Abstract
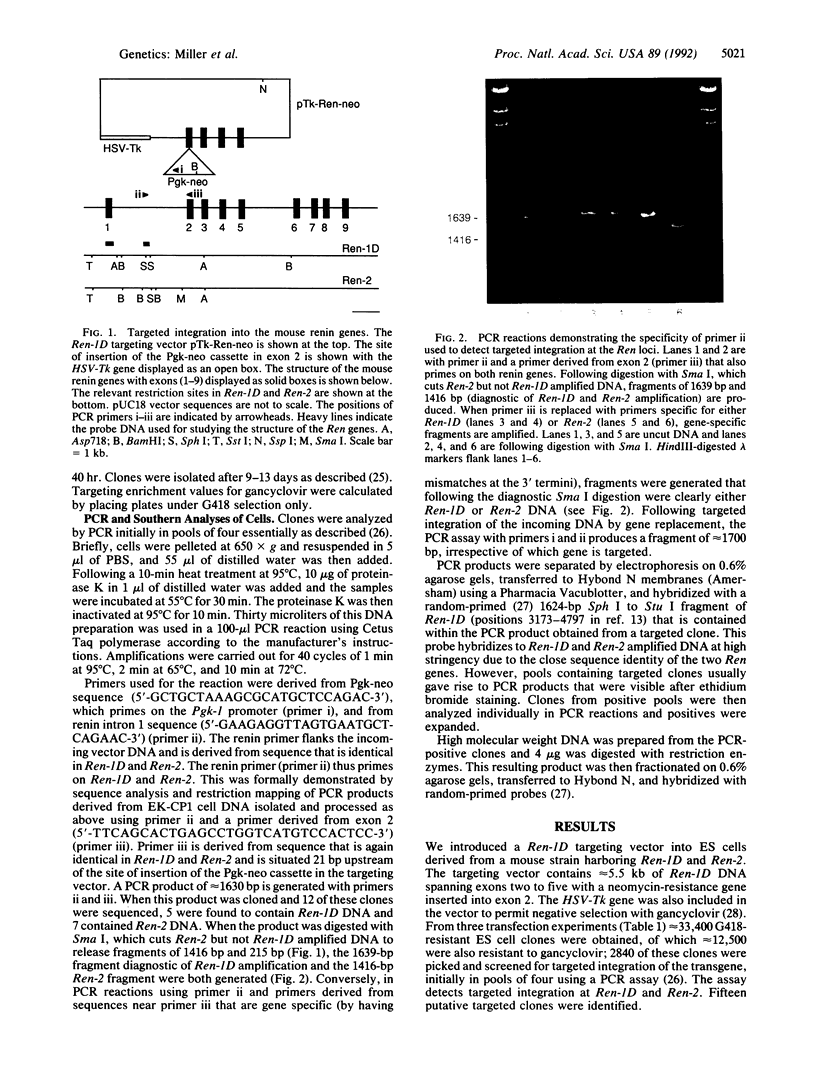
We have introduced a Ren-1D targeting vector into embryonic stem cells containing the two highly homologous mouse renin genes Ren-1D and Ren-2. Using a polymerase chain reaction (PCR) screen designed to detect targeted integration at Ren-1D and Ren-2, we isolated 15 targeted embryonic stem cell clones, all of which had undergone a gene conversion event at the Ren-1D locus. We did not isolate any clones in which the incoming DNA had recombined with Ren-2. Over the region encompassed by our transgene, Ren-1D and Ren-2 display greater than 95% homology. Our results suggest that the machinery driving gene targeting by means of homologous recombination in mammalian cells is capable of distinguishing between these two sequences. Construction of transgenic mice with the embryonic stem cells reported here carrying a mutated renin gene will permit a greater understanding of the functions of the Ren-1D and Ren-2 gene products and their relative contribution to cardiovascular homeostasis.
