Abstract
Keratoconus (KC) is a non-inflammatory thinning and protrusion of the cornea in which the cornea assumes a conical shape. Complex etiology of this condition at present remains an enigma. Although environmental factors have been involved in KC pathogenesis, strong underlining genetic susceptibility has been proven. The lack of consistent findings among early genetic studies suggested a heterogeneity and complex nature of the genetic contribution to the development of KC. Recently, genome-wide linkage studies (GWLS) and genome-wide association studies (GWAS) were undertaken. Next-generation sequencing (NGS)-based genomic screens are also currently being carried out. Application of these recently developed comprehensive genetic tools led to a much greater success and increased reproducibility of genetic findings in KC. Involvement of the LOX gene identified through GWLS has been confirmed in multiple cohorts of KC patients around the world. KC susceptibility region located at the 2q21.3 chromosomal region near the RAB3GAP1 gene identified through GWAS was independently replicated. Rare variants in the ZNF469 gene (mutated in corneal dystrophy Brittle Cornea Syndrome) and in the TGFBI gene (mutated in multiple corneal epithelial–stromal TGFBI dystrophies) have been repeatedly identified in familial and sporadic KC patients of different ethnicities. Additional comprehensive strategies using quantitative endophenotypes have been successfully employed to bring further understanding to the genetics of KC. Additional genetic determinants including the COL5A1 gene have been identified in the GWAS of KC-related trait central corneal thickness. These recent discoveries confirmed the importance of the endophenotype approach for studying complex genetic diseases such as KC and showed that different connective tissue disorders may have the same genetic determinants.
Keywords: Keratoconus, Genetics, Complex disease, Genetic variation, Linkage, Genetic association, Sequencing, Corneal dystrophy, Genotyping
Background
Keratoconus (KC) is a complex corneal condition characterized by progressive corneal thinning and steepening resulting in moderate to marked visual impairment [1]. The disease is relatively common; it affects approximately 300,000 people in the U.S. [2, 3] and is one of the three top indications for corneal transplantation in the U.S. and worldwide [4].
KC most commonly affects children. It is often detected at puberty and is progressive until the third to fourth decade of life, when it usually arrests [1]. As the cornea thins and steepens, it assumes a conical shape, causing increasingly myopic and astigmatic vision. The decrease in visual acuity can first be addressed with glasses, then later with rigid gas permeable contact lenses. If the disease continues to progress, the contact lens wear becomes gradually more intolerable, and corneal transplantation is indicated to restore vision. These visual changes can also significantly decrease quality of life for KC patients, especially when the patient has been affected for more than a decade, and as the visual acuity of the fellow “better” eye decreases [5].
The progression of the disease is caused by a decrease in the biomechanical strength of the cornea, which is composed primarily of stacked collagen and keratocytes [6]. Current research suggests a complex etiology for the disease including a genetic predisposition [3, 7, 8]. Studies have shown that a positive family history greatly increases the odds of a patient being diagnosed with KC [9–12].
There is also a possible association of KC with other genetic conditions such as inflammatory bowel disease (IBD) [13], Familial Mediterranean Fever (FMF) [14], rare chromosomal abnormalities including those associated with Down syndrome [15], and diabetes mellitus (DM), for which DM patients have a lower incidence of KC [16–18].
However, isolated KC with no associations is by far the most common presentation seen by a practicing clinician [1, 8]. The identification of genes responsible for this type of KC has been the main focus of many studies done by many research groups around the world. Significant progress has been made towards identifying subclinical phenotypic markers suitable for genetic studies by videokeratography and optical coherence tomography, including both anterior and posterior elevation and pachymetric data. As will be shown below, several genes have been implicated across these studies, including genes coding for various collagens and related to extracellular matrix production; still, many others seem to only be tangentially related to these processes. Genetic research into the etiology of the disease will improve the clinician’s ability to predict and ultimately prevent KC in patients.
In the main part below followed by Table 1 and Fig. 1, this paper will summarize the current status of research into the genetics of KC.
Table 1.
KC genes and identified variants
| Gene | Function | CHR | Variant (s) | Method (Reference) | Variant Location | Ref |
|---|---|---|---|---|---|---|
| LOX | Lysyl oxidase, participates in collagen cross-linking | 5q23.2 | rs10519694 | GWLS/LD/FM | Intron | [25] |
| rs1800449/rs2288393 | GWLS/LD/FM | Missense | [25, 29] | |||
| TG | ||||||
| rs41407546 | S | Missense | UN | |||
| rs2956540 | GWLS/LD/FM | Intron | [25, 29–31] | |||
| TG | ||||||
| COL5A1 | Collagen type V, alpha-1 chain, part of fibril-forming corneal collagen | 9q34.2-q34.3 | rs1536482 | GWLS/FM/CCT GWAS | 5′ near gene | [74, 111] |
| rs7044529 | GWLS/FM/CCT GWAS | Intron | [74, 108, 111] | |||
| CAST | Calpain/calpastatin, proteolytic degradation | 5q15 | rs4434401 | GWLS/FM | Intron | [37] |
| RAB3GAP1 | Rab GTPase activating protein, regulates exocytosis | 2q21.3 | rs4954218 | GWAS | 5′ near gene | [56, 60] |
| HGF | Hepatocyte growth factor, involved in corneal wound healing | 7q21.1 | rs3735520 | GWAS, TG | 1 KB promoter | [29, 54] |
| rs1014091 | GWAS, S | 1 KB promoter | [54, 55] | |||
| rs17501108 | S | 1 KB promoter | [55] | |||
| rs2286194 | S | intron | [55] | |||
| FNDC3B | Fibronectin, extracellular matrix protein | 3q26.31 | rs4894535 | CCT GWAS | Intron | [74] |
| FOXO1 | Transcription factor | 13q14.1 | rs2721051 | CCT GWAS, LA CCT GWAS | 3′ near gene | [74, 108] |
| TGFBI | Transforming growth factor beta induced | 5q31.1 | Multiple rare variants | S | Exon | [66, 67] |
| ZNF469 | Transcription factor, regulates corneal collagen structure and synthesis | 16q24.2 | rs9938149 | CCT GWAS, TG | 3′ near gene | [74, 112] |
| Multiple rare variants | S | Exon | [62–64] | |||
| DOCK9 | Dedicator of cytokinesis 9, Guanine nucleotide-exchange factor | 13q32.3 | c.2262A > C p.Gln754His | GWLS/S | Missense | [41] |
| MPDZ-NF1B | Not available | 9p23 | rs1324183 | CCT GWAS, TG | Intergenic | [30, 74, 112] |
| WNT10A | Member of WNT gene family of secreted signaling proteins | 2q35 | rs121908120 | CCT GWAS | Missense | [113] |
| ZEB1 | Zinc finger transcription factor | 10p11.22 | c.1920G > T; p.Gln640His | S | Missense | [84, 85] |
| SOD1 | Superoxide dismutase 1, cytoplasmic antioxidant enzyme | 21q22.11 | Multiple SNVs, deletion | S | Intron | [99, 115] |
| IL1A | Interleukin 1alpha, cytokine | 2q13 | rs2071376 | TG, S | Intron | [91, 119] |
| IL1B | Interleukin 1beta, cytokine | 2q13 | rs1143627 | TG, S | Promoter | [119–121] |
| rs16944 | TG, S | Promoter | [119–121] | |||
| COL4A3 | Collagen type IV, alpha-3 chain, structural part of corneal membranes | 2q36.3 | Multiple SNVs | S | Missense | [123] |
| COL4A4 | Collagen type IV, alpha-4 chain, structural part of corneal membranes | 2q36.3 | Multiple SNVs | S | Missense | [123, 124] |
| VSX1 | Visual system homeobox 1, transcription factor | 20p11.2 | Multiple SNVs | S | Missense, silent, intronic | [133] |
Table abbreviations: KC = keratoconus; CHR = chromosome; GWLS = genome-wide linkage study; GWAS = genome-wide association study; LD = linkage disequilibrium; FM = fine mapping; S = sequencing; TG = targeted genotyping; CCT = central corneal thickness; SNV = single nucleotide variant; LA = Latino; UN = unpublished data
Fig. 1.
