Abstract
IMPORTANCE
Chronic traumatic encephalopathy (CTE) refers to pathologic changes that have been found in some individuals with a history of repetitive traumatic impact to the head (hereinafter referred to as head trauma). These changes cannot be assessed during the clinical evaluation of a living patient.
OBSERVATIONS
The neuropathologic features, taxonomy, history, role of biomarkers in diagnosis, and existing criteria of CTE are reviewed. Previous criteria have been proposed to approach the living patient; however, a unified, specific approach is needed for the practicing clinician. We propose a new diagnostic construct for the clinical syndrome associated with repetitive exposure to head trauma: traumatic encephalopathy syndrome. This clinical paradigm will provide the framework for a diagnosis of probable, possible, and unlikely traumatic encephalopathy syndrome, with included discussion regarding the minimum exposure, nature of the clinical course, and additional clinical features needed for diagnosis.
CONCLUSIONS AND RELEVANCE
While prospective longitudinal studies are ongoing to further elucidate the association of exposure to head trauma, clinical features, and the development of pathologic changes, a corresponding clinical construct for diagnosis is necessary.
The current definition of chronic traumatic encephalopathy (CTE) is based on pathologic changes that do not have a direct clinical application. Although the term traumatic encephalopathy syndrome (TES) has been described previously when referring to the clinical syndrome associated with exposure to repetitive traumatic impact to the head (hereinafter referred to as head trauma),1,2 it has been applied in a very general sense, used to refer to a wide variety of clinical outcomes after brain trauma. The construct of TES, however, should be very carefully and precisely elucidated. Traumatic encephalopathy syndrome will be used in this review to refer to a progressive neurodegenerative disease that may occur as a result of previous, most often cumulative, head trauma. Use of TES is not meant to include the acute or postacute manifestations of a single concussion, postconcussion syndrome, or moderate to severe traumatic brain injury (TBI). The term CTE will be used when referring to pathologic findings, which may or may not be associated with a clinical syndrome. Recently, the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) study2 was proposed to investigate the clinicopathologic correlation in patients with CTE findings and will analyze the brains and spinal cords of 300 deceased individuals during the next 4 years. Using clinical criteria based on those proposed by Montenigro et al1 and consensus neuropathologic criteria, this group will retrospectively correlate pathologic findings with clinical features2 to provide insight into their association. In contrast to the UNITE study, our proposed criteria are clinically based and can be applied prospectively and provide a framework to guide clinical diagnosis. This framework is intended to inform clinical practice, allowing consistency across practice groups, and in the future can be used to establish whether a consistent pathologic feature is associated with TES. These criteria should be viewed as companion criteria to those of the UNITE study, providing a much needed clinical framework for current management. The proposed criteria herein are not intended to be a construct for prediction of underlying CTE pathologic changes. The framework is intended to be used by physicians for individuals with a history of exposure to head trauma who present with neurocognitive problems lasting longer than 2 years. Given the evolving nature of CTE and TES research, we expect that these working criteria will be updated over time.
Neuropathologic Features of CTE
Tremendous work has been performed during the last 10 years to understand this newly identified pathologic entity.3–7 Two groups have published proposed criteria that comment on the gross and microscopic features seen in their respective case series.8–10 These reports have laid important groundwork, which led to the first National Institutes of Health consensus conference in February 2015 to develop guidelines for the pathologic diagnosis of CTE.11,12 Gross pathologic changes include atrophy of the gray and white matter, diencephalon, and mammillary bodies; enlargement of the third ventricle and the frontal and temporal horns of the lateral ventricles; prominent perivascular spaces; depigmentation of the locus coeruleus and substantia nigra; and fenestrated or cavum septum pellucidum. Microscopic findings include deposition of p-tau protein as neurofibrillary tangles and neuropil threads with preferential involvement of the superficial layers of the associational neocortex and along blood vessels in the depths of the cortical sulci. Pathologic changes in TAR DNA-binding protein 43 and inflammation are also seen with advancing neuropathologic stages.8–10 Because of these findings, CTE has been described as predominantly a tauopathy, with an abnormal amount and distribution of tau in the brain.
