Abstract
The use of nucleic acid detection for HIV type 1 (HIV-1) detection is strongly recommended in infants <18 months of age, in whom serology is unreliable. This study evaluated the Cobas AmpliPrep/Cobas TaqMan HIV-1 Qualitative Test v2.0 (TaqMan HIV-1 Qual Test, v2.0), a dual-target total nucleic acid real-time PCR assay. The limit of detection (LOD) of the new test in plasma and dried blood spots (DBS) was determined with the 2nd International HIV-1 RNA WHO standard. The specificity of the assay was tested with EDTA plasma (n = 1,301) and DBS from HIV-negative adults (n = 1,000). The sensitivity was determined using HIV-1-positive samples (n = 169 adult EDTA plasma, n = 172 adult DBS, and n = 100 infant DBS) that included group M, subtypes A to H, CRF01_AE, CRF02_AG, and groups O and N. All positive specimens and a subset of the negative specimens were also tested with the Abbott RealTime HIV-1 Qual assay (RealTime). The LOD of the TaqMan assay was 20 copies/ml in plasma and 300 copies/ml in DBS, with specificities of 99.8% in plasma and 99.9% in DBS. The TaqMan assay results were 100% concordant with RealTime results in EDTA plasma samples and in 100 HIV-1-negative adult DBS. Among 172 HIV-1-positive DBS from adults, the TaqMan assay showed positive results for all DBS while RealTime missed five DBS with low target concentrations. Infant DBS results were 100% concordant. The improved sensitivity of the Cobas AmpliPrep/Cobas TaqMan HIV-1 Qualitative Test, v2.0, compared to current commercially available assays may enable earlier diagnosis and treatment in adults and infants. The dual-target test may ensure HIV-1 detection even if a mutation is present in one of the two target regions. The DBS sample matrix facilitates virological testing in remote areas.
INTRODUCTION
Globally, 35.3 million people were living with HIV infection at the end of 2012 (1). Sub-Saharan Africa remains the most severely affected region, with approximately 1 in 20 adults infected with HIV. This accounts for 71% of the people living with HIV worldwide (1). Although access to early infant diagnosis of HIV is improving, in 2011 only 35% of infants born to HIV-positive mothers were tested within 2 months of birth (1). The Joint United Nations Programme on HIV/AIDS (UNAIDS) call for action has set new targets for 2020, the first of which is for 90% of those infected with HIV to know their status. Improved assays like the one evaluated in this study should assist in increasing access to HIV diagnosis, particularly in a vulnerable pediatric population that requires nucleic acid-based testing (2). Transmission of HIV occurs through sexual contact, exposure to infected blood products (3), and vertical mother-to-child transmission (4). For many infected infants, the progression of AIDS is rapid, and thus, early antiretroviral therapy is crucial for survival. In a randomized, controlled clinical trial in South Africa, it was demonstrated that early HIV diagnosis and early antiretroviral therapy could reduce early infant mortality by 76% and slow HIV progression by 75% (5). Access to HIV testing and early confirmation of a pediatric infection are thus critical for immediate access to treatment and care programs. Nucleic acid testing is particularly important for early diagnosis of infants ≤18 months of age. In this population, serologic tests are unreliable for the identification of true virologic status, because passively transferred maternal HIV type 1 (HIV-1) antibodies may be detectable in the HIV-exposed infant for up to 18 months of life (6) or longer in some instances (7). Thus, the World Health Organization (WHO) strongly recommends that HIV virological testing be used to diagnose HIV infections in infants and children below 18 months of age (8). The regions that are most severely affected by the HIV epidemic, such as sub-Saharan Africa, are often faced with severe resource constraints limiting access to virological testing. The difficulties of cold-chain transfer of collected blood samples from remote areas to centralized laboratories and the lack of technicians with phlebotomy skills remain significant challenges, while the costs of laboratory equipment and the need for skilled technicians further limit the availability of routine testing. The use of dried whole-blood spots (DBS) addresses the sample collection and transport challenges. The Cobas AmpliPrep/Cobas TaqMan HIV-1 Qualitative Test v2.0 (TaqMan HIV-1 Qual Test, v2.0) is an in vitro diagnostic (IVD), total nucleic acid amplification test for the qualitative detection of HIV-1 DNA and RNA (or total nucleic acid [TNA]) in human plasma or DBS using automated specimen processing followed by automated amplification and detection. The assay is indicated for individuals (adults or children) who are suspected to be actively infected with HIV-1. Detection of HIV-1 TNA is indicative of infection with HIV-1. In adults, the test may be used as an aid in the diagnosis of HIV-1 infection (9). The TaqMan HIV-1 Qualitative Test, v2.0, was developed based on the original version (version 1.0) and shares many aspects of the sample preparation and the general reagent complement used in the quantitative TaqMan HIV-1 Test, v2.0. This report presents an assessment of the TaqMan HIV-1 Qual Test, v2.0, using the 2nd International HIV-1 RNA WHO standard, NIBSC code 97/65041, HIV-1 subtype B, and clinical specimens, including group M, subtypes A H, CRF01_AE, group O, and group N. This new assay demonstrates improved performance in both plasma and dried blood spots.
