Abstract
Toxoplasmosis is an infection caused by the protozoan parasite Toxoplasma gondii that can lead to severe sequelae in the fetus during pregnancy. Definitive serologic diagnosis of the infection during gestation is made mostly by detecting T. gondii-specific antibodies, including IgG and IgM, individually in a single serum sample by using commercially available kits. The IgA test is used by some laboratories as an additional marker of acute infection. Most of the commercial tests have failed to reach 100% correlation with the reference method, the Sabin-Feldman dye test for the detection of Toxoplasma IgG antibodies. For Toxoplasma IgM and IgA antibodies, there is no reference method and their evaluation is done by comparing the results of one assay to those of another. There is a need for multiplexed assay platforms, as the serological diagnosis of T. gondii infection does not rely on the detection of a single Ig subtype. Here we describe the development of a plasmonic gold chip with vast fluorescence enhancement in the near-infrared region for simultaneous detection of IgG, IgM, and IgA antibodies against T. gondii in an ∼1-μl serum or whole-blood sample. When 168 samples were tested on this platform, IgG antibody detection sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were all 100%. IgM antibody detection achieved 97.6% sensitivity and 96.9% specificity with a 90.9% PPV and a 99.2% NPV. Thus, the nanoscience-based plasmonic gold platform enables a high-performance, low-cost, multiplexed assay requiring ultrasmall blood volumes, paving the way for the implementation of universal screening for toxoplasmosis infection during gestation.
INTRODUCTION
Toxoplasma gondii is a protozoan parasite capable of infecting virtually all warm-blooded animals. Infection in humans is due primarily to the ingestion of contaminated food or water and is generally asymptomatic (1). However, in fetuses and immunocompromised patients (e.g., AIDS patients or patients with transplants or cancer or undergoing immunosuppressive therapies), the infection can result in high morbidity and mortality rates. Indeed, primary infection with T. gondii acquired during gestation may lead to miscarriage or severe sequelae in the fetus (2). In immunocompromised patients, acute infection or reactivation of a latent infection may cause life-threatening syndromes such as toxoplasmic encephalitis, pneumonia, or disseminated disease (3). It is thus important to screen these particular populations for T. gondii infection in order to take appropriate measures. In some countries, monthly prenatal serological screening is performed for all pregnant women whether or not they are considered at risk for T. gondii infection (4, 5). In countries with a low prevalence of T. gondii infection, screening of pregnant women at high risk is recommended (6). This screening allows timely detection of maternal primary infection and leads to preventive or therapeutic intervention in order to decrease the risk of significant ocular and neurological manifestations. In immunocompromised patients, knowledge of the Toxoplasma serological status of patients is of utmost importance for prophylactic measures and early treatment of patients with clinical manifestations suggestive of toxoplasmosis. In most nonreference laboratories, the diagnosis is performed by detecting IgG and IgM in the serum of patients by commercially available methods. While the reference method for the detection of Toxoplasma IgG is the Sabin-Feldman dye test, only a few laboratories use it because it is difficult to set up, time-consuming, and relatively expensive (7, 8). Most commercial tests compare their results with those of the Sabin-Feldman IgG dye test without reaching 100% correlation; moreover, the IgG dye test detects IgG earlier than other methods (9–12). For Toxoplasma IgM and IgA antibodies, there is to date no reference method and their evaluation is done by comparing one assay to another (9, 12–16). Positivity for IgM antibodies is often considered a marker of acute infection, as they appear in the first week following infection (3). However, IgM antibody positivity should be interpreted with caution, as it can persist for years after infection and there are also false-positive IgM test results (3, 8, 17). IgA test results are used by some laboratories as an additional marker of acute infection in the diagnosis of congenital toxoplasmosis in newborns and could also be used as a marker of reactivation in immunocompromised patients (13, 18, 19).
The serological diagnosis of T. gondii infection does not rely on a sole subtype of Ig detection. Indeed, detection of IgG and IgM should be performed for each Toxoplasma serology test, with IgA status providing additional information regarding acute infection or reactivation. In the case of positive IgG, IgM, and IgA results, there is a high likelihood of acute Toxoplasma infection, whereas in the case of positive IgG and IgA and negative IgM results, reactivation is suspected (3, 19–21). Thus, there is a need to detect several subtypes of Ig in a single assay. To date, no platform is capable of detecting T. gondii IgG, IgM, and IgA simultaneously in the same assay. To facilitate this goal, a multiplexed platform with high assay precision is needed.