Full text
PDF
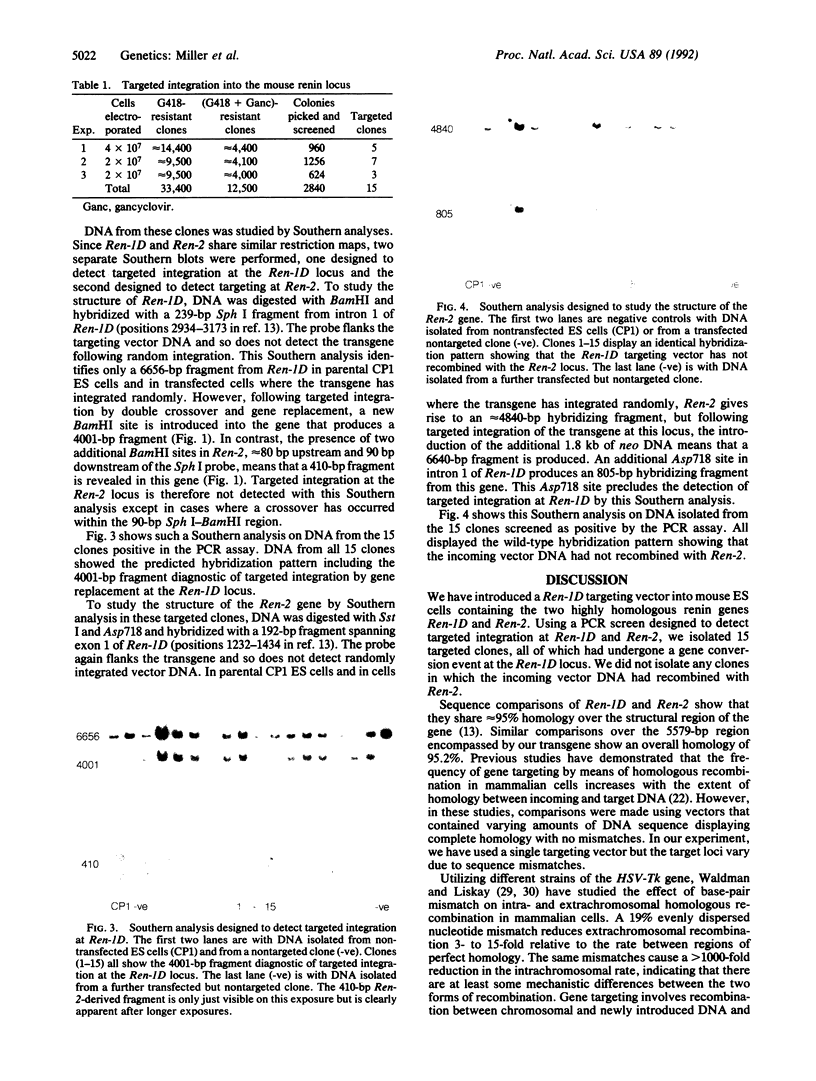


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel K. J., Gross K. W. Close physical linkage of the murine Ren-1 and Ren-2 loci. Nucleic Acids Res. 1988 Mar 25;16(5):2111–2126. doi: 10.1093/nar/16.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adra C. N., Boer P. H., McBurney M. W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60(1):65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Bing J., Poulsen K., Hackenthal E., Rix E., Taugner R. Renin in the submaxillary gland: a review. J Histochem Cytochem. 1980 Aug;28(8):874–880. doi: 10.1177/28.8.7003007. [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Mullins L. J., George H., Smith G., Brooks J., Pioli D., Brammar W. J. The nucleotide sequence of a mouse renin-encoding gene, Ren-1d, and its upstream region. Gene. 1989 Dec 7;84(1):91–104. doi: 10.1016/0378-1119(89)90143-1. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Reith A. D., Brammar W. J. A retroviral provirus closely associated with the Ren-2 gene of DBA/2 mice. Nucleic Acids Res. 1984 Nov 26;12(22):8579–8593. doi: 10.1093/nar/12.22.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Gross K. W., Piccini N., Wilson C. M. Evolution and variation of renin genes in mice. Genetics. 1984 Nov;108(3):651–667. doi: 10.1093/genetics/108.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker M., Tronik D., Rougeon F. Extra-renal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5155–5158. doi: 10.1073/pnas.86.13.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J. Potential for genetic manipulation of mammals. Mol Biol Med. 1989 Dec;6(6):557–565. [PubMed] [Google Scholar]
- Fabian J. R., Field L. J., McGowan R. A., Mullins J. J., Sigmund C. D., Gross K. W. Allele-specific expression of the murine Ren-1 genes. J Biol Chem. 1989 Oct 15;264(29):17589–17594. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Field L. J., Gross K. W. Ren-1 and Ren-2 loci are expressed in mouse kidney. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6196–6200. doi: 10.1073/pnas.82.18.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J., Philbrick W. M., Howles P. N., Dickinson D. P., McGowan R. A., Gross K. W. Expression of tissue-specific Ren-1 and Ren-2 genes of mice: comparative analysis of 5'-proximal flanking regions. Mol Cell Biol. 1984 Nov;4(11):2321–2331. doi: 10.1128/mcb.4.11.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm I., Ollo R., Panthier J. J., Rougeon F. Evolution of aspartyl proteases by gene duplication: the mouse renin gene is organized in two homologous clusters of four exons. EMBO J. 1984 Mar;3(3):557–562. doi: 10.1002/j.1460-2075.1984.tb01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. S., Sheng M., Greenberg M. E., Kolodner R. D., Papaioannou V. E., Spiegelman B. M. Targeting of nonexpressed genes in embryonic stem cells via homologous recombination. Science. 1989 Sep 15;245(4923):1234–1236. doi: 10.1126/science.2506639. [DOI] [PubMed] [Google Scholar]