MATERIALS AND METHODS
Qualitative assays.
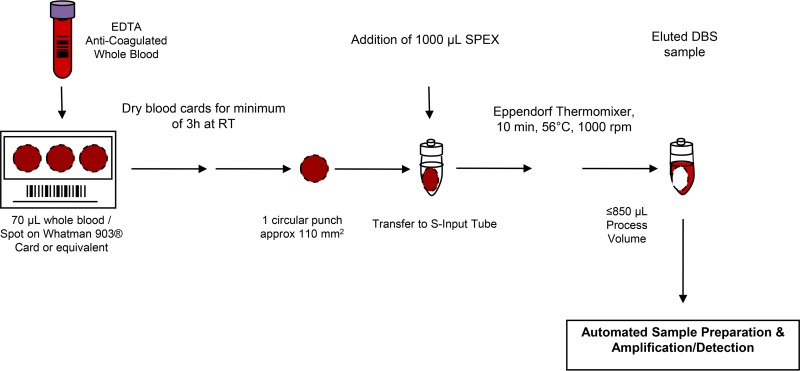
The Abbott RealTime HIV-1 Qual assay (Abbott Molecular, Des Plaines, IL, USA) and the TaqMan HIV-1 Qual Test v2.0 (Roche Diagnostics, Indianapolis, IN, USA) were performed according to the manufacturer's instructions. The preanalytical steps in the DBS workflow for the TaqMan HIV-1 Qual Test v2.0 are outlined in Fig. 1. Both assays are standardized to the World Health Organization International Standard (WHO-IS) for HIV-1 RNA (2nd International HIV-1 RNA WHO standard, NIBSC code 97/65041, HIV-1 subtype B).
FIG 1.
Overview of Cobas AmpliPrep/Cobas TaqMan HIV-1 Qualitative Test, v2.0, and workflow preanalytics of DBS. RT, room temperature.
Changes made within the TaqMan HIV-1 Qual Test, v2.0.
Key changes to improve assay sensitivity include an improved sample preparation method and reagents from the TaqMan HIV-1 v2.0 Quant assay, using the dual-target assay design. Among the modifications included to improve the assay specificity were chemical modifications of the primer 3′ ends and an optimized software algorithm to assess cycle threshold (CT), cutoff criteria, and minimum relative fluorescence intensity (RFI min) (10, 11).
Sample collection and preparation.
At all study sites (Luxembourg Institute of Health, Luxembourg; the University of the Witwatersrand, Wits Medical School Virology Laboratory, Johannesburg, South Africa; and Roche Diagnostics International AG, Rotkreuz, Switzerland), fresh whole blood was collected in EDTA tubes for the analytical and clinical studies. DBS were prepared from whole-blood samples within 4 days of the blood draw. Whole blood (70 μl) was spotted onto demarcated circles on a DBS Whatman 903 Neonatal STD EU card (GE Healthcare, Westborough, MA, USA) or on Munktell TFN specimen collection cards (Munktell Filter AB, Falun, Sweden) (preperforated). The DBS cards were dried for at least 3 h and stored at room temperature in individual Ziplock bags containing a desiccant for up to 4 months before analysis. Within 6 h of collection, EDTA plasma was obtained following separation by centrifugation and then stored at −80°C. In South Africa, the specimen analysis was conducted per ethics clearance certificate M121151 from the University of the Witwatersrand Human Ethics Committee, Johannesburg, South Africa, as well as ethics reference no. 257/2013 from the University of Tshwane, Faculty of Health Sciences Research Ethics Committee, Pretoria, South Africa.
