Abstract
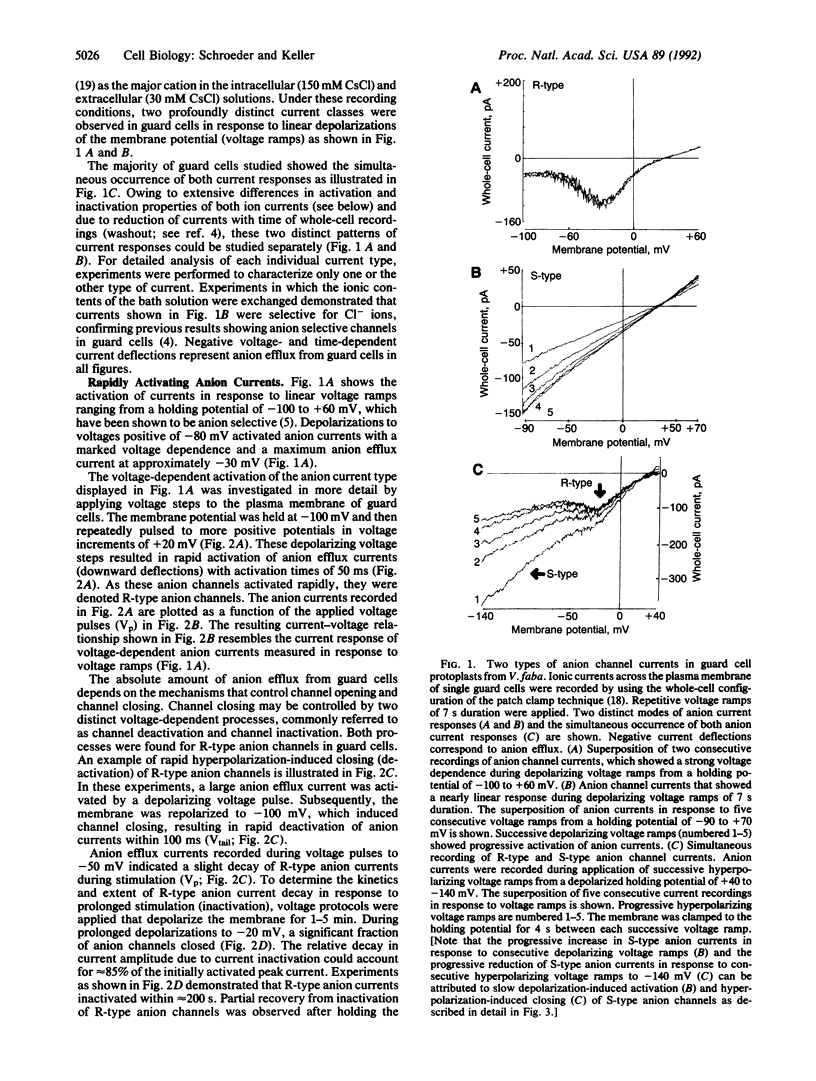
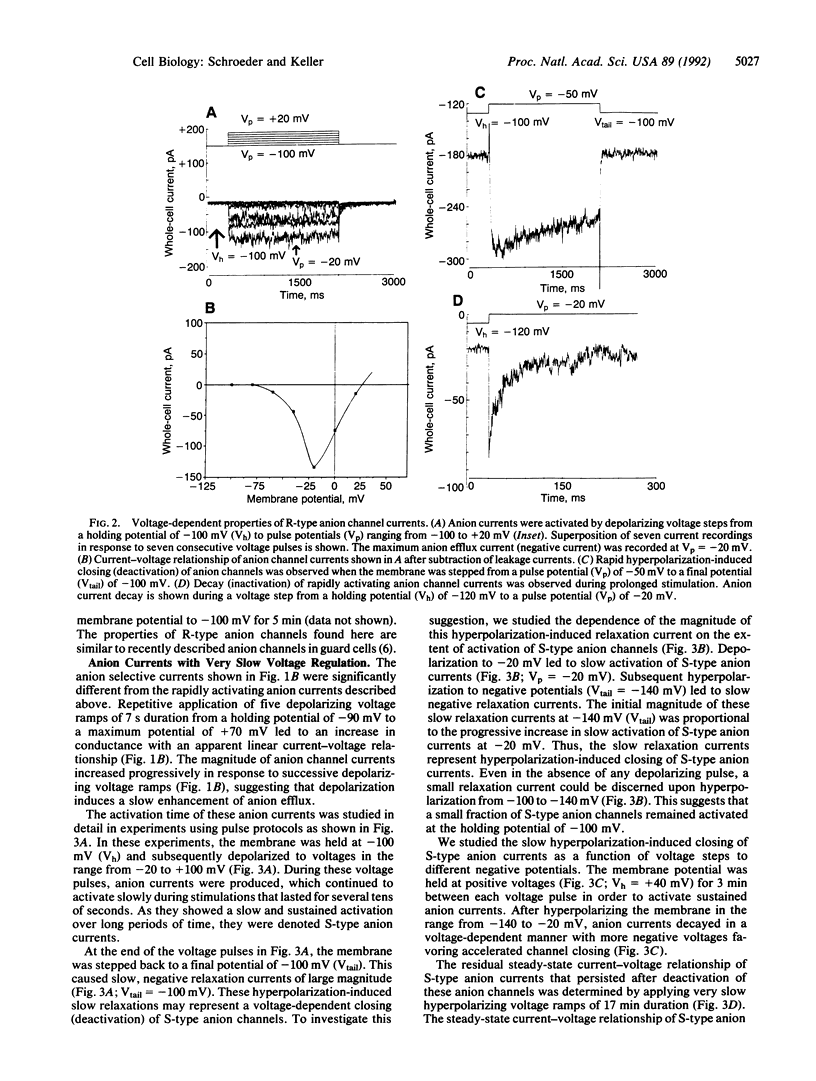
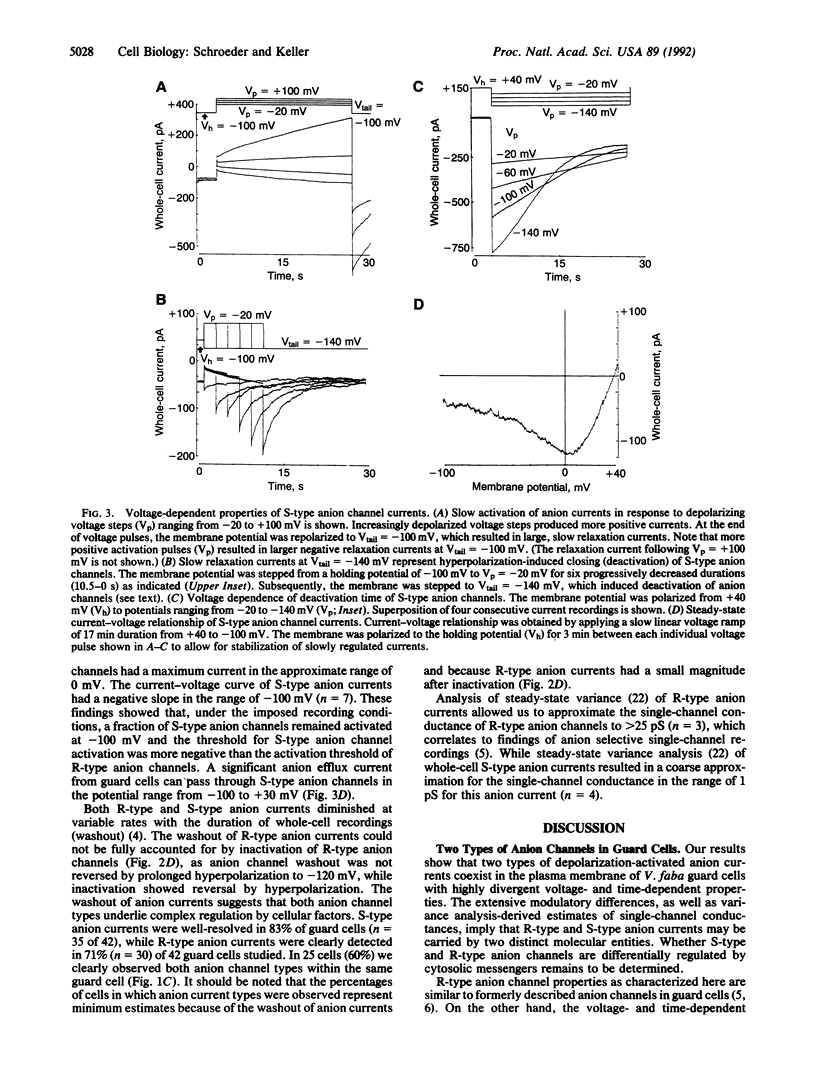
Transpirational water loss by plants is reduced by closing of stomatal pores in the leaf epidermis. Anion channels in the plasma membrane of guard cells may provide a key molecular mechanism for control of stomatal closing in leaves. However, central questions regarding the regulation, diversity, and function of anion channels in guard cells and other higher plant cells remain unanswered. We show here that two highly distinct types of depolarization-activated anion currents operate in the plasma membrane of Vicia faba guard cells. One described type of anion channel was activated rapidly within 50 ms by depolarization, inactivated during prolonged stimulation, and deactivated rapidly at hyperpolarized potentials (R-type anion current). The other depolarization-activated anion current showed extremely slow voltage-dependent activation and deactivation (S-type anion current) and lacked inactivation. The distinct voltage and time dependencies of R-type and S-type anion channels suggest that they may play a role during depolarization-associated signal transduction in higher plant cells and that these anion channels may contribute to different processes in the regulation