The existence of CTE as currently described is not universally accepted in the neuropathology community. Recent research supports the concept that some deposition of tau is associated with normal human aging and does not necessarily indicate a pathologic entity.13 Some studies also suggest that some tau aggregates may even have a protective role in vivo.14,15 This supposition raises the question of whether the presence of tau is truly a marker of clinicopathologic features, with some authors suggesting that tau may indeed be a marker of neuronal injury after head trauma but that its association with clinical symptoms may not be causal.16 Last, postmortem studies of symptomatic, retired professional athletes have described pathologic changes consistent with neurodegenerative diseases other than CTE or mixed pathologic changes with features consistent with CTE and another neurodegenerative disease, making delineation of the clinical and pathologic entities difficult.17,18 Larger prospective studies that include matched controls are necessary to clarify the relationship between the clinical syndrome of TES and underlying pathologic features.
Taxonomy and History of TES
Punch drunk syndrome and dementia pugilistica are terms coined in the 1920s and 1930s to describe a clinical syndrome associated with repetitive head trauma in boxers.19, 20 The term chronic traumatic encephalopathy was later introduced when a similar syndrome was described in nonboxers.21 Recent debate has questioned whether the clinicopathologic entity originally described as dementia pugilistica falls within a spectrum of CTE-TES or represents a distinct clinical entity. Gardner and colleagues22 described TES, referred to in their report as modern CTE, as a clinically distinct entity from dementia pugilistica, or classic CTE. Compared with classic CTE, modern CTE was believed to be associated with less prominent motor and cerebellar features, a decreased rate of exposure to head trauma, symptom onset at an earlier age, and increased likelihood of progression.22 However, the force exposure in boxers differs greatly from that of the other contact sports included in the definition of modern CTE by Gardner et al22 and may alone be sufficient to explain observed clinicopathologic differences between classic and modern CTE. The increased incidence of motor features (eg, dysarthria, ataxia, disequilibrium, and tremor) observed in boxers compared with other contact sport athletes may reflect the mechanics and number of traumatic impacts experienced. This conclusion is supported by the observation that the severity of the condition has been shown to be directly related to the length of the boxer’s career and the number of bouts.23
The early reports inboxers19, 20 and a publication by Stern et al24 have suggested the following 2 distinct clinical presentations for TES: (1) earlier age at onset with initial mood and behavior disturbance and (2) later age at onset with initial cognitive impairment. However, the observation that almost all of those individuals with mood and behavioral disturbances at onset demonstrated cognitive impairment at some point supports the concept of a spectrum of presentations rather than distinct subtypes.24 In addition, the behavioral predominant group may have had an alternative psychiatric explanation for their symptoms that is unrelated to head trauma exposure, and their symptoms should be considered separately from TES. These distinctions of classic and modern CTE and 2 distinct clinical presentations of TES are based on a limited number of cases. Definitive classification into classic vs modern CTE or clinical subtypes of TES is difficult to support at this time. Rigid diagnostic categories will likely prove insufficient to accurately capture the underlying CTE-TES disease spectrum.
Role of Biomarkers in the Diagnosis of TES
Research investigating possible associative biomarkers for CTE-TES is ongoing, but at the present time, no reliable predictive biomarkers exist. Candidate biomarkers generally fall under the categories of serum or cerebrospinal fluid assays; various forms of advanced neuroimaging, such as positron emission tomographic imaging of accumulated tau; and genetic polymorphisms. Biomarkers hold the promise of furthering our understanding of the clinical and pathologic spectrum of TES, and we hope that biomarkers will play a major role in the clinical diagnosis of TES in the future. However, at present, the diagnosis of TES should be based on a clinical construct until the predictive abilities of these biomarkers are more clearly established.
