Abstract
Background
In patients with Polycystic Ovarian Syndrome (PCOS), resolution of infertility is an important goal of treatment. Wedge resection of the ovaries described as a means to achieve this was practiced in the middle of twentieth century. With the advent of endoscopic surgery, surgical approach for the same condition has been modified. Multi point biopsy, multiple needle puncture, electofulguration and laser fulguration are being tried in the context of PCOS. This project was taken up to evaluate the scope of electo fulguration in clomiphene resistant PCOS.
Methods
Forty patients who did not show sonographic evidence of ovulation with clomiphene citrate (CC) 100mg OD for 05 days in two cycles were subjected to laparoscopy. The patients who did not show any pelvic factor for infertility were alternately assigned to electro - fulguration treatment of ovaries or no fulguration during laparoscopy. These were designated as ‘Lap EC’ & ‘Only CC’ group respectively. For ‘Only CC’ group’ stimulation with CC was continued for four cycles with a higher dose 150 mg OD for 05 days. Lap EC group were subjected to CC 100mg OD for 5 days for two cycles in case of non achievement of ovulation in the first two drug free cycles following EC. Folliculometry, HCG administration and Intra Uterine Insemination (IUI) was performed for both groups.
Results
Total percentage of ovulatory cycles were 51.8% in EC group compared to 5.26% in the CC group. Overall pregnancy rate of 30% was achieved in the Lap EC group as compared to only 10% in the CC group (p<0.05).
Conclusion
Laparoscopic electrofulguration of ovaries increases the chances of ovulation and conception. This being a cheaper one time procedure as compared to other expensive ovulation inducing agents, should be the preferred mode and the primary procedure wherever polycystic ovaries are encountered while evaluating a case of infertility by laparoscopy.
Key Words: Infertility, PCOS, Electrofulguration
Introduction
The Polycystic Ovarian Syndrome (PCOS) remains an incompletely understood entity that appears with regularity in the practice of most gynaecologists. PCOS is characterized by the presence of enlarged ovaries with multiple small cysts in a hypervascularised androgen secreting stroma with signs of androgen excess (hirsutism, acne) and cycle disturbances (oligomenorrhoea / amenorrhoea) [1]. As the presenting symptoms of this groups of patients is often infertility due to chronic anovulation, restoration of ovulatory function assumes paramount importance.
Although the polycystic ovary was described in text books from the early part of twentieth century the condition was considered untreatable and best left alone. Treatment in North America developed only in the fourth decade and initially consisted of laparotomy with bilateral ovarian wedge resection [2,3]. As concerns regarding post operative adhesions surfaced and ovulation inducing agents became available, medical induction of ovulation became the dominant form of treatment. Clomiphene citrate (CC) is usually the first line of therapy. In case of poor response to CC, powerful gonadotrophins are used. Close monitoring in such cycle is essential to obviate hyper stimulation. High cost of gonadotrophins is another limiting factor.
In recent years, there is a renewed interest in surgical approach, because of the rapidly expanding field of operative laparoscopy. Though the mechanisms responsible for the ovulation inducing properties of a destructive procedure are ill understood, use of simple mechanical puncture of ovaries, laser treatment as well as electrofulguration are being tried by various workers [4,5]. The simplicity of one time procedure of electrofulguration can give it a place in the armamentarium of gynaecologists. With this background, this project was undertaken to evaluate the role of laparoscopic electrofulguration of the ovaries in patients of PCOS who had shown a poor response to CC.
Material and Method
The study was carried out in a tertiary care hospital for a period of 18 months from Jan 1999 to Jun 2000 on women with polycystic ovarian syndrome. After history and clinical examination, Trans Vaginal Sonography (TVS), Hystero salpingography (HSG), endocrinological profile: LH, FSH, Prolactin and TSH and Husband's Seminogram were carried out in all patients selected for the study. The criteria for inclusion in the study were presenting complaints of infertility, menstrual irregularities in the form of oligomenorrhoea / amenorrhoea, enlarged cystic ovaries on ultrasound, and an elevated LH on day 3 of the cycle. Patients having any other obvious cause for infertility like male factors, uterine malformation or gross tubo – peritoneal factor on HSG were excluded from the study.
