Significance
Most previous studies on intracellular signaling during neurite guidance were performed in the context of unidirectional neurite growth. They could not address the molecular basis of directional outgrowth of multiple neurites mainly because of the lack of a good model system. Using a pair of bipolar neurons in the nematode Caenorhabditis elegans, we found that distinct sets of intracellular molecules are required for neurite extension toward the anterior and the posterior. Moreover, signaling pathways that promote neurite extension in different directions antagonize each other to achieve balanced growth. Therefore, our study offers an in vivo example for a long-standing concept that spatially selective activation of intracellular signaling molecules could enable a diverse range of neuronal growth patterns.
Keywords: neurite growth, guanine nucleotide exchange factors, Rac GTPases
Abstract
Although previous studies have identified many extracellular guidance molecules and intracellular signaling proteins that regulate axonal outgrowth and extension, most were conducted in the context of unidirectional neurite growth, in which the guidance cues either attract or repel growth cones. Very few studies addressed how intracellular signaling molecules differentially specify bidirectional outgrowth. Here, using the bipolar PLM neurons in Caenorhabditis elegans, we show that the guanine nucleotide exchange factors (GEFs) UNC-73/Trio and TIAM-1 promote anterior and posterior neurite extension, respectively. The Rac subfamily GTPases act downstream of the GEFs; CED-10/Rac1 is activated by TIAM-1, whereas CED-10 and MIG-2/RhoG act redundantly downstream of UNC-73. Moreover, these two pathways antagonize each other and thus regulate the directional bias of neuritogenesis. Our study suggests that directional specificity of neurite extension is conferred through the intracellular activation of distinct GEFs and Rac GTPases.
During development, axons and dendrites emerge from neuronal cell bodies and navigate toward their target. Previous studies of neurite guidance have focused on identifying both extracellular guidance molecules and their receptors (reviewed in ref. 1) and intracellular effectors that regulate cytoskeleton dynamics and growth cone movement (reviewed in ref. 2). Although several signaling cascades connect the activation of various ligand-bound receptors to the remodeling of the actin and microtubule cytoskeletons, they do so through similar second messengers (i.e., kinases, phosphatases, GTPases) (3, 4) and thus do not address how the directed outgrowth of multiple neurites occurs. Presumably, distinct intracellular pathways mediate neurite outgrowth and extension in different directions, but what these pathways are remains unclear.
One potential means of regulating differential outgrowth is the use of the many members of the Rho family of small GTPases [Rac (including Rac1, Rac2, Rac3, and RhoG), Cdc42, and Rho] and the guanine nucleotide exchange factors (GEFs) that activate these GTPases (5). Studies using in vitro cultured mammalian neurons suggest that Rac1 (activated by the GEFs Tiam1 and Dock180), RhoG (activated by the first GEF domain of Trio), and Cdc42 promote axonal outgrowth and extension by regulating the actin and microtubule cytoskeleton (6, 7). In vivo studies in Drosophila and Caenorhabditis elegans suggest that Rac GTPases have overlapping functions in the control of axon growth and guidance and that the Trio GEF is essential for Rac activities in the nervous system (8–10). In contrast, Rho and its downstream effector ROCK negatively regulate axon growth in mammals (11, 12) and suppress dendritic extension in Drosophila (13).
Although some studies suggest that small GTPases and GEFs may have different effects on axonal and dendritic growth (14–16), virtually no study has addressed the directional specificity of those signaling molecules for neurites with similar properties in the context of bidirectional growth. Here, we use the bipolar PLM neurons in C. elegans to investigate this question. The posteriorly located, bilaterally symmetric PLM neurons are two of the six mechanosensory touch receptor neurons (TRNs) (17). The PLM neurons have two sensory neurites that grow under different directions; both contain the MEC-4 transduction channel (18) (Fig. S1) and have bundles of the 15-protofilament microtubules that are present only in the TRNs and are required for mechanosensation (19, 20). In addition, both neurites are capable of forming synapses if partners are physically close (21). In contrast to the PLM neurons, the four remaining TRNs (the two anterior ALM, the AVM, and the PVM neurons) are monopolar.
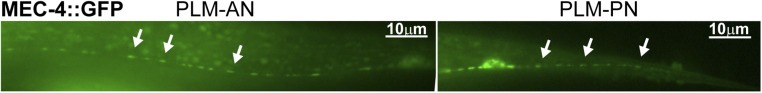
Fig. S1.
The MEC-4::GFP fusion protein, expressed from a mec-4p::mec-4::GFP translational reporter, is localized in puncta along both PLM-AN and PLM-PN. Arrows point to some of the fluorescent puncta.
As part of a genetic screen for mutants with morphologically abnormal TRNs (to be reported elsewhere), we identified mutants defective in differential outgrowth of the two PLM neurites. In particular, we found that GEF proteins UNC-73/Trio and TIAM-1 promote neuronal extension toward the anterior and the posterior, respectively, and that the Rac subfamily GTPase CED-10/Rac1 is activated by TIAM-1, whereas CED-10 and MIG-2/RhoG act redundantly downstream of UNC-73. Moreover, the two pathways promoting growth in opposing directions antagonize each other to control neurite morphogenesis. Thus, our study suggests that intracellular signaling pathways confer directional specificity on neurite extension through the activation of distinct GEFs and Rac GTPases.
Results
UNC-73/Trio Promotes Anteriorly Directed Neurite Extension.