Recently, a new near-infrared (NIR) region fluorescence-enhancing plasmonic gold microarray platform was developed to detect multiple antibodies in serum (22–25). The unique capabilities of the platform, including a high signal-to-background ratio, broad dynamic range, and high sensitivity, are due to fluorescence enhancement by an underlying nanostructured gold film in the 550- to 900-nm range by up to ∼100-fold (23–25). Such drastic signal enhancement by nanoengineered gold structures has enabled an ∼2-log increase in the dynamic range and sensitivity of fluorescence detection methods and assays. Moreover, multiplexed detection can be easily implemented on the plasmonic gold film to detect a panel of antibodies over an array of spatially defined antigen spots, with multicolor capability in the visible-to-NIR region (500- to 900-nm) window to simultaneously detect IgG, IgM, and IgA subtypes of each antibody in the same run. All of these tests can be accomplished with a single drop of serum or whole blood. Such a technology is unique and ideally suited for serological screening for infectious diseases with greatly improved sensitivity and specificity, a rapid turnaround, and a low cost. Here, taking advantage of the advanced features of plasmonic gold, we demonstrate the simultaneous detection of IgG, IgM, and IgA antibodies to T. gondii in ∼1-μl serum and whole-blood samples. By multiplexing the assay with a small sample volume, we have developed a toxoplasmosis test that matches the performance of the dye test used by reference laboratories at a small percentage of the cost, thus opening a door to universal screening in the United States, as well as in much-less-developed countries.
MATERIALS AND METHODS
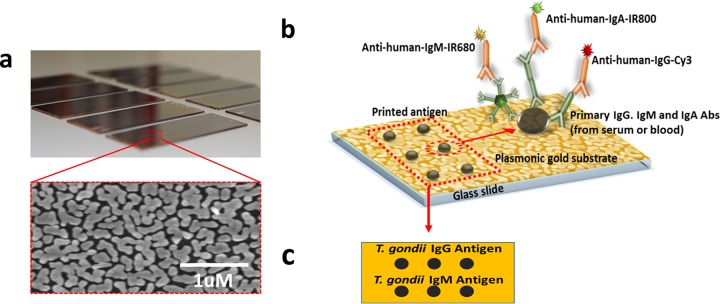
Plasmonic gold film on glass slides and antigen selection.
A plasmonic gold film composed of tortuous gold nanoislands was fabricated on glass slides through a solution phase growth method as previously described, resulting in a surface packed with gold nanoislands with plasmon resonance in the NIR region and abundant nanoscale gaps (Fig. 1a) (23). We generated and optimized T. gondii antigen microarrays on such nanostructured plasmonic gold films. A commercial antigen mixture (Toxoplasma IgG; BioCheck, Inc.) having sufficient binding to IgA antibodies was selected for the detection of T. gondii IgG and IgA antibodies. T. gondii antigen mixture M, produced in our Palo Alto Medical Foundation Toxoplasmosis Serology Laboratory (PAMF-TSL; a reference laboratory for toxoplasmosis in the United States), was used to detect T. gondii IgM antibodies. Antigen mixture M was prepared as described previously and is currently used for the detection of IgM by enzyme-linked immunosorbent assay (ELISA) at the PAMF-TSL (15).
FIG 1.
The plasmonic gold chip allows the detection of individual immunoglobulin isotypes in a single ultrasmall-volume sample. (a) Electron micrograph demonstrating gold islands and abundant nanogaps in the gold plasmonic film (b) Schematic illustrating the spatial relationship of the platform's gold plasmonic surface, the T. gondii antibody-specific antigens immobilized on the film by robotic printing, the primary antibodies (Abs) from diluted human serum or blood, and the detection antibodies conjugated with different fluorophores. (c) Schematic layout of the orientation of the two T. gondii antigens printed on plasmonic gold chips for each sample.
Antigen microarray printing and microarray protein assay.
The two T. gondii antigens were immobilized on plasmonic gold chips by robotic array printing (Nano-Plotter 2.1; GeSIM) at 0.45 mg/ml in triplicate spots at specific locations (Fig. 1b and c). The chips were covered by multiarray chambers (ProPlate; Grace Bio-Labs), and up to 16 individual arrays can be assayed on each chip. Five percent bovine serum albumin (Sigma-Aldrich) in phosphate-buffered saline (1× PBS; GE Healthcare Life Sciences) was then used to block the chips for 1 h. We diluted 1-μl human serum or whole-blood samples 100-fold in fetal bovine serum (GE Healthcare Life Sciences), applied them to the printed antigen spots on the Au chips, and incubated them. After 1 h of incubation at room temperature, the human IgG, IgM, and IgA antibodies from the patient samples were captured on the antigen spots and the chips were washed three times with PBST (PBS–0.05% Tween 20 [Sigma-Aldrich]). The Au chips were then incubated for 30 min with a mixture of 2 nM Cy3-labeled anti-human IgG secondary antibody, IRDye680-labeled anti-human IgM secondary antibody, and IRDye800-labeled anti-human IgA secondary antibody (all unlabeled goat anti-human IgG, IgM, and IgA antibodies and Cy3 NHS ester were from Vector Laboratories, and IRDye 680LT NHS ester and IRDye 800CW NHS ester were from LI-COR Biosciences). After incubation, the chips were washed three times with PBST, once with PBS, and once with deionized water and dried with compressed air. Through this sandwich microarray assay process (Fig. 1b) on the plasmonic gold film, T. gondii-specific antibodies were captured by the respective target antigens and the antibody subtypes were quantified by the fluorescence of the dye labels on the corresponding secondary antibodies.