All patients received CC 100mg OD from day 2 to day 6 of the cycle. TVS was used to monitor the response from day 10. If patient did not show any dominant follicle by day 15 in two consecutive cycles she was subjected to laparoscopy. Identification of any additional factors on laparoscopy (i.e endometriosis / PID) qualified as a criteria of exclusion. The patients who had not ovulated with CC and had not shown any pelvic factor for infertility were alternately assigned to electrofulguration treatment or no fulguration during laparoscopy. These were designated as ‘Lap EC’ & ‘Only CC’ group respectively.
After general anaesthesia and endotracheal intubation, the laparoscope was introduced subumbilically. Karlstorz, Hopkins straight forward zero degree telescope was used, with a view diameter of 10 mm, length of 33 cm, model number 26033 AP. To puncture abdomen, Karl Storz trocar and cannula was used, having a length of 10.5 cm, diameter of 11 mm, model number 30103 KP. Trocars of 6 mm diameter were introduced into each of the iliac fossae for diathermy probe and the grasping forceps (30421 MP Karl Storz: diameter = 5mm; length = 36 cm; insulated). Diathermy equipment used was Valley Lab. Unipolar, 50hz current was used. Needle point diathermy probe was inserted to one cm depth and current was applied for 2 seconds at 4-8 points in each ovary (Fig. 1, Fig. 2). Normal saline was introduced into the pouch of Douglas to enhance ovarian cooling after electocoagulation. After electrocoagulation each ovary was lowered into the pool of saline.
Fig. 1.
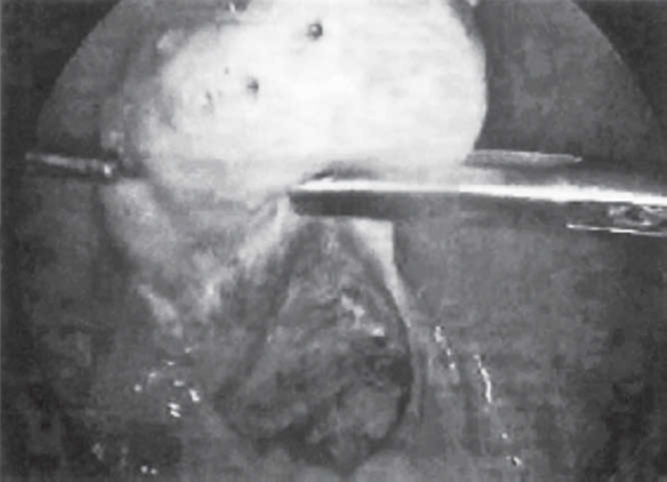
Laparoscopic electrofulguration in PCOS
Fig. 2.
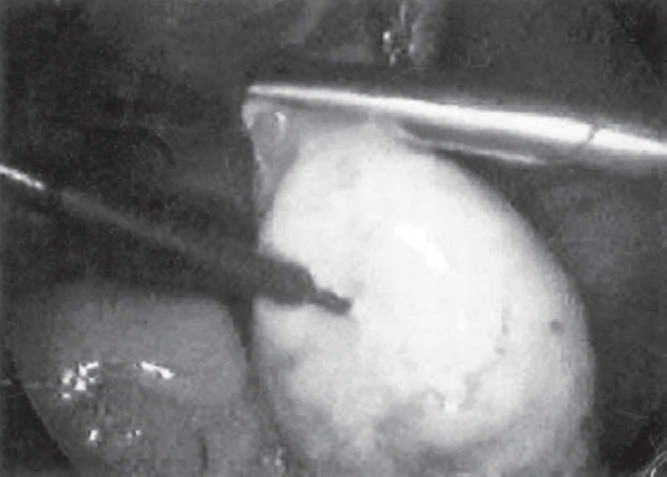
Electrofulguration in progress
On return of menses following laparoscopy, Lap EC group were not given any drug for next two cycles, but TVS was carried out from day 10 to look for evidence of ovulation. In case of non achievement of ovulation / conception in the first two cycles following EC, CC was restarted in the doses of 100mg OD for the next two cycles. For ‘only CC’ group stimulation with CC was continued for four cycles with a higher dose of 150mg OD for 5days. TVS was done for all the patients from day 10. Human Chorionic Gonadotrophin (HCG) 5,000 IU was administered on demonstration of a dominant follicle of 18mm. Intra Uterine Insemination (IUI) was performed on demonstration of sonographic evidence of ovulation. Pregnancy was diagnosed by velocit test in case of missing of period.
