelithiasis derived by cholecystectomy; F: Axial plane of T2-w MRI showing the same case in D but now implanted with a human gallstone (arrow); G: Coronal plane of T2-w MRI displaying the same case in F with a gallstone (arrow); H: Coronal plane of T2-w MRI two hypointense gallstones (arrows) located in a hyperintense VGB in another case. S denotes stomach on MR images. CBD: Common bile duct; VGB: Virtual gallbladder; MRI: Magnetic resonance imaging.
Implantation of human gallstones in the VGB to create a cholelithiasis model: Gallstones of cholesterol, pigment and mixed contents of 1-10 mm in diameter were collected from patients after cholecystectomy (Figure 2E). Stones were disinfected by immersion in 70% ethanol for 5 min and rinsed with sterile physiological saline. Thirty days following CDB ligation, under the same anesthetic and laparotomic procedures as in the first surgery, the abdomen of CBD-ligated rats was reopened. The VGB was identified and the yellowish bile was aspirated using a syringe with a 27 gauge needle. The needle hole was extended to a length 5 mm by using a pair of microsurgical scissors. Depending on the actual size of the VGB and the purpose of the study, 1-3 gallstones could be inserted into the cavity (Figure 2F-2H). The opening was then closed with a suture ligation and the animal was left to recover from the surgery.
Assay of total serum bilirubin
For all animals, serum bilirubin was determined on the day before of the CDB ligation and on day 1, 3, 7, 14, 21, 28 and 60 after operation (Figures 1 and 3). Fresh blood samples were collected in VACUETTE® heparin tubes by cutting a small section of the distal tail with a fresh scalpel blade. A droplet of Histoacryl® topical adhesive (Braun, Tuttlingen, Germany) was applied on the skin incision to stop bleeding. For serum separation, the blood samples were allowed to coagulate in the dark to prevent enzyme degradation. The samples were then centrifuged at 4000 rpm for 10 min at 4 °C using a 4226 ALC Centrifuge (Milan, Italy). The supernatant containing the serum was transferred to 1.5 mL micro-centrifuge Eppendorf® Safe-Lock® vials. With a Hitachi automatic analyzer (Tokyo, Japan), analysis of total serum bilirubin (TSB) was carried out.
Figure 3.
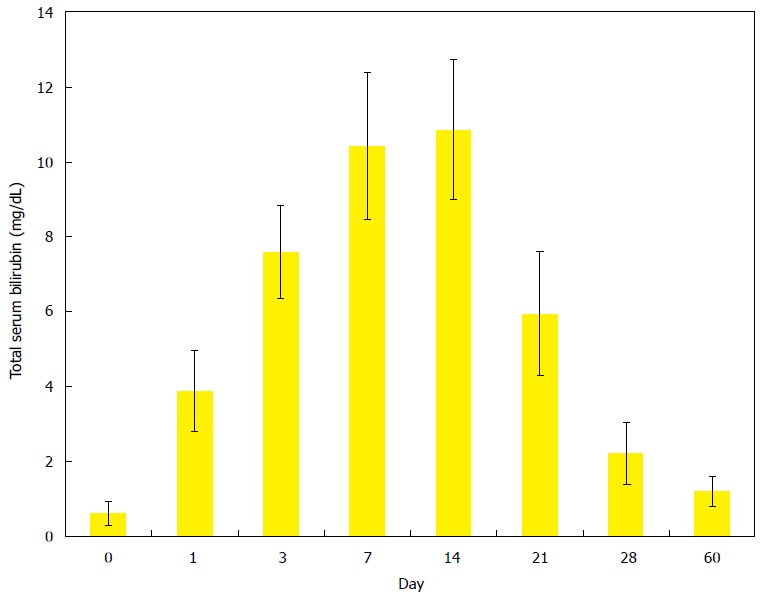
Evolving changes of total serum bilirubin levels in rats after ligation of common bile duct to induce a rat model of virtual gallbladder and cholelithiasis. Total bilirubin levels reached the highest values between the days 7 and 14 that and then rapidly dropped to the values close to the normal range after day 28 till day 60.