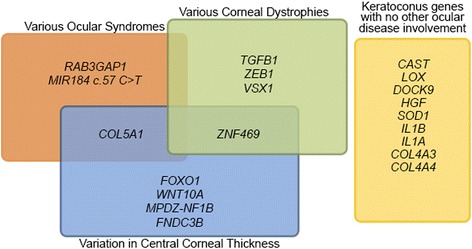
Keratoconus genes and their involvement in other ocular diseases
Review
Genes identified through genome-wide linkage studies (GWLS)
GWLS denotes genotyping families affected by a certain disease using a collection of genetic markers across the genome, and examining how those genetic markers segregate with the disease across multiple families. GWLS, also called linkage studies, were applied successfully to identify genetic variants that contribute to rare disorders like familial breast cancer [19], Huntington disease [20], cystic fibrosis [21], and others (for a comprehensive review see [22]). For decades, these studies were generally conducted using 300–400 microsatellite markers spaced at 10–20 centimorgans (cM) apart. These multiallelic markers were robust and highly informative; however, their genotyping was a time-consuming process. Shortly after single nucleotide polymorphisms (SNPs) were discovered to be abundant polymorphic markers uniformly distributed throughout the human genome [23], dense SNP arrays quickly became the genotyping platform of choice due to the highly unparalleled interrogation and accurate scoring. Testing of genotyping data also evolved from being based on model-based (recessive, dominant, etc.) to robust non-parametric alternatives [24].
LOX
One of the most significant recent developments in the field of KC genetics is the identification of polymorphisms in the LOX (collagen crosslinking enzyme lysyl oxidase) gene that is potentially responsible for a linkage signal at the 5q32-q33 chromosomal region identified by a two-stage GWLS using hundreds of polymorphic microsatellite markers (state-of-the-art-technology available at the time) and the nonparametric method of analysis [25]. After looking at biological functions of hundreds of known or predicted genes in five linkage regions, LOX was found to be the most promising candidate among plausible KC candidate genes [26]. LOX initiates the cross-linking of collagens and elastin by catalyzing oxidative deamination of the epsilon-amino group in certain lysine and hydroxylysine residues [27]. LOX defects can potentially lead to the reduction of cross-linking of collagen fibers of the corneal stroma thus leading to biomechanical weakening of the cornea. Despite the fact that an early study in a group of Italian patients failed to identify LOX mutations [28], further extensive genotyping in multiple samples of independently collected KC patients around the world confirmed the effect of SNP rs2956540 in LOX in Czech KC cases of European descent [29], Chinese cases [30], Iranian cases [31], and in a recent meta-analysis of published studies [32]. LOX involvement is also supported by functional data that showed its attenuation in corneal epithelium of KC patients at levels corresponding to disease severity [33] and revealed changes in LOX distribution and its decreased activity in KC corneas [34].
CAST
Two independent GWLS, one in a single extended KC family and another using multiple unrelated families with KC, mapped a KC locus to a genomic region located at 5q14.3-q21.1 [26, 35]. This region overlaps the CAST gene encoding calpastatin, the inhibitor of calpains (non-lysosomal intracellular proteases), which was considered a likely candidate based on the robust presence in the mammalian eye [36], which was further confirmed by in silico analysis of EST (expressed sequence tags) databases of human eye tissues [37]. This analysis showed the presence of different CAST isoforms in different parts of the eye (cornea, lens, pterygium) as well as a potential difference in their distribution in KC cornea ESTs as compared with those from normal corneal tissues [37]. In addition, higher levels of calpain small subunit-1 protein were found by protein profiling in the epithelia of KC corneas [38]. Initial linkage findings using microsatellite markers were further confirmed by genotyping of high-density SNPs in and around the CAST gene in family and case-control panels of patients with KC followed by comprehensive linkage and association analysis [37, 39]. Both studies found CAST SNPs to be significantly associated with KC.
DOCK9
One previously reported chromosomal region at 13q32 linked to KC in Ecuadorian families [40] was sequenced in 51 individuals and 105 matching controls. This mutation screen identified a possible functionally important mutation c.2262A > C (p.Gln754His, rs191047852) in the DOCK9 gene (dedicator of cytokinesis 9), which segregated with phenotype in one large Ecuadorian family [41]. This particular change was absent in other tested families and in controls, however it is present, albeit with extremely low frequency (minor allele frequency (MAF) = 0.00002; Minor Allele Count = 3) in 61,000 individuals collected around the world and sequenced by The Exome Aggregation Consortium (ExAC). The pathogenic nature of this change is supported by functional investigation, suggesting that it results in the aberrant splicing of the DOCK9 gene that leads to exon skipping, resulting in the introduction of a premature stop codon, disrupting the functional domains of DOCK9 protein that may alter the biological role of DOCK9 as an activator of Cdc42 (cell division cycle 42), an important regulator of corneal wound repair [42].
Other loci
Reports on familial KC have proposed both dominant and recessive modes of inheritance, while most families do not fit any typical mode of inheritance [1]. To date, the following gene loci for KC have been identified using GWLS methodology worldwide: 1p36.23-36.21, 2p24, 2q13, 3p14-q13, 5q14.3-q21.1, 5q21.2, 5q32-q33, 8q13.1-q21.11, 9q34, 13q32, 14q11.2, 14q24.3, 15q15.1, 15q22.33-24.2, 16q22.3-q23.1, and 20p13-p12.2, 20q12 [26, 35, 40, 43–51]. Potential mutations in the IL1RN (interleukin 1 receptor antagonist) and SLC4A11 (solute carrier family 4, sodium borate transporter, member 11) genes have been identified in Ecuadorian family linked to the 2q13-q14.3 and 20p13-p12 regions [51].
Genome-wide association studies (GWAS)
For many years, linkage analysis or GWLS was the primary tool used for the genetic mapping of Mendelian and complex traits with familial aggregation. However, over the last ten years, GWAS have evolved into a powerful tool for investigating the genetic architecture of human genetic diseases, especially complex and common genetic traits. Analytical methods used for GWAS are based on interrogating SNPs, single base-pair changes in the DNA sequence that were found to occur with high frequency in the human genome [23]. SNPs are by far the most abundant and common form of genetic variation in the human genome. Many SNPs are present in a large proportion of human populations [52]. A SNP with allele frequency significantly altered between the case and the control group is considered to be associated with the trait. Minimizing the false positive rate is the most important consideration for GWAS. Thus, genome-wide significance threshold of a nominal p-value < 5 × 10−8 has been established [53]. Well-designed GWAS would also include a replication and analyses that include consideration of the joint as well as the individual discovery and replication datasets.
HGF
Two major GWAS were undertaken almost in parallel and identified new candidate genes for KC. The first one using pooled DNA from an Australian cohort of KC samples and two-step confirmation procedure using two independent case-control cohorts) identified promoter polymorphism SNP rs3735520 in the HGF (hepatocyte growth factor) gene [54]. Effect of HGF SNP rs3735520 was confirmed in a panel of unrelated Czech KC cases of European descent [29]. Interestingly, extensive analysis of HGF SNPs in addition to the Australian KC population identified multiple associated SNPs [55].
RAB3GAP1
The second GWAS used discovery cohort of 222 Caucasian KC patients and 3324 matched controls and two independent confirmation panels, a case-control panel of 304 cases and 518 controls and a family panel of 307 subjects (146 affected) in 70 families. A novel SNP rs4954218 in the RAB3GAP1 (RAB3 GTPase activating protein catalytic subunit) gene was identified [56]. Mutations in RAB3GAP1 gene are associated with the Warburg Micro syndrome in patients of different ethnic backgrounds [57–59]. Notably, identified polymorphism SNP rs4954218 was independently replicated in an unrelated cohort of Australian Caucasian KC patients thus providing further validity to this novel locus [60].