Results
Both groups were matched as regards to age and pre operative hormone status (Table 1, Table 2).
Table 1.
Age distribution and age of menarche
| Age (years) | Lap EC group (n=20) | Only CC group (n=20) |
|---|---|---|
| < 20 | 0 | 0 |
| 20-30 | 15 | 16 |
| >30 | 5 | 4 |
| Mean age (years) | 26.3 | 26.75 |
| Mean age of menarche (years) | 13.05 | 12.6 |
Table 2.
Pre-operative hormone status of patients in both groups
| Hormone | Lap EC group | CC group | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| LH (mlU/ml) | 26.8 | 4.08 | 22.0-40.0 | 30.2 | 5.21 | 23.5-48.4 |
| FSH (mlU/ml) | 6.9 | 1.75 | 2.4-12.6 | 8.7 | 0.91 | 6.1-10.4 |
| PRL (ng/ml) | 19.2 | 3.10 | 17.1-28.8 | 18.4 | 2.65 | 13.9-27.1 |
The mean time of onset of menses in the patients treated with fulguration was 18 days following surgery. The corresponding figure for the untreated group was 32 days. The figures for ovulatory cycles, conception and miscarriage have been given in Table 3. The results of laparoscopic electrocoagulation were very encouraging as compared to the patients receiving only CC. Expressed as cycles, 20 out of 38 cycles (52.6%) were ovulatory in the first two months in the Lap EC group. Reintroduction of CC in 8 patients who did not ovulate spontaneously after Lap EC resulted in 8 ovulatory cycles. Thus total ovulatory cycles in the Lap EC group with and without clomiphene was 51.8% compared to only 5.26% (4 out of 76) in the ‘Only CC’ group. 4 patients conceived (20%) with only Lap EC therapy while a further 2 (10%) conceived with the addition of CC. Thus an overall pregnancy rate of 30% was achieved in the ‘Lap EC’ group as compared to only two pregnancy (10%) in the ‘only CC’ group (p<0.05). One pregnancy in CC group and two pregnancies in EC group ended in spontaneous abortion in the first trimester.
Table 3.
Clinical Responses to treatment in patients with PCOS
| Diagnosis | Patients | No of cycles | No of ovulations | % cycles ovulatory | Pregnancies | Miscarriage |
|---|---|---|---|---|---|---|
| CC Group | ||||||
| CC 150 mg for 5 days | 20 | 76 | 04 | 5.26 | 2 | 1 |
| Lap EC Group | ||||||
| LapEC only | 20 | 38 | 20 | 52.6 | 4 | 2 |
| Lap EC + CC 100 | 8 | 16 | 8 | 50 | 2 | − |
| Total | 28 | 54 | 28 | 51.8 | 6 | 2 |
Discussion
For all those patients of PCOS who are found to be having a poor response to Clomiphene Citrate, the choice of treatment rests between medical ovulation induction using higher doses, higher stimulants like gonadotrophins or the use of laparoscopic surgical method. Potential advantages of the surgical methods include multiple ovulatory cycles from a single treatment and elimination of the risk of Ovarian Hyper Stimulation Syndrome (OHSS). Additionally, high cost and intensive monitoring associated with gonadotropin therapy can be avoided.
The first report on laparoscopic surgery in patients with PCOS resorted to ovarian biopsy in an effort to decrease the ovarian bulk [6]. As the use of electrocautery at laparoscopy increased, it was used to create thermal damage of the excess ovarian stroma. As no tissue was removed, less raw surface and bleeding resulted and less were the chances of future adhesion formation. The classic laparoscopic method was described by Gjonnaess [7]. Daniell and Miller first described the use of lasers in PCOS patients [5]. A summary of conceptions following laparoscopic ovarian drilling has been compiled by Donesky and Adashi [2]. Following laparoscopic surgery, almost 80% have ovulated and 60% have conceived. The results of our study is less than the above compilation because of a short follow up period. However, even these rates are distinctly better than those patients who were continued with higher doses of CC.