Existing Clinical Criteria for TES
Jordan,25 Victoroff,26 and Montenigro et al1 have proposed clinical diagnostic criteria for TES. Jordan25 proposed clinical diagnostic criteria for TES (termed CTE in that report) with classifications of definite, probable, possible, and improbable TES based on clinical features and the presence or absence of corresponding pathologic changes. Definite TES included neurologic signs that are consistent with CTE (described as behavioral disturbance, cognitive dysfunction, and/or motor-related symptoms) and pathologic confirmation of tau deposition. Probable TES was defined as “two or more of the following conditions: cognitive and/or behavioral impairment; cerebellar dysfunction; pyramidal tract disease or extrapyramidal disease.”25 (p6) Although not a required element in his criteria, Jordan25 further proposed that a diagnosis of probable CTE could be supported by abnormal neuroimaging findings on positron emission tomography, single-photon emission tomography, structural magnetic resonance imaging, or diffusion-tensor imaging. Strengths of this framework include distinguishing between postconcussion syndrome and TES, a discussion of the possibility of mixed pathologic features that contribute to an individual’s clinical presentation (eg, CTE plus Alzheimer disease), and allowance for the limitations of current biomarker research. This framework includes a definite category based on the presence of pathologic changes with clinical presentation and comments on the supportive nature of abnormal imaging findings in the diagnosis that are each difficult to support at this time.
Victoroff26 proposed the need for operational clinical criteria to allow for consistency between physicians and to help advance research regarding the association of CTE and TES. The review by Victoroff26 enumerated the signs and symptoms of 92 boxers and 4 American football players from 1928 to 2010. From the review, Victoroff26 proposed criteria for diagnosis of clinically probable and clinically possible TES. Clinically probable TES required a history of head trauma exposure, persistence of symptoms for longer than 2 years, lack of another diagnosis to otherwise explain the signs and symptoms, and the presence of at least 2 symptoms (ie, speech, mood, or behavioral disturbance) and 3 signs (ie, ataxia, memory loss, and dysarthria).26 One strength of this framework was the absence of a classification of definite TES with the implied understanding that determination of clinically definite TES was not possible at that time. Another strength was the omission of biomarkers from the clinical diagnostic criteria. Victoroff26 acknowledged that the most important limitations of his study include the difficulty of retrospective case reports, the lack of specificity of the signs and symptoms of TES, and awareness of the fundamental difficulty of establishing pathologic and clinical criteria for this entity in parallel. In addition, this framework was created based on data available from boxers who, given different force exposure, may not represent the entire spectrum of TES.
Montenigro et al1,27 reviewed published cases of pathologic CTE to further describe the clinical spectrum of TES and to propose criteria for use in research design, which were not intended to be used clinically for the evaluation of living patients. The authors used data from 202 cases of male athletes with histories of repetitive head trauma who met review criteria for possible, probable, and neuropathologically confirmed CTE. Boxers represented 70% of this cohort, and 97 cases (48.0%) predated 1970.
Based on their review, Montenigro et al1,27 proposed diagnostic criteria for TES using only those clinical signs and symptoms that were present in more than 70%of reported cases of CTE, requiring repetitive head trauma, persistence of symptoms for longer than 1 year, and absence of another neurologic disorder that could otherwise account for the symptoms. In addition, at least 1 core clinical feature (ie, cognitive, behavioral, or mood disturbance) and 2 supportive features (ie, impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs, progressive decline, or delayed onset) were required for a TES diagnosis. Several modifiers were added to the definition, including behavioral or mood variant, cognitive variant, mixed variant, or TES dementia; progressive course, stable course, or unknown or inconsistent course; and with or without motor features. The criteria then sought to establish the likelihood of CTE (underlying pathologic change) based on meeting criteria for TES and the presence or absence of positive biomarkers (including findings on magnetic resonance imaging or positron emission tomography and cerebrospinal fluid changes). The authors favored sensitivity over specificity in diagnosis, which is appropriate for their intention, namely, the use of these criteria in research studies to better understand the TES-CTE spectrum. However, this design decision results in necessary limitations (eg, increased false-positive findings) in the clinical setting. Montenigro et al1, 27 proposed that delay of onset and progression were not required features for diagnosis, which limits the ability of these criteria to distinguish postconcussion syndrome or chronic neurocognitive impairment after moderate or severe TBI from TES. Last, Montenigro et al1, 27 proposed guidelines for probable, possible, and unlikely CTE (underlying pathologic change) based on meeting criteria for TES, which is difficult to support at this time.