Genes involved in KC and other corneal dystrophies
TGFBI
TGF beta-induced protein (TGFBIP) is an extracellular protein that mediates cell adhesion to collagen, laminin and fibronectin and proteoglycans, such as decorin and biglycan with expression changes triggered by the activation of the TGFB signaling pathway [61]. Transcript coding for TGFBI (previously called BIGH3) was the second most abundant transcript identified in the cDNA library constructed from KC corneas [62]. TGFBI gene mutations have been frequently identified in patients with corneal epithelial–stromal TGFBI dystrophies, a group of heterogeneous conditions that are characterized by the progressive loss of corneal transparency [63] resulting in the corneal abnormalities witnessed in transgenic mice [64, 65]. Recently, potential mutations in TGFBI was identified in Chinese [66] and in Polish KC patients [67]. The TGFBI protein has been identified in primary amyloid deposits of hereditary corneal dystrophies and in secondary corneal amyloidosis of diverse etiologies [68] as well as in corneal stromal amyloid deposits in KC patients [69]. Increased levels of TGFBI protein have been identified in corneas of patients with Fuchs’ endothelial corneal dystrophy (FECD) [70, 71]. However, not all analyzed KC patients showed association with polymorphisms in the TGFBI gene [72].
ZNF469
Brittle cornea syndrome (BCS) is an autosomal recessive generalized connective tissue disorder associated with extreme corneal thinning (220–450 μm) and a high risk of corneal rupture. Homozygous mutations in the ZNF469 (zinc finger protein 469) gene coding for a transcriptional factor containing zinc finger domains were found in patients with BCS type 1 [73]. The common genetic variant rs9938149 in ZNF469 was found to confer increased KC risk [74] and influence CCT (central corneal thickness) in the general population [74–76]. In addition, extensive sequencing of this gene in KC patients of different ethnicities by various research groups identified significant enrichment of a number of potentially pathogenic ZNF469 alleles [77–79]. These missense variants were found in 23 % of 43 KC patients from New Zealand, one-half of which were Maori or Polynesian [78] and in 12.5 % of three European cohorts with isolated KC (two from the United Kingdom, and one from Switzerland [79]). However, in contrast to previous studies, recent sequencing analysis of ZNF469 in Polish patients with KC and high myopia and Polish individuals without ocular abnormalities found no significant enrichment of any sequence variants in ZNF469 [80]. Based on these results together with the lack of evidence for the functional impact of the variants, ZNF469 involvement remains contentious at this time.
ZEB1
Mutations in the ZEB1 (zinc finger E-box binding homeobox 1) gene are repeatedly found in patients with posterior polymorphous corneal dystrophy type 3 (PPCD3) [81, 82] and seem to result in variable ocular phenotypes [83]. In particular, a unique coding mutation c.1920G > T (p.Gln640His) in this gene has been first identified in a family with KC and FECD [84] and later in a patient with triple corneal dystrophy consisting of KC, epithelial basement membrane corneal dystrophy (EBMCD) and FECD [85] thus, further supporting mutational spectrum of ZEB1 with a unique genotype/phenotype correlation.
VSX1
The VSX1 (visual system homeobox 1) gene belongs to a family of homeodomain transcription factors that are thought to control cell differentiation in craniofacial and ocular development, making it a promising functional candidate gene for KC pathogenesis of various corneal dystrophies [86, 87]. Various VSX1 gene variants have been proposed to be the genetic cause of KC in several sporadic and familiar cases [87], Italian patients [88], Iranian patients [89], Korean [90], and Chinese [91] patients, as well as in cases with PPCD (reviewed in [92]). However, no evidence of association with VSX1 variants was identified in subsequent recent research studies with large patient cohorts and recently developed genotyping methods that allow for simultaneous interrogation of hundreds of thousands of independent SNPs providing information on common genomic variation [93–99]. In addition, extending previously identified VSX1 variants to additional populations and samples provided evidence of their benign nature [100, 101]. Current evidence seems to largely support a limited role for VSX1 in KC pathogenesis.
Use of endophenotype CCT to identify additional genes and variants
Variation in CCT is one of the most highly heritable human traits [102, 103]. Reduced CCT is often associated with KC [104, 105]. Several recently performed GWAS identified a number of genomic loci associated with differences in CCT that revealed differences in genetic determinants of CCT between ethnic groups, and evaluated the relevance of CCT-associated loci to KC susceptibility [74–76, 106–108].
COL5A1
Since the corneal stroma is composed of collagen fibrils, it is not surprising that a number of genomic loci associated with CCT contain genes that code for various collagens, such as COL1A1 and COL1A2 [109], and COL8A2 [76]. The most evidence, however, points to the involvement of the COL5A1 gene coding for type V collagen subunit 1 in both CCT variation [76, 106, 108, 110] and KC [74, 111]. However, in-depth analysis of variants in KC families as well as in sporadic cases showed that while some KC patients carrying minor alleles of these variants do have thinner corneas, others do not, highlighting the complex relationship between genetic variation in COL5A1, corneal thinning and KC development [111].
FNDC3B, FOXO1, MPDZ-NF1B
CCT-associated variants rs4894535 located in the FNDC3B gene, rs2721051 near the FOXO1 gene, and rs1324183 located between the MPDZ and NF1B genes have been found to be associated with KC in large multinational cohorts of KC patients and controls [74]. SNP rs1324183 was further associated with an increased risk of KC in Chinese cases [30] and in the Australian population [112].
WNT10A
The identified CCT variants only explain about 8 % of the variability of the trait [75]. One possible component of the missing heritability is low frequency variants. The published GWAS of CCT to date focus primarily on common variants (i.e., MAF >5 %); however, when putative rare functional coding exome variants from the Illumina Human Exome array were evaluated, a novel rare WNT10A exonic variant (rs121908120), which increases the risk of KC by decreasing corneal thickness, was identified [113]. This variant is located in a gene 437 kb away from the USP37 gene, previously associated with CCT, and completely accounts for the signal previously seen at USP37. It increases the risk of KC two times. WNT10A (wingless-type MMTV integration site family member 10A) belongs to the WNT gene family. This family consists of structurally related genes encoding secreted signaling molecules that have been implicated in important developmental processes, including regulation of cell fate and patterning during corneal development [114].
Other potentially involved genes
SOD1
The SOD1 (superoxide dismutase 1) gene has been proposed and repeatedly investigated as a candidate gene for KC with published data supporting [99, 115] as well as refuting [28, 40, 89, 93, 116] its involvement. The sometimes identified increased levels of oxidative stress markers in corneas from patients with KC [117, 118] suggest that defects in the SOD1 gene, encoding a major cytoplasmic antioxidant enzyme that metabolizes superoxide radicals, might be involved in the development of this disease. However, lack of data supporting such genetic involvement suggests a possibility that said oxidative stress may be an end product of other pathologic processes caused by defects in other genes.
IL1B, IL1A
IL1B (interleukin 1 beta) promoter polymorphisms and IL1A (interleukin 1 alpha) intronic polymorphism rs2071376 have been suggested to play roles in KC susceptibility due to significant differences in allelic frequency between groups of KC patients and controls in Han Chinese [91, 119], Korean [120], and Japanese [121] populations. However, the same polymorphism showed no evidence of association in the Turkish population [122].
COL4A3, COL4A4
Genes coding for collagens COL4A3 (type IV collagen alpha3, COL4A4 (type IV collagen alpha4) have been suggested as well [123, 124]; however, additional studies in multiple populations found no association with these SNPs [91, 125] or found evidence of their extensive presence in the normal population [126].