LOD of the TaqMan HIV-1 Qual Test v2.0 assay.
Limit-of-detection (LOD) comparisons conducted with an EDTA plasma single target (HIV-1 gag gene) as used in TaqMan HIV-1 Qualitative Test, v1.0, and with dual targets (HIV-1 gag gene and long terminal repeat [LTR] region) as used in TaqMan HIV-1 Qualitative Test, v2.0, have been performed at five concentration levels (100, 50, 25, 12.5, 6.3, and 3.1 copies [cp]/ml) using the 1st International HIV-1 RNA WHO standard, NIBSC code 97/656, HIV-1 subtype B, diluted in HIV-1-negative human EDTA plasma. One copy of HIV-1 RNA is equivalent to 1.7 ± 0.1 IU. Probit (probability logistic regression) analysis was performed in a research test that is similar but not identical to the IVD test used in the remainder of this study.
The limit of detection of the TaqMan HIV-1 Qual Test, v2.0, was determined by testing the 2nd International HIV-1 RNA WHO standard, NIBSC code 97/65041, HIV-1 subtype B, diluted in HIV-1-negative human EDTA plasma or whole blood for DBS. One copy of HIV-1 RNA is equivalent to 1.7 ± 0.1 IU. The limit of detection was determined using three different reagent lots. Three dilution series were analyzed for each reagent lot. A total of approximately 126 replicates per concentration level were tested for EDTA plasma, and a total of approximately 189 replicates per concentration level were tested for DBS.
Specificity of the TaqMan HIV-1 Qual Test, v2.0.
The specificity of the TaqMan HIV-1 Qual Test, v2.0, was determined with two reagent lots by analysis of HIV-1-negative EDTA plasma and DBS specimens from routine blood donors. A total of 1,301 individual EDTA negative plasma specimens and 1,000 negative DBS were tested. Discrepant samples were retested in duplicate and resolved using hemi-nested PCR (hnPCR) (for method description, see below).
Method of correlation of the performance of TaqMan HIV-1 Qual Test v2.0 to that of Abbott RealTime HIV-1 Qual assay in EDTA plasma.
The performance of the TaqMan HIV-1 Qual Test, v2.0, was compared to that of the Abbott RealTime HIV-1 Qual assay by analyzing 169 undiluted confirmed HIV-1-positive and 100 confirmed HIV-1-negative clinical EDTA plasma specimens from adults. The specimen subtypes included HIV-1 group M subtypes A to H and were analyzed at the external site in Luxembourg. A total of 169 positive samples that ranged between 1.0E + 02 and 5.0E + 05 copies per ml (cp/ml), including samples with contents that were close to the limit of detection of the TaqMan HIV-1 Qual Test, v2.0, were evaluated.
Method of correlation of the performance of TaqMan HIV-1 Qual Test v2.0 to that of Abbott RealTime HIV-1 Qual assay in DBS.
The performance of the TaqMan HIV-1 Qual Test, v2.0, was compared to that of the Abbott RealTime HIV-1 Qual assay by analysis of a total of 172 undiluted, confirmed HIV-1-positive clinical DBS specimens from adult blood donors; 99 prospectively collected, undiluted HIV-1-positive clinical early infant (age ≤18 months) DBS specimens; 100 DBS specimens from healthy HIV-1-negative adult donors; and 201 specimens from HIV-1-negative infants (age, ≤18 months). The HIV-1-positive specimens comprised HIV-1 group M subtypes A to H, group O and N (group O and N for adult DBS only), and were analyzed in Luxembourg.
hnPCR.