MRI of the VGB and cholelithiasis
Before and after each surgical procedure, all rats underwent serial MRI scans to monitor the progressive expansion of the CBD (Figure 2C and 2D) and to detect the presence of gallstone(s) in the VGB (Figure 2F-2H, Figure 4A and Figure 5A). Briefly, under gas anesthesia using 2% isoflurane (Halocarbon, River Edge, NJ, United States) mixed with 20% of O2 and 80% of air, MRI was conducted at a clinical 3.0T whole body MR magnet (Trio; Siemens, Erlangen, Germany) with a maximum gradient capability of 45 mT/m with an 8-channel phased array wrist coil (in vivo, Latham, NY, United States). T2 weighted turbo spin echo images were acquired at both transverse and coronal planes of 1 mm thickness with the following parameters as TR/TE = 4300/69 ms, flip angle = 140°, turbo factor = 13, field of view = 77 mm × 85 mm, matrix = 414 × 512, number of averages = 6 and total imaging time of 3 min 9 s.
Figure 4.
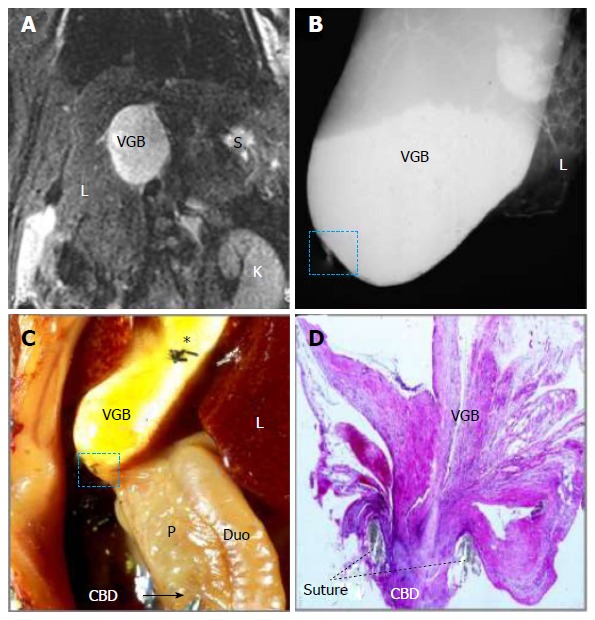
Magnetic resonance imaging, microcholangiography and histomorphology of a rat on day 14 after surgically induced cholestasis towards creating a model of virtual gallbladder. A: Coronal T2-w MRI shows an oval-shaped hyperintense dilated CBD or VGB with L, K and S denoting the liver, kidney and stomach respectively; B: Digital microcholangiography displays a hyperdense dilated CBD or VGB with L denoting the liver; C: Laparotomic view shows the dilated CBD or VGB with L, P and Duo denoting the liver, pancreas and duodenum respectively; note the transparent distal CBD as indicated by a needle tip and an arrow, suggesting absent bile flow, and asterisk indicates where the needle hole was closed by suture ligation; D: Photomicrograph of hematoxylin and eosin stained slide of the ligature (dashed square on B and C) shows a complete CBD obstruction separating the VGB and distal CBD (original magnification × 100). MRI: Magnetic resonance imaging; CBD: Common bile duct; VGB: Virtual gallbladder.
Figure 5.
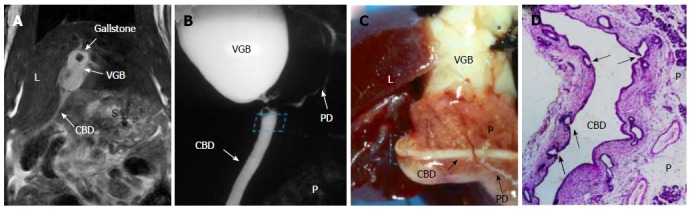
Magnetic resonance imaging, microcholangiography and histomorphology of a rat on day 60 after surgically induced cholelithiasis. A: Coronal T2-w MRI shows a hypointense gallstone located in a hyperintense VGB and distal nondilated CBD as a hyperintense line with L and S denoting the liver and stomach; B: Digital microcholangiography displays both the hyperdense VGB and distal nondilated CBD bridged in-between by an pancreatic ductule with P denoting the pancreas; C: Macroscopy views the VGB with L denoting the liver, note the distal nondilated and barium-filled CBD imbedded in the pancreas (P) and a branching ductule bridging the ligation between the proximal and distal CBD, suggesting resumed bile flow, asterisk indicates where the needle hole was closed by suture ligation; D: Photomicrograph of hematoxylin and eosin stained slide of the distal CBD near the ligature (dashed square on B and C) demonstrates multiple proliferated glandular ductules (arrows) that contribute to the eventual bypass of the biliary obstruction between the VGB and distal CBD (original magnification × 100). MRI: Magnetic resonance imaging; CBD: Common bile duct; VGB: Virtual gallbladder; PD: Pancreatic duct.