Rare recurring mutation in miR184 (microRNA184) in families with KC and cataracts
MicroRNAs (miRNAs) bind to complementary sequences within the 3′ untranslated region (UTR) of mRNAs from hundreds of target genes, leading either to mRNA degradation or suppression of translation. Germline sequence variants in mature miRNAs are extremely rare possibly due to the extreme conservation and importance of mature miRNAs as well as its tremendously small size (18–25 base pairs). A heterozygous c.57 C > T mutation in the seed region of MIR184 (miR-184) was found to be responsible for familial severe KC combined with early-onset anterior polar cataract in the Northern Irish family [127]. The same mutation was later identified in an unrelated family with the EDICT (endothelial dystrophy, iris hypoplasia, congenital cataract, and stromal thinning) syndrome [128] and most recently in a five-generation family with cataracts and varying corneal abnormalities including severe KC and non-ectatic corneal thinning from Galicia, Spain [129]. Interestingly, genetic ancestry testing of the Spanish family strongly suggested that the c.57 C > T MIR184 mutation arose independently in the Galician and Northern Irish families and thus represents the first observation of the recurrent germline mutation in the microRNA gene leading to the genetic disease described [130]. No mutation(s) within the stem loop of MIR184 in isolated KC cases was detected in two independent screens, suggesting that mutations in MIR184 are more relevant to cases of KC associated with other ocular abnormalities [131, 132].
Discussion
Major strides had been made in the understanding of KC genetics. However, more research is needed to make biological connections between already identified KC genes as well as new genes. Several KC research groups around the world are designing and performing high-throughput studies in familial and sporadic KC patients to accomplish this task. These studies will include genotyping large cohorts of well-characterized ethnically homogenous patients and large groups of ethnically matched controls using the most comprehensive genomic chips containing up to 2.5 million independent SNPs. In addition, outgoing development and dropping prices for the next-generation sequencing (NGS) based whole genome screens are becoming more of a reality. Such screens especially in families with KC and in thoroughly selected KC patients with extreme phenotypic features can identify and test rare or low frequency variants that cannot be tested with chip technology.
As KC often begins by affecting vision, and thereby quality of life at a young age, being able to diagnose and arrest the progress of the disease at an earlier stage will aid clinicians in treating KC patients. Currently, KC is diagnosed by evaluating a variety of non-genetic metrics, such as corneal topography and pachymetry. A thorough understanding of the genetic contribution to the disease progression will increase the certainty of a KC diagnosis and allow that diagnosis to be made sooner. It may also bring additional options for the treatment of this disorder. As technology progresses and genetic screening becomes simpler and more cost effective, ophthalmologists may find value in testing suspected KC patients for the genetic variations discussed in this paper. This knowledge could enable the general ophthalmologist to understand the disease etiology such that he or she can diagnose more easily and potentially screen patients’ family members, thereby proactively caring for the disease as it develops.
Conclusion
Although the genetic etiology of KC remains to be comprehensively defined, recent GWLS and GWAS have made significant progress in identifying genetic variation that is strongly correlated with the disease. SNPs associated with the following genes have been implicated: LOX, CAST, DOCK9, IL1RN, SLC4A11, HGF, RAB3GAP1, TGFBI, ZNF469, ZEB1, VSX1, COL5A1, COL4A3, COL4A4, FNDC3B, FOXO1, MPDZ-NF1B, WNT10A, SOD1, IL1B, IL1A, in addition to the microRNA MIR184. Notably, not all analyses of each of these genes completely confirm their role in KC pathogenesis. Rather, it is likely that KC can result from abnormalities in several biochemical pathways for which the interactions have not yet been outlined. Genetic analyses that document these associations will eventually elucidate this connection.
Abbreviations
CCT, central corneal thickness; cM, centimorgan; GWAS, Genome-wide association study; GWLS, Genome-wide linkage study; KC, Keratoconus; LD, Linkage disequilibrium; NGS, next-generation sequencing
Acknowledgements
This work was supported by The Eye Defects Research Foundation Inc., the Skirball Fund for Molecular Ophthalmology and National Eye Institute grant R01-09052 (Y.S.R).
Authors’ contributions
YB contributed to the design of the manuscript, performed literature searches, drafted manuscript, and contributed to its revisions. BM contributed to the drafting of the manuscript. YSR made substantial contribution to conception, design and critical revisions of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/S0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–73. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen K, Hjortdal J, Pihlmann M, Corydon TJ. Update on the keratoconus genetics. Acta Ophthalmol. 2013;91(2):106–13. doi: 10.1111/j.1755-3768.2012.02400.x. [DOI] [PubMed] [Google Scholar]
- 4.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016;134(2):167–73. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 5.Sahebjada S, Fenwick EK, Xie J, Snibson GR, Daniell MD, Baird PN. Impact of keratoconus in the better eye and the worse eye on vision-related quality of life. Invest Ophthalmol Vis Sci. 2014;55(1):412–6. doi: 10.1167/iovs.13-12929. [DOI] [PubMed] [Google Scholar]
- 6.Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus. Eye (Lond) 2014;28(2):189–95. doi: 10.1038/eye.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler J, Hauser MA, Afshari NA, Allingham RR, Liu Y. The Genetics of Keratoconus: A Review. Reprod Syst Sex Disord. 2012;(Suppl 6). pii: 001. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3686480/. [DOI] [PMC free article] [PubMed]
- 8.Rabinowitz YS. The genetics of keratoconus. Ophthalmol Clin North Am. 2003;16(4):607–20. doi: 10.1016/S0896-1549(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 9.Gordon-Shaag A, Millodot M, Kaiserman I, Sela T, Barnett Itzhaki G, Zerbib Y, et al. Risk factors for keratoconus in Israel: a case–control study. Ophthalmic Physiol Opt. 2015;35(6):673–81. doi: 10.1111/opo.12237. [DOI] [PubMed] [Google Scholar]
- 10.Naderan M, Shoar S, Rezagholizadeh F, Zolfaghari M, Naderan M. Characteristics and associations of keratoconus patients. Cont Lens Anterior Eye. 2015;38(3):199–205. doi: 10.1016/j.clae.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Karimian F, Aramesh S, Rabei HM, Javadi MA, Rafati N. Topographic evaluation of relatives of patients with keratoconus. Cornea. 2008;27(8):874–8. doi: 10.1097/ICO.0b013e31816f5edc. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Yang H, Rabinowitz YS. Longitudinal study of keratoconus progression. Exp Eye Res. 2007;85(4):502–7. doi: 10.1016/j.exer.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tréchot F, Angioi K, Latarche C, Conroy G, Beaujeux P, Andrianjafy C, et al. Keratoconus in inflammatory bowel disease patients: a cross-sectional study. J Crohns Colitis. 2015;9(12):1108–12. doi: 10.1093/ecco-jcc/jjv151. [DOI] [PubMed] [Google Scholar]