Florescence signal analysis.
To quantify anti-Toxoplasma IgG, IgM, and IgA captured on the antigen spots on plasmonic gold chips, we used a GenePix 4000B scanner (Molecular Devices) to detect the Cy3–anti-human IgG signal and a LI-COR Odyssey scanner (LI-COR Biosciences) to scan the IRDye680–anti-human IgM and IRDye800–anti-human IgA signals. The microarray fluorescence-scanned images were analyzed by GenePix 6.1, and fluorescence signals at the antigen spots were quantified to probe the relative amounts of the three subtypes of T. gondii-specific antibody detected in each patient's serum or blood sample. On each Au chip, positive and negative standard controls for the three subtypes of antibodies were also included for assay quality control and signal normalization. Each patient's final result was quantified by a mean fluorescence intensity (MFI) ratio obtained by dividing the actual MFI of spots by the MFI of the spots of the standard positive-control sample. The same positive- and negative-control samples were used for signal normalization for all of the samples measured throughout this work.
Origin of serum and whole blood.
The serum and the control samples used in this study came from the PAMF-TSL Biobank. The retrospective use of the serum was approved by an institutional review board-approved waiver of consent and waiver of authorization. We used ultrasmall sample volumes for multiplexed and multicolor antibody detection on the plasmonic gold platform, and the results were compared side by side to standard IgG dye test results and IgM and IgA double-sandwich ELISA results for T. gondii-specific antibodies developed at the PAMF-TSL (15, 26, 27). The following titers were considered positive at the PAMF-TSL: IgG dye test, ≥1:16; IgM, ≥2; IgA, ≥2.1 (15, 26, 27). The IgM and IgA ELISAs both had a “gray zone” for the samples near the cutoff titers, and there were two serum samples in the IgM ELISA gray zone (with test results between 1.7 and 1.9) and four serum samples in the IgA ELISA gray zone (with test results between 1.9 and 2). Since none of these gray zone samples had an interpretation of acute infection or reactivation, we categorized them as negative in our analysis of results.
The whole-blood and serum sample pairs were provided by five volunteers for comparison of the test results based on serum samples and whole blood. All of the serum and whole-blood samples were tested blind on the plasmonic gold platform.
Statistical analysis.
The VassarStats Website for Statistical Computation was used for the statistical analyses. The K coefficient (Cohen's kappa coefficient) was used to compare the results of the plasmonic gold platform with those from the PAMF-TSL. Better agreement between the plasmonic gold platform and the PAMF-TSL results would lead to an increased kappa coefficient. Perfect agreement would equate to a kappa coefficient of 1, and chance agreement would equate to a kappa coefficient of 0. Kappa coefficients between 0.81 and 0.99 are considered nearly perfect, those between 0.61 and 0.80 indicate substantial agreement, and those between 0.41 and 0.60 indicate moderate agreement (28).
RESULTS
Choice of dye for secondary detection antibody.
The plasmonic gold platform could enhance the fluorescence of multiple dyes with nonoverlapping emission spectra such as Cy3, IRDye680, and IRDye800 (22–25). This multicolor capacity in the 500- to 900-nm visible-to-NIR spectral window allowed simultaneous detection of the IgG, IgM, and IgA antibody subtypes in the same sample with high signal-to-noise ratios and a broad ∼4.5-log dynamic range of the signals for all three colors and antibody subtypes. Since IgA and IgM were the less abundant antibody subtypes in human serum, we particularly assigned IRDye800 and IRDye680 with 50- to 100-fold fluorescence signal-to-noise ratio enhancement to accurately report their signals. The visible Cy3 label was used to detect the more abundant IgG antibodies in human serum and blood samples, with a signal-to-noise ratio enhancement of ∼3- to 5-fold over conventional substrates (25).
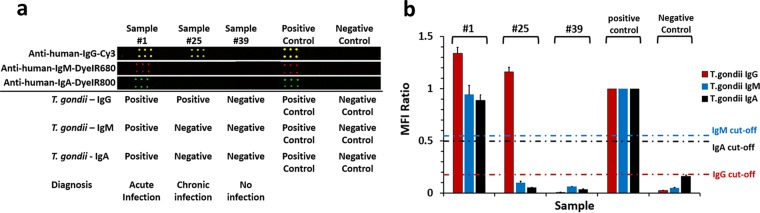
Multiplexed detection of T. gondii antibodies from ultrasmall volumes of human serum.