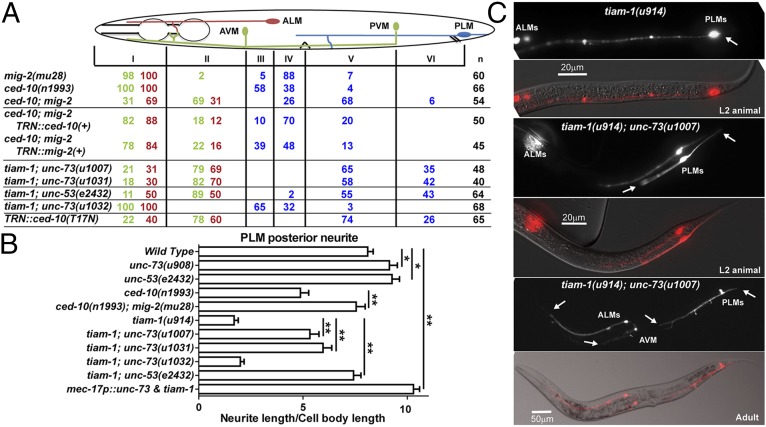
Previously, we found that unc-73/Trio regulates axonal guidance in the TRNs (22). We isolated a new unc-73 allele (u908, Fig. S2) in our recent screen and found that mutations in unc-73 led to the specific shortening of TRN anteriorly directed neurites (ANs). unc-73 encodes multiple isoforms of a guanine nucleotide exchange factor that is homologous to a mammalian triple functional domain protein (TRIO). The different isoforms of UNC-73 contain a RacGEF domain, a RhoGEF domain, or both. One of the isoforms, UNC-73B, which contains only the RacGEF domain, specifically activates Rac pathways and affects axonal guidance, cell migration, muscle arm extension, and phagocytosis (9, 23–25). In contrast, a different isoform, UNC-73E, which contains only the RhoGEF domain, activates Rho GTPases and regulates both cell migration and locomotion (26, 27). We found that missense mutations u908 and rh40 and splice donor mutation e936, which all alter UNC-73B, resulted in shortened ANs from both the PLM and ALM. The PLM-AN often stopped before reaching the PVM cell body, the ALM-AN did not extend past the first pharyngeal bulb, and the AVM-AN stopped extending anteriorly after branching into the nerve ring (Fig. 1; the PVM-AN was not scored because it runs together with AVM-AN in the ventral cord and the AN ending could not be easily identified). Mutations ce362 and ev802, which alter unc-73E, did not cause similar defects (Fig. 1C).
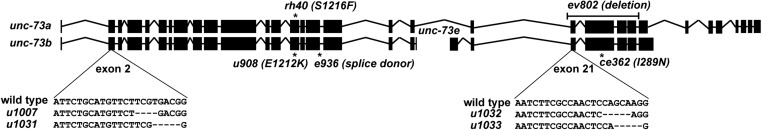
Fig. S2.
Gene structures for unc-73a, -b, and -e isoforms and the molecular lesions in various unc-73 alleles. u908 was isolated from our screen as a mutant having TRN morphological defects. u1007, u1031, u1032, and u1033 were created using CRISPR/Cas9-mediated genome editing in animals that carried the tiam-1(u914) allele (28). Guide RNAs were designed to target exon 2 to inactivate unc-73B and exon 21 to inactivate unc-73E. Frameshift-causing deletions in each of the two isoforms were identified by genotyping.
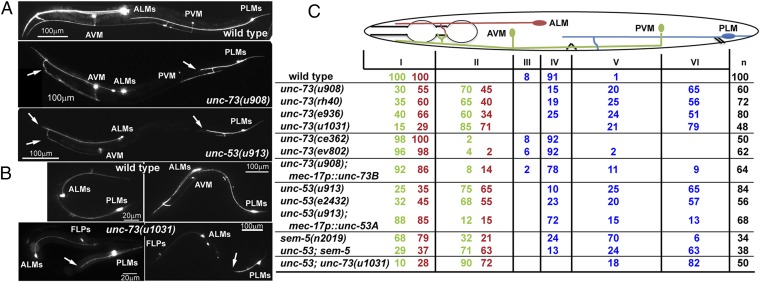
Fig. 1.
UNC-73 and UNC-53 promote anteriorly directed neurite extension. (A) Adult TRN morphologies, visualized by RFP expressed from the mec-17 promoter, in wild-type, unc-73(u908), and unc-53(914) animals. Arrows here and elsewhere point to the premature ends of the neurites. (B) TRN morphologies in wild-type or unc-73(u1013) animals at the second larval stage (Left) and early adult stage (Right). mec-3p::RFP was used to label the TRNs and FLPs in unc-73(u1013) mutants. (C) The percentage of TRN anterior neurites terminating in specific zones (red numbers for ALM, green numbers for AVM, and blue numbers for PLM). The animal body is divided into six zones along the anterior–posterior axis. Zone I occupies the area from the nose to the posterior end of the first pharyngeal bulb; AVM and ALM cell bodies, vulva, PVM, and PLM cell bodies define the boundaries from zone II to zone VI. Determined by a χ2 test, results of unc-53(e2432); unc-73(u1031) were not significantly different (P > 0.05) from those of unc-73(u1031) single mutants, and the difference between unc-53(e2432); sem-5(n2019) and unc-53(e2432) was not significant.