- 14.Kosker M, Arslan N, Alp MY, Ozisler C, Acar M, Dogan AS, et al. Association between Keratoconus and familial Mediterranean fever in Turkey. Cornea. 2016;35(1):77–80. doi: 10.1097/ICO.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 15.Papoulidis I, Papageorgiou E, Siomou E, Oikonomidou E, Thomaidis L, Vetro A, et al. A patient with partial trisomy 21 and 7q deletion expresses mild Down syndrome phenotype. Gene. 2014;536(2):441–3. doi: 10.1016/j.gene.2013.11.078. [DOI] [PubMed] [Google Scholar]
- 16.Naderan M, Naderan M, Rezagholizadeh F, Zolfaghari M, Pahlevani R, Rajabi MT. Association between diabetes and keratoconus: a case–control study. Cornea. 2014;33(12):1271–3. doi: 10.1097/ICO.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 17.Kuo IC, Broman A, Pirouzmanesh A, Melia M. Is there an association between diabetes and keratoconus? Ophthalmology. 2006;113(2):184–90. doi: 10.1016/j.ophtha.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Woodward MA, Blachley TS, Stein JD. The association between sociodemographic factors, common systemic diseases, and Keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology. 2016;123(3):457–65. doi: 10.1016/j.ophtha.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–9. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 20.Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306(5940):234–8. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 21.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 22.Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease. Science. 2008;322(5903):881–8. doi: 10.1126/science.1156409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genomes Project C, Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES. Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet. 1996;58(6):1347–63. [PMC free article] [PubMed] [Google Scholar]
- 25.Bykhovskaya Y, Li X, Epifantseva I, Haritunians T, Siscovick D, Aldave A, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case–control studies. Invest Ophthalmol Vis Sci. 2012;53(7):4152–7. doi: 10.1167/iovs.11-9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Rabinowitz YS, Tang YG, Picornell Y, Taylor KD, Hu M, et al. Two-stage genome-wide linkage scan in keratoconus sib pair families. Invest Ophthalmol Vis Sci. 2006;47(9):3791–5. doi: 10.1167/iovs.06-0214. [DOI] [PubMed] [Google Scholar]
- 27.Hämäläinen ER, Jones TA, Sheer D, Taskinen K, Pihlajaniemi T, Kivirikko KI. Molecular cloning of human lysyl oxidase and assignment of the gene to chromosome 5q23.3-31.2. Genomics. 1991;11(3):508–16. doi: 10.1016/0888-7543(91)90057-L. [DOI] [PubMed] [Google Scholar]
- 28.De Bonis P, Laborante A, Pizzicoli C, Stallone R, Barbano R, Longo C, et al. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol Vis. 2011;17:2482–94. [PMC free article] [PubMed] [Google Scholar]
- 29.Dudakova L, Palos M, Jirsova K, Stranecky V, Krepelova A, Hysi PG, et al. Validation of rs2956540:G > C and rs3735520:G > A association with keratoconus in a population of European descent. Eur J Hum Genet. 2015;23(11):1581–3. doi: 10.1038/ejhg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao XD, Chen P, Chen ZL, Li SX, Wang Y. Evaluating the association between Keratoconus and reported genetic loci in a Han Chinese population. Ophthalmic Genet. 2015;36(2):132–6. doi: 10.3109/13816810.2015.1005317. [DOI] [PubMed] [Google Scholar]
- 31.Hasanian-Langroudi F, Saravani R, Validad MH, Bahari G, Yari D. Association of Lysyl oxidase (LOX) polymorphisms with the risk of Keratoconus in an Iranian population. Ophthalmic Genet. 2015;36(4):309–14. doi: 10.3109/13816810.2014.881507. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Zhang L, Hong J, Wu D, Xu J. Association of common variants in LOX with Keratoconus: a meta-analysis. PLoS One. 2015;10(12):e0145815. doi: 10.1371/journal.pone.0145815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shetty R, Sathyanarayanamoorthy A, Ramachandra RA, Arora V, Ghosh A, Srivatsa PR, et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol Vis. 2015;21:12–25. doi: 10.3390/molecules21010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudakova L, Liskova P, Trojek T, Palos M, Kalasova S, Jirsova K. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res. 2012;104:74–81. doi: 10.1016/j.exer.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Tang YG, Rabinowitz YS, Taylor KD, Li X, Hu M, Picornell Y, et al. Genomewide linkage scan in a multigeneration Caucasian pedigree identifies a novel locus for keratoconus on chromosome 5q14.3-q21.1. Genet Med. 2005;7(6):397–405. doi: 10.1097/01.GIM.0000170772.41860.54. [DOI] [PubMed] [Google Scholar]
- 36.Persson H, Kawashima S, Karlsson JO. Immunohistochemical localization of calpains and calpastatin in the rabbit eye. Brain Res. 1993;611(2):272–8. doi: 10.1016/0006-8993(93)90513-M. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Bykhovskaya Y, Tang YG, Picornell Y, Haritunians T, Aldave AJ, et al. An association between the calpastatin (CAST) gene and keratoconus. Cornea. 2013;32(5):696–701. doi: 10.1097/ICO.0b013e3182821c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph R, Srivastava OP, Pfister RR. Differential epithelial and stromal protein profiles in keratoconus and normal human corneas. Exp Eye Res. 2011;92(4):282–98. doi: 10.1016/j.exer.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Bykhovskaya Y, Li X, Taylor KD, Haritunians T, Rotter JI, Rabinowitz YS. Linkage analysis of high-density SNPs confirms Keratoconus locus at 5q chromosomal region. Ophthalmic Genet. 2016;37(1):109–10. doi: 10.3109/13816810.2014.889172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajecka M, Radhakrishna U, Winters D, Nath SK, Rydzanicz M, Ratnamala U, et al. Localization of a gene for keratoconus to a 5.6-Mb interval on 13q32. Invest Ophthalmol Vis Sci. 2009;50(4):1531–9. doi: 10.1167/iovs.08-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czugala M, Karolak JA, Nowak DM, Polakowski P, Pitarque J, Molinari A, et al. Novel mutation and three other sequence variants segregating with phenotype at keratoconus 13q32 susceptibility locus. Eur J Hum Genet. 2012;20(4):389–97. doi: 10.1038/ejhg.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karolak JA, Rydzanicz M, Ginter-Matuszewska B, Pitarque JA, Molinari A, Bejjani BA, et al. Variant c.2262A > C in DOCK9 Leads to Exon Skipping in Keratoconus Family. Invest Ophthalmol Vis Sci. 2015;56(13):7687–90. doi: 10.1167/iovs.15-17538. [DOI] [PubMed] [Google Scholar]
- 43.Burdon KP, Coster DJ, Charlesworth JC, Mills RA, Laurie KJ, Giunta C, et al. Apparent autosomal dominant keratoconus in a large Australian pedigree accounted for by digenic inheritance of two novel loci. Hum Genet. 2008;124(4):379–86. doi: 10.1007/s00439-008-0555-z. [DOI] [PubMed] [Google Scholar]
- 44.Hutchings H, Ginisty H, Le Gallo M, Levy D, Stoesser F, Rouland JF, et al. Identification of a new locus for isolated familial keratoconus at 2p24. J Med Genet. 2005;42(1):88–94. doi: 10.1136/jmg.2004.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brancati F, Valente EM, Sarkozy A, Fehèr J, Castori M, Del Duca P, et al. A locus for autosomal dominant keratoconus maps to human chromosome 3p14-q13. J Med Genet. 2004;41(3):188–92. doi: 10.1136/jmg.2003.012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bisceglia L, De Bonis P, Pizzicoli C, Fischetti L, Laborante A, Di Perna M, et al. Linkage analysis in keratoconus: replication of locus 5q21.2 and identification of other suggestive Loci. Invest Ophthalmol Vis Sci. 2009;50(3):1081–6. doi: 10.1167/iovs.08-2382. [DOI] [PubMed] [Google Scholar]