We first tested a small set of 56 patient serum samples (∼1-μl sample volume) to evaluate the capability of our multicolor T. gondii antibody assay on the plasmonic gold platform and to establish positive and negative cutoff values for each of the three antibody subtypes. Indeed, in a patient's serum sample previously confirmed to be positive for Toxoplasma IgG, IgM, and IgA antibodies at the PAMF-TSL, we observed high positive Cy3–anti-human IgG, IRDye680–anti-human IgM, and IRDye800–anti-human IgA signal levels on the printed antigen spots (Fig. 2a, patient 1). Using this multicolor detection scheme, we were able to clearly identify and quantify the signals for T. gondii IgG, IgM, and IgA in each sample (Fig. 2). Assay results obtained with the plasmonic gold platform successfully identified patients without T. gondii infection showing very low signal levels in all antibody subtypes (e.g., sample 39 in Fig. 2a), patients with suspected acute infection who tested positive for IgG, IgM, and IgA (e.g., sample 1 in Fig. 2a), and patients with chronic infection who tested positive for IgG and negative for IgM and IgA (sample 25 in Fig. 2a). The fluorescence signals of the three subtypes of antibodies in each of the 56 samples were quantified and normalized to the IgG, IgM, and IgA MFI signals of a positive-control sample (Fig. 2b). The resulting signals (as normalized ratios) were analyzed, and cutoff values for the three subtypes were selected (IgG, 0.18; IgM, 0.55; IgA, 0.5) such that the multiplexed plasmonic gold assay could best differentiate positive and negative samples (Fig. 2b) and match the diagnostic results of the IgG dye test and the IgM and IgA ELISA from the PAMF-TSL. These cutoffs were then used in the analysis of test results from a larger set of patient samples and were proven to be suitable for the determination of T. gondii IgG-, IgM-, and IgA-positive or -negative status.
FIG 2.
Fluorescence mapping result (a) and signal quantification on plasmonic gold chips (b) for simultaneous detection of all three subtypes of T. gondii-specific antibodies in typical serum samples selected from the population of 168 patients with acute (patient 1) or chronic (patient 25) infection or no infection (patient 39) and in positive- and negative-control samples. The error bars represent errors among signals of the triplicate spots for each antigen shown in panel a.
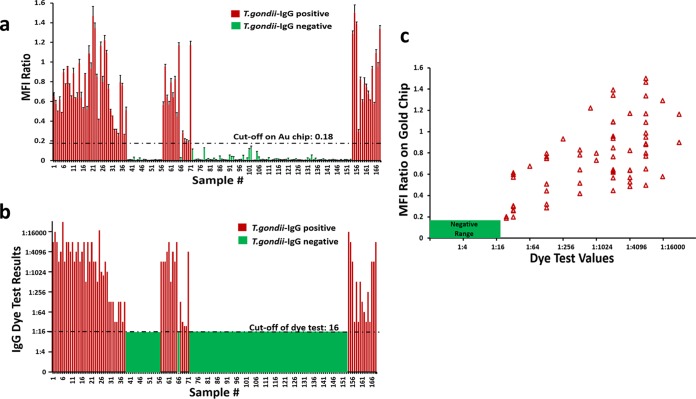
Plasmonic gold chip and IgG dye tests have the same sensitivity and specificity.
We tested a larger set of 168 patient serum samples on plasmonic gold chips and measured T. gondii-specific IgG, IgM, and IgA antibodies simultaneously by using only 1 μl of each sample. The results of the IgG dye test, IgM ELISA, and IgA ELISA from the PAMF-TSL were used as gold standards for comparison and validation of those obtained with the plasmonic gold platform. Since IgG is the most important indicator of T. gondii infection, quantitative fluorescence signals corresponding to Toxoplasma IgG of all 168 patients measured on gold chips were plotted on the same graph (Fig. 3a) with the corresponding dye test results (Fig. 3b). Of the 168 serum samples tested, 103 had negative dye test titers of <1:16 and 65 had positive dye test titers of ≥1:16. We found that the plasmonic gold chip results displayed perfect correlation with the dye test results obtained with the same 65 samples detected as positive for T. gondii IgG and the rest of the test population with negative IgG results (Fig. 3c). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of IgG detection by gold chip were all 100% for the 168 patients tested (Table 1). Interestingly, the plasmonic gold platform was able to detect even the very low IgG dye test results of 23 serum samples with titers between 1:24 and 1:512. Moreover, the simultaneous detection of IgM and IgA on gold chips also had excellent sensitivity (97.6 and 90.9% for IgM and IgA, respectively) and specificity (96.9 and 95.2% for IgM and IgA, respectively) (Table 1).
FIG 3.
(a) Signal quantification (bar chart) on plasmonic gold chips for T. gondii-specific IgG antibody detection in 168 samples. (b) Dye test results for T. gondii-specific IgG antibody detection in the same 168 samples. (c) Significant correlation between the results of T. gondii-specific IgG detection on plasmonic gold chips and by dye test. The R2 and P values of this correlation are 0.2 and 0.003, respectively, indicating a significant positive relationship between the IgG MFI ratio on gold chips and dye test values.
TABLE 1.