Furthermore, we generated a unc-73B null allele, u1031, by creating a frameshift-causing 5-bp deletion in the second exon of unc-73B using CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9-mediated genome editing (Fig. S2) (28). This null mutation produced sterile adults, which was not observed in animals carrying missense mutations, as well as a strong uncoordinated phenotype. The anteriorly directed neurite growth was also strongly suppressed in unc-73B(u1031) animals (Fig. 1C). Although the u1031 mutation also affected unc-73A, which contains both RacGEF and RhoGEF domains, the morphological defects in the mutants could be rescued by specifically expressing the unc-73B(+) from the TRN-specific mec-17 promoter (Fig. 1C). Therefore, we refer to u1031 as an unc-73B allele in this context. The expression of unc-73 in the TRNs was previously reported (29). Together, these results indicate that the Rac-specific GEF domain of UNC-73 promotes neurite extension toward the anterior in a cell-autonomous manner.
van Haren et al. (30) reported that TRIO complexed with NAV1 (neuronal Navigator 1) to regulate neurite extension by binding to the plus end of growing microtubules in cultured mouse hippocampal neurons. UNC-53, the C. elegans homolog of Navigators, is also required for TRN outgrowth and guidance (31), and we found a specific shortening of TRN-ANs in unc-53 mutants (Fig. 1C). Mutations in sem-5/Grb2, which encodes an adaptor protein for UNC-53/NAVs (32), also resulted in shorter TRN-ANs; the loss of sem-5 did not enhance the phenotype of unc-53, suggesting that they work together (Fig. 1C). The TRN morphological defects in the unc-73 unc-53 double mutants were similar to unc-73 null single mutants, suggesting that TRIO and NAVs act in the same pathway to promote neurite growth.
TIAM-1 Promotes Posteriorly Directed Neurite Extension.
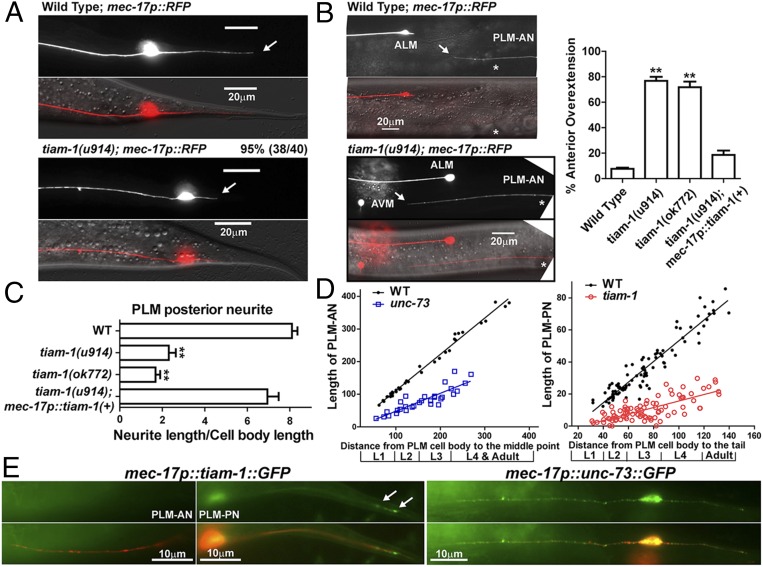
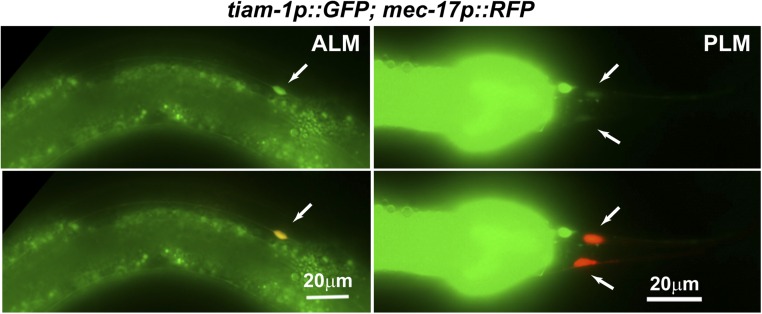
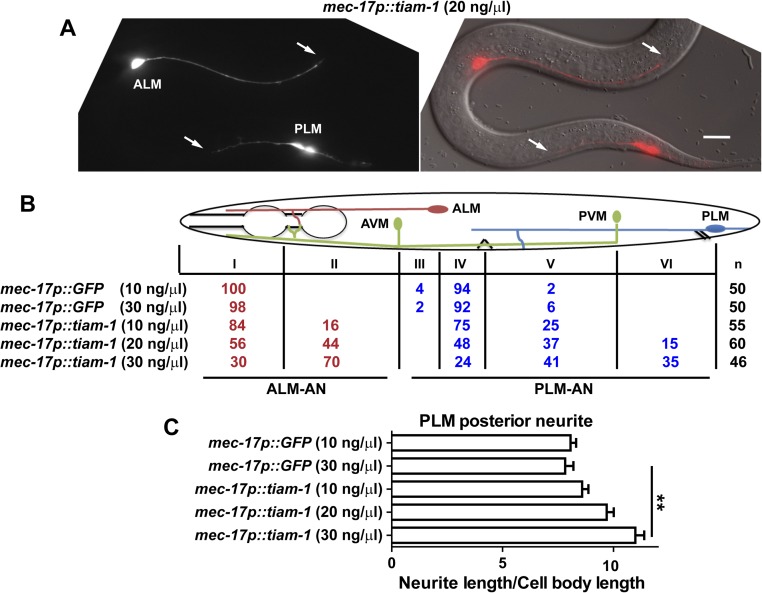
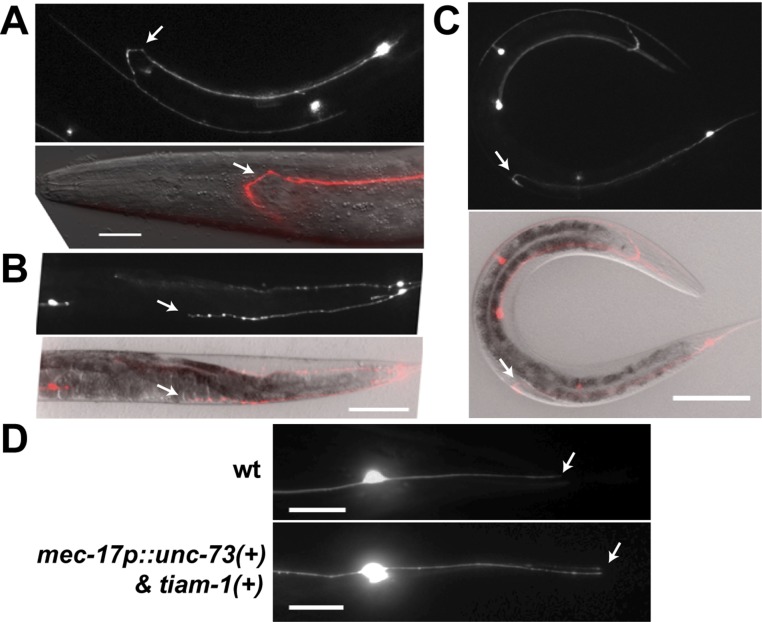
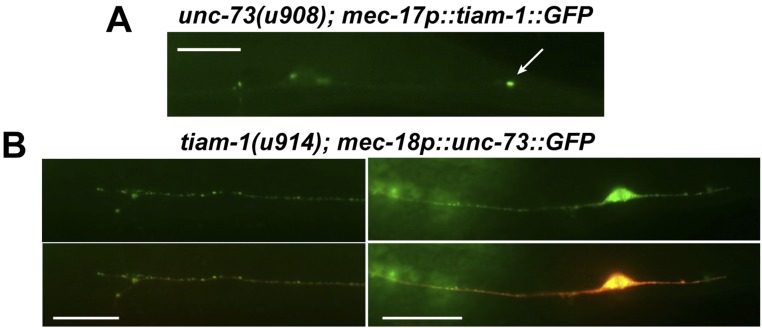
The growth of the PLM posterior neurite requires TIAM-1 (homolog of mammalian T-cell lymphoma Invasion And Metastasis 1), another Rac GEF (33). Both the tiam-1 nonsense allele u914 (R570*, isolated in our screen) and the deletion allele ok772 caused significant shortening of the PLM-PN and overextension of the PLM-AN (Fig. 2 A–C). Expression of tiam-1(+) cDNA from the TRN-specific mec-17 promoter rescued these defects. Although the tiam-1 promoter reporter was expressed in both ALM and PLM neurons (Fig. S3), we did not