- 47.Liskova P, Hysi PG, Waseem N, Ebenezer ND, Bhattacharya SS, Tuft SJ. Evidence for keratoconus susceptibility locus on chromosome 14: a genome-wide linkage screen using single-nucleotide polymorphism markers. Arch Ophthalmol. 2010;128(9):1191–5. doi: 10.1001/archophthalmol.2010.200. [DOI] [PubMed] [Google Scholar]
- 48.Hughes AE, Dash DP, Jackson AJ, Frazer DG, Silvestri G. Familial keratoconus with cataract: linkage to the long arm of chromosome 15 and exclusion of candidate genes. Invest Ophthalmol Vis Sci. 2003;44(12):5063–6. doi: 10.1167/iovs.03-0399. [DOI] [PubMed] [Google Scholar]
- 49.Tyynismaa H, Sistonen P, Tuupanen S, Tervo T, Dammert A, Latvala T, et al. A locus for autosomal dominant keratoconus: linkage to 16q22.3-q23.1 in Finnish families. Invest Ophthalmol Vis Sci. 2002;43(10):3160–4. [PubMed] [Google Scholar]
- 50.Fullerton J, Paprocki P, Foote S, Mackey DA, Williamson R, Forrest S. Identity-by-descent approach to gene localisation in eight individuals affected by keratoconus from north-west Tasmania. Australia Hum Genet. 2002;110(5):462–70. doi: 10.1007/s00439-002-0705-7. [DOI] [PubMed] [Google Scholar]
- 51.Nowak DM, Karolak JA, Kubiak J, Gut M, Pitarque JA, Molinari A, et al. Substitution at IL1RN and deletion at SLC4A11 segregating with phenotype in familial keratoconus. Invest Ophthalmol Vis Sci. 2013;54(3):2207–15. doi: 10.1167/iovs.13-11592. [DOI] [PubMed] [Google Scholar]
- 52.International HapMap C, Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467(7311):52–8. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pe’er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32(4):381–5. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
- 54.Burdon KP, Macgregor S, Bykhovskaya Y, Javadiyan S, Li X, Laurie KJ, et al. Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Invest Ophthalmol Vis Sci. 2011;52(11):8514–9. doi: 10.1167/iovs.11-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahebjada S, Schache M, Richardson AJ, Snibson G, Daniell M, Baird PN. Association of the hepatocyte growth factor gene with keratoconus in an Australian population. PLoS One. 2014;9(1):e84067. doi: 10.1371/journal.pone.0084067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Bykhovskaya Y, Haritunians T, Siscovick D, Aldave A, Szczotka-Flynn L, et al. A genome-wide association study identifies a potential novel gene locus for keratoconus, one of the commonest causes for corneal transplantation in developed countries. Hum Mol Genet. 2012;21(2):421–9. doi: 10.1093/hmg/ddr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris-Rosendahl DJ, Segel R, Born AP, Conrad C, Loeys B, Brooks SS, et al. New RAB3GAP1 mutations in patients with Warburg Micro Syndrome from different ethnic backgrounds and a possible founder effect in the Danish. Eur J Hum Genet. 2010;18(10):1100–6. doi: 10.1038/ejhg.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, et al. Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat Genet. 2005;37(3):221–3. doi: 10.1038/ng1517. [DOI] [PubMed] [Google Scholar]
- 59.Asahina M, Endoh Y, Matsubayashi T, Fukuda T, Ogata T. Novel RAB3GAP1 compound heterozygous mutations in Japanese siblings with Warburg Micro syndrome. Brain Dev. 2016;38(3):337–40. doi: 10.1016/j.braindev.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Bae HA, Mills RA, Lindsay R, Phillips T, Coster D, Mitchell P, et al. Replication and meta-analysis of candidate loci identified variation at RAB3GAP1 associated with keratoconus. Invest Ophthalmol Vis Sci. 2013;54(7):5132–5. doi: 10.1167/iovs.13-12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engler C, Chakravarti S, Doyle J, Eberhart CG, Meng H, Stark WJ, et al. Transforming growth factor-beta signaling pathway activation in Keratoconus. Am J Ophthalmol. 2011;151(5):752–9. doi: 10.1016/j.ajo.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabinowitz YS, Dong L, Wistow G. Gene expression profile studies of human keratoconus cornea for NEIBank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest Ophthalmol Vis Sci. 2005;46(4):1239–46. doi: 10.1167/iovs.04-1148. [DOI] [PubMed] [Google Scholar]
- 63.Kannabiran C. Genetics of corneal endothelial dystrophies. J Genet. 2009;88(4):487–94. doi: 10.1007/s12041-009-0067-1. [DOI] [PubMed] [Google Scholar]
- 64.Bustamante M, Tasinato A, Maurer F, Elkochairi I, Lepore MG, Arsenijevic Y, et al. Overexpression of a mutant form of TGFBI/BIGH3 induces retinal degeneration in transgenic mice. Mol Vis. 2008;14:1129–37. [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss JS, Møller HU, Aldave AJ, Seitz B, Bredrup C, Kivela T, et al. IC3D classification of corneal dystrophies--edition 2. Cornea. 2015;34(2):117–59. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 66.Guan T, Liu C, Ma Z, Ding S. The point mutation and polymorphism in keratoconus candidate gene TGFBI in Chinese population. Gene. 2012;503(1):137–9. doi: 10.1016/j.gene.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 67.Karolak JA, Polakowski P, Szaflik J, Szaflik JP, Gajecka M. Molecular Screening of Keratoconus Susceptibility Sequence Variants in VSX1, TGFBI, DOCK9, STK24, and IPO5 Genes in Polish Patients and Novel TGFBI Variant Identification. Ophthalmic Genet. 2016;37(1):37–43. doi: 10.3109/13816810.2014.926375. [DOI] [PubMed] [Google Scholar]
- 68.Suesskind D, Auw-Haedrich C, Schorderet DF, Munier FL, Loeffler KU. Keratoepithelin in secondary corneal amyloidosis. Graefes Arch Clin Exp Ophthalmol. 2006;244(6):725–31. doi: 10.1007/s00417-005-0153-x. [DOI] [PubMed] [Google Scholar]
- 69.Tai TY, Damani MR, Vo R, Rayner SA, Glasgow BJ, Hofbauer JD, et al. Keratoconus associated with corneal stromal amyloid deposition containing TGFBIp. Cornea. 2009;28(5):589–93. doi: 10.1097/ICO.0b013e31818c9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jurkunas UV, Bitar M, Rawe I. Colocalization of increased transforming growth factor-beta-induced protein (TGFBIp) and Clusterin in Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2009;50(3):1129–36. doi: 10.1167/iovs.08-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weller JM, Zenkel M, Schlotzer-Schrehardt U, Bachmann BO, Tourtas T, Kruse FE. Extracellular matrix alterations in late-onset Fuchs’ corneal dystrophy. Invest Ophthalmol Vis Sci. 2014;55(6):3700–8. doi: 10.1167/iovs.14-14154. [DOI] [PubMed] [Google Scholar]
- 72.Udar N, Kenney MC, Chalukya M, Anderson T, Morales L, Brown D, et al. Keratoconus--no association with the transforming growth factor beta-induced gene in a cohort of American patients. Cornea. 2004;23(1):13–7. doi: 10.1097/00003226-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Abu A, Frydman M, Marek D, Pras E, Nir U, Reznik-Wolf H, et al. Deleterious mutations in the Zinc-Finger 469 gene cause brittle cornea syndrome. Am J Hum Genet. 2008;82(5):1217–22. doi: 10.1016/j.ajhg.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Y, Vitart V, Burdon KP, Khor CC, Bykhovskaya Y, Mirshahi A, et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013;45(2):155–63. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Y, Dimasi DP, Hysi PG, Hewitt AW, Burdon KP, Toh T, et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010;6(5):e1000947. doi: 10.1371/journal.pgen.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vithana EN, Aung T, Khor CC, Cornes BK, Tay WT, Sim X, et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Genet. 2011;20(4):649–58. doi: 10.1093/hmg/ddq511. [DOI] [PubMed] [Google Scholar]