The criteria proposed by Montenigro et al1,27 were then adapted for the recently proposed UNITE study.2 This study proposes analyzing the brains and spinal cords of 300 deceased individuals with a history of repetitive head trauma during the next 4 years. The investigators will use consensus neuropathologic data for diagnosis of CTE and an adaptation of the criteria of Montenigro et al1,27 to perform a retrospective study of the clinical features of those individuals who meet minimum head trauma exposure criteria and the consensus neuropathologic criteria of CTE. The UNITE study is designed for retrospective identification of individual diagnostic features that are associated with CTE pathologic features. The study proposes to apply the criteria of Montenigro et al1,27 regarding core and supportive clinical features and subtype and course modifiers. The UNITE study is limited by ascertainment bias, retrospective clinical evaluation, and absence of a control population and as such will not be able to speak to the specificity of CTE pathologic features for the presence of clinically meaningful disease.
Our criteria differ from the UNITE study’s approach in that the criteria proposed herein rely on clinical features in a population exposed to repetitive head trauma to provide a clinical diagnosis of TES without the goal of predicting underlying pathologic changes. Without well-designed prospective studies, the assumption that CTE pathologic features are caused by repetitive exposure to head trauma and that these pathologic changes cause clinically evident symptoms and signs remains unproven.
The existing clinical criteria have been essential contributions to the discussion of TES; however, all rely on retrospectively obtained, highly selective reference populations, which limits their ability to fully address the spectrum of the disease and to clarify the association of TES and CTE. As a function of favoring sensitivity over specificity, existing criteria also permit a diagnosis of TES when only behavioral or mood symptoms exist, which may occur in primary psychiatric disease. Research has shown significant crossover in symptoms and presentation between patients with primary psychiatric disorders and neurodegenerative disease, so caution must be exercised in diagnosis in these patients.28–30 Although inclusion of these cases with behavioral or mood symptoms in the research design makes sense, specificity of diagnosis should be favored in the clinical setting.
Proposed Clinical Criteria
A wide spectrum of symptoms and signs has been described for TES, creating a clear need for a working clinical definition. The proposed criteria (Box) are broadly consistent with previous definitions and emphasize that cognitive dysfunction, behavioral symptoms, and mood changes are core features of TES.1 At this time, clinical criteria should emphasize diagnostic specificity over sensitivity. Because behavioral and mood symptoms are common, significant false-positive diagnoses can be made if their presence alone is sufficient for the diagnosis of TES. A diagnosis of TES should be considered when a patient presents with persistent neurocognitive problems that last longer than 2 years in the setting of prior head trauma exposure. The relative confidence with which a diagnosis of TES can be made depends on the number and type of symptoms and signs present. The proposed clinical criteria present a construct of probable, possible, and unlikely TES. Owing to current knowledge gaps regarding the relationship of TES to the underlying pathologic changes of CTE, a definite TES category is not proposed at this time, and these criteria are not intended to predict pathologic change.
Box. Proposed Clinical Diagnostic Criteria for TES.
Required Features
Persistence of symptoms for longer than 2 yearsa,b
No other neurologic disorder is more likely to account for all the clinical featuresa,b
History of head trauma exposure, typically associated with history of concussion, although may be limited to subconcussive traumaa,b
Head trauma exposure is repetitive in naturea
Demonstrated progressive coursea,b
Delayed symptom onseta
Self-report or observer report of cognitive dysfunction, confirmed with objective cognitive decline documented by results of formal neuropsychological testinga,c
Supportive Features
Emotional dysregulation: including depression, anxiety, agitation, aggression, paranoid ideation, deterioration of interpersonal relationships, and suicidality
Behavioral change: including violence, poor impulse control, socially inappropriate behavior, avolition, apathy, change in personality, and comorbid substance abuse
Motor disturbance: including bradykinesia, tremor, rigidity, gait instability, dysarthria, dysphagia, ataxia, and gaze disturbance
Abbreviation: TES, traumatic encephalopathy syndrome.
aA diagnosis of probable TES requires persistence of symptoms for longer than 2 years, no other neurologic disorder that is more likely to account for all the clinical features, history of traumatic impact exposure that is repetitive in nature, delayed symptom onset, a progressive course, cognitive decline, and at least 1 of the supportive features listed above.
bPossible TES requires persistence of symptoms for longer than 2 years, no other neurologic disorder that is more likely to account for all the clinical features, history of head trauma exposure, progressive course, and at least 1 supportive feature. Unlikely TES would not meet minimum diagnostic criteria for possible TES and may include individuals in whom another neurodegenerative disease or psychiatric disorder is likely.
cCognitive decline typically affects more than 1 domain (executive, visuospatial, memory, and language).