Postmortem microcholangiography and histomorphology
Rats were euthanized by intravenous injection of an overdosed Nembutal. The abdomen was opened with a skirt incision to expose all abdominal visceral organs (Figure 2B). Using a method similar to that reported previously[18], the VGB without or with implanted gallstone(s) was punctured with a needle-syringe to aspirate the bile. Barium sulphate suspension (Micropaque; Guerbet, France) was injected to fill in the cavity, which was closed by suture ligation. The hepatic, biliopancreatic and duodenum were entirely excised and digitally radiographed (Mammomat Inspiration; Siemens, Germany) at 26 kV and 15 mA (Figure 4B and Figure 5B), followed by fixation in 10% formaldehyde for histomorphology (Figure 4C, 4D and Figure 5C, 5D).
RESULTS
General conditions
All 20 rats tolerated well the anesthesia and surgery. However, during early pilot stage, 4 rats failed to form the final cholelithiasis model, one rat died of cholestasis before bile flow recovery, one rat did not show CBD dilatation probably due to invalid obstruction because of a too distal ligation site, and other two died of biliary peritonitis due to the bile leakage after needle suture, instead of suture ligation, to close the incision after gallstone implantation. Thus, the remaining 16 rats were included in this study (4 for cholestasis only and 12 for cholelithiasis) and sacrificed at designated time points for postmortem examinations. No gender difference was noticed regarding experimental procedures and outcomes.
Rat model of VGB
A VGB was successfully established by ligation of CBD, which proved to be safe and was confirmed by in vivo MRI, normalized total bilirubin, microcholangiography and histology (Figures 2-5). The entire procedure from induction of anesthesia to closure of skin incision took approximately 15 min. During the first 3 wk after surgery, the animals showed signs of jaundice including yellowish skin and sclera as well as whitish feces, which worsened on day 7 to 14 but gradually diminished afterwards. The CBD-ligated rats maintained fair life quality with normal physical/behavioral manifestations without apparent loss of body weight. As shown in Table 1, a wide diversity in the dimension of VGB was found, ranging from 0.5 to 4.0 cm in diameter and the collected bile juice varied from 0.8 to 7.2 mL.
Table 1.
Magnetic resonance imaging measurements and drained bile volumes from rats with cholestasis (n = 4) and cholelithiasis (n = 12)
| No. of rats | VGB diameter (cm) | Bile volume (mL) | Incidence (%) |
| 3 | 3.1-4.0 | 7.2 ± 1.9 | 18.8 |
| 6 | 2.1-3.0 | 3.8 ± 1.4 | 37.5 |
| 5 | 1.1-2.0 | 2.6 ± 1.2 | 31.3 |
| 2 | 0.5-1.0 | 0.8 ± 0.3 | 12.5 |
VGB: Virtual gallbladder.
Rat model of cholelithiasis
All the 12 rats survived the anesthesia and surgical procedures for gallstone implantation in the VGBs. Each surgery took about 30 min, somewhat longer than the first operation due to intraperitoneal adhesion. After VGB isolation and bile aspiration, the gallstone(s) were successfully implanted, as seen on MRI (Figure 2F-2H and Figure 5A). No complications such as bleeding, enterocutaneous fistula, infection or sepsis, tissue damage or organ injury were observed. Minimum intra-abdominal adhesions between the liver and intestinal loops near the VGB were encountered, which were however manageable by blunt separation maneuver. Altogether 20 gallstones varying 1-10 mm in size were implanted, i.e., 3, 2 and 1 stone(s) in 2, 4 and 6 rats respectively.