Hemi-nested PCR (hnPCR) analysis was applied to confirm a positive test result as an HIV-1-specific reaction. The analysis consists of amplification of an aliquot of the primary amplicon with two master mix (MMx) amplification primer sets. The amplification mixes did not contain Uracil-N glycosylase (UNG), as this would have destroyed any potential PCR product. In the first PCR, using MMx set 1, only one region of the complete target amplicon is amplified using the original forward primer and a reverse primer, based on the sequence of the probe. If the result obtained with this MMx set is positive, giving a PCR product of the same base pair length as that seen with a confirmed positive sample, the possibility remains that the initial reaction was not HIV-1 specific. To confirm the positive result as HIV-1 specific, the primary PCR product is amplified in a second amplification reaction with MMx set 2 using the original reverse primer and a forward primer, based on the sequence of the probe. If the result obtained is also positive, this acts as confirmation that the target sequence was present in the primary PCR sample, ratifying that the reaction was HIV-1 specific. For each viral target, two different MMx sets are used, and HIV-1 is tested with 4 different MMx sets (HIV-1M LTR set 1 and set 2 and HIV-1M GAG set 1 and set 2).
RESULTS
Effect of dual target on limit of detection.
Adding a second target per viral genome increased the sensitivity of the assay. This was tested by comparing the limit of detection of a single-target viral load test to that of a dual-target viral load test, using Probit analysis (Table 1). The single-target assay reached a limit of detection of 62.9 cp/ml (95% confidence range, 37.7 to 160.6 cp/ml), whereas the dual-target “research” assay reached a limit of detection of 22.8 cp/ml (95% confidence range, 16.5 to 39.1).
TABLE 1.
Limit-of-detection comparison in EDTA plasma using the 1st International HIV-1 RNA WHO Standard, NIBSC code 97/656, HIV-1 subtype B, diluted in HIV-1-negative human EDTA plasmaa
| Nominal input (no. of HIV-1 RNA copies/ml) | Single target (HIV-1 gag gene)b |
Dual target (HIV-1 gag gene and LTR region)c |
||||
|---|---|---|---|---|---|---|
| No. of replicates | No. of positives | Positivity rate (%) | No. of replicates | No. of positives | Positivity rate (%) | |
| 100 | 21 | 21 | 100 | 30 | 30 | 100 |
| 50 | 21 | 20 | 95.2 | 30 | 30 | 100 |
| 25 | 21 | 16 | 76.2 | 30 | 29 | 96.7 |
| 12.5 | 21 | 14 | 66.7 | 30 | 25 | 83.3 |
| 6.3 | 21 | 5 | 23.8 | 30 | 13 | 43.3 |
| 3.1 | 20 | 7 | 35 | 30 | 8 | 26.7 |
Results obtained with a single target (HIV-1 gag gene) as used in TaqMan HIV-1 Qualitative Test, v1.0, and with a dual target (HIV-1 gag gene and LTR region) as used in TaqMan HIV-1 Qualitative Test, v2.0, are compared. One copy of HIV-1 RNA is equivalent to 1.7 ± 0.1 IU.
Probit analysis showed a 95% hit rate of 62.9 copies (cp)/ml (95% confidence interval, 37.7 to 160.6 cp/ml).
Probit analysis showed a 95% hit rate of 22.8 cp/ml (95% confidence interval, 16.5 to 39.1 cp/ml).
Clinical limit of detection.
The limit of detection of the TaqMan HIV-1 Qual Test, v2.0, was determined by testing the 2nd International HIV-1 RNA WHO standard, NIBSC code 97/65041, HIV-1 subtype B, diluted in HIV-1-negative human EDTA plasma or whole blood for DBS. The evaluation was performed according to CLSI guideline EP17-A2 (12). The combined results for all three lots tested for EDTA plasma and DBS are shown in Table 2 and Table 3 and demonstrate that the TaqMan HIV-1 Qual Test, v2.0, detected HIV-1 RNA at concentrations of 16.5 cp/ml (95% confidence interval, 14.3 to 19.8 cp/ml) in EDTA plasma and 221.8 cp/ml (95% confidence interval, 195.6 to 260.5 cp/ml) in DBS, respectively, as determined by Probit analysis.
TABLE 2.