Investigation of competing causes of a patient’s symptoms before concluding a diagnosis of TES is essential. Where other clinical syndromes may overlap, physicians must focus on the identification and treatment of these other potential causes before assigning a diagnosis of TES. Competing nonexclusive causes may include but are not limited to attention-deficit disorder or attention-deficit/hyperactivity disorder, sleep disturbance (including obstructive sleep apnea), psychiatric disorders, medication effect or abuse, substance abuse, migraine or chronic pain disorders, and other neurodegenerative diseases. Each of these diagnoses should be considered, diagnosed if applicable, and treated before diagnosis of TES.
When TES is a diagnostic consideration, the individual should undergo assessment by serial history and physical examination for evidence of progression. The magnitude of progression necessary to meet a TES diagnosis has yet to be defined, and therefore clinical judgment must be used to determine whether the magnitude of progression is greater than expected for age and comorbidities. We believe that TES is distinct from the immediate static neurocognitive decrement (also referred to as chronic neurologic impairment) that can be seen after a single moderate or severe TBI because TES exhibits progressive deterioration over time.31, 32
The proposed criteria are intended to create a diagnostic framework for the clinical evaluation of TES (Figure). We propose that a clinical diagnosis of probable TES requires the following features: (1) persistence of symptoms for longer than 2 years; (2) history of head trauma exposure that is repetitive in nature; (3) no other neurodegenerative, neurologic, or psychiatric condition that is more likely to explain the patient’s symptoms; (4) progressive course; (5) delayed symptom onset; and 6) self-report or observer report of cognitive dysfunction in combination with objective evidence of cognitive decline on results of formal neuropsychological testing. A probable TES diagnosis would also include at least 1of the following supportive features: emotional dysregulation (eg, depression, anxiety, agitation, aggression, paranoid ideation, deterioration of interpersonal relationships, and suicidality), behavioral change (eg, violence, poor impulse control, socially inappropriate behavior, avolition, apathy, and personality change), or motor disturbance (tremor, rigidity, bradykinesia, gait instability, dysarthria, dysphagia, ataxia, and gaze disturbance). Possible TES also requires (1) persistence of symptoms for longer than 2 years; (2) history of head trauma exposure; (3) no other neurodegenerative, neurologic, or psychiatric condition that is more likely to explain the patient’s symptoms; and (4) a progressive course as well as at least 1 supportive feature listed above. Possible TES does not require cognitive decline, repetitive head trauma exposure, or delayed onset of symptoms. Unlikely TES would not meet minimum diagnostic criteria for possible TES, and another neurodegenerative disease, a psychiatric disorder, or another diagnosis may be responsible for the clinical presentation in such individuals.
Figure. Initial Clinical Approach to the Patient With Neurocognitive Problems and Possible Traumatic Encephalopathy Syndrome (TES).
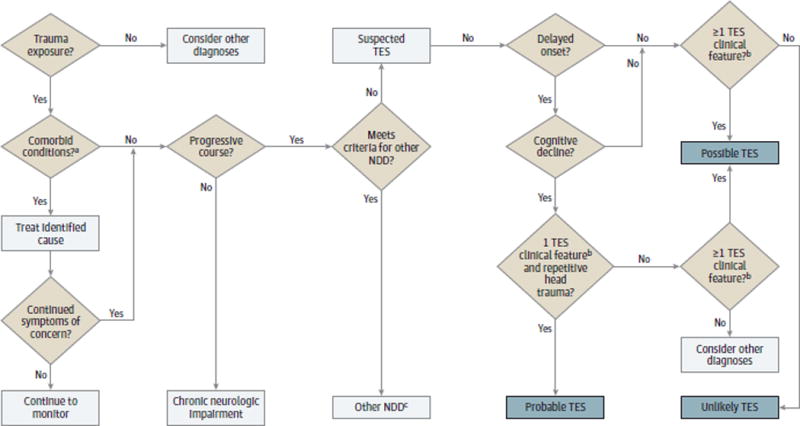
This model includes patients with a duration of neurocognitive problems for longer than 2 years. For a patient to be considered for a diagnosis of TES, the neurocognitive complaints or decline must be beyond the expected course given the individual’s age and other medical issues. This flowchart is intended to provide a framework for the practicing physician; clinical judgment and assessment remain a necessary aspect of the diagnostic pathway. NDD indicates neurodegenerative disease.