MRI findings
On coronal and transverse T2-w MRI, 2-3 wk after ligation, a dilated CBD or VGB formed a hyperintense hollow organ of pear, circular or cylindrical shapes located under the liver at the right upper quadrant region of the abdomen, simulating the organ of gallbladder (Figures 2C, 2D, 4A and 5A). The patent distal CBD was also occasionally visible (Figure 5A). T2-w MRI of this rat model of cholelithiasis typically displayed hypointense gallstone(s) located in a hyperintense dilated CBD or VGB (Figure 2F-2H and Figure 5A).
Serum biochemical parameters
Figure 3 depicts the changes of the serum total bilirubin concentrations in function of time from CBD-ligated rats on the day before the surgery for CDB ligation and on day 1, 3, 7, 14, 21, 28 and 60 after operation. Before CDB ligation, the total bilirubin values were averaged at a normal level of 0.6 ± 0.3 mg/dL. On the successive days after surgery, these values increased up to 10.9 ± 1.9 mg/dL on day 14, but rapidly declined afterwards to the level close to the normal range, i.e., 2.2 ± 0.8 mg/dL and 1.2 ± 0.4 mg/dL on day 28 and 60, respectively.
Microcholangiographic findings
On digital microcholangiography, the dilated CBD or VGB appeared hyperdense due to the instilled barium suspension (Figures 4B and 5B). The 4 rats with hyperbilirubinemia or cholestasis sacrificed before day 21 failed to display the opacified distal CBD (Figure 4B), whereas all 12 rats of cholelithiasis displayed both the VGB and patent distal CBD due to anastomosis formation between biliopancreatic ducts (Figure 5B).
Histomorphological findings
At autopsy, the dilated CBD appeared as a hollow organ filled with the bile that was substituted by barium sulphate suspension for microcholangiography, suggestive of a VGB (Figures 4C and 5C). Initially there was no bile flow in the distal CBD due to ligation induced cholestasis (Figure 4C), but with time the biliary obstruction was resolved by collateral formation (Figure 5C). Microscopically, while the CBD was completely obstructed in certain cases (Figure 4D), in other cases multiple proliferated biliary ductules could be found around the CBD and pancreatic tissue, which bypassed the ligation (Figure 5D). These findings were consistent with those from MRI (Figure 5A) and microcholangiography (Figure 5B).
DISCUSSION
Cholelithiasis refers to the gallstone formation in the biliary tract, usually in the gallbladder. Animal models of biliary infection and cholestasis have been applied in research for etiology and diagnosis of cholelithiasis as well as drug intervention and diet modification for the treatment of the disease. These models are often poorly manageable, inefficient, expensive, unreliable, and hepatotoxic or their applications are hardly translational to human scenarios. In the present study, we have strived to establish and validate a rat model of human gallstones potentially useful in research on cholelithiasis or choledocholithiasis especially for developing effective diagnostic and therapeutic techniques. This rat model of cholelithiasis can be realized by two sequential steps: (1) creating a VGB in rats by chronic expansion of the CBD into a cystic cavity after CBD ligation; and (2) implanting human gallstone(s) in the VGB with resumed biliary flow and efficient intestinal discharge, as proven by the in vivo MRI findings (Figure 5A), serum bilirubin tests (Figure 3), microcholangiography (Figure 5B) and histomorphology (Figure 5C and D). Overall, this rat model of cholelithiasis turned out to be feasible for preparations and reliable for validation. Recently a novel optical imaging agent for diagnosis and differential diagnosis of human gallstones has been identified by using this animal model (paper submitted).
Unlike experimental gallstone models in the previous studies conducted on gallbladder-bearing species such as ground squirrels[19], mice[20], dogs, cats, guinea pigs, rabbits, sheep, pigs and cattle[12], our experiments were conducted on rats that lack a gallbladder. The rat specie was chosen due to its amplest availability, lowest cost, easiest housing and handling, in addition to its feasibility of being imaged by using clinical scanners[14]. However, because of its natural deficiency[12], a structure resembling a gallbladder in both morphology and functionality with rats deems mandatory to further study cholelithiasis. In concordance with previous studies[17,21], after ligation the proximal CBD increased its diameter with a noticeably thickened wall due to the evolving bile stasis. Studies showed that the diameters of the CBD could enlarge from 0.3 to 8 mm in only 5 d owing to bile accumulation[21], and further dilate to a few centimeters in 2-3 wk[18]. This can explain the dimensions up to 40 mm of the VGB with a large volume of bile collected in our model rats 4 wk after CBD ligation.