Destruction of ovarian stromal elements and release of androgen rich follicular fluid from the puncture of sub capsular cysts causes a fall in local and circulating levels of androgens. Decrease in substrate for follicular aromatase and damage to the follicle walls itself, produces a fall in circulating oestradiol levels. This releases pituitary from the negative feedback on FSH and positive feedback on LH.
The changes in hormone levels after laparoscopic electrocoagulation have been studied in detail by various authors. Serum levels of testosterone was found reduced significantly after cautery [8]. Concerning the behavior of DHEAS serum levels while some have found no change after surgery, Naether et al [9] found a significant reduction in DHEAS levels. The FSH levels have been found to rise after surgery causing fall in LH: FSH ratio[10]. Regarding post operative LH levels most authors have found a reduction [8,10]. Inhibin levels fall significantly immediately after ovarian cautery [11]. It is believed that all these changes make the follicular environment more responsive and ovulation ensues [12].
Some authors have found a correlation between the number of points cauterized and the ovulation rate. Gjonnaes reported highest ovulation rates with more than 10 points, but the difference between 6 and 10 points was marginal [7]. We had used maximum 8 points and did not find any difference between 6 and 8 point approach.
Since the adhesion formation is directly related to the raw ovarian surface produced, it is generally agreed that the number of punctures be restricted to minimum necessary. Armar et al. recommended only four diathermy points per ovary [8]. Gjonnaess later used only 6-8 and Greenblatt used 8 points per ovary [13,14]. As adhesions have been noticed in equal rates after the use of laser, it has been suggested by Naether et al that if laparoscopic treatment of the ovaries is considered, electrocoagulation seems to be cheaper and easier technique [9,14]. However, better ovulation rates with the use of Halmiun yag laser as brought out in a recent report can not be denied [15].
Ovarian wedge resection (OWR) went out of use as adhesion formation was a significant sequelae. Hence the place of laparoscopic electrocoagulation needs to be evaluated on this point. Gjonaess [14] had found translucent adhesions in some patients subjected to electrocautery but judged them of limited consequence to the tubo-ovaian relationship. Animal research on pigs suggested that one major effect of laparoscopic electrocoagulation of ovaries is an increase in ovarian blood supply and adhesion formation which are classified as mild to minimal [9]. The data available reveals that adhesions formation is not a rare occurrence following laparoscopic electrocoagulation. However, the significance of minimal adhesions to fertility is not proven. Gurgan et al performed a prospective randomized controlled study and concluded that, although minimal adhesion formation is undeniable, they are not possibly of sufficient severity to prevent conceptions [16]. 37.5% pregnancies ended in abortion in the current study. Spontaneous abortions occur in about 30% of pregnancies after ovulation induction in women with PCOS, regardless of the treatment used. The most likely reason for the increased rate of early pregnancy loss in PCOS is relatively elevated levels of LH observed in many of these women which impairs the oocyte quality [17].
It can be concluded that laparoscopic electrocoagulation of ovaries increases the chances of ovulation and conception. Being a much cheaper one time procedure as compared to gonadotrophins, it should be the preferred mode of therapy for all cases of clomiphene resistant polycystic ovarian disease. It can also be recommended as a primary procedure wherever PCOS is encountered while evaluating a case of infertility by laparoscopy. The altered micro environment makes the ovaries more responsive to stimulation both exogenously and endogenously.
