Abstract
Many fermentation volatiles important to wine aroma potentially arise from yeast metabolism of hexose sugars, but assessing the relative importance of these pathways is challenging due to high endogenous hexose substrate concentrations. To overcome this problem, gas chromatography combustion isotope ratio mass spectrometry (GC-C-IRMS) was used to measure high-precision 13C/12C isotope ratios of volatiles in wines produced from juices spiked with tracer levels (0.01–1 APE) of uniformly labeled [U-13C]-glucose. The contribution of hexose to individual volatiles was determined from the degree of 13C enrichment. As expected, straight-chain fatty acids and their corresponding ethyl esters were derived almost exclusively from hexoses. Most fusel alcohols and their acetate esters were also majority hexose-derived, indicating the importance of anabolic pathways for their formation. Only two compounds were not derived primarily from hexoses (hexanol and isobutyric acid). This approach can be extended to other food systems or substrates for studying precursor–product relationships.
Keywords: GC-C-IRMS, compound specific isotope analysis, CSIA, volatile analyses, wine
INTRODUCTION
Hundreds of volatile compounds have been identified in wine, of which about 70 appear to be critical for wine aroma.1 A small subset of these key aroma compounds are so-called “primary” odorants that are detectable in the grape and extracted during fermentation, for example, rotundone (“black pepper” in Syrah) and methyl anthranilate (“grapey/foxy” in Concord).2,3 However, many important wine aroma compounds are “secondary”; that is, they appear during fermentation either due to de novo biosynthesis by yeast or bacteria or through metabolism of grape-derived precursor compounds such as glycoconjugates.4 “Tertiary” odorants may also arise during storage through several mechanisms, including acid-catalyzed transformations, oak contact, or microbial spoilage.
Identifying precursors or formation mechanisms for wine components during or after fermentation is of interest to wine researchers, as this knowledge can lead to improved strategies for predicting the concentrations of various compounds in finished wines. Frequently, this is accomplished by addition of a putative precursor to an appropriate media. For example, limonene and α-terpeniol were added to model wines to evaluate the potential of these compounds to serve as precursors for 1,8-cineole (“eucalyptus” odor).5 In another experiment, a glycoconjugate-enriched extract was added to a model juice medium prior to fermentation to quantify the contribution of yeast metabolic activity to the release of volatile aglycones.6 Such approaches are appropriate for tracking must precursors at low initial concentrations, but are less useful for compounds with high endogenous concentrations (e.g., sugars, amino acids) where additions large enough to effect measurable changes in volatiles create the risk of altering microbial physiology. For example, the fusel alcohols (fermentation-derived alcohols with more than two carbons, e.g., isoamyl alcohol) and their corresponding acetate esters are important wine odorants, as the alcohols are associated with off-aromas at high concentrations and the acetate esters are credited with enhancing fruity aromas common to young wines.7 Many of the fusel alcohols are thought to be formed during alcoholic fermentation by one of two pathways related to amino acid metabolism: (i) catabolism of grape amino acids, characterized by deamination of amino acids to α-keto acids followed by decarboxylation to an aldehyde and, finally, reduction to a fusel alcohol (the Ehrlich pathway);8 or (ii) production of α-keto acids during amino acid biosynthesis from sugars, which may then be degraded to fusel alcohols as described above (the anabolic pathway.)7 Changes in must nitrogen composition can alter fusel alcohol production,9–11 but it is not always evident if changes in the Ehrlich or anabolic pathways, or both, are responsible for this outcome. To complicate matters further, some fusel alcohols (e.g., β-phenylethanol) exist as glycoconjugates in grapes and can serve as additional sources of these compounds in wine.6
Stable isotope tracers can be used as an alternative to adding unlabeled substrates in the evaluation of precursor–product relationships. Tracer studies have been widely used in food chemistry, for example, in studies of precursors of Maillard reaction products,12 and have occasionally been extended to studies of the fate and origin of wine components. In one work, deuterated isobutyric acid and ethyl isobutyrate spikes were used to study their formation in wine.13 However, to yield detectable changes using a typical GC-MS system, stable isotope tracers must be added at a concentration of at least >0.5% (and generally higher) of the endogenous concentration,14 which may be prohibitively expensive and, as mentioned above, create the risk of affecting fermentation physiology. Radioisotopes can be employed at lower concentrations due to their low natural abundance, with labeled compounds typically detected following off-line separation via a scintillation counter. In wine, [3H]-malvidin glucoside has been used as a model to track the fate of anthocyanins before and after fermentation,15 and [U-14C]-glucose, -leucine, and -isoleucine have been used to follow volatile production in grain mash fermentations.16 In practice, radioisotopes are rarely used as tracers in food chemistry studies because of associated hazards.
An alternative to conventional tracer experiments with GCMS is high-precision isotope ratio measurement by gas chromatography combustion isotope ratio mass spectroscopy (GC-C-IRMS). In GC-C-IRMS, organic compounds are combusted to CO2 following their elution from a GC, and the isotopomers of CO2 (m/z 44, 45, 46) are measured by IRMS for each peak.14 GC-C-IRMS has been used for natural abundance studies of wine, primarily for studies on authenticity and adulteration. For example, 13C/12C ratios can be used to detect illegal addition of cane sugar (derived from a C4 plant) to grape juice (derived from a C3 plant) prior to fermentation and, in combination with D/H and 18O/16O ratios, can serve as geographical fingerprints for wine provenance.17 The major advantage of GC-C-IRMS for tracer studies is that it can achieve much higher precison than conventional GC-MS; in practice, 13C enrichments as low as ~0.1 atom percent excess (APE) can be utilized for tracer studies.18 GC-C-IRMS has been used for tracer studies in a number of disciplines where a sizable endogenous pool of a potential precursor exists and large degrees of enrichment are not possible for economic or other practical reasons.14 For example, GC-C-IRMS has been used to monitor the fate and metabolism of dietary omega-3 fatty acids in animal studies.19 GC-C-IRMS has also been used in conjunction with 13CO2 labeling studies of chamber-grown grapevines to determine the timing of biosynthesis of glycoconjugate precursors,20 but to our knowledge GC-C-IRMS has not been used for tracer studies of wine fermentations or in related areas of food processing.