a Includes obstructive sleep apnea, migraine, mood disorder, substance abuse, medication effect, and “worried well” (ie, individuals who do not have a medical disorder but may visit a physician owing to psychological distress or need for reassurance).
b Include emotional dysregulation, behavioral change, or motor disturbance.
c Consider trauma-accelerated NDD vs typical NDD.
Discussion of Proposed Criteria
These criteria provide a guide for approaching the management of this unique patient population. However, their application still requires careful clinical judgment on the part of the physician.
No data exist regarding the minimum number or the severity of incidents of head trauma necessary to develop TES, so the proposed criteria do not specify a minimum threshold of exposure. The typical history of a patient with TES is expected to include repetitive head trauma exposure and a history of 1 or more concussions. Studies have suggested that the risk for TES is likely a dose-dependent entity, related to the number of incidents and the severity of head trauma experienced.33 Most published cases of neuropathologically confirmed CTE have had a history of repetitive exposure associated with 1 or more diagnosed mild TBIs,9 although Goldstein et al34 have reported cases of pathologic CTE in the setting of only 2 known head injuries without otherwise being exposed to repetitive head trauma. A breadth of literature also exists on military blast injury and cases in which a single blast injury was associated with persistent deficits in neurophysiologic function, learning, and memory as well as pathologic CTE.34,35 Whether a single mild head trauma event is sufficient to induce subsequent neurodegeneration is unknown; however, pathologic studies after a single moderate or severe TBI have found levels of neurofibrillary tangles and tau deposition that are significantly higher than in age-matched controls.36 If neurodegeneration after a single mild TBI is possible, we would expect this outcome to be a very rare entity and not to be emphasized as a common presentation of TES. Evidence also suggests that athletes who have been exposed to repetitive subconcussive head trauma without sustaining concussion can develop pathologic changes consistent with CTE, so a diagnosed concussion is not a required feature of TES.9
Individuals with a history of head trauma exposure can develop progressive neurologic deterioration owing to other more common neurodegenerative diseases such as Alzheimer disease, frontotemporal dementia, vascular dementia, or dementia with Lewy bodies. These presentations can be difficult to differentiate; however, each neurodegenerative disease has diagnostic criteria that include a distinct pattern of presenting features, clinical course, imaging findings, and neuropsychological deficits. Although prior studies have supported TBI as a risk factor for the development of neurodegenerative disease,36–40 the contribution of head trauma exposure to the age at onset, clinical course, and severity of other neurodegenerative diseases is unknown. A history of head trauma exposure could conceivably lead to an earlier onset of symptoms or result in an accelerated or more severe clinical course. More research is required to investigate the relationship between a single head trauma event or repetitive head trauma exposure and the development or acceleration of neurodegenerative diseases other than TES. This possibility of trauma-accelerated neurodegenerative disease should be considered distinct from TES.
A delay in symptom onset from the time of head trauma exposure is an important feature distinguishing TES from acute concussion and postconcussion syndrome. The expected or mean latency period for TES is unknown but has been reported in previous studies to range from 8 to 20 years.8,9 Concomitant postconcussion syndrome and TES may coexist, masking the delayed onset of TES. McKee et al6 have described cases of athletes who were already symptomatic at the time of their retirement and were subsequently found to have pathologic changes consistent with CTE on postmortem examination.