A recovery period of four weeks was chosen, because shortly after surgery the rats showed limited activity with weak mechanical strength of the CBD. In animals, persistent obstruction of CBD may cause cholestatic liver injury, hyperbilirubinemia, portal hypertension, ascites, endocrine disorders, hepatic encephalopathy and skeletal fragility (osteopenia)[22-24]. Interestingly, especially in rats, after a course of about 4 wk of CBD obstruction, they progressively recovered from cholestasis with a diminishing serum bilirubin level, improved jaundice symptoms, and gain in body weight and strength. This recovery response could be explained by the unique proliferative changes of the biliopancreatic duct tree after a complete CDB-ligation in rats[18,25]. Contrary to humans, in which complete biliary obstruction can be life threatening[26], most of such cholestatic rats eventually survived due to formation of sufficient biliary collaterals, which has been widely referred in the literature[18,25,27]. Indeed, the CDB-ligated rats have been used as a model of typical and selective cholangiocytic proliferation, which is characterized by an increased biliary pressure that triggers newly formed capillary networks or peribiliary capillary plexuses surrounding the intrahepatic bile duct branches over time[27,28]. It seems that native bile ducts, the preexisting bile capillary and proliferated ductules jointly play a significant role in the transport mechanism of biliary substances, secretion and reabsorption of water and electrolytes[29]. Therefore, this phenomenon might explain the recovery of the CBD-ligated rats during thirty days after a complete CBD obstruction in rats. Moreover, a period of thirty days also allowed the natural absorption of the intra-abdominal fibrous adhesion caused by the first CBD-ligation surgery. As a consequence, during the second surgery for stone implantation, the exposure of and access to the newly formed VGB became easier with less complications. Furthermore, during this elongated period, the thickened “gallbladder” wall due to tissue proliferation eased incision and closure for stone implantation and prevention of bile leakage.
The second step for preparing the rat model of cholelithiasis is to embed human gallstone(s) into the VGB of rats 4 wk after CBD-ligation. Human gallstones were categorized into 3 common types as cholesterol, pigment and mixed stones[30]. Therefore, the rat cholelithiasis model introduced here would be suitable in studies on diagnostic or therapeutic interventions with chemical drugs that could undergo the bile excretion and further interact with a particular component of the stone from human patients as exemplified in our recent study[17].
By direct implantation of human gallstones, we intended to overcome the limitations with the previous models of gallstone disease such as gallbladder infection, production of biliary stasis, and diet composition modifications[12]. Gallbladder concretions were generated in rabbits subjected to infection with different bacteria such as coliform, vibrio cholera, typhoid bacilli, and streptococci isolated from tonsils and human gallbladders[12,31]. Biliary stasis based on CBD ligation in combination with bacteria inoculation resulted in more reliable formation of gallstone than bacterial injection alone[32]. Gallbladder trauma along with bacterial infection has been also proved to be effective to cause gallstones[33]. However, these bacterial methods generally require serial inoculations and longer time. In the case of the bile stasis methods, they could be unreliable and inconsistent. The gallstone production by dietary modifications appeared successful with numerous dietary schemes including cholesterol and sunflower seed oil in rabbits[12], cholesterol rich foods[34], diet rich in sucrose (74.3%) with low lipid content in hamsters[35], and ground chow either with 1% cholesterol or 0.5% cholic acid in mice[36]. However, the dietary methods were frequently hepatotoxic and some animals poorly tolerated the diet, and the models were time consuming and inconsistent[12]. The most rapid and reliable system seemed to be the dihydrocholesterol-rabbit model leading to cholelithiasis within fifteen days without apparent toxicity in the liver. However, the gallstone composition was dissimilar to that in humans, thus not translational for many applications. The formation of human-type gallstones by feeding guinea pigs with modocoll and cholestyramine was also tried with satisfactory frequency and quickness without hepatotoxicity[12], which however was more expensive than other small rodent models[13].