In this work, we report the use of GC-C-IRMS as a novel approach to follow the path of a stable isotope tracer during the alcoholic fermentation of hexose. Uniformly labeled [U-13C]-glucose was added to a grape must prior to fermentation at trace levels (0.01, 0.1, and 1 APE), and its contribution to a diverse range of fermentation volatiles (i.e., fusel alcohols, fatty acids, and their associated esters) was assessed. This technique can be used as a more general approach for studying the origin and fate of compounds in food systems while minimizing tracer concentration.
MATERIALS AND METHODS
Fermentations
Fermentations were conducted using Riesling juice sourced from the Cornell research vineyards in Lansing, NY, USA, during the 2010 harvest. Fruit (450 kg) was received by the Cornell Vinification and Brewing Technology Laboratory, where it was crushed, destemmed, basket pressed, and settled overnight at ambient conditions. Approximately 30 L was siphoned into two 20 L Nalgene containers (Thermo Fisher Scientific, Waltham, MA, USA) and frozen at −20 °C. Before use, the juice was thawed in a 40 °C water bath for 1 h and mixed by inversion. Glucose, fructose, and yeast assimilable nitrogen (YAN) were quantified by enzymatic colorimetric methods using a Chemwell 2910 Multianalyzer (Unitech Scientific, Hawaiian Gardens, CA, USA). To supplement the initial juice YAN content of 40 mg N/L, diammonium hydrogen phosphate (674 mg/L) and Fermaid K (Lallemand, Santa Rosa, CA, USA) (250 mg/L) were added to yield a final YAN concentration of 214 mg N/L. Yeast (EC1118, Lallemand) was rehydrated in 40 °C spring water (Crystal Rock, Watertown, CT, USA) with 40 g/L GoFerm (Scott Laboratories, Petaluma, CA, USA), and the juice (5 L) was inoculated at a rate of 0.3 g/L. Aliquots of 541 g (500 mL) of inoculated must were then added to 1 L Pyrex media bottles (Corning Inc., Tewksbury, MA, USA). One treatment served as a control (no [U-13C]-glucose added), and uniformly labeled [U-13C]-glucose (Cambridge Isotope Laboratories, Andover, MA, USA) was added at one of three levels: 1.0 APE (1.0150 g/500 mL), 0.1 APE (0.1015 g/500 mL), or 0.01 APE (0.0102 g/500 mL). Fermentations were carried out in triplicate for each of the four treatments (three tracer levels + one control). The fermenters were topped with a three-piece airlock with a floating bubbler (Buon Vino Manufacturing, Cambridge, ON, Canada). Fermentations were conducted over a 10 day period at ambient temperatures in a dark cabinet and were measured for residual sugar after all fermenters had stopped producing CO2. At completion, all fermentations had residual sugar (glucose, fructose) values of <0.5 g/ L. The wine was stabilized by the addition of 50 mg/L of SO2 and then frozen and stored at −20 °C until needed for further analysis.
Sample Preparation
Volatile components were extracted from wine using the method described by Ortega and others.21 To a 15 mL screw-capped borosilicate glass centrifuge tube were added 4.5 g of (NH4)2SO4, 3 mL of wine, 7 mL of water, and 0.2 mL of dichloromethane (DCM). For GC-MS quantification 15 μL of a 140 μg/mL solution of 2-octanol was added to the sample as in internal sample; this step was omitted for the GC-C-IRMS analysis. The samples were mixed for 1 h using a carousel rotating at 20 rpm to invert the tubes and then centrifuged at 2500g for 10 min. Approximately 1.5 mL of the emulsified bottom layer was recovered with a glass Pasteur pipet, transferred to a 2.2 mL microfuge tube, and centrifuged for 4 min at 14000g. Finally, 100 μL of the DCM layer was transferred to an Agilent GC autosampler vial with a 250 μL microvolume insert.
Quantification and Identification of Volatiles by GC-MS
Compounds in the DCM extracts were identified and quantified by gas chromatography–mass spectrometry (GC-MS). An HP 6890 GC with a split/splitless inlet (Agilent Technologies, Palo Alto, CA, USA) and autoinjector was coupled to an Agilent 5795 quadrupole mass selective detector (MSD). A 60 m × 0.32 mm × 0.25 μm Aglient HP-INNOWax column (polyethylene glycol) was used. The GC conditions were as follows: initial head pressure at 8.57 psi with a column flow of 1.6 mL/min in constant flow mode, inlet at 250 °C, 2 μL splitless injection, and total flow through the injector of 84.8 mL/ min. The oven parameters were as follows: initial temperature 35 °C (held for 2 min) ramped to 40 °C at 5 °C/min (held 5 for min), ramped to 200 °C at 3 °C/min (held for 5 min), and ramped to 250 °C at 25 °C/min (held for 20 min). The MSD was operated with an ionization energy of 70 eV with the electron multiplier voltage of 1700 V. The MSD was scanned over the mass range of m/z 33–300. Chemstation MSD (Agilent Technologies) was used for data processing. Compound identification was performed by matching retention times and mass spectra of peaks to authentic standards, and peak areas were normalized to that of the 2-octanol internal standard. Calibration curves were generated from the analysis of known concentrations of pure standards spiked into a model wine. Calibration curves for all 26 compounds had an r2 > 0.99. Quantifier ions and retention times for each compound are listed in Table 1.
Table 1.