- 77.Davidson AE, Borasio E, Liskova P, Khan AO, Hassan H, Cheetham ME, et al. Brittle cornea syndrome ZNF469 mutation carrier phenotype and segregation analysis of rare ZNF469 variants in familial keratoconus. Invest Ophthalmol Vis Sci. 2015;56(1):578–86. doi: 10.1167/iovs.14-15792. [DOI] [PubMed] [Google Scholar]
- 78.Vincent AL, Jordan CA, Cadzow MJ, Merriman TR, McGhee CN. Mutations in the zinc finger protein gene, ZNF469, contribute to the pathogenesis of keratoconus. Invest Ophthalmol Vis Sci. 2014;55(9):5629–35. doi: 10.1167/iovs.14-14532. [DOI] [PubMed] [Google Scholar]
- 79.Lechner J, Porter LF, Rice A, Vitart V, Armstrong DJ, Schorderet DF, et al. Enrichment of pathogenic alleles in the brittle cornea gene, ZNF469, in keratoconus. Hum Mol Genet. 2014;23(20):5527–35. doi: 10.1093/hmg/ddu253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karolak JA, Gambin T, Rydzanicz M, Szaflik JP, Polakowski P, Frajdenberg A, et al. Evidence against ZNF469 being causative for keratoconus in Polish patients. Acta Ophthalmol. 2016;94(3):289–94. doi: 10.1111/aos.12968. [DOI] [PubMed] [Google Scholar]
- 81.Liskova P, Palos M, Hardcastle AJ, Vincent AL. Further genetic and clinical insights of posterior polymorphous corneal dystrophy 3. JAMA Ophthalmol. 2013;131(10):1296–303. doi: 10.1001/jamaophthalmol.2013.405. [DOI] [PubMed] [Google Scholar]
- 82.Evans CJ, Liskova P, Dudakova L, Hrabcikova P, Horinek A, Jirsova K, et al. Identification of six novel mutations in ZEB1 and description of the associated phenotypes in patients with posterior polymorphous corneal dystrophy 3. Ann Hum Genet. 2015;79(1):1–9. doi: 10.1111/ahg.12090. [DOI] [PubMed] [Google Scholar]
- 83.Liskova P, Filipec M, Merjava S, Jirsova K, Tuft SJ. Variable ocular phenotypes of posterior polymorphous corneal dystrophy caused by mutations in the ZEB1 gene. Ophthalmic Genet. 2010;31(4):230–4. doi: 10.3109/13816810.2010.518577. [DOI] [PubMed] [Google Scholar]
- 84.Lechner J, Dash DP, Muszynska D, Hosseini M, Segev F, George S, et al. Mutational spectrum of the ZEB1 gene in corneal dystrophies supports a genotype-phenotype correlation. Invest Ophthalmol Vis Sci. 2013;54(5):3215–23. doi: 10.1167/iovs.13-11781. [DOI] [PubMed] [Google Scholar]
- 85.Mazzotta C, Traversi C, Raiskup F, Rizzo CL, Renieri A. First identification of a triple corneal dystrophy association: keratoconus, epithelial basement membrane corneal dystrophy and fuchs’ endothelial corneal dystrophy. Case Rep Ophthalmol. 2014;5(3):281–8. doi: 10.1159/000367937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semina EV, Mintz-Hittner HA, Murray JC. Isolation and characterization of a novel human paired-like homeodomain-containing transcription factor gene, VSX1, expressed in ocular tissues. Genomics. 2000;63(2):289–93. doi: 10.1006/geno.1999.6093. [DOI] [PubMed] [Google Scholar]
- 87.Héon E, Greenberg A, Kopp KK, Rootman D, Vincent AL, Billingsley G, et al. VSX1: a gene for posterior polymorphous dystrophy and keratoconus. Hum Mol Genet. 2002;11(9):1029–36. doi: 10.1093/hmg/11.9.1029. [DOI] [PubMed] [Google Scholar]
- 88.Bisceglia L, Ciaschetti M, De Bonis P, Campo PA, Pizzicoli C, Scala C, et al. VSX1 mutational analysis in a series of Italian patients affected by keratoconus: detection of a novel mutation. Invest Ophthalmol Vis Sci. 2005;46(1):39–45. doi: 10.1167/iovs.04-0533. [DOI] [PubMed] [Google Scholar]
- 89.Saee-Rad S, Hashemi H, Miraftab M, Noori-Daloii MR, Chaleshtori MH, Raoofian R, et al. Mutation analysis of VSX1 and SOD1 in Iranian patients with keratoconus. Mol Vis. 2011;17:3128–36. [PMC free article] [PubMed] [Google Scholar]
- 90.Jeoung JW, Kim MK, Park SS, Kim SY, Ko HS, Wee WR, et al. VSX1 gene and keratoconus: genetic analysis in Korean patients. Cornea. 2012;31(7):746–50. doi: 10.1097/ICO.0b013e3181e16dd0. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Jin T, Zhang X, Wei W, Cui Y, Geng T, et al. Common single nucleotide polymorphisms and keratoconus in the Han Chinese population. Ophthalmic Genet. 2013;34(3):160–6. doi: 10.3109/13816810.2012.743569. [DOI] [PubMed] [Google Scholar]
- 92.Aldave AJ, Han J, Frausto RF. Genetics of the corneal endothelial dystrophies: an evidence-based review. Clin Genet. 2013;84(2):109–19. doi: 10.1111/cge.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stabuc-Silih M, Strazisar M, Hawlina M, Glavac D. Absence of pathogenic mutations in VSX1 and SOD1 genes in patients with keratoconus. Cornea. 2010;29(2):172–6. doi: 10.1097/ICO.0b013e3181aebf7a. [DOI] [PubMed] [Google Scholar]
- 94.Aldave AJ, Yellore VS, Salem AK, Yoo GL, Rayner SA, Yang H, et al. No VSX1 gene mutations associated with keratoconus. Invest Ophthalmol Vis Sci. 2006;47(7):2820–2. doi: 10.1167/iovs.05-1530. [DOI] [PubMed] [Google Scholar]
- 95.Tang YG, Picornell Y, Su X, Li X, Yang H, Rabinowitz YS. Three VSX1 gene mutations, L159M, R166W, and H244R, are not associated with keratoconus. Cornea. 2008;27(2):189–92. doi: 10.1097/ICO.0b013e31815a50e7. [DOI] [PubMed] [Google Scholar]
- 96.Liskova P, Ebenezer ND, Hysi PG, Gwilliam R, El-Ashry MF, Moodaley LC, et al. Molecular analysis of the VSX1 gene in familial keratoconus. Mol Vis. 2007;13:1887–91. [PMC free article] [PubMed] [Google Scholar]
- 97.Tanwar M, Kumar M, Nayak B, Pathak D, Sharma N, Titiyal JS, et al. VSX1 gene analysis in keratoconus. Mol Vis. 2010;16:2395–401. [PMC free article] [PubMed] [Google Scholar]
- 98.Abu-Amero KK, Kalantan H, Al-Muammar AM. Analysis of the VSX1 gene in keratoconus patients from Saudi Arabia. Mol Vis. 2011;17:667–72. [PMC free article] [PubMed] [Google Scholar]
- 99.Moschos MM, Kokolakis N, Gazouli M, Chatziralli IP, Droutsas D, Anagnou NP, et al. Polymorphism Analysis of VSX1 and SOD1 Genes in Greek Patients with Keratoconus. Ophthalmic Genet. 2015;36(3):213–7. doi: 10.3109/13816810.2013.843712. [DOI] [PubMed] [Google Scholar]
- 100.Dash DP, George S, O’Prey D, Burns D, Nabili S, Donnelly U, et al. Mutational screening of VSX1 in keratoconus patients from the European population. Eye. 2010;24(6):1085–92. doi: 10.1038/eye.2009.217. [DOI] [PubMed] [Google Scholar]
- 101.Eran P, Almogit A, David Z, Wolf HR, Hana G, Yaniv B, et al. The D144E substitution in the VSX1 gene: a non-pathogenic variant or a disease causing mutation? Ophthalmic Genet. 2008;29(2):53–9. doi: 10.1080/13816810802008242. [DOI] [PubMed] [Google Scholar]
- 102.Dimasi DP, Burdon KP, Craig JE. The genetics of central corneal thickness. Br J Ophthalmol. 2010;94(8):971–6. doi: 10.1136/bjo.2009.162735. [DOI] [PubMed] [Google Scholar]
- 103.Toh T, Liew SH, MacKinnon JR, Hewitt AW, Poulsen JL, Spector TD, et al. Central corneal thickness is highly heritable: the twin eye studies. Invest Ophthalmol Vis Sci. 2005;46(10):3718–22. doi: 10.1167/iovs.04-1497. [DOI] [PubMed] [Google Scholar]
- 104.Colin J, Sale Y, Malet F, Cochener B. Inferior steepening is associated with thinning of the inferotemporal cornea. J Refract Surg. 1996;12(6):697–9. doi: 10.3928/1081-597X-19960901-11. [DOI] [PubMed] [Google Scholar]