- Jones C. A., Sigmund C. D., McGowan R. A., Kane-Haas C. M., Gross K. W. Expression of murine renin genes during fetal development. Mol Endocrinol. 1990 Mar;4(3):375–383. doi: 10.1210/mend-4-3-375. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim R. G., Seidah N. G., Rougeon F. N-linked glycosylation affects the processing of mouse submaxillary gland prorenin in transfected AtT20 cells. Eur J Biochem. 1991 Jun 1;198(2):535–540. doi: 10.1111/j.1432-1033.1991.tb16047.x. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Miller C. C., Carter A. T., Brooks J. I., Lovell-Badge R. H., Brammar W. J. Differential extra-renal expression of the mouse renin genes. Nucleic Acids Res. 1989 Apr 25;17(8):3117–3128. doi: 10.1093/nar/17.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. J., Peters J., Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990 Apr 5;344(6266):541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- Mullins J. J., Sigmund C. D., Kane-Haas C., Gross K. W., McGowan R. A. Expression of the DBA/2J Ren-2 gene in the adrenal gland of transgenic mice. EMBO J. 1989 Dec 20;8(13):4065–4072. doi: 10.1002/j.1460-2075.1989.tb08590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthier J. J., Holm I., Rougeon F. The mouse Rn locus: S allele of the renin regulator gene results from a single structural gene duplication. EMBO J. 1982;1(11):1417–1421. doi: 10.1002/j.1460-2075.1982.tb01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Huang H. V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986 Mar;112(3):441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tronik D., Dreyfus M., Babinet C., Rougeon F. Regulated expression of the Ren-2 gene in transgenic mice derived from parental strains carrying only the Ren-1 gene. EMBO J. 1987 Apr;6(4):983–987. doi: 10.1002/j.1460-2075.1987.tb04848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Dependence of intrachromosomal recombination in mammalian cells on uninterrupted homology. Mol Cell Biol. 1988 Dec;8(12):5350–5357. doi: 10.1128/mcb.8.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Differential effects of base-pair mismatch on intrachromosomal versus extrachromosomal recombination in mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5340–5344. doi: 10.1073/pnas.84.15.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Cherry M., Taylor B. A., Wilson J. D. Genetic and endocrine control of renin activity in the submaxillary gland of the mouse. Biochem Genet. 1981 Jun;19(5-6):509–523. doi: 10.1007/BF00484623. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Erdös E. G., Wilson J. D., Taylor B. A. Location on chromosome 1 of Rnr, a gene that regulates renin in the submaxillary gland of the mouse. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5623–5626. doi: 10.1073/pnas.75.11.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Taylor B. A. Genetic regulation of thermostability of mouse submaxillary gland renin. J Biol Chem. 1982 Jan 10;257(1):217–223. [PubMed] [Google Scholar]