Volatile Composition of Wines, in Milligrams per Liter, Produced by Fermentations with Different Levels of [U-13C]-Glucose Enrichmenta
| atom percent excess of hexose (APEhex) |
||||||
|---|---|---|---|---|---|---|
| compound | RTb | Q ionc | 0 | 0.01 | 0.1 | 1.0 |
| ethanol | nad | 43 | 114848 | 112466 | 114059 | 118289 |
| isobutyl acetate | 10.591 | 43 | 0.14 | 0.11 | 0.09 | 0.08 |
| ethyl butyrate | 11.571 | 71 | 0.23 | 0.25 | 0.21 | 0.19 |
| ethyl isovalerate | 13.383 | 57 | 0.04 | 0.05 | 0.03 | 0.07 |
| isobutyl alcohol | 14.297 | 43 | 30.70 | 28.25 | 27.77 | 26.25 |
| 1-butanol | 16.875 | 56 | 1.45 | 1.42 | 1.44 | 1.45 |
| isoamyl acetate | 18.744 | 43 | 3.41 | 2.98 | 2.46 | 2.55 |
| isoamyl alcohol | 20.300 | 55 | 74.07 | 102.70 | 84.88 | 81.49 |
| ethyl hexanoate | 21.413 | 88 | 0.34 | 0.35 | 0.27 | 0.28 |
| hexyl acetate | 23.332 | 43 | 0.15 | 0.17 | 0.18 | 0.15 |
| ethyl lactate | 26.774 | 43 | 5.65b | 16.97a | 13.84a | 12.45a |
| 1-hexanol | 27.144 | 56 | 0.74 | 0.97 | 0.83 | 0.76 |
| ethyl octanoate | 30.802 | 88 | 0.21 | 0.38 | 0.23 | 0.24 |
| ethyl 3-hydroxybutyrate | 34.474 | 43 | 0.13 | 0.18 | 0.15 | 0.14 |
| propanoic acid | 35.567 | 74 | 3.73 | 4.32 | 3.36 | 3.00 |
| isobutyric acid | 36.723 | 43 | 2.11 | 2.29 | 1.82 | 1.51 |
| butyric acid | 39.111 | 60 | 0.30 | 0.15 | 0.13 | 0.16 |
| ethyl decanoate | 39.228 | 88 | 1.34a | 1.98b | 1.39a | 1.16a |
| isovaleric acid | 40.685 | 60 | 0.64 | 0.86 | 0.75 | 0.65 |
| diethyl succinate | 40.815 | 101 | 0.12 | 0.22 | 0.18 | 0.18 |
| methionol | 42.374 | 61 | 1.59 | 2.60 | 2.23 | 1.96 |
| phenylethyl acetate | 45.970 | 104 | 0.37 | 0.34 | 0.29 | 0.27 |
| hexanoic acid | 47.107 | 60 | 3.57 | 4.52 | 3.84 | 3.36 |
| β-phenylethanol | 49.401 | 91 | 15.88 | 22.19 | 18.82 | 18.92 |
| octanoic acid | 54.254 | 60 | 5.82 | 6.19 | 5.68 | 4.52 |
| decanoic acid | 60.752 | 60 | 2.78a | 0.93b | 1.09b | 0.88b |
Letters within a row indicate significant difference using the Bonferroni family error rate α = 0.05; if no letters are present, then no significant differences were observed for that compound.
RT = retention time in minutes.
Qion = quantifier ion by GC-MS.
Measured using direct injection method.
Due to its coelution with the extraction solvent DCM, ethanol was quantified separately via a direct injection method.22 Wine was filtered through a 0.25 μm filter and 100 μL diluted with 900 μL of water; 1 μL of this sample was then injected at a 50:1 split into a 250 °C inlet. The oven parameters were as follows: initial 35 °C (held for 2 min) ramped to 40 °C at 5 °C/min (held for 5 min), ramped to 70 °C at 5 °C/min (held for 5 min), and ramped to 250 °C at 25 °C/min (held for 7 min).
Carbon Isotope Ratio Analysis of Wine Volatiles by GC-C-IRMS
The design and operation of the GC-C-IRMS system are described in more detail elsewhere.23 Briefly, the HP 6890 GC-MS described above was coupled to a Thermo MAT 253 IRMS (Bremen, Germany) via a specially designed combustion interface. The IRMS was tuned for high linearity with the conduction limit open three full turns and operated at a source pressure of 9.3 × 10−7 Torr and an accelerating potential of 9.5 kV. The measured absolute sensitivity was ~1600 molecules/ion. Data were collected and analyzed using ISODAT 3.0 (Thermo Scientific). The GC column was connected to an online microcombustion reactor via a four-way rotary valve, which permitted solvent diversion to the MSD and sample introduction into IRMS. The microcombustion reactor was constructed from a continuous fused silica capillary (50 cm × 0.53 mm i.d.) hand packed with three 15 cm × 0.1 mm wires (one Cu, one Pt, and one Ni wire) in the center of the capillary. The capillary portion containing the wires was mechanically retained in a 30 cm × 0.8 mm i.d. alumina tube with metal fittings (Valco Instruments, Houston, TX, USA) on both ends. The exposed ends of the capillary were connected to the interface with press-tight fittings. The alumina tube was maintained at 950 °C using a 30 cm Thermcraft tube furnace (Winston-Salem, NC, USA), which had a 15 cm heated zone. Water generated due to combustion was removed from the system using a Nafion water trap (dimensions = 10 cm × 0.8 mm i.d.) immediately following the combustion reactor. The open-split consisted of the 0.6 m × 0.075 mm capillary connected to the IRMS inlet at one end, with the other end directly inserted into the postwater trap transfer line. For analysis of wine volatiles, excluding ethanol, a 60 m × 0.32 mm × 0.25 μm Agilent HP-INNOWax column described above was used. The GC conditions were as follows: initial head pressure at 8.57 psi with a column flow of 1.6 mL/min in constant flow mode, inlet at 250 °C, 2 μL splitless injection, and total flow through the injector of 84.8 mL/min. The oven parameters were as follows: initial 35 °C (held for 2 min) ramped to 40 °C at 5 °C/min (held for 5 min), ramped to 200 °C at 3 °C/min (held for 5 min), and ramped to 250 °C at 25 °C/min (held for 20 min).
Because of ethanol's coelution with the extraction solvent, DCM, it was characterized by GC-C-IRMS separately from the other measured compounds. Sample preparation, injection technique, and GC conditions were the same as described for GC-MS.