observe any morphological defects in tiam-1–deficient ALM neurons. However, overexpression of tiam-1 in the TRNs caused the elongation of the PLM-PN and premature termination of the ALM-AN and PLM-AN in a dose-dependent manner (Fig. S4). These results suggest that TIAM-1 acts cell-autonomously to promote posteriorly directed neurite growth and to suppress anteriorly directed extension. Consistent with its positive effect on PLM-PN growth, TIAM-1::GFP localized specifically to the distal region of PLM-PN and was not found in PLM-AN (Fig. 2E). Antibody staining against GFP in animals carrying the mec-17p::tiam-1::GFP transgene failed to detect any signal in PLM-AN either. Although we could not exclude the possibility that the amount of TIAM-1 in the anterior was below our detection limit, the effect of TIAM-1 loss on the overextension of PLM-AN is likely to be indirect. In contrast, UNC-73::GFP, which rescued the unc-73 mutant phenotype, was found as puncta along both PLM-AN and PLM-PN, suggesting that UNC-73 is ubiquitously present in the neuron but its growth-promoting activity is limited to the anterior neurites. Nevertheless, we cannot rule out that overexpression of UNC-73::GFP caused it to be found in the PLM-PN.
Fig. 2.
TIAM-1 promotes posteriorly directed neurite growth. (A) The shortening of PLM-PN in tiam-1 mutants compared with wild-type animals. Arrows indicate the end of PLM-PN. (B) Overextension of PLM-AN in tiam-1 animals and the percentage of PLM-AN passing the ALM cell body. Asterisks in the images indicate the position of the vulva, and the arrows point to where PLM-AN terminates. (C) The length of PLM-PN in tiam-1 animals. (D) The lengths (in μm) of PLM-AN and PLM-PN in wild-type, unc-73(u1031), and tiam-1(u914) animals during different developmental stages. The distance from the PLM cell body to the middle point (the position of vulva in L4 and adults) or the tip of the tail was used as a measurement of developmental progress. Linear regression (solid lines) was applied to these data. The slope indicates the growth rate of neurites relative to the extension of the worm body. For WT PLM-AN, the slope = 1.1 ± 0.02 and r2 = 0.99 (goodness of fit); for unc-73 PLM-AN, the slope = 0.55 ± 0.06 and r2 = 0.77; for WT PLM-PN, the slope = 0.65 ± 0.02 and r2 = 0.90; for tiam-1 PLM-PN, the slope = 0.2 ± 0.02 and r2 = 0.55. (E) The localization of TIAM-1::GFP and UNC-73::GFP fusion proteins expressed specifically in the TRNs from L1 animals that carry the mec-17p::RFP transgene. Arrows point to the position of TIAM-1::GFP in the distal region of PLM-PN.