There exist advantages and disadvantages or limitations with this cholelithiasis model in rats. First, the model was not intended for studying the formation or etiology of gallstones, instead it is for validating diagnostic or therapeutic agents potentially interactive with human gallstones after systemically administration, hepatic uptake or metabolism and biliary excretion. Second, the model appears complex to make, but all rats tolerated the twice surgical procedures with a high success rate. Third, indeed the ligation of the CBD induced cholestatic liver injury, cholangiocellular proliferation, ductular reaction and fibrosis up to the development of biliary cirrhosis from seven days to weeks after surgery. But, in rodents bile flow does resume with time as proven by gradually normalized serum hepatic enzymatic parameters[18], which closely simulates cholelithiatic patients with chronic and repeated onset of cholestasis. Thus, this rat model of human gallstones with background histological alterations could be suitable for certain clinically relevant translational research[17].
Technically, the high success rate of this model was assured by two major factors. First, the cholestasis induced by CBD ligation in rats is only transient as evidenced by normalization of serum bilirubin level and resumed bile flow seen on micro-cholangiography[18]. Secondly, after implantation of human gallstones, careful closure of VGB wall proved crucial to avoid any bile leakage that is a lethal complication.
In conclusion, we have introduced a rat model of cholelithiasis with human gallstones, which appears to be an experimental model with certain desirable characteristics[12]. It is a relatively simple and cost-effective method that offers the possibility of studying already formed human gallstones in rats. It was not toxic since no special diet different to the standard rodent chow is required. Another practical aspect is the time consumed for the model of gallstones. In contrast to previous reports in which the formation can occur in up to a year[12], the proposed animal model can be invariably ready by around sixty days. Most importantly it may offer translational results for clinically relevant applications since it contains human gallstones.
COMMENTS
Background
The clinical syndrome cholelithiasis refers to the presence of gallstones in the biliary tract, usually in the gallbladder, with a global incidence in up to 20% of the adult population. The risk factors for developing gallstones include gender, age, geographic location, genes, ethnicity, metabolic conditions, obesity, diet and alcohol or drug consumption. Cholelithiasis can trigger acute cholecystitis leading to severe abdominal pain, jaundice and secondary infections as well as more life-threatening complications include acute cholangitis and pancreatitis with increased risk of gallbladder cancer.
Research frontiers
Over the past decades, research on diagnosis, prevention and treatment of cholelithiasis has been a popular focus of medical community. By using different methods including gallbladder infection, induced biliary stasis, and modified diet, animal models of cholelithiasis have been created to resemble human pathophysiology. However, these models are often cost-ineffective and unreliable with a low rate of gallstone formation or marked hepatotoxicity. In addition, their gallstone compositions may differ from that in humans, making it difficult to extrapolate experimental findings to clinic applications.
Innovations and breakthroughs
Both the mouse and the rat are the most common experimental animals. However, unlike the mouse that has a gallbladder but has more difficulties in imaging studies, the rat by nature does not have a gallbladder, which has hampered research on imaging gallstones. To tackle this problem, the authors first induced a virtual gallbladder in rats by ligation of the common bile duct. Then they implanted gallstone(s) collected from human patients into the virtual gallbladder to create a cholelithiatic model in rats, which was validated by in vivo magnetic resonance imaging and evolving serum bilirubin levels, and ex vivo microcholangiography and histology.
Applications
This rat cholelithiasis model is deemed useful in certain translational research such as fluorescent visualization of gallstones for laparoscopic detection and differential diagnosis, as well as in studying cholelithiolysis by chemicals that are systemically administered, taken up by hepatocytes and excreted via the bile.
Peer-review
This is an original research paper describing the methodology for establishing and validating a rat model of cholelithiasis, which therefore falls well into the scope of this journal.