ISODAT 3.0 was used to calculate isotope ratios in delta notation; 17O corrections were made using the Santrock and Hayes method.24 Background was determined using the individual background determination with a 5 s moving linear regression, whereas peak detection was set to a slope of 0.5 mV/s for peak start and 0.4 mV/s for peak end. For calibration, CO2 gas pulses were admitted directly to the IRMS source from a Conflo III device (Thermo Scientific). CO2 was supplied by a pressurized tank during each GC-C-IRMS run for isotope ratio standardization, with three 30 s pulses at the beginning and three at the end of each chromatogram. The isotope ratio of the CO2 standard gas was traceable to the international standard Vienna Pee Dee Belemnite (VPDB) (RVPDB = 0.011180) by running calibration standards containing fatty acid methyl ester (FAME) 17:0 and FAME 21:0 with known isotope ratios determined by off-line dual-inlet IRMS.25 This FAME standard was run at the beginning and end of each day's runs to determine the “apparent” isotope ratio of the CO2 gas pulses, which in turn could be used to calculate the isotope ratios of wine components during analytical runs. This calibration approach addresses fractionation in the GC-C-IRMS system because the FAME standards go through the same path as the wine components (i.e., GC, online combustion, water trap, open split, IRMS).25 Isotope ratios are reported as δ13CVPDB in units of “per mil” (‰) and based on the International System of Units (SI), where δ13CVPDB is defined as
| (1) |
Rx and RVPDB represent the 13C/12C isotope ratio of an analyte compound X and the VPDB standard, respectively.
Bulk Analysis of Grape Must Carbon Isotope Ratio by Elemental Analyzer-IRMS (EA-IRMS)
A 2 mL aliquot of the frozen Riesling juice (no [U-13C]-glucose added) was submitted to the Cornell Stable Isotope Laboratory (COIL) for EA-IRMS analysis. Five microliters of juice taken from a 2 mL frozen aliquot was used for bulk stable isotope analysis using a continuous flow system.26 The sample was combusted at 900 °C in a Carlo Erba NC2500 elemental analyzer, and resultant CO2 was transferred via a Conflo IV interface (Thermo Scientific) to a Delta V isotope ratio mass spectrometer (Thermo Scientific) for carbon isotope analysis. Isotope ratios are reported in delta notation, as described above.
Statistical Analysis
All linear regression analysis and ANOVA calculations were carried out using MiniTab (Minitab, Reading, MA, USA) statistical analysis software.
Isotope Addition and APE Calculations
APE is defined in eq 2
| (2) |
where FHEXe is the atom fraction of the enriched hexose and FHEXn is the atom fraction of the native hexose pool. FHEXe is calculated by the amount of [U-13C]-glucose added to the system. The mass balance equation (eq 3) can be rearranged to eq 4, where FULG is the atom fraction of uniformly labeled glucose and mULG is the mass.
| (3) |
| (4) |
For tracer level additions of [U-13C]-glucose, the atom fraction of uniformly labeled glucose (FULG) = 1, the mass fraction (mHEXn/mHEXe) will be ~1, and eq 4 simplifies to
| (5) |
Combining eqs 2 and 5 yields eq 6 and provides a good approximation for the APE of the enriched hexose pool (APEHEX) as a function of the amount of [U-13C]-glucose added to the fermentation and the original hexose concentration.
| (6) |
Calculating the Percentage of Carbon Derived from Hexose in Volatile Compounds
The observed APE of each volatile (APEobsd) was calculated from experimental data for each volatile in each tracer fermentation, where RE is the 13C/12C ratio in the enriched sample and RN is the mean 13C/12C ratio of the corresponding compound in the natural abundance fermentations:14
| (7) |
To determine the percent of each volatile compound that originated from hexose substrate, (APEobsd) was assumed to be related to the APE of the hexose pool (APEhex) by eq 8.
| (8) |
APEhex is a factor of the tracer level (eq 6). The apparent fractionation factor, α, can arise from kinetic isotope effects and can potentially vary among compounds. However, in most biochemical processes, α is expected to range from between 0.98 and 1.02, and assuming α = 1 will introduce negligible error. Equation 8 can be simplified and rearranged to solve for the percent of carbon in a given volatile derived from hexose sugars (eq 9).
| (9) |
RESULTS AND DISCUSSION
Volatile Composition of Fermentations
A summary of the average concentration of volatile compounds by level of enrichment is given in Table 1. Mean concentrations of each compound were within the range of values reported previously in the analysis of wine samples.21 Individual ANOVAs were calculated for each of the 28 compounds by level of enrichment. To account for potential type 1 error due to the number of comparisons, the Bonferroni correction was applied using a family error rate of α = 0.05. Whereas most compounds did not show significant differences, ethyl lactate, ethyl decanoate, and decanoic acid show significant differences between the control fermentation and the enriched fermentations. These differences may have arisen because the control fermentation was conducted at a different time from the enriched fermentations, rather than as an effect of enrichment treatment. In particular, production of midchain fatty acids such as decanoic acid and their corresponding ethyl esters is known to be affected by variation in oxygen status during fermentation.27 However, for all other compounds, the lack of difference indicates that labeling does not perturb the system.
Native 13C/12 C Ratios
Table 2 reports the native isotope ratios of 19 volatile compounds in the experimental Riesling wine, expressed as δ13C values. Literature reports on compound-specific isotope ratios of wine volatiles other than ethanol are relatively rare,28 and to our knowledge our work represents the largest number reported to date. Significant differences were observed in natural abundance isotope ratios among volatile compounds, and δ13C values ranged from −13 to −35‰. Fewer compounds were characterized by GC-CIRMS than were quantified by GC-MS because the combustion step of GC-C-IRMS converts all compounds to CO2; thus, coeluting peaks cannot be resolved as they can be in GC-MS through use of a unique m/z ion to quantify each compound.14 Precision, calculated as the standard deviation (SD) of δ13C values (‰) from fermentation replicates, ranged from ±0.1‰ (ethanol) to ±7.5‰ (hexyl acetate), with a mean value of ±1.9‰. This precision is lower than that for benchmark values for high-precision GC-C-IRMS, SD (δ13C) < 0.4‰,26 because it reflects biological variability among the fermentation replicates rather than analytical variability alone. We observed much better precision, SD (δ13C) < 1.4‰, for analytical replicates of the same fermentation samples. The higher precision observed for ethanol likely reflects the fact that it is the major fermentation product throughout alcoholic fermentation, and, thus, should be less prone to fractionation. Furthermore, the sample preparation did not require extraction. By comparison, many of the other volatiles in Table 2 account for <0.1% of the initial hexose substrate and thus may be more sensitive to changes in conditions among fermentation replicates.
Table 2.