Limit of detection in EDTA plasma of the TaqMan HIV-1 Qualitative Test, v2.0, using the 2nd International HIV-1 RNA WHO Standard, NIBSC code 97/65041, HIV-1 subtype B, diluted in HIV-1-negative human EDTA plasmaa
| Nominal input (HIV-1 RNA cp/ml) | No. of replicates | No. of positives | Positivity rate (%) |
|---|---|---|---|
| 60 | 126 | 126 | 100 |
| 40 | 186 | 185 | 99 |
| 30 | 126 | 125 | 99 |
| 20 | 126 | 124 | 98 |
| 15 | 59 | 53 | 90 |
| 10 | 126 | 108 | 86 |
| 5 | 125 | 66 | 53 |
| 0 | 126 | 0 | 0 |
One copy of HIV-1 RNA is equivalent to 1.7 ± 0.1 IU. Probit analysis showed a 95% hit rate of 16.5 cp/ml (95% confidence interval, 14.3 to 19.8 cp/ml).
TABLE 3.
Limit of detection in DBS of the TaqMan HIV-1 Qualitative Test, v2.0, using the 2nd International HIV-1 RNA WHO Standard, NIBSC code 97/65041, HIV-1 subtype B, diluted in HIV-1-negative whole blood for DBSa
| Nominal input (HIV-1 RNA cp/ml) | No. of replicates | No. of positives | Positivity rate (%) |
|---|---|---|---|
| 500 | 189 | 189 | 100 |
| 400 | 187 | 185 | 99 |
| 300 | 188 | 183 | 97 |
| 150 | 188 | 166 | 88 |
| 75 | 188 | 108 | 57 |
| 0 | 188 | 0 | 0 |
One copy of HIV-1 RNA is equivalent to 1.7 ± 0.1 IU. Probit analysis showed a 95% hit rate of 221.8 cp/ml (95% confidence interval, 195.6 to 260.5 cp/ml).
Specificity.
Five of the 1,301 individual confirmed HIV-1-negative EDTA plasma specimens returned a positive HIV-1 result. All five initially positive samples were retested with the TaqMan HIV-1 Qual Test, v2.0, in duplicate and yielded negative results. In addition, all five samples were tested for a specific HIV-1 signal using hemi-nested PCR (hnPCR). Three samples were shown to be HIV-1 specific via hnPCR and were excluded for specificity analysis. Two samples could not be confirmed to be HIV-1 specific via hnPCR. After resolution of discrepant samples, a total of 1,298 EDTA plasma samples were included, achieving a specificity of 99.9% (one-sided lower 95% confidence limit, >99.5%). Three of the 1,000 individual confirmed HIV-1-negative DBS specimens yielded a positive HIV-1 result. All three initially positive samples were retested in duplicate and yielded negative results. In addition, all three samples were tested for a specific HIV-1 signal using hemi-nested PCR (hnPCR). Two samples were shown to be HIV-1 specific via hnPCR and were excluded for specificity analysis. One sample could not be confirmed to be HIV-1 specific via hnPCR. After resolution of discrepant samples, a total of 998 DBS samples were included, achieving a specificity of 99.8% (one-sided lower 95% confidence limit, >99.5%).
Method correlation of TaqMan HIV-1 Qual Test v2.0 versus Abbott RealTime HIV-1 Qual assay.
Method correlation results are summarized in Table 4 for the confirmed positive specimens and in Table 5 for the confirmed negative specimens. Correlation to the Abbott RealTime HIV-1 Qual assay in human plasma showed 100% agreement (n = 169 positive HIV-1 clinical specimens; n = 100 HIV-1-negative individual donors). Correlation with the Abbott RealTime HIV-1 Qual assay was also tested in both adult and infant DBS specimens. Using DBS from adults, an agreement of 100% in HIV-1-negative (n = 100) specimens was obtained. Of 172 confirmed HIV-1-positive DBS from adults, the new TaqMan HIV-1 Qual Test v2.0 assay showed positive results for all DBS while the Abbott RealTime HIV-1 Qual assay missed five DBS with a low target concentration. The five confirmed HIV-1-positive DBS from adults that tested positive on TaqMan HIV-1 Qual Test v2.0 and negative on the Abbott RealTime HIV-1 Qual assay could be confirmed to be positive using nested PCR (see Table 6). Testing of infant DBS specimens showed 100% concordance (n = 100 HIV-1-positive clinical specimens; n = 200 HIV-1-negative specimens from infants).