Footnotes
Supported by Flanders Research Foundation (FWO-42865); the KU Leuven Molecular Small Animal Imaging Center MoSAIC (KUL EF/05/08); the center of excellence in vivo molecular imaging research (IMIR); KU Leuven projects IOF-HB/08/009 and IOF-HB/12/018; the European Union (Asia-Link CfP 2006-EuropeAid/123738/C/ACT/Multi-Proposal No. 128-498/111); the National Natural Science Foundation of China No. 81071828; and Jiangsu Province Natural Science Foundation No. BK2010594. The corresponding author is currently a Bayer Lecture Chair holder.
Institutional review board statement: This experimental research was approved by the Ethical Committee of Medical School, KU Leuven, Belgium.
Conflict-of-interest statement: None of the authors have any conflict of interest.
Data sharing statement: No additional data available for this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 22, 2016
First decision: April 15, 2016
Article in press: June 2, 2016
P- Reviewer: Hang B, Wang Y S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci. 2007;52:1313–1325. doi: 10.1007/s10620-006-9107-3. [DOI] [PubMed] [Google Scholar]
- 2.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157–169, vii. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Bodmer M, Brauchli YB, Krähenbühl S, Jick SS, Meier CR. Statin use and risk of gallstone disease followed by cholecystectomy. JAMA. 2009;302:2001–2007. doi: 10.1001/jama.2009.1601. [DOI] [PubMed] [Google Scholar]
- 4.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 5.Qiao T, Ma RH, Luo XB, Yang LQ, Luo ZL, Zheng PM. The systematic classification of gallbladder stones. PLoS One. 2013;8:e74887. doi: 10.1371/journal.pone.0074887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172–187. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amigo L, Zanlungo S, Mendoza H, Miquel JF, Nervi F. Risk factors and pathogenesis of cholesterol gallstones: state of the art. Eur Rev Med Pharmacol Sci. 1999;3:241–246. [PubMed] [Google Scholar]
- 8.US National Library of Medicine, Medical Encyclopedia. Acute cholecystitis. [updated 2016 Mar 20] Available from: http://www.nlm.nih.gov/medlineplus/ency/article/000264.htm.
- 9.Csendes A, Burdiles P, Maluenda F, Diaz JC, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg. 1996;131:389–394. doi: 10.1001/archsurg.1996.01430160047008. [DOI] [PubMed] [Google Scholar]
- 10.Buyukasik K, Toros AB, Bektas H, Ari A, Deniz MM. Diagnostic and therapeutic value of ERCP in acute cholangitis. ISRN Gastroenterol. 2013;2013:191729. doi: 10.1155/2013/191729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitale GC. Early management of acute gallstone pancreatitis. Ann Surg. 2007;245:18–19. doi: 10.1097/01.sla.0000250967.32581.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freston JW, Bouchier IA. Experimental cholelithiasis. Gut. 1968;9:2–4. doi: 10.1136/gut.9.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comp Med. 2008;58:324–340. [PMC free article] [PubMed] [Google Scholar]
- 14.Ni Y, Wang H, Chen F, Li J, DeKeyzer F, Feng Y, Yu J, Bosmans H, Marchal G. Tumor models and specific contrast agents for small animal imaging in oncology. Methods. 2009;48:125–138. doi: 10.1016/j.ymeth.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Kara ME. The anatomical study on the rat pancreas and its ducts with emphasis on the surgical approach. Ann Anat. 2005;187:105–112. doi: 10.1016/j.aanat.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 17.Miranda Cona M, Liu YW, Hubert A, Yin T, Feng YB, de Witte P, Waelkens E, Jiang YS, Zhang J, Mulier S, et al. Differential diagnosis of gallstones by using Hypericin as a fluorescent optical imaging agent. World J Gastroenterol. 2016:In press. doi: 10.3748/wjg.v22.i29.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni Y, Lukito G, Marchal G, Cresens E, Yu J, Petré C, Baert AL, Fevery J. Potential role of bile duct collaterals in the recovery of the biliary obstruction: experimental study in rats using microcholangiography, histology, serology and magnetic resonance imaging. Hepatology. 1994;20:1557–1566. doi: 10.1002/hep.1840200627. [DOI] [PubMed] [Google Scholar]