- 105.Steele TM, Fabinyi DC, Couper TA, Loughnan MS. Prevalence of Orbscan II corneal abnormalities in relatives of patients with keratoconus. Clin Experiment Ophthalmol. 2008;36(9):824–30. doi: 10.1111/j.1442-9071.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- 106.Vitart V, Bencić G, Hayward C, Skunca Herman J, Huffman J, Campbell S, et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum Mol Genet. 2010;19(21):4304–11. doi: 10.1093/hmg/ddq349. [DOI] [PubMed] [Google Scholar]
- 107.Cornes BK, Khor CC, Nongpiur ME, Xu L, Tay WT, Zheng Y, et al. Identification of four novel variants that influence central corneal thickness in multi-ethnic Asian populations. Hum Mol Genet. 2012;21(2):437–45. doi: 10.1093/hmg/ddr463. [DOI] [PubMed] [Google Scholar]
- 108.Gao X, Gauderman WJ, Liu Y, Marjoram P, Torres M, Haritunians T, et al. A genome-wide association study of central corneal thickness in Latinos. Invest Ophthalmol Vis Sci. 2013;54(4):2435–43. doi: 10.1167/iovs.13-11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dimasi DP, Chen JY, Hewitt AW, Klebe S, Davey R, Stirling J, et al. Novel quantitative trait loci for central corneal thickness identified by candidate gene analysis of osteogenesis imperfecta genes. Hum Genet. 2010;127(1):33–44. doi: 10.1007/s00439-009-0729-3. [DOI] [PubMed] [Google Scholar]
- 110.Hoehn R, Zeller T, Verhoeven VJ, Grus F, Adler M, Wolfs RC, et al. Population-based meta-analysis in Caucasians confirms association with COL5A1 and ZNF469 but not COL8A2 with central corneal thickness. Hum Genet. 2012;131(11):1783–93. doi: 10.1007/s00439-012-1201-3. [DOI] [PubMed] [Google Scholar]
- 111.Li X, Bykhovskaya Y, Canedo AL, Haritunians T, Siscovick D, Aldave AJ, et al. Genetic association of COL5A1 variants in keratoconus patients suggests a complex connection between corneal thinning and keratoconus. Invest Ophthalmol Vis Sci. 2013;54(4):2696–704. doi: 10.1167/iovs.13-11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahebjada S, Schache M, Richardson AJ, Snibson G, MacGregor S, Daniell M, et al. Evaluating the association between keratoconus and the corneal thickness genes in an independent Australian population. Invest Ophthalmol Vis Sci. 2013;54(13):8224–8. doi: 10.1167/iovs.13-12982. [DOI] [PubMed] [Google Scholar]
- 113.Cuellar-Partida G, Springelkamp H, Lucas SE, Yazar S, Hewitt AW, Iglesias AI, et al. WNT10A exonic variant increases the risk of keratoconus by decreasing corneal thickness. Hum Mol Genet. 2015;24(17):5060–8. doi: 10.1093/hmg/ddv211. [DOI] [PubMed] [Google Scholar]
- 114.Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/β-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52(7):4734–41. doi: 10.1167/iovs.10-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Udar N, Atilano SR, Brown DJ, Holguin B, Small K, Nesburn AB, et al. SOD1: a candidate gene for keratoconus. Invest Ophthalmol Vis Sci. 2006;47(8):3345–51. doi: 10.1167/iovs.05-1500. [DOI] [PubMed] [Google Scholar]
- 116.Al-Muammar AM, Kalantan H, Azad TA, Sultan T, Abu-Amero KK. Analysis of the SOD1 Gene in Keratoconus Patients from Saudi Arabia. Ophthalmic Genet. 2015;36(4):373–5. doi: 10.3109/13816810.2014.889173. [DOI] [PubMed] [Google Scholar]
- 117.Kenney MC, Chwa M, Atilano SR, Tran A, Carballo M, Saghizadeh M, et al. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005;46(3):823–32. doi: 10.1167/iovs.04-0549. [DOI] [PubMed] [Google Scholar]
- 118.Arnal E, Peris-Martinez C, Menezo JL, Johnsen-Soriano S, Romero FJ. Oxidative stress in keratoconus? Invest Ophthalmol Vis Sci. 2011;52(12):8592–7. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Wei W, Zhang C, Zhang X, Liu M, Zhu X, et al. Association of Interleukin-1 Gene Single Nucleotide Polymorphisms with Keratoconus in Chinese Han Population. Curr Eye Res. 2016;41(5):630–5. doi: 10.3109/02713683.2015.1045083. [DOI] [PubMed] [Google Scholar]
- 120.Kim SH, Mok JW, Kim HS, Joo CK. Association of -31 T > C and −511 C > T polymorphisms in the interleukin 1 beta (IL1B) promoter in Korean keratoconus patients. Mol Vis. 2008;14:2109–16. [PMC free article] [PubMed] [Google Scholar]
- 121.Mikami T, Meguro A, Teshigawara T, Takeuchi M, Uemoto R, Kawagoe T, et al. Interleukin 1 beta promoter polymorphism is associated with keratoconus in a Japanese population. Mol Vis. 2013;19:845–51. [PMC free article] [PubMed] [Google Scholar]
- 122.Palamar M, Onay H, Ozdemir TR, Arslan E, Egrilmez S, Ozkinay F, et al. Relationship between IL1beta-511C > T and ILRN VNTR polymorphisms and keratoconus. Cornea. 2014;33(2):145–7. doi: 10.1097/ICO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 123.Stabuc-Silih M, Ravnik-Glavac M, Glavac D, Hawlina M, Strazisar M. Polymorphisms in COL4A3 and COL4A4 genes associated with keratoconus. Mol Vis. 2009;15:2848–60. [PMC free article] [PubMed] [Google Scholar]
- 124.Saravani R, Hasanian-Langroudi F, Validad MH, Yari D, Bahari G, Faramarzi M, et al. Evaluation of possible relationship between COL4A4 gene polymorphisms and risk of keratoconus. Cornea. 2015;34(3):318–22. doi: 10.1097/ICO.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 125.Abu-Amero KK, Helwa I, Al-Muammar A, Strickland S, Hauser MA, Allingham RR, et al. Case-control association between CCT-associated variants and keratoconus in a Saudi Arabian population. J Negat Results Biomed. 2015;14:10. doi: 10.1186/s12952-015-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kokolakis NS, Gazouli M, Chatziralli IP, Koutsandrea C, Gatzioufas Z, Peponis VG, et al. Polymorphism analysis of COL4A3 and COL4A4 genes in Greek patients with keratoconus. Ophthalmic Genet. 2014;35(4):226–8. doi: 10.3109/13816810.2014.946055. [DOI] [PubMed] [Google Scholar]
- 127.Hughes AE, Bradley DT, Campbell M, Lechner J, Dash DP, Simpson DA, et al. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am J Hum Genet. 2011;89(5):628–33. doi: 10.1016/j.ajhg.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iliff BW, Riazuddin SA, Gottsch JD. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Invest Ophthalmol Vis Sci. 2012;53(1):348–53. doi: 10.1167/iovs.11-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bykhovskaya Y, Caiado Canedo AL, Wright KW, Rabinowitz YS. C.57 C > T Mutation in MIR 184 is Responsible for Congenital Cataracts and Corneal Abnormalities in a Five-generation Family from Galicia, Spain. Ophthalmic Genet. 2015;36(3):244–7. doi: 10.3109/13816810.2013.848908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bykhovskaya Y, Seldin MF, Liu Y, Ransom M, Li X, Rabinowitz YS. Independent origin of c.57 C > T mutation in MIR184 associated with inherited corneal and lens abnormalities. Ophthalmic Genet. 2015;36(1):95–7. doi: 10.3109/13816810.2014.977491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abu-Amero KK, Helwa I, Al-Muammar A, Strickland S, Hauser MA, Allingham RR, et al. Screening of the Seed Region of MIR184 in Keratoconus Patients from Saudi Arabia. Biomed Res Int. 2015;2015:604508. doi: 10.1155/2015/604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lechner J, Bae HA, Guduric-Fuchs J, Rice A, Govindarajan G, Siddiqui S, et al. Mutational analysis of MIR184 in sporadic keratoconus and myopia. Invest Ophthalmol Vis Sci. 2013;54(8):5266–72. doi: 10.1167/iovs.13-12035. [DOI] [PubMed] [Google Scholar]
- 133.Burdon KP, Vincent AL. Insights into keratoconus from a genetic perspective. Clin Exp Optom. 2013;96(2):146–54. doi: 10.1111/cxo.12024. [DOI] [PubMed] [Google Scholar]