Comparison of IgG dye test, IgM ELISA, and IgA ELISA results from the PAMF-TSL with test results obtained with 168 serum samples on plasmonic gold chips
| Ig subtype tested on Au chips and result | No. of serum samples tested in TSL |
Au chip % sensitivity | Au chip % specificity | Au chip % agreement | % K coefficient | Au chip % PPV | Au chip % NPV | |
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
| IgG | ||||||||
| Positive | 65 | 0 | 100 (93–100)a | 100 (95.5–100) | 100 (97.2–100) | 1 (1–1) | 100 (93–100) | 100 (95.5–100) |
| Negative | 0 | 103 | ||||||
| IgM | ||||||||
| Positive | 40 | 4 | 97.6 (85.6–99.9) | 96.9 (91.6–99) | 97 (92.8–98.9) | 0.92 (0.85–0.99) | 90.9 (77.4–97) | 99.2 (94.9–100) |
| Negative | 1 | 123 | ||||||
| IgA | ||||||||
| Positive | 20 | 7 | 90.9 (69.4–98.4) | 95.2 (90–97.9) | 94.6 (89.7–97.4) | 0.78 (0.65–0.92) | 74.1 (53.4–88.1) | 98.6 (94.4–99.8) |
| Negative | 2 | 139 | ||||||
Values in parentheses are 95% confidence intervals.
We tested all of the 168 patient samples for IgG, IgM, and IgA against T. gondii antigen in three independent experiments on various days and on different gold chips and obtained excellent reproducibility in the sensitivity and specificity of the multiplex, multicolor assay on the plasmonic gold chips, and the measurement coefficient of variation was ∼15%. We also used our chosen toxoplasmosis antigens to test for antibodies to other infectious disease pathogens such as cytomegalovirus, rubella virus, and herpesvirus, and we did not observe any cross-reactivity issues, indicating a high specificity of our chosen toxoplasmosis antigens for T. gondii infection.
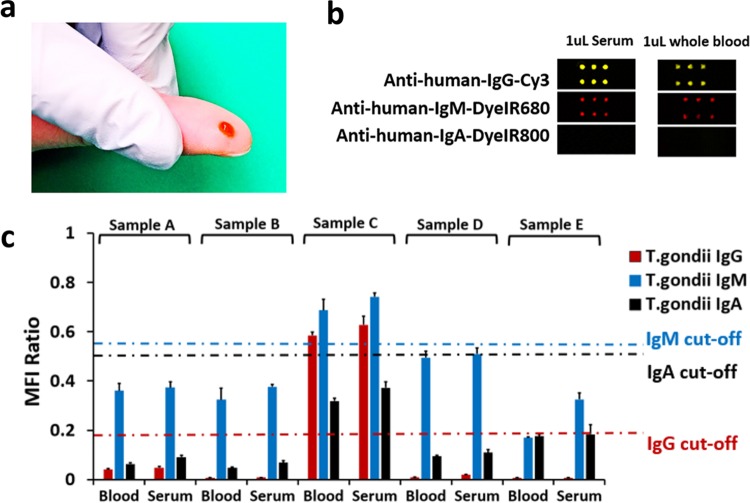
Multiplexed detection of T. gondii antibodies in ultrasmall volumes of whole blood.
The plasmonic-gold-based assay was also promising in detecting T. gondii IgG, IgM, and IgA antibodies in ultrasmall volumes of ∼1 μl of human whole blood (Fig. 4a). The results of antibody detection in whole-blood samples were highly consistent with those obtained by using serum samples from the same patient (Fig. 4b and c). There was also an excellent correlation between the whole-blood test results and IgG dye test and IgM and IgA ELISA data from the PAMF-TSL, as only sample C tested positive for both IgG and IgM. The whole-blood assay results need to be further confirmed with a larger sample set.
FIG 4.
(a) An ultrasmall volume of whole blood (e.g., a blood sample obtained by finger pricking) is needed to detect all three T. gondii-specific antibodies. (b) Fluorescence mapping results comparing signals on plasmonic gold chips tested with whole-blood and serum samples from a typical patient positive for T. gondii IgG and IgM antibodies. (c) Quantification of signals (bar chart) and complete match of the serum and whole-blood test results of five independent patients (A to E). The blood test results of the five samples on plasmonic gold chip reached 100% sensitivity, specificity, and agreement with the serum test results from the PAMF-TSL.
DISCUSSION
The annual global burden of congenital toxoplasmosis has been estimated at ∼190,000 cases, corresponding to an incidence of 1.5 newborns per 1,000 live births (29). In congenitally infected infants and immunocompromised patients, T. gondii can be responsible for severe ocular and neurological complications (3). Thus, there is a real need to integrate T. gondii screening into public health policies and services worldwide (29). However, because of the current complexity and price of the assays proposed, this screening has not yet been implemented worldwide, including the United States (30). In our study, we have demonstrated that fluorescence-enhancing plasmonic gold substrates can facilitate highly sensitive multiplexed tests for detecting T. gondii antibody subtypes in a drop of serum or whole blood. We achieved the simultaneous detection of three subtypes of human antibodies (IgG, IgM, and IgA) to T. gondii in ultrasmall sample volumes by using three fluorophores in the visible and NIR regions with nonoverlapping emission spectra. Fluorescence signal enhancement enabled three-color antibody tests with a high signal-to-noise ratio and broad signal dynamic range for detecting the three antibody subtypes with three different fluorophores. The relatively low abundance of IgA and IgM was detected with high sensitivity by choosing the fluorophores with the highest fluorescence enhancement on the plasmonic gold platform. Thus, we established that the plasmonic gold platform is a highly sensitive, multiplexed, and multicolor approach to the detection of multiple antibodies and antibody subtypes in a minute amount of sample, including serum and whole blood.