The proposed TES diagnostic criteria require that self-reported or observer-reported cognitive dysfunction must be confirmed with objective results of formal neuropsychological testing, but a specific pattern of neuropsychological deficits on testing has not yet been established. Cognitive dysfunction affecting multiple domains (ie, executive, visuospatial, memory, and language) would support probable TES, whereas cognitive dysfunction solely affecting 1 domain would be most consistent with possible TES. Previous Alzheimer disease literature41 suggests that multiple-domain dysfunction in individuals with mild cognitive impairment is more likely to progress to Alzheimer dementia. Based on this hypothesis, we extrapolate that multidomain involvement is more likely to predict the development of neurodegenerative disease. To diagnose TES, the neurocognitive deficits must be beyond those expected based on the individual’s age and other medical comorbidities. Although behavioral change and emotional dysregulation may present before cognitive dysfunction in some cases of TES, the proposed criteria favor specificity and therefore do not permit a diagnosis of TES in the absence of cognitive dysfunction. As the field evolves, it may become possible to link biomarkers or pathologic findings to specific clinical features of TES, at which time these criteria may be modified.
Conclusions
While ongoing research efforts attempt to further clarify the association of head trauma exposure, clinical symptoms, and neuropathologic changes, physicians should have clinical criteria that can be applied to the evaluation and diagnosis of TES. The work of McKee et al,6 Omaluet al,3–5, 8 Jordan,25 Victoroff,26 and Montenigro et al1, 27 has been invaluable in initiating the conversation regarding CTE and the clinical presentation of TES. Concurrently, our proposed diagnostic criteria seek to continue this conversation by providing a viable model for practicing physicians to diagnose TES. As the field progresses, these criteria are expected to evolve. We emphasize the importance of careful clinical judgment and specificity of diagnosis. Last, caution is emphasized in giving an individual a diagnosis of TES given the implications of bestowing a diagnosis of an untreatable progressive condition.
Footnotes
Author Contributions
Dr. Reams had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Administrative, technical, or material support: Lorincz, Kutcher.
Study supervision: Lorincz, Kutcher.
Conflict of Interest Disclosures
Dr. Eckner reported receiving research funding from grant 1 K23 HD078502-01A1 from the National Institutes of Health (NIH), the National Collegiate Athletics Association, the US Department of Defense, the University of Michigan Injury Center, and the Foundation for Physical Medicine and Rehabilitation.
Dr. Paulson reported receiving support from the NIH and has a research contract with Ionis Pharmaceuticals.
Dr. Kutcher reported serving as a consultant for ElMindA, the National Basketball Association, the National Hockey League Players’ Association, and the National Football League Players’ Association.
No other disclosures were reported.
Additional Contributions
James F. Burke, MD, University of Michigan assisted with editing and multiple reviews of the manuscript. No compensation was given.
Contributor Information
Nicole Reams, Email: nreams@northshore.org, nikkikaris@gmail.com.
James T. Eckner, Email: jeckner@med.umich.edu.
Andrea A. Almeida, Email: almeidaa@med.umich.edu.
Andrea L. Aagesen, Email: aquandt@med.umich.edu.
Bruno Giordani, Email: giordani@med.umich.edu.
Hank Paulson, Email: henryp@med.umich.edu.
Matthew L. Lorincz, Email: lorincz@med.umich.edu.
Jeffrey S. Kutcher, Email: jkutcher@med.umich.edu.