δ13C Values in Natural Abundance Control Fermentationsa
| compound | n | δ13CVPDB (‰) | SD (‰) | groupb |
|---|---|---|---|---|
| acetic acid | 3 | –13.48 | 2.25 | A |
| isobutyric acid | 3 | –17.78 | 3.78 | A, B |
| decanoic acid | 3 | –22.84 | 1.84 | B, C |
| isoamyl alcohol | 3 | –24.12 | 1.69 | B, C D |
| hexanoic acid | 3 | –24.24 | 1.29 | B, C, D |
| isobutyl alcohol | 3 | –24.47 | 0.40 | B, C, D |
| octanoic acid | 3 | –24.63 | 1.19 | B, C, D |
| ethyl octanoate | 3 | –24.80 | 1.97 | B, C, D |
| ethyl lactate | 2 | –25.84 | 0.46 | B, C, D, E, F |
| 1-butanol | 3 | –25.89 | 2.03 | C, D, E |
| ethyl hexanoate | 3 | –25.94 | 1.88 | C, D, E |
| ethyl 3-hydroxybutyrate | 3 | –27.02 | 2.74 | C, D, E, F |
| ethanol | 3 | –27.47 | 0.10 | C, D, E, F |
| isoamyl acetate | 3 | –28.58 | 2.15 | C, D, E, F |
| hexyl acetate | 3 | –29.15 | 7.55 | C, D, E, F |
| methionol | 3 | –29.40 | 0.59 | C, D, E, F |
| β–phenylethanol | 3 | –31.29 | 0.86 | D, E, F |
| phenylethyl acetate | 3 | –33.97 | 1.66 | F |
| 1-hexanol | 2 | –34.19 | 0.21 | E, F |
Results are for means and standard deviations of fermentation replicates.
Compounds with different letters differ at p < 0.05 level (Tukey's test).
The isotope ratio of ethanol (δ13C = −27.47‰) was within the range typically observed for wine29 and was depleted with respect to the isotope ratio for the bulk grape juice (δ13C = −26.57‰). The depletion of ethanol and concurrent enrichment of CO2 with respect to the sugar substrate has been previously reported and is believed to occur because ethanol is derived from the C-1, C-2, C-5, and C-6 positions of hexoses, which are depleted with respect to the C-3 and C-4 positions.30 Several short- and mid-chain fatty acids (acetic, isobutyric, and decanoic) were enriched with respect to ethanol, as has been previously reported.28 These volatile fatty acids are produced by yeast toward the end of fermentation.31 Because lighter isotopes of sugars are metabolized preferentially during fermentation,32 the observed effect may be due to enrichment of the hexose pool by the time the majority of these fatty acids are formed. However, as mentioned under Materials and Methods, this fractionation effect is small and constant and thus will not compromise results from labeling studies. Most other volatiles, including fusel alcohols, ethyl esters, and several acetate esters, did not differ significantly in isotope ratio from ethanol. The most depleted compound measured was 1-hexanol (δ13C = −34.19‰). As discussed in the next section, this is likely because hexanol is primarily derived from the 13C-depleted grape lipid fraction29 as opposed to being synthesized by yeast from sugars. Phenylethyl acetate was also significantly depleted with respect to ethanol, although the reason for this was unclear.
Determination of Hexose Contribution to Volatiles through Tracer Experiments
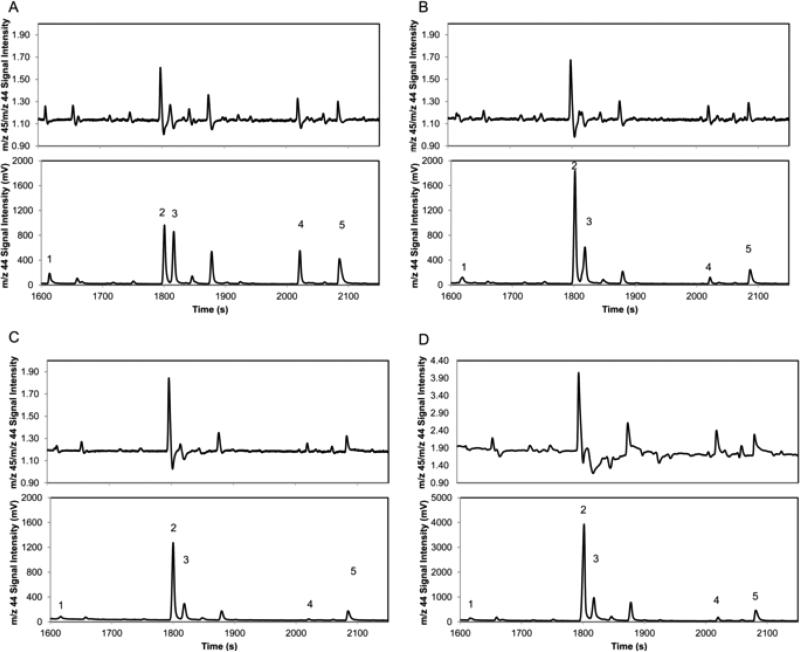
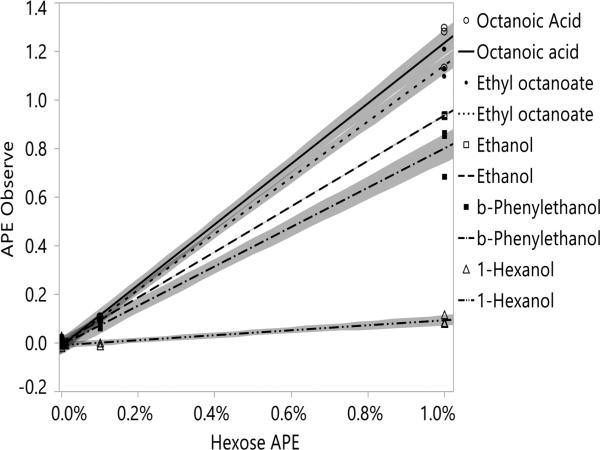
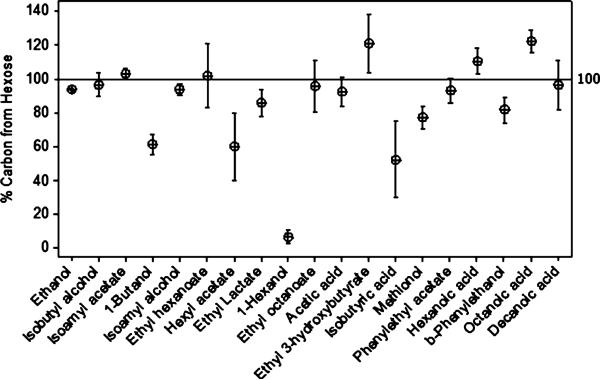
Typical chromatograms obtained at different levels of [U-13C]-glucose enrichment are shown in Figure 1. With increasing enrichment of the hexose pool the m/z 45 trace increased relative to the m/z 44 trace for compounds such as ethyl lactate that derive most of their carbon from sugar. The 45/44 ratio for compounds that are derived primarily from grape compounds other than sugars, such as hexanol, did not change significantly. APEobsd of volatile compounds in each fermentation experiment were calculated using eq 7, and the percent of carbon derived from the hexose pool was calculated by plotting APEobsd versus APEhex (eq 9). Representative plots are shown in Figure 2 for octanoic acid, ethyl octanoate, ethanol, β-phenylethanol, and 1-hexanol, where the slope represents the percent of carbon within a compound derived from the hexose pool. Figure 3 and Supplementary Table 1 in the Supporting Information report the fraction of carbon derived from hexose in each volatile compound. The error associated with measurements of percent hexose in individual compounds follows a similar pattern to what was observed in natural abundance studies.16 The contribution of the hexose pool to ethanol, which is present in large quantities and represents a major fermentation product, can be determined with high confidence (94 ± 0.5%), whereas the error associated with low-concentration volatiles such as ethyl 3-hydroxybutyrate increases by an order of magnitude or greater.