Conflicts of Interest
None identified
References
- 1.Balen AH, Conway GS, Kalstas G. Polycystic ovarian syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2705–2712. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 2.Donesky BW, Adashi EY. Surgically induced ovulation in polycystic ovarian syndrome: wedge resection revisited in the age of laparoscopy. Fertl Steril. 1995;63:439–463. doi: 10.1016/s0015-0282(16)57408-1. [DOI] [PubMed] [Google Scholar]
- 3.Stein JF, Leventhal ML. Amenorrhoea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- 4.Armar NA, Lachelin GC. Laparoscopic ovarian diathermy. An effective treatment for anti estrogen resistant anovulatory infertility in women with polycystic ovaries. Br J Obstet Gynecol. 1993;100:161–164. doi: 10.1111/j.1471-0528.1993.tb15214.x. [DOI] [PubMed] [Google Scholar]
- 5.Daniel JF, Milter N. Polycystic ovaries treated by laser vaporization. Fertil Steril. 1989;51:232–236. doi: 10.1016/s0015-0282(16)60482-x. [DOI] [PubMed] [Google Scholar]
- 6.Neuwirth RS. A method of bilateral ovarian biopsy at laparoscopy in infertility and chronic anovulation. Fertil Steril. 1972;23:361–366. doi: 10.1016/s0015-0282(16)38948-8. [DOI] [PubMed] [Google Scholar]
- 7.Gjonnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil Steril. 1984;41:20–25. doi: 10.1016/s0015-0282(16)47534-5. [DOI] [PubMed] [Google Scholar]
- 8.Armar NA, McGarrigle HHG, Honour J. Laparoscopic ovarian diathermy in the management of anovulatory infertility in women with polycystic ovaries: L endocrine changes and clinical outcome. Steril. 1990;58:45–49. doi: 10.1016/s0015-0282(16)53214-2. [DOI] [PubMed] [Google Scholar]
- 9.Naether OGJ, Fischer R, Weise HC. Laparoscopic electrocoagulation of the ovarian surface in infertile patients with polycystic ovaian disease. Fertil steril. 1993;60:88–94. doi: 10.1016/s0015-0282(16)56042-7. [DOI] [PubMed] [Google Scholar]
- 10.Rossmanith WG, Keckstein J, Spatzier C, Lauritzen The impact of ovarian laser surgery on the gonadotrophin secretion in women with polycystic ovarian disease. Clin Endocrinol (Oxf) 1991;34:223–230. doi: 10.1111/j.1365-2265.1991.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 11.Campo S, Felli A, Lamanna MA, Barini A Garcea N. Endocrine changes and clinical outcome after laparoscopic ovarian resection in women with polycystic ovaries. Hum Reprod. 1993;8:359–363. doi: 10.1093/oxfordjournals.humrep.a138051. [DOI] [PubMed] [Google Scholar]
- 12.Klovacs G, Buckler H, Bangah M. Treatment of anovulation due to polycystic ovarian syndrome by laparoscopic ovarian electocautery. Br J Obstet Gynaecol. 1991;98:30–35. doi: 10.1111/j.1471-0528.1991.tb10307.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt Em, Casper RF. Adhesion formation after laparoscopic ovarian cautery for polycystic ovarian syndrome: lack of correlation with pregnancy rate. Fertil Steril. 1993;60:766–770. [PubMed] [Google Scholar]
- 14.Gjonnaess H. Ovarian electrocautery in the treatment of women with polycystic ovary syndrome (PCOS) Actal Obstetric et Gynaecology Scandinavia. 1994;73:407–412. doi: 10.3109/00016349409006253. [DOI] [PubMed] [Google Scholar]
- 15.Laparoscopic treatment of polycystic ovaries with holmium yag laser. Fertil Steril. 2002;77:852–853. doi: 10.1016/s0015-0282(01)03278-2. [DOI] [PubMed] [Google Scholar]
- 16.Gurgan T, Kisisci H, Yarali H. Evaluation of adhesion formation after laparoscopic treatment of polycystic ovarian disease. Fertil Steril. 1991;56:1176–1178. doi: 10.1016/s0015-0282(16)54737-2. [DOI] [PubMed] [Google Scholar]
- 17.Regan L, Owen J, Jacobs HS. Hypersecretion of leutinising hormone, infertility and miscarriage. Lancet. 1990;336:1141–1144. doi: 10.1016/0140-6736(90)92765-a. [DOI] [PubMed] [Google Scholar]