Fig. S3.
The expression of tiam-1 reporter qyEx115[tiam-1p::GFP] in ALM and PLM neurons. Arrows indicate the cell body positions. The prominent GFP in the Right panels is from the posterior intestine.
Fig. S4.
Overexpression of tiam-1 induces premature termination of TRN anterior neurites. (A) Shortening of the anterior neurites in animals overexpressing tiam-1 in TRN neurons. Arrows point to prematurely terminated ALM-AN and PLM-AN. (B) Percentage of ALM-AN and PLM-AN terminating at specific zones. n is the number of cells examined. (C) Length of PLM-PN in animals carrying various transgenes. Error bars represent the SEM, and double asterisks indicate P < 0.01. (Scale bar, 20 μm.)
UNC-73 and TIAM-1 Regulate Neurite Elongation Throughout Development.
The morphogenesis of PLM neurons can be divided into two phases: (i) the initial outgrowth of the two neurites and the establishment of a bipolar shape in the late embryonic stages and (ii) the elongation of the two neurites throughout the larval stages. UNC-73 and TIAM-1 are required for the latter extension phase because their mutants showed persistent neurite growth defects from the first larval stage to adulthood (Fig. 2D); the two GEFs are unlikely to be involved in the initial neurite outgrowth, as the capacity of the neurons to form two opposing neurites was not affected in the mutants.
Heiman and Shaham proposed a “retrograde extension” mechanism for dendritic growth in C. elegans amphid neurons, showing that neurites can grow by anchoring their distal tips to specific locations and later being stretched as the cell body migrates away during larval development (34). UNC-73 and TIAM-1 are not likely to act similarly in PLM neurons because the neurites in unc-73 and tiam-1 mutants showed substantial growth throughout development but with a much lower growth rate than the wild-type neurites (SI Results). Therefore, GEFs most probably regulate active neurite extension by affecting growth cone migration.
CED-10/Rac1 Promotes Posterior Neurite Growth Downstream of TIAM-1.
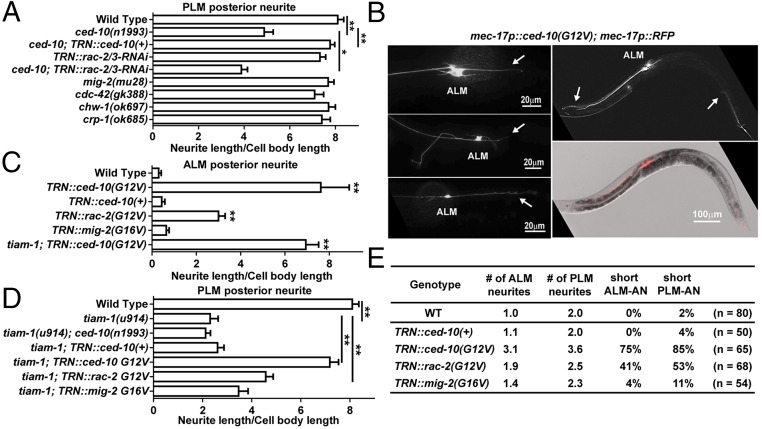
Functionally, both UNC-73 and TIAM-1 activate Rac subfamily GTPases by stimulating the exchange of GDP for GTP; in in vitro nucleotide exchange assays, UNC-73B activates CED-10/Rac1 and MIG-2/RhoG (35), and TIAM-1 activates human Rac1 (33). C. elegans has three active Rac subfamily genes: ced-10/Rac1, rac-2, and mig-2/RhoG. Mutations in ced-10/Rac1 caused a significant reduction in the length of PLM-PN (Fig. 3A). This phenotype was enhanced when we expressed interfering hairpin RNAs targeting the third and fourth exons of rac-2 from a TRN-specific promoter. [This RNAi experiment would also reduce the expression of the pseudogene rac-3, which is 98% identical to rac-2 and appears to be a nonfunctional locus (36).] Therefore, TIAM-1, CED-10/Rac1, and RAC-2 were needed for the production of the PLM posterior neurite. Moreover, tiam-1; ced-10 double mutants have a phenotype similar to tiam-1 single mutants, supporting that Rac1 functions in the same pathway as TIAM-1 in promoting posteriorly directed extension (Fig. 3D). Mutations in another Rac gene, mig-2, or in the Cdc-42 genes (cdc-42, chw-1, and crp-1) did not alter PLM morphology (Fig. 3A).
Fig. 3.
CED-10/Rac1 promotes posteriorly directed neurite outgrowth. (A) The length of PLM-PN in various strains. (B) TRN morphologies in animals expressing constitutively active CED-10(G12V) from the mec-17 promoter. (Left panels) Morphological transformation of ALM neurons; arrows indicate the ectopic ALM-PN. (Right panels) The premature termination of ALM-AN and PLM-AN; arrows point to the ends of the anterior neurites. (C and D) The length of ALM-PN and PLM-PN in various strains. (E) The average number of ALM and PLM neurites emanating from the cell bodies; the percentage of TRNs with shortened anterior neurites.