Figure 1.
IRMS chromatograms of wine extract at different levels of 13C enrichment: (A) control; (B) 0.01% [U-13C]-glucose; (C) 0.1% [U-13C]-glucose; (D) 1.0% [U-13C]-glucose. The m/z 44 and 45 signals represent CO2 ions containing 12C and 13C, respectively, from aroma compounds after separation and combustion. The top trace of each chromatogram depicts the 45/44 ratio. Peak identification: 1, hexyl acetate; 2, ethyl lactate; 3, hexanol; 4, ethyl octanoate; 5, acetic acid.
Figure 2.
Linear regression of observed APE versus hexose APE. Shaded areas represent the 95% confidence interval.
Figure 3.
Contribution of hexose-derived carbon to each compound. The mean is indicated by the circles, and the error bars represent the 95% confidence interval.
The contribution of hexoses to ethanol (94%) was somewhat surprising, as it indicated that a small amount of non-hexose substrate contributed to ethanol formation. Excluding the possibility of impure standards or experimental error, one likely explanation is that fermentable sugars other than hexoses contributed to ethanol. In particular, sucrose is reported to be present in musts at concentrations of 2–10 g/kg,33 or up to 5% of the hexose concentration in our work. Unfortunately, sucrose was not measured, and it was not possible to evaluate this hypothesis.
Like ethanol, nearly all wine volatiles measured in our studies were derived primarily from hexoses (Figure 3 and Supporting Information Supplementary Table 1). Notably, fusel alcohols associated with amino acid metabolism (e.g., methionol, β-phenylethanol, isoamyl alcohol, isobutyl alcohol) were >75% hexose derived. The acetate esters (isoamyl acetate, phenylethyl acetate) are formed by acetylation of fusel alcohols and were also derived primarily from hexoses. This indicates that the major contributor to these fusel alcohols is the anabolic pathway, in which carbon skeletons are synthesized de novo from hexoses,7 as opposed to catabolism of amino acids via the Ehrlich pathway.8 A third potential source for β-phenylethanol is from juice in either a free or glycosylated form, although, based on data from a previous study, this could account for only 3% of the total β-phenylethanol observed.34 To our knowledge, this is the first time that the relative contributions of different pathways to fusel alcohols in a wine fermentation have been evaluated. Whereas the values observed here are likely dependent on fermentation conditions such as yeast strain, our observation that the anabolic pathway is dominant is consistent with the amounts of fusel alcohols produced and the typical concentrations of amino acids in must available for Ehrlich degradation. For example, we observed ca. 100 mg/L of isoamyl alcohol in wine, whereas typical leucine concentrations in juice are reported to be <25 mg/L.35 Although glucose labeling studies have not, to our knowledge, been previously performed on wine fermentations, [U-14C]-glucose has been used to track production of fusel alcohols in grain mash fermentations.16 In the earlier work, 25–45% of fusel alcohols were derived from glucose (no replicates), substantially less than in our current study. The discrepancy may be because the mash fermentation was performed at lower gravity, which would have decreased the relative importance of the anabolic pathway once amino acid sources were exhausted.
The high contribution (>90%) of the hexose pool to straight-chain fatty acids (hexanoic, octanoic, and decanoic) and their corresponding ethyl esters (ethyl hexanoate and ethyl octanoate) was as expected; these compounds are known to be produced by yeast fatty acid metabolism and, in the case of ethyl esters, through subsequent esterification with ethanol.7 Interestingly, two of the fatty acids (octanoic and hexanoic) and ethyl 3-hydroxybutyrate had apparent hexose contributions slightly but significantly greater than 100% (103−121%). Because these semipolar compounds demonstrated slight tailing and because GC-C-IRMS peak tails are 13C depleted, hexose contributions of more than 100% may be an analytical artifact. Unlike the straight-chain fatty acids, only about half of the branched-chain isobutyric acid was derived from the hexose pool (Supporting Information Supplementary Table 1). Branched-chain fatty acids have been shown to decrease with supplementation of must with ammonia salts,36 suggesting that they may arise from catabolism of amino acids.37 This result may indicate that a significant portion of isobutyric acid arises from valine catabolism with the fermentation conditions employed.