TABLE 4.
Correlation of TaqMan HIV-1 Qual Test, v2.0, to the Abbott RealTime HIV-1 Qual assay in confirmed HIV-1-positive EDTA plasma and DBS samples
| Sample category | Result for TaqMan HIV-1 Qual Test, v2.0 | No. of samples with indicated Abbott RealTime HIV-1 Qual assay result |
|
|---|---|---|---|
| Positive | Negative | ||
| Confirmed HIV-1-positive EDTA plasma (n = 169) | Positive | 169 | 0 |
| Negative | 0 | 0 | |
| Confirmed HIV-1-positive adult and infant DBS (n = 272) | Positive | 267 | 5 |
| Negative | 0 | 0 | |
TABLE 5.
Correlation of TaqMan HIV-1 Qualitative Test, v2.0, to the Abbott RealTime HIV-1 Qual assay in confirmed HIV-1-negative EDTA plasma and DBS samples
| Sample category | Result for TaqMan HIV-1 Qual Test, v2.0 | No. of samples with indicated Abbott RealTime HIV-1 Qual assay result |
|
|---|---|---|---|
| Positive | Negative | ||
| Confirmed HIV-1-negative EDTA plasma (n = 100) | Positive | 0 | 0 |
| Negative | 0 | 100 | |
| Confirmed HIV-1-negative adult and baby DBS (n = 300) | Positive | 0 | 0 |
| Negative | 0 | 300 | |
TABLE 6.
Characterization of five noncorrelating confirmed HIV-1-positive DBS samples that tested negative in the Abbott RealTime HIV-1 Qual assay
| Sample | EDTA plasma viral load (cp/ml) in TaqMan HIV-1 Test, v2.0 | Genotype | No. of positive results/total no. of tests |
|
|---|---|---|---|---|
| TaqMan HIV-1 Qual Test, v2.0 | Abbott RealTime HIV-1 Qual Assay | |||
| 1 | 1,355 | C | 3/3 | 0/3 |
| 2 | 1,364 | C | 3/3 | 1/3 |
| 3 | 1,604 | C | 3/3 | 1/3 |
| 4 | 1,648 | C | 3/3 | 1/3 |
| 5 | 1,318 | C | 3/3 | 1/3 |
Reliability of the TaqMan HIV-1 Qual Test v2.0 and Abbott RealTime assays.
The Roche assay demonstrated better run and sample reliability than the Abbott RealTime comparator assay (Table 7), where reliability was calculated as given below:
| 1 |
| 2 |
TABLE 7.
Reliability of TaqMan HIV-1 Qualitative Test, v2.0, and Abbott RealTime HIV-1 Qual assay at South African trial site
| Reliability category | % reliability (no. of valid runs or samples/total no. of runs or samples) |
|
|---|---|---|
| TaqMan HIV-1 Qual Test, v2.0 | Abbott RealTime HIV-1 Qual | |
| Runs | 97 (37/38) | 88 (30/34) |
| Samples | 94 (486/515) | 85 (486/571) |
The Roche assay requires only one spot per test, permitting multiple testing attempts using a typical DBS card that collects approximately 70 μl of whole blood. Taking the data together, use of the Roche assay will significantly reduce losses to follow-up due to testing failures. A summary of the TaqMan HIV-1 Qual Test, v1.0, TaqMan HIV-1 Qual Test, v2.0, and RealTime Qual assays is provided in Table 8.
TABLE 8.