Currently, the detection of all three immunoglobulin isotypes of T. gondii is done mostly by carrying out three individual tests that require much larger sample amounts. This was the first time a multicolor microarray on plasmonic gold chips was used for infectious disease detection and diagnosis. Using this multicolor detection scheme, we were able to differentiate patients with suspected acute infections from those with chronic infections with sensitivity and specificity matching those of well-established assays performed in the National Reference Laboratory for toxoplasmosis in the United States (PAMF-TSL). This opens up the opportunity for the plasmonic gold platform to be used as new research tool, potentially replacing the current diagnostic tests for T. gondii and enable mass screening in the United States and other regions or countries.
The results of IgG detection on the plasmonic gold platform perfectly matched the results of the IgG dye test, which is considered the reference method for IgG detection (9, 12, 31). In addition to the multiplex capacity and the small sample volume, this perfect correlation represents an important advantage as it will allow us to have a test with results equivalent to those of the IgG dye test without all of the inconvenience of its complex implementation and interpretation (7, 8). Comparing the results of IgM detection on the plasmonic gold platform to those of the PAMF-TSL IgM ELISA, we reached an agreement of 97%, which is very good compared to the assays performed in nonreference laboratories in the United States (17). A recent study found that only 49% of the IgM-positive samples tested in nonreference laboratories were determined to be positive at the PAMF-TSL, suggesting that the T. gondii IgM tests in nonreference laboratories were possibly inferior to the plasmonic gold IgM test with ∼97% agreement with the PAMF-TSL result (17). Moreover, the IgM PPV of the plasmonic gold platform was 90.9%, which is much higher than that found in some commercial laboratories with a PPV of 6% (32). To date, there is no reference method for IgM detection and the commercially available assays have different sensitivities and specificities according to comparisons with each other. In our study, we obtained high sensitivity and specificity and also good PPV and NPV compared to the PAMF-TSL IgM ELISA.
IgA detection gives additional information about a patient's T. gondii serological status. IgA positivity associated with IgG and IgM is in favor of acute infection. IgA test results seem to be less sensitive than IgM test results but give a higher specificity and PPV for the diagnosis of acute infection (13, 33, 34). Moreover, IgA detection is important for the diagnosis of congenital toxoplasmosis and could also be useful in immunocompromised patients in the case of reactivation (8, 19, 21, 27, 33). In our study, we obtained very good sensitivity and specificity of IgA test results (90.9 and 95.2%, respectively). Thus, with the simultaneous detection of three subtypes of antibodies, the plasmonic gold platform can be used to perform Toxoplasma serology screening and also, in the case of IgG, IgM, and IgA positivity, could give additional information regarding the likelihood of acute infection.
In addition to the advantages of the multicolor assay for the detection of various subclasses of antibodies, the plasmonic gold chip platform offers other properties superior to those of current diagnostic methods such as the ability to use ultrasmall human sample volumes for assay processing. The IgG dye test and IgM and IgA ELISAs require several milliliters of serum, making it necessary for patients to get venous blood draws, and then, depending on the need for confirmatory testing, the blood samples will be transported among various laboratory facilities. In contrast, the plasmonic gold platform can use ultrasmall volumes of samples, including whole blood, which could enable the use of samples obtained by finger pricking for point-of-care diagnostics and thus entirely alter the current approach.
To date, the sole commercial multiplexed test uses the BioPlex 2200 system (Bio-Rad), which is based on Luminex technology, but a single run cannot detect, as our assay does, all three subtypes (IgG, IgM, and IgA) of antibodies (35). The volume of serum required is at least 50 μl, and the high cost of this technology is unsuited for mass screening. Indeed, among the main stumbling blocks to the implementation of universal screening of pregnant women for toxoplasmosis and other infectious diseases are the fragmentation and high cost of existing assays (30). On the other hand, according to Stillwaggon et al., universal screening is a cost-saving strategy even in countries with a low incidence of congenital toxoplasmosis, including the United States (30). With plasmonic gold chips, T. gondii IgG, IgM, and IgA tests can be completed within 2 h at $10 per patient. This is due to the very small quantities of reagents required in addition to the high multiplexing capacity of this nanotechnology-based platform. Thus, the plasmonic gold platform has the advantages of high affordability; high assay performance, matching that of the well-established but fragmented tests in the reference laboratories; and ease of implementation in any diagnostic laboratory facility. Likewise, the platform developed is not only a screening test but also, with the multiplexed (IgG, IgM, and IgA) capacity and high correlation with the IgG dye test and IgM and IgA ELISA, less confirmatory testing would be necessary, which could make the plasmonic gold assay an effective and cost-saving diagnostic test.