Only one compound, 1-hexanol, was derived primarily from non-hexose sources. Although hexanol is generally present at concentrations of ≥1 mg/L in wine, alcoholic fermentation of juice-like media in the absence of non-sugar-grape derived compounds results in no detectable hexanol.6 Hexanol is detectable in grape must and can also be produced during fermentation by the reduction of C6 aldehydes and unsaturated C6 alcohols.38 These C6 compounds are largely derived from enzymatic oxidation of unsaturated fatty acids during grape crushing.39 Unsaturated fatty acids, along with other lipids, are known to be 13C-depleted in plants,40 a result which correlates with our previous observation that native hexanol is depleted as compared to other wine volatiles (Table 2). Hexyl acetate, formed by enzymatic esterification of hexanol,41 is enriched to the level predicted by the mass balance of acetic acid and hexanol (Table 2).
Recommended Enrichment Levels for GC-C-IRMS Studies of Fermentations
The precision of GC-C-IRMS depends not only on the precision of the analytical instrumentation but also on the concentration of the compound of interest, the 13C enrichment level, and the degree of biological variability. The fermentations were enriched with 0.01, 0.1, and 1.0% [U-13C]-glucose, corresponding to roughly +11, +110, and +1100‰ maximal possible enrichments in volatiles, respectively. The average SD values for the three enrichment treatments were 1.7, 6.4, and 53.4‰ across increasing enrichment treatments. Defining the limit of detection as 3 times the noise, the smallest contribution of hexose to a volatile compound would be APEobsd = 46, 17, and 15 for the 0.01, 0.1, and 1.0 APE treatments, although this will vary among compounds. Because of the marginal improvements associated with using 1.0 APE and the high costs of [U-13C]-glucose, using 0.1 APE provides the best balance of cost and variability. For 500 mL fermentations containing 100 g of hexose per 500 mL of must, generating 0.1 APE requires 0.1 g of [U-13C]-glucose, or $15 per fermentation at current prices of $150 per gram of [U-13C]-glucose. Lower tracer levels may be possible, but will require limiting variability across replicates.
In conclusion, this study is the first evaluation of a method utilizing GC-C-IRMS to trace the origin of volatile compounds during the fermentation of grape juice into wine by enriching the hexose pool with 13C. Under the conditions studied, the majority of fermentation volatiles studied, including most esters, fatty acids, and alcohols, derive at least 50% of their carbon from hexoses. Hexanol was the only compound observed to be majority (>90%) derived from grape compounds other than sugars. Using 0.1 APE of [U-13C]-glucose provides the optimal balance between precision and cost. In future studies, the effects of varying physiological conditions, such as temperature, soluble solids concentration, and nutrient status, on the relative importance of different pathways could be quantified by this approach, assuming adequate analytical performance. This method could also be applied to other complex food systems, such as Maillard reactions, to clarify the contributions of different pathways while minimizing costs.
Supplementary Material
Footnotes
ASSOCIATED CONTENT
Supporting Information
Supplementary Table 1. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Ferreira V. Volatile aroma compounds and wine sensory attributes. In: Reynolds AG, editor. Managing Wine Quality. Woodhead Publishing; Oxford, UK: 2010. [Google Scholar]
- 2.Siebert TE, Wood C, Elsey GM, Pollnitz AP. Determination of rotundone, the pepper aroma impact compound, in grapes and wine. J. Agric. Food Chem. 2008;56:3745–3748. doi: 10.1021/jf800184t. [DOI] [PubMed] [Google Scholar]
- 3.Nelson R, Acree T, Lee C, Butts R. Methyl anthranilate as an aroma constituent of American wine. J. Food Sci. 1977;42:57–59. [Google Scholar]
- 4.Baumes R. Wine aroma precursors. In: Moreno-Arribas MV, Polo MC, editors. Wine Chemistry and Biochemistry. Springer Science + Business Media; Berlin, Germany: 2009. p. 251. [Google Scholar]
- 5.Capone DL, Van Leeuwen K, Taylor DK, Jeffery DW, Pardon KH, Elsey GM, Sefton MA. Evolution and occurrence of 1,8-cineole (eucalyptol) in Australian wine. J. Agric. Food Chem. 2011;59:953–959. doi: 10.1021/jf1038212. [DOI] [PubMed] [Google Scholar]
- 6.Ugliano M, Bartowsky EJ, McCarthy J, Moio L, Henschke PA. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J. Agric. Food Chem. 2006;54:6322–6331. doi: 10.1021/jf0607718. [DOI] [PubMed] [Google Scholar]
- 7.Ugliano M, Henschke PA. Yeasts and wine flavor. In: Moreno-Arribas MV, Polo MC, editors. Wine Chemistry and Biochemistry. Springer Science + Business Media; Berlin, Germany: 2009. p. 314. [Google Scholar]
- 8.Hazelwood LA, Daran JM, van Maris AJA, Pronk JT, Dickinson JR. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Orte P, Ibarz M, Cacho J, Ferreira V. Addition of amino acids to grape juice of the Merlot variety: effect on amino acid uptake and aroma generation during alcoholic fermentation. Food Chem. 2006;98:300–310. [Google Scholar]
- 10.Hernández-Orte P, Ibarz M, Cacho J, Ferreira V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005;89:163–174. [Google Scholar]
- 11.Garde-Cerdan T, Ancin-Azpilicueta C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. Lebensm.-Wiss. Technol. 2008;41:501. [Google Scholar]
- 12.Schieberle P. The Carbon Module Labeling (CAMOLA) technique: a useful tool for identifying transient intermediates in the formation of Maillard-type target molecules. Ann. N.Y. Acad. Sci. 2005;1043:236–248. doi: 10.1196/annals.1333.029. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Maroto MC, Schneider R, Baumes R. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005;53:3503–3509. doi: 10.1021/jf048157o. [DOI] [PubMed] [Google Scholar]
- 14.Brenna JT. High-precision gas isotope ratio mass spectrometry: recent advances in instrumentation and biomedical applications. Acc. Chem. Res. 1994;27:340–346. [Google Scholar]