Summary of performance data for the TaqMan HIV-1 Qualitative Test, v2.0, Qualitative Test, v1.0, and Abbott RealTime HIV-1 Qual assay for both EDTA plasma and DBS methods
| Assay | Performance claim target | Sensitivity (cp/ml) | % specificity | Target region | Subtype detection | Input vol | Calibration | Sample prepn |
|---|---|---|---|---|---|---|---|---|
| TaqMan HIV-1 Test, v2.0 | EDTA plasma | 16.5 | 99.8 (n = 1,298) | Dual target: gag gene and LTR region | Group M subtypes A, B, C, D, CRF01-AE, F, G, subtype H and group N, group O | 850 μl | No calibration at customer site required | Automated TaqMan |
| DBS | 221.8 | 99.9 (n = 998) | 1 DBS | Manual (preanalytics) followed by automated TaqMan | ||||
| TaqMan HIV-1 Test, v1.0 (RUO)a | EDTA plasma | 514 | Not determined | Single target: gag gene | Group M subtypes A to N | 100 μl | No calibration at customer site required | Automated TaqMan |
| DBS | 1,090 | 100 (n = 496) | 1 DBS | Manual (preanalytics) followed by automated TaqMan | ||||
| Abbott RealTime HIV-1 Qual | EDTA plasma | 110 | 100 (n = 550) | Single target: integrase region of polymerase gene | Group M subtypes A, B, C, D, CRF01-AE, F, CRF02-AG, G, subtype H and group N, group O | 200 μl | Lot-specific calibration at customer site required | Manual (m2000sp) |
| DBS | 2,500 | 100 (n = 550) | 2 DBS | Manual (m2000sp) |
RUO, research use only.
DISCUSSION
Sensitivity is of paramount importance for qualitative HIV PCR assays designed for diagnostic purposes both in infants and in the acute infection scenario in adults. The viral loads of HIV-infected infants may start out low at birth and increase over time (13). Therefore, a more sensitive HIV PCR test can lead to earlier, more accurate diagnosis (14), which can minimize the occurrence of potentially irreversible immune damage before therapy is initiated, improve linkage to care, and thus reduce the loss of infected infants to follow-up. Many individuals tested for HIV may have had some antiretroviral exposure, reducing their DNA and RNA viral loads. A more sensitive HIV PCR assay can help detect infections in these patients.
We have demonstrated that the TaqMan HIV-1 Qual Test version v2.0 has increased sensitivity compared to the previously determined sensitivity for the v1.0 assay (15). The Probit 95% LOD for the v2.0 test is 16.5 cp/ml in plasma samples, compared to 514 cp/ml for the v1.0 test. The LOD for a DBS sample for the v2.0 test is 221.8 cp/ml compared to 1,090 cp/ml for the v1.0 test. In infants and those with acute infection, this represents a clinically relevant improvement in performance.
The improved sensitivity is most likely attributable to an increase in the total sample volume that the assay can process. The previous test version required prelysis of plasma samples with SPEX buffer, limiting the effective sample input volume to <100 μl (15). Since this step is no longer required, 850 μl of sample volume can be used in the assay. In addition, the assay uses a dual-target approach (16), which doubles the number of effective targets per virus genome, improving the likelihood of detection. This concept was validated in a direct comparison of the sensitivity of a single-target viral load test to that of a dual-target viral load test (Table 1).
In conclusion, the new TaqMan HIV-1 Qual Test, v2.0, offers improved sensitivity over the other assays evaluated in this study (Table 8), which should facilitate the earlier diagnosis and treatment of HIV-infected infants and allow further reductions in HIV-related morbidity and mortality. Antiretroviral therapy of a longer duration, using a greater variety of antiretrovirals, is being used for both maternal and infant prophylaxis. This type of therapy would effectively lower the viral loads in infants who do become infected. Therefore, increased sensitivity is becoming increasingly important in improving diagnosis of HIV infection in infants. The reliability of the test, with the need for only one DBS per test, should result in fewer test failures that would require follow-up and repeated phlebotomy.
ACKNOWLEDGMENTS
We are grateful for the contributions of Zukiswa Mahlumba, Livhuwani Nxumalo, Samia Regaia, Jean-Yves Servais, Diana Thamke, Jan Furrer, Martin Hesse, and Suzana Simovic. We acknowledge Ahmad Haeri Mazanderani from the NHLS—University of Pretoria for the kind provision of a portion of pediatric clinical specimens. We thank Christian Simon, Lucia Hans, and Gayle Sherman for comments on the manuscript.
Funding Statement
Roche Diagnostics, Ltd., Switzerland, provided partial funding and the necessary reagents used in this study. S.P.T., B.S., and P.B. are employed by Roche. W.S. and S.C. received no specific grant from any funding agency in the public or not-for-profit sectors.