- 19.Xu QW, Shaffer EA. The potential site of impaired gallbladder contractility in an animal model of cholesterol gallstone disease. Gastroenterology. 1996;110:251–257. doi: 10.1053/gast.1996.v110.pm8536864. [DOI] [PubMed] [Google Scholar]
- 20.Xu GQ, Xu CF, Chen HT, Liu S, Teng XD, Xu GY, Yu CH. Association of caveolin-3 and cholecystokinin A receptor with cholesterol gallstone disease in mice. World J Gastroenterol. 2014;20:9513–9518. doi: 10.3748/wjg.v20.i28.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyot C, Combe C, Desmoulière A. The common bile duct ligation in rat: A relevant in vivo model to study the role of mechanical stress on cell and matrix behaviour. Histochem Cell Biol. 2006;126:517–523. doi: 10.1007/s00418-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 22.Weinreb M, Pollak RD, Ackerman Z. Experimental cholestatic liver disease through bile-duct ligation in rats results in skeletal fragility and impaired osteoblastogenesis. J Hepatol. 2004;40:385–390. doi: 10.1016/j.jhep.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Ackerman Z, Weinreb M, Amir G, Pollak RD. Bone mineral metabolism and histomorphometry in rats with cholestatic liver disease. Liver. 2002;22:166–172. doi: 10.1046/j.0106-9543.2002.01566.x. [DOI] [PubMed] [Google Scholar]
- 24.Tripodi V, Contin M, Fernández MA, Lemberg A. Bile acids content in brain of common duct ligated rats. Ann Hepatol. 2012;11:930–934. [PubMed] [Google Scholar]
- 25.Gaudio E, Franchitto A, Pannarale L, Carpino G, Alpini G, Francis H, Glaser S, Alvaro D, Onori P. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12:3546–3552. doi: 10.3748/wjg.v12.i22.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lygidakis NJ, Carlei F. Structural and functional biliary tree changes secondary to extrahepatic biliary obstruction. Endoscopy. 1989;21 Suppl 1:321–323. doi: 10.1055/s-2007-1012981. [DOI] [PubMed] [Google Scholar]
- 27.Nakano S, Haratake J, Hashimoto H. Alterations in bile ducts and peribiliary microcirculation in rats after common bile duct ligation. Hepatology. 1995;21:1380–1386. [PubMed] [Google Scholar]
- 28.Ohtani O, Murakami T. Peribiliary portal system in the rat liver as studied by the injection replica scanning electron microscopic method. Scanning electron microscopy. Part 11. Chicago: SEM Inc; 1978. pp. 241–244. [Google Scholar]
- 29.Kono N, Nakanuma Y. Ultrastructural and immunohistochemical studies of the intrahepatic peribiliary capillary plexus in normal livers and extrahepatic biliary obstruction in human beings. Hepatology. 1992;15:411–418. doi: 10.1002/hep.1840150310. [DOI] [PubMed] [Google Scholar]
- 30.Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Simeone DM, Gilbert R. Gallstone Incidence and Risk Factors. Upchurch. Greenfield’s Surgery: Scientific Principles and Practice. 5th ed. Wolters Kluwer; 2010. [Google Scholar]
- 31.Rosenow EC. The etiology of cholecystitis and gallstones and their production by the intravenous injection of bacteria. J Infect Dis. 1916;19:527–556. [Google Scholar]
- 32.Copher GH, Illingworth CFW. Experimental study of the factor of biliary stasis in the production of gallstones. Surg Gynec Obstet. 1928;46:658–659. [Google Scholar]
- 33.Cushing H. Observations upon the origin of gallbladder infections and upon the experimental formation of gallstones. Johns Hopkins Bulletin. 1899;10:166–170. [Google Scholar]
- 34.Okey R. Gallstone formation and intake of B vitamins in cholesterol-fed guinea pig. Proc Soc Exp Biol (NY) 1942;51:349–350. [Google Scholar]
- 35.Dam H, Christensen F. Alimentary production of gallstones in hamsters. Acta Pathol Microbiol Scand. 1952;30:236–242. doi: 10.1111/j.1699-0463.1952.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 36.Caldwell FT, Levitsky K, Rosenberg B. Dietary production and dissolution of cholesterol gallstones in the mouse. Am J Physiol. 1965;209:473–478. doi: 10.1152/ajplegacy.1965.209.3.473. [DOI] [PubMed] [Google Scholar]