The plasmonic gold platform can also be easily upgraded for multiplexed detection of the serology of several infectious diseases combined such as the ToRCH panel (T. gondii, herpesvirus, cytomegalovirus, and rubella virus) plus HIV and hepatitis B virus. Antibodies to these major pathogens and their IgG, IgM, and IgA subtypes can be simultaneously detected in a single droplet of serum or whole blood, with high test performance matching that of gold-standard individual tests based on large sample volumes. Such a capability will offer new opportunities for mass detection serology allowing the screening of patients at an affordable cost.
ACKNOWLEDGMENTS
C. Pomares thanks the Philippe Foundation, the Bourse BPCA et Association les Amis de la Faculté de Médecine de Nice, and the Association Recherche et Développement en Pathologie Infectieuse et Tropicale. X. Li thanks Bo Zhang of the Stanford Chemistry department for helpful advice.
Footnotes
For a commentary on this article, see doi:10.1128/JCM.00913-16.
REFERENCES
- 1.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Montoya JG, Remington JS. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 47:554–566. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 3.Robert-Gangneux F, Dardé M-L. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prusa AR, Kasper DC, Pollak A, Olischar M, Gleiss A, Hayde M. 2015. Amniocentesis for the detection of congenital toxoplasmosis: results from the nationwide Austrian prenatal screening program. Clin Microbiol Infect 21:191.e1-8. doi: 10.1016/j.cmi.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. 2009. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- 6.Paquet C, Yudin M. 2013. Toxoplasmosis in pregnancy: prevention, screening and treatment. J Obstet Gynaecol Can 35:78–81. (In English and French.) doi: 10.1016/S1701-2163(15)31053-7. [DOI] [PubMed] [Google Scholar]
- 7.Sabin AB, Feldman HA. 1948. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoon parasite (Toxoplasma). Science 108:660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- 8.Murat J-B, Hidalgo HF, Brenier-Pinchart M-P, Pelloux H. 2013. Human toxoplasmosis: which biological diagnostic tests are best suited to which clinical situations? Expert Rev Anti Infect Ther 11:943–956. doi: 10.1586/14787210.2013.825441. [DOI] [PubMed] [Google Scholar]
- 9.Prusa A-R, Hayde M, Unterasinger L, Pollak A, Herkner KR, Kasper DC. 2010. Evaluation of the Roche Elecsys Toxo IgG and IgM electrochemiluminescence immunoassay for the detection of gestational Toxoplasma infection. Diagn Microbiol Infect Dis 68:352–357. doi: 10.1016/j.diagmicrobio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Sickinger E, Gay-Andrieu F, Jonas G, Schultess J, Stieler M, Smith D, Hausmann M, Stricker R, Stricker R, Dhein J, Braun H-B. 2008. Performance characteristics of the new ARCHITECT Toxo IgG and Toxo IgG Avidity assays. Diagn Microbiol Infect Dis 62:235–244. doi: 10.1016/j.diagmicrobio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Petersen E, Borobio MV, Guy E, Liesenfeld O, Meroni V, Naessens A, Spranzi E, Thulliez P. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J Clin Microbiol 43:1570–1574. doi: 10.1128/JCM.43.4.1570-1574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gay-Andrieu F, Fricker-Hidalgo H, Sickinger E, Espern A, Brenier-Pinchart M-P, Braun H-B, Pelloux H. 2009. Comparative evaluation of the ARCHITECT Toxo IgG, IgM, and IgG Avidity assays for anti-Toxoplasma antibody detection in pregnant women sera. Diagn Microbiol Infect Dis 65:279–287. doi: 10.1016/j.diagmicrobio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Kodym P, Machala L, Rohácová H, Sirocká B, Malý M. 2007. Evaluation of a commercial IgE ELISA in comparison with IgA and IgM ELISAs, IgG avidity assay and complement fixation for the diagnosis of acute toxoplasmosis. Clin Microbiol Infect 13:40–47. doi: 10.1111/j.1469-0691.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- 14.Murat J-B, Dard C, Fricker Hidalgo H, Dardé M-L, Brenier-Pinchart M-P, Pelloux H. 2013. Comparison of the Vidas system and two recent fully automated assays for diagnosis and follow-up of toxoplasmosis in pregnant women and newborns. Clin Vaccine Immunol 20:1203–1212. doi: 10.1128/CVI.00089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naot Y, Remington JS. 1980. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J Infect Dis 142:757–766. doi: 10.1093/infdis/142.5.757. [DOI] [PubMed] [Google Scholar]
- 16.Nascimento FS, Suzuki LA, Rossi CL. 2008. Assessment of the value of detecting specific IgA antibodies for the diagnosis of a recently acquired primary Toxoplasma infection. Prenat Diagn 28:749–752. doi: 10.1002/pd.2052. [DOI] [PubMed] [Google Scholar]