We next tested whether elevating Rac1 activity changed TRN morphology by expressing alleles of Rac genes [ced-10(G12V), rac-2(G12V), and mig-2(G16V)] encoding constitutively active proteins (36) in the TRNs. As in a previous study of Rac1 function in the PDE neurons (37), constitutively active CED-10 [CED-10(G12V)] induced the production of many lamellipodia-like protrusions from the plasma membrane, multiple short ectopic neurites from the cell body, and abnormal branches from the main TRN processes (Fig. 3 B and E). Nevertheless, the most common (85%, n = 65) and dramatic phenotype resulting from the expression of CED-10(G12V) was the production of a long ectopic ALM posterior neurite (Fig. 3 B and C). The ability of CED-10(G12V) to promote the generation of ALM-PN did not depend on the presence of tiam-1 (Fig. 3C). In PLM neurons, hyperactive mutations in ced-10 restored the PLM-PN in tiam-1 mutants (Fig. 3D). These results suggest that CED-10 acts downstream of TIAM-1 to promote the growth of the posterior neurite. Constitutively active CED-10 also led to the shortening of both ALM-AN and PLM-AN (Fig. 3B, Right), which indicates that the promotion of posteriorly directed neurite growth by TIAM-1 and Rac1 results in the inhibition of anterior extension.
Importantly, overexpression of wild-type ced-10(+) did not cause a similar phenotype, indicating that the activity but not the level of CED-10 protein is crucial for posterior outgrowth. Constitutively active RAC-2 had a similar but weaker effect (Fig. 3), confirming that CED-10 is the major Rac GTPase regulating TRN neurite extension. Constitutively active MIG-2/RhoG, however, produced very little change in the TRN morphology in the wild-type animals and failed to rescue the outgrowth defects of tiam-1 mutants (Fig. 3).
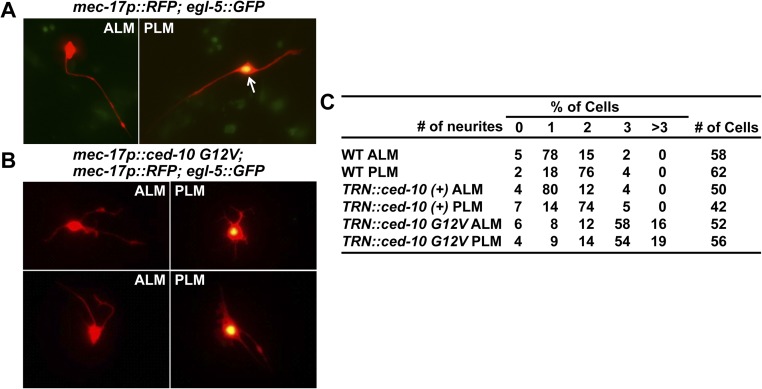
CED-10/Rac1–mediated regulation of neurite morphology appeared to be intrinsic because the morphology of TRNs cultured in vitro was also controlled by Rac1 activity. Wild-type ALM and PLM neurons were morphologically distinct in culture: 78% of the ALM neurons had only one prominent neurite, whereas 76% of the PLM neurons had two neurites (Fig. S5A). When CED-10(G12V) was expressed in the TRNs, both ALM and PLM grew more than two neurites, although their average length was shorter than those of the wild-type cells (Fig. S5 B and C). Thus, increased CED-10 activity resulted in the transformation of cell shapes and the ectopic growth of multiple neurites.
Fig. S5.
In vitro cultured ALM and PLM neurons of wild-type and transgenic animals expressing ced-10(G12V) from TRN-specific mec-17 promoter. (A) ALM neurons labeled with mec-17p::RFP in cytoplasm—a TRN marker—but not the nuclear egl-5::GFP—a PLM marker (57) have one long neurite. The arrow indicates the cell bodies that express both GFP and RFP. PLM neurons that are doubly labeled have two neurites growing in the opposing directions. (B) ALM and PLM neurons expressing ced-10(G12V) have multiple short neurites, abnormal cell shape, and increased spread area of the cell body. The average length of these neurites (9.5 μm for TRN::ced-10 G12V ALM and 8.2 μm for TRN::ced-10 G12V PLM) was shorter than that of neurites in cultured wild-type TRNs (26.4 μm for ALM and 19.3 μm for PLM). (C) The percentage of cells having 0, 1, 2, 3, and >3 neurites.
CED-10/Rac1 and MIG-2/RhoG Redundantly Regulate Anteriorly Directed Elongation.
ced-10 and mig-2 act redundantly downstream of unc-73 in the regulation of cell division and migration (38). Although neither mig-2 nor ced-10 single mutants had anterior extension defects, we found that ced-10; mig-2 double mutants had shorter TRN-ANs, most obviously in the PLM and AVM neurons (Fig. 4A). These morphological defects could be rescued by expressing either ced-10(+) or mig-2(+), suggesting that CED-10 and MIG-2 redundantly promote anteriorly directed extension.
Fig. 4.