- 15.Zimman A, Waterhouse AL. Incorporation of malvidin-3-glucoside into high molecular weight polyphenols during fermentation and wine aging. Am. J. Enol. Vitic. 2004;55:139–146. [Google Scholar]
- 16.Reazin G, Scales H, Andreasen A. Mechanism of major congener formation in alcoholic grain fermentations. J. Agric. Food Chem. 1970;18:585–589. [Google Scholar]
- 17.Reid LM, O'Donnell CP, Downey G. Recent technological advances for the determination of food authenticity. Trends Food Sci. Technol. 2006;17:344–353. [Google Scholar]
- 18.Asche S, Michaud AL, Brenna JT. Sourcing organic compounds based on natural isotopic variations measured by high precision isotope ratio mass spectrometry. Curr. Org. Chem. 2003;7:1527–1543. [Google Scholar]
- 19.Goodman KJ, Brenna JT. High sensitivity tracer detection using high-precision gas chromatography-combustion isotope ratio mass spectrometry and highly enriched uniformly carbon-13 labeled precursors. Anal. Chem. 1992;64:1088–1095. doi: 10.1021/ac00034a004. [DOI] [PubMed] [Google Scholar]
- 20.Baumes R, Wirth J, Bureau S, Gunata Y, Razungles A. Biogeneration of C13-norisoprenoid compounds: experiments supportive for an apo-carotenoid pathway in grapevines. Anal. Chim. Acta. 2002;458:3–14. [Google Scholar]
- 21.Ortega C, Lopez R, Cacho J, Ferreira V. Fast analysis of important wine volatile compounds: development and validation of a new method based on gas chromatographic-flame ionisation detection analysis of dichloromethane microextracts. J. Chromatogr., A. 2001;923:205–214. doi: 10.1016/s0021-9673(01)00972-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Choong Y, Su N, Lee M. A rapid method for determination of ethanol in alcoholic beverages using capillary gas chromatography. J. Food Drug Anal. 2003;11:133–140. [Google Scholar]
- 23.Zhang Y, Tobias HJ, Brenna JT. Steroid isotopic standards for gas chromatography-combustion isotope ratio mass spectrometry (GCC-IRMS). Steroids. 2009;74:369–378. doi: 10.1016/j.steroids.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Santrock J, Studley SA, Hayes J. Isotopic analyses based on the mass spectra of carbon dioxide. Anal. Chem. 1985;57:1444–1448. doi: 10.1021/ac00284a060. [DOI] [PubMed] [Google Scholar]
- 25.Caimi RJ, Houghton LA, Brenna JT. Condensed-phase carbon isotopic standards for compound-specific isotope analysis. Anal. Chem. 1994;66:2989–2991. doi: 10.1021/ac00090a030. [DOI] [PubMed] [Google Scholar]
- 26.Brenna JT, Corso TN, Tobias HJ, Caimi RJ. High-precision continuous-flow isotope ratio mass spectrometry. Mass Spectrom. Rev. 1997;16:227–258. doi: 10.1002/(SICI)1098-2787(1997)16:5<227::AID-MAS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Zamora F. Biochemistry of alcoholic fermentation. In: Moreno-Arribas MV, Polo MC, editors. Wine Chemistry and Biochemistry. Springer Science + Business Media; Berlin, Germany: 2009. p. 3. [Google Scholar]
- 28.Spitzke ME, Fauhl-Hassek C. Determination of the 13C/12C ratios of ethanol and higher alcohols in wine by GC-C-IRMS analysis. Eur. Food Res. Technol. 2010;231:247–257. [Google Scholar]
- 29.Hobbie EA, Werner RA. Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol. 2004;161:371–385. doi: 10.1111/j.1469-8137.2004.00970.x. [DOI] [PubMed] [Google Scholar]
- 30.Rossmann A, Butzenlechner M, Schmidt H. Evidence for a nonstatistical carbon isotope distribution in natural glucose. Plant Physiol. 1991;96:609–614. doi: 10.1104/pp.96.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viegas CA, Rosa MF, Sá-Correia I, Novais JM. Inhibition of yeast growth by octanoic and decanoic acids produced during ethanolic fermentation. Appl. Environ. Microbiol. 1989;55:21–28. doi: 10.1128/aem.55.1.21-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunkeler D, Andersen N, Aravena R, Bernasconi SM, Butler BJ. Hydrogen and carbon isotope fractionation during aerobic biodegradation of benzene. Environ. Sci. Technol. 2001;35:3462–3467. doi: 10.1021/es0105111. [DOI] [PubMed] [Google Scholar]
- 33.Margalit Y. In: Concepts in Wine Chemistry. Crum JD, editor. Wine Appreciation Guild; San Francisco, CA, USA: 2004. [Google Scholar]
- 34.Ugliano M, Moio L. Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal. Chim. Acta. 2008;621:79–85. doi: 10.1016/j.aca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Ough C, Huang Z, An D, Stevens D. Amino acid uptake by four commercial yeasts at two different temperatures of growth and fermentation: effects on urea excretion and reabsorption. Am. J. Enol. Vitic. 1991;42:26–40. [Google Scholar]
- 36.Vilanova M, Ugliano M, Varela C, Siebert T, Pretorius IS, Henschke PA. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007;77:145–157. doi: 10.1007/s00253-007-1145-z. [DOI] [PubMed] [Google Scholar]
- 37.Styger G, Prior B, Bauer FF. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011;38:1145–1159. doi: 10.1007/s10295-011-1018-4. [DOI] [PubMed] [Google Scholar]
- 38.Herraiz T, Herraiz M, Reglero G, Martin-Alvarez PJ, Cabezudo MD. Changes in the composition of alcohols and aldehydes of C6 chain length during the alcoholic fermentation of grape must. J. Agric. Food Chem. 1990;38:969–972. [Google Scholar]
- 39.Joslin W, Ough C. Cause and fate of certain C6 compounds formed enzymatically in macerated grape leaves during harvest and wine fermentation. Am. J. Enol. Vitic. 1978;29:11–17. [Google Scholar]
- 40.Chikaraishi Y, Naraoka H, Poulson SR. Hydrogen and carbon isotopic fractionations of lipid biosynthesis among terrestrial (C3, C4 and CAM) and aquatic plants. Phytochemistry. 2004;65:1369–1381. doi: 10.1016/j.phytochem.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Dennis EG, Keyzers RA, Kalua CM, Maffei SM, Nicholson EL, Boss PK. Grape contribution to wine aroma: production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012;60:2638–2646. doi: 10.1021/jf2042517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.