- 17.Dhakal R, Gajurel K, Pomares C, Talucod J, Press CJ, Montoya JG. 2015. Significance of a positive Toxoplasma immunoglobulin M test result in the United States. J Clin Microbiol 53:3601–3605. doi: 10.1128/JCM.01663-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert RE, Thalib L, Tan HK, Paul M, Wallon M, Petersen E, European Multicentre Study on Congenital Toxoplasmosis. 2007. Screening for congenital toxoplasmosis: accuracy of immunoglobulin M and immunoglobulin A tests after birth. J Med Screen 14:8–13. doi: 10.1258/096914107780154440. [DOI] [PubMed] [Google Scholar]
- 19.Pinon JM, Foudrinier F, Mougeot G, Marx C, Aubert D, Toupance O, Niel G, Danis M, Camerlynck P, Remy G. 1995. Evaluation of risk and diagnostic value of quantitative assays for anti-Toxoplasma gondii immunoglobulin A (IgA), IgE, and IgM and analytical study of specific IgG in immunodeficient patients. J Clin Microbiol 33:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Press C, Montoya JG, Remington JS. 2005. Use of a single serum sample for diagnosis of acute toxoplasmosis in pregnant women and other adults. J Clin Microbiol 43:3481–3483. doi: 10.1128/JCM.43.7.3481-3483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. 2011. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J 30:1056–1061. doi: 10.1097/INF.0b013e3182343096. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Kumar RB, Dai H, Feldman BJ. 2014. A plasmonic chip for biomarker discovery and diagnosis of type 1 diabetes. Nat Med 20:948–953. doi: 10.1038/nm.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabakman SM, Lau L, Robinson JT, Price J, Sherlock SP, Wang H, Zhang B, Chen Z, Tangsombatvisit S, Jarrell JA, Utz PJ, Dai H. 2011. Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range. Nat Commun 2:466. doi: 10.1038/ncomms1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Yang J, Zou Y, Gong M, Chen H, Hong G, Antaris AL, Li X, Liu C-L, Chen C, Dai H. 2014. Plasmonic micro-beads for fluorescence enhanced, multiplexed protein detection with flow cytometry. Chem Sci 5:4070–4075. doi: 10.1039/C4SC01206B. [DOI] [Google Scholar]
- 25.Koh B, Li X, Zhang B, Yuan B, Lin Y, Antaris AL, Wan H, Gong M, Yang J, Zhang X, Liang Y, Dai H. 2016. Visible to near-infrared fluorescence enhanced cellular imaging on plasmonic gold chips. Small 12:457–465. doi: 10.1002/smll.201502182. [DOI] [PubMed] [Google Scholar]
- 26.Desmonts G, Remington JS. 1980. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol 11:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepick-Biek P, Thulliez P, Araujo FG, Remington JS. 1990. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis 162:270–273. doi: 10.1093/infdis/162.1.270. [DOI] [PubMed] [Google Scholar]
- 28.Viera AJ, Garrett JM. 2005. Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363. [PubMed] [Google Scholar]
- 29.Torgerson PR, Mastroiacovo P. 2013. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ 91:501–508. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stillwaggon E, Carrier CS, Sautter M, McLeod R. 2011. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis 5:e1333. doi: 10.1371/journal.pntd.0001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiter-Owona I, Petersen E, Joynson D, Aspöck H, Dardé ML, Disko R, Dreazen O, Dumon H, Grillo R, Gross U, Hayde M, Holliman R, Ho-Yen DO, Janitschke K, Jenum PA, Naser K, Olszewski M, Thulliez P, Seitz HM. 1999. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull World Health Organ 77:929–935. [PMC free article] [PubMed] [Google Scholar]
- 32.Garry DJ, Elimian A, Wiencek V, Baker DA. 2005. Commercial laboratory IgM testing for Toxoplasma gondii in pregnancy: a 20-year experience. Infect Dis Obstet Gynecol 13:151–153. doi: 10.1080/10647440500148024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel B, Young Y, Duffy K, Tanner RP, Johnson J, Holliman RE. 1993. Immunoglobulin-A detection and the investigation of clinical toxoplasmosis. J Med Microbiol 38:286–292. doi: 10.1099/00222615-38-4-286. [DOI] [PubMed] [Google Scholar]
- 34.Arcavi M, Orfus G, Griemberg G. 1997. Diagnosis of toxoplasmosis by joint detection of immunoglobulin A and immunoglobulin M. J Clin Microbiol 35:1450–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guigue N, Menotti J, Hamane S, Derouin F, Garin YJ-F. 2014. Performance of the BioPlex 2200 flow immunoassay in critical cases of serodiagnosis of toxoplasmosis. Clin Vaccine Immunol 21:496–500. doi: 10.1128/CVI.00624-13. [DOI] [PMC free article] [PubMed] [Google Scholar]