Mutations in unc-73 suppress the shortening of PLM-PN in tiam-1 mutants. (A) The percentage of TRN anterior neurites terminating in various zones (red numbers for ALM, green numbers for AVM, and blue numbers for PLM). Determined by a χ2 test, the results of tiam-1(u914); unc-73(u1031) animals were significantly different (P < 0.05) from that of unc-73(u1031) single mutants (data in Fig. 1) for PLM anterior extension; the difference between tiam-1; unc-53(e2432) and unc-53(e2432) was also significant. However, the results for ALM-AN and AVM-AN were not significantly different between tiam-1; unc-73 double mutants and unc-73 animals. (B) The length of PLM-PN in the strains is indicated. (C) TRN morphology in L2 animals and adults of tiam-1; unc-73 double mutants. Arrows point to neurite ends.
Unexpectedly, we found that mutations in mig-2 suppressed the PLM posterior extension defects in ced-10 mutants (Fig. 4B). PLM-PN in ced-10; mig-2 double mutants was significantly longer than that in ced-10 single mutants and was similar to the wild type. These results suggest that the suppression of anteriorly directed neurite extension in ced-10; mig-2 double mutants simultaneously promoted outgrowth toward the posterior.
Antagonism Between Anteriorly and Posteriorly Directed Neurite Extensions.
Because mutations in unc-73 and tiam-1 differed in their effects on PLM neurite extension, we examined the PLM morphology in tiam-1; unc-73 double mutants. Because unc-73 and tiam-1 genes are physically adjacent on the same chromosome, we recreated the unc-73B null mutations (the u1007 and u1031 alleles) (Fig. S2) in animals carrying the tiam-1(u914) allele using the CRISPR method. The loss of unc-73B strongly rescued the posterior extension defects found in tiam-1 single mutants, and the tiam-1; unc-73 double mutants had less severe anterior growth defects compared with unc-73 null animals, although PLM-AN was still significantly shortened compared with the wild type (Fig. 4; compare with Fig. 1). As controls, the u1032 and u1033 alleles, which affect unc-73E, had no effects on the Tiam-1 phenotype (Fig. 4 A and B). Similarly, tiam-1; unc-53 had restored PLM-PN and still shorter PLM-AN; again, the PLM-AN defect was less severe in the double mutants than in unc-53 single mutants (Fig. 4 A and B). These results suggest that the loss of UNC-73 or UNC-53 causes down-regulation of the growth signal for the anterior neurites and the derepression of posterior outgrowth and that the lack of TIAM-1 also partially reactivates the neurite growth toward the anterior. In the absence of both GEFs, PLM neurons adopt a default bipolar shape with two prominent neurites. Interestingly, mutation of tiam-1 did not rescue the shortening of the anterior neurite in the monopolar ALM and AVM of unc-73 animals, although tiam-1 is expressed in these neurons (Fig. 4A).
The mutual suppression between UNC-73 and TIAM-1 in PLM was not mediated by the changes in localization. TIAM-1::GFP still localized to the distal region of the PLM-PN in unc-73 mutants, and the localization of UNC-73::GFP in PLM-AN and PLM-PN was not affected in tiam-1 mutants (Fig. S6). These data support the hypothesis that the posterior-specific TIAM-1 could functionally override the presence of UNC-73 in PLM-PN to promote posteriorly directed extension. In fact, when both UNC-73 and TIAM-1 were overexpressed in the TRNs, PLM-PN became elongated compared with the wild-type neurite despite the excessive amount of UNC-73 (Fig. 4B). Although most (92%; n = 85) of the TRN anterior neurites overexpressing the two GEFs had a normal length, moderate shortening of ALM-AN and PLM-AN was observed in the remaining 8% of the cells (Fig. S7).
Fig. S6.
The morphology of TRNs overexpressing unc-73 and tiam-1. Animals carrying the uIs134[mec-17p::RFP] transgene as a TRN marker were injected with mec-17p::unc-73(+) and mec-17p::tiam-1(+) (each at 30 ng/μL). Although 92% (n = 85) of the TRNs showed a generally normal morphology, 8% of the TRNs had shorter anterior neurites. (A) ALM-AN did not extend over the branching point as the wild-type neurites. (Scale bar, 20 μm.) Arrows here and elsewhere in the figure point to the premature ends of the neurites. (B and C) PLM-AN was shortened and did not extend to the position of the vulva. (Scale bar, 100 μm.) (D) The PLM-PN was elongated upon the overexpression of both unc-73 and tiam-1.
Fig. S7.
The localizations of UNC-73 and TIAM-1 are independent of each other. (A) TIAM-1::GFP expressed from the TRN-specific mec-17 promoter was still localized to the distal region (arrow) of the PLM-PN in unc-73B(u908) mutants. (B) UNC-73::GFP expressed from the TRN-specific mec-18 promoter was found in both PLM-AN and PLM-PN in tiam-1 mutants, similar to the localization in wild-type animals. (Scale bar, 20 μm.)
The phenotype of tiam-1; unc-73 double mutants was similar to that of ced-10; mig-2 animals, suggesting that CED-10 acts downstream of TIAM-1 and that UNC-73 activates both MIG-2 and CED-10. The expression of a dominant-negative CED-10/Rac1(T17N) mutant (39) also reproduced the tiam-1; unc-73 double phenotype presumably by sequestering both UNC-73 and TIAM-1 from their substrates (Fig. 4B). This result supports that CED-10 contributes to both anterior and posterior growth, although it is essential posteriorly and redundant anteriorly.
UNC-73 and TIAM-1 also Promote Directional Neurite Elongation in Other Neurons.