REFERENCES
- 1.UNAIDS. 2013. Global update on HIV treatment 2013: results, impact and opportunities: WHO report in partnership with UNICEF and UNAIDS. http://www.unaids.org/sites/default/files/media_asset/20130630_treatment_report_en_0.pdf.
- 2.UNAIDS. 2014. 90-90-90—an ambitious treatment target to help end the AIDS epidemic. http://www.unaids.org/en/resources/documents/2014/90-90-90.
- 3.Curran JW, Jaffe HW, Hardy AM, Morgan WM, Selik RM, Dondero TJ. 1988. Epidemiology of HIV infection and AIDS in the United States. Science 239:610–616. doi: 10.1126/science.3340847. [DOI] [PubMed] [Google Scholar]
- 4.Lehman DA, Farquhar C. 2007. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol 17:381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 5.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA; CHER Study Team . 2008. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, Bolton KD. 2005. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J 24:993–997. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez M, Ludwig DA, Khan SS, Chaparro AA, Rivera DM, Cotter AM, Scott GB. 2012. Has highly active antiretroviral therapy increased the time to seroreversion in HIV exposed but uninfected children? Clin Infect Dis 55:1255–1261. doi: 10.1093/cid/cis662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. 2010. WHO recommendations on the diagnosis of HIV infection in infants and children. http://apps.who.int/iris/bitstream/10665/44275/2/9789241599085_eng_Annexes.pdf. [PubMed]
- 9.Roche. 2013. Cobas AmpliPrep/Cobas Taqman HIV-1 Qualitative Test, version 2.0 [instruction for use]. Roche Molecular Systems Inc, Branchburg, NJ. [Google Scholar]
- 10.Maritz J, Preiser W, van Zyl GU. 2012. Establishing diagnostic cut-off criteria for the COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative test through validation against the Amplicor DNA test v1.5 for infant diagnosis using dried blood spots. J Clin Virol 53:106–109. doi: 10.1016/j.jcv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Maritz J, van Zyl GU, Preiser W. 2014. Irreproducible positive results on the Cobas AmpliPrep/Cobas TaqMan HIV-1 Qual test are different qualitatively from confirmed positive results. J Med Virol 86:82–87. doi: 10.1002/jmv.23811. [DOI] [PubMed] [Google Scholar]
- 12.CLSI. 2005. Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline— 2nd ed CLSI, Wayne, PA. [Google Scholar]
- 13.Steketee RW, Abrams EJ, Thea DM, Brown TM, Lambert G, Orloff S, Weedon J, Bamji M, Schoenbaum EE, Rapier J, Kalish ML; the New York City Perinatal HIV Transmission Collaborative Study. 1997. Early detection of perinatal human immunodeficiency virus (HIV) type 1 infection using HIV RNA amplification and detection. J Infect Dis 175:707–711. doi: 10.1093/infdis/175.3.707. [DOI] [PubMed] [Google Scholar]
- 14.Lilian RR, Kalk E, Bhowan K, Berrie L, Carmona S, Technau K, Sherman GG. 2012. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol 50:2373–2377. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens W, Erasmus L, Moloi M, Taleng T, Sarang S. 2008. Performance of a novel human immunodeficiency virus (HIV) type 1 total nucleic acid-based real-time PCR assay using whole blood and dried blood spots for diagnosis of HIV in infants. J Clin Microbiol 46:3941–3945. doi: 10.1128/JCM.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sizmann D, Glaubitz J, Simon CO, Goedel S, Buergisser P, Drogan D, Hesse M, Kroh M, Simmler P, Dewald M, Gilsdorf M, Fuerst M, Ineichen R, Kirn A, Pasche P, Wang ZJ, Weisshaar S, Young K, Haberhausen G, Babiel R. 2010. Improved HIV-1 RNA quantitation by Cobas (R) AmpliPrep/Cobas (R) TaqMan (R) HIV-1 Test, v2.0 using a novel dual-target approach. J Clin Virol 49:41–46. doi: 10.1016/j.jcv.2010.06.004. [DOI] [PubMed] [Google Scholar]