of stomatal movements. In particular, the slow and sustained nature of S-type anion channel activation revealed here leads us to hypothesize that S-type anion channels may provide a central molecular mechanism for control of stomatal closing, which is accompanied by long-term anion efflux and depolarization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt M. R., Thiel G., Trentham D. R. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1990 Aug 23;346(6286):766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Fricker M. D., Read N. D., Trewavas A. J. Role of Calcium in Signal Transduction of Commelina Guard Cells. Plant Cell. 1991 Apr;3(4):333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Read N. D., Trewavas A. J. Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature. 1990 Aug 23;346(6286):769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Busch H., Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990 Dec;9(12):3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen R., Satter R. L. Rhythmic and phytochrome-regulated changes in transmembrane potential in samanea pulvini. Nature. 1975 May 29;255(5507):408–410. doi: 10.1038/255408a0. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Wilson K. J. Properties of Single K and Cl Channels in Asclepias tuberosa Protoplasts. Plant Physiol. 1987 Oct;85(2):413–418. doi: 10.1104/pp.85.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Fang H. H. Inward-rectifying K+ channels in guard cells provide a mechanism for low-affinity K+ uptake. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11583–11587. doi: 10.1073/pnas.88.24.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989 May;14(5):187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I. K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J Gen Physiol. 1988 Nov;92(5):667–683. doi: 10.1085/jgp.92.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding E. P., Cosgrove D. J. Large plasma-membrane depolarization precedes rapid blue-light-induced growth inhibition in cucumber. Planta. 1989;178:407–410. [PubMed] [Google Scholar]
- Terry B. R., Tyerman S. D., Findlay G. P. Ion channels in the plasma membrane of Amaranthus protoplasts: one cation and one anion channel dominate the conductance. J Membr Biol. 1991 May;121(3):223–236. doi: 10.1007/BF01951556. [DOI] [PubMed] [Google Scholar]
- Ullrich C. I., Novacky A. J. Electrical Membrane Properties of Leaves, Roots, and Single Root Cap Cells of Susceptible Avena sativa: Effect of Victorin C. Plant Physiol. 1991 Mar;95(3):675–681. doi: 10.1104/pp.95.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]