References
- 1.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimer’s Research & Therapy. 2014;6(5):68. doi: 10.1186/s13195-014-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mez J, Solomon TM, Daneshvar DH, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alz Res & Ther. 2015;7:62. doi: 10.1186/s13195-015-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–34. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 4.Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006 Nov;59(5):1086–92. doi: 10.1227/01.NEU.0000245601.69451.27. [DOI] [PubMed] [Google Scholar]
- 5.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010;6(3):130–36. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 6.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. Journal of Neuropathology and Experimental Neurology. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6(2):244–54. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 8.Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183. doi: 10.1227/NEU.0b013e318212bc7b. [DOI] [PubMed] [Google Scholar]
- 9.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mez J, Stern RA, McKee AC. Chronic Traumatic Encephalopathy: where are we and where are we going? Curr Neurol Neurosci Rep. 2013;13:407. doi: 10.1007/s11910-013-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25(3):35–64. doi: 10.1111/bpa.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee A, Alvarez V, Bieniek K, et al. Preliminary Results of the NINDS/NIBIB Consensus Meeting to Evaluate Pathological Criteria for the Diagnosis of CTE (P2.178) Neurology. 2015;84(14, Supplement P2.178) [Google Scholar]
- 13.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–66. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ, Yang Y, Zhang JY, Wang Q, Xu H, Liao FF, Wang JZ. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A. 2007;104:3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karantzoulis S, Randolph C. Modern Chronic Traumatic Encephalopathy in Retired Athletes: What is the Evidence? Neuropsychol Rev. 2013;23:350–360. doi: 10.1007/s11065-013-9243-4. [DOI] [PubMed] [Google Scholar]
- 17.Hazrati L, Tartaglia MC, Diamandis P, et al. Absence of chronic traumatic encephalopathy in retired football players with multiple concussions and neurological symptomatology. Front Hum Neurosci. 2013;7:222. doi: 10.3389/fnhum.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak LA, Smith GG, Reyes PF. Dementia in a retired world boxing champion: case report and literature review. Clin Neuropathol. 2013;28:275–280. [PubMed] [Google Scholar]
- 19.Martland HS. Punch Drunk. Journal of the American Medical Association. 1928;91(15):1103–1107. [Google Scholar]
- 20.Millspaugh JA. Dementia pugilistica. US Naval Medicine Bulletin. 1937;35:297–303. [Google Scholar]
- 21.Miller H. Mental after-effects of head injury. Proc R Soc Med. 1966;59:257–261. doi: 10.1177/003591576605900327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner A, Iverson GL, McCrory P. Chronic traumatic encephalopathy in sport: a systematic review. Br J Sports Med. 2014;48(2):84–90. doi: 10.1136/bjsports-2013-092646. [DOI] [PubMed] [Google Scholar]
- 23.Roberts A. Brain damage in boxers: a study of prevalence of traumatic encephalopathy among ex-professional boxers. London: Pitman Medical Scientific Publishing Co; 1969. [Google Scholar]
- 24.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. 2013;9:222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- 26.Victoroff J. Traumatic encephalopathy: review and provisional research diagnostic criteria. NeuroRehabilitation. 2013;32:211–224. doi: 10.3233/NRE-130839. [DOI] [PubMed] [Google Scholar]
- 27.Montenigro PH, Bernick C, Cantu RC. Clinical features of repetitive traumatic brain injury and chronic traumatic encephalopathy. Brain Pathol. 2015;25(3):304–17. doi: 10.1111/bpa.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan HM, Stolwyk R, Neath J, et al. Neurocognitive similarities between severe chronic schizophrenia and behavioural variant frontotemporal dementia. Psychiatry Res. 2015;225(3):658–66. doi: 10.1016/j.psychres.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Landqvist Waldö M, Gustafson L, Passant U, Englund E. Psychotic symptoms in frontotemporal dementia: a diagnostic dilemma? Int Psychogeriatr. 2014;9:1–9. doi: 10.1017/S1041610214002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borek LL, Friedman JH. Treating psychosis in movement disorder patients: a review. Expert Opin Pharmacother. 2014;15(11):1553–64. doi: 10.1517/14656566.2014.918955. [DOI] [PubMed] [Google Scholar]
- 31.Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine PositionStatement: Concussion in Sport. Clin J Sport Med. 2013;23:1–18. doi: 10.1097/JSM.0b013e31827f5f93. [DOI] [PubMed] [Google Scholar]
- 32.Jordan BD. Chronic Traumatic Encephalopathy and Other Long-term Sequelae. Continuum (Minneap Minn) 2014;20(6):1588. doi: 10.1212/01.CON.0000458972.94013.e1. [DOI] [PubMed] [Google Scholar]
- 33.McCrory P, Zazryn T, Cameron P. The evidence for chronic traumatic encephalopathy in boxing. Sports Med. 2007;37(6):467–476. doi: 10.2165/00007256-200737060-00001. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alz & Dementia. 2012;10(3):S242–S253. doi: 10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neursourg Psychiatry. 2003;74(7):857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166:810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: A case-control study. Neurology. 2003;60:1610–1615. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- 40.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a Predictor of Conversion From Mild Cognitive Impairment to Alzheimer’s Disease. J Am Geriatr Soc. 2014;62(4):679–684. doi: 10.1111/jgs.12742. [DOI] [PubMed] [Google Scholar]