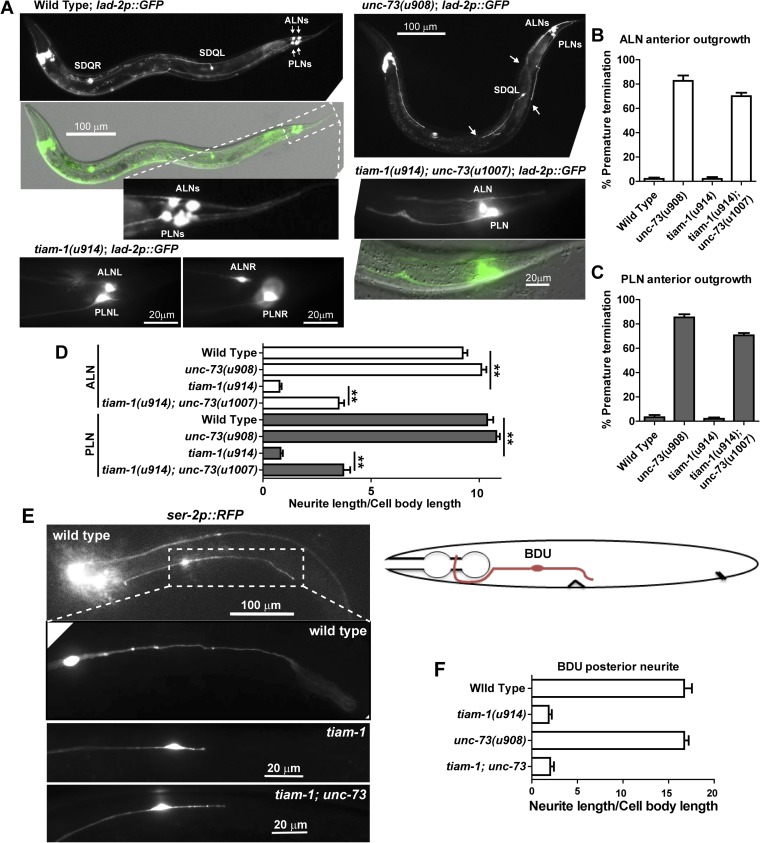
In addition to PLM neurons, UNC-73 and TIAM-1 also regulated the elongation of the anterior and posterior neurites, respectively, in ALNL/R and PLNL/R neurons, two pairs of bilaterally symmetrical neurons located at the tail region (SI Results; Fig. S8). Antagonism between the two GEFs was also observed in these neurons. Moreover, in the bipolar BDU neurons, which are located in the anterior half of the animal, TIAM-1 specifically promoted the posteriorly directed neurite extension (Fig. S8). These results suggest that GEFs generally control the directional specificity of neurite extension along the anterior–posterior (A–P) axis.
Fig. S8.
UNC-73 and TIAM-1 promote directional neurite growth in ALN, PLN, and BDU neurons. (A) The morphology of ALN and PLN neurons, labeled by GFP expressed from lad-2 promoter, in wild-type, tiam-1, unc-73, and unc-73 tiam-1 animals. lad-2p::GFP also labels SDQ neurons and several head neurons. In the unc-73(u908) panel, arrows point to the premature termination of the anterior neurites of ALN, PLN, and SDQL neurons. (B and C) Percentage of ALN and PLN neurons with defects in anteriorly directed neurite extension. (D) Length of ALN-PN and PLN-PN in various strains. Error bars represent the SEM, and double asterisks indicate P < 0.01. (E) Significant shortening of BDU-PN in tiam-1 animals. A diagram of the bipolar BDU neurons shows that BDU-PN terminates around the vulva. (F) The length of BDU-PN in various strains.
SI Results
UNC-73 and TIAM-1 Are Unlikely to Regulate Neurite Extension by Affecting Neurite Anchoring.
There are two possible mechanisms for the UNC-73– and TIAM-1–mediated extension of PLM neurites. First, the neurites may actively extend throughout development in parallel with the elongation of the animal body; second, like the dendrites of C. elegans amphid neurons (34), PLM neurites may grow by anchoring their distal tips to specific locations and being passively stretched as the distance increases between the cell body and the anchoring position as the animal grows. This second mechanism, known as “retrograde extension” (34), is unlikely because the unc-73 PLM-AN and tiam-1 PLM-PN continue to grow, albeit more slowly than the wild-type neurites (Fig. 2D; the slope is an indicator of neurite growth rate). If UNC-73 and TIAM-1 are required for neurite tip anchoring to a target location, the mutant neurites would have been insensitive to larval development and not grow at all; if the tips are attached to an incorrect position, the growth rate for the neurites would have been similar between mutants and wild-type animals. Neither of these two predictions was supported by the data. Therefore, UNC-73 and TIAM-1 most probably promote active neurite extension toward different directions by affecting the migration and guidance of the growth cone.
UNC-73 and TIAM-1 also Promote Directional Neurite Elongation in Other Neurons.
The GEFs needed for directional growth of the PLM neurons also act in other bipolar neurons. ALNL/R and PLNL/R neurons are two pairs of bilaterally symmetrical neurons located at the tail region; their anterior neurites terminated prematurely in unc-73B and not unc-73E mutants, and their posterior neurites were considerably shorter in tiam-1 mutants (Fig. S8 A–D). The anterior ALN and PLN neurites in tiam-1; unc-73 double mutants showed similar but less penetrant defects compared with the unc-73 single, and mutations in unc-73 partially restored the posterior neurites in tiam-1 mutants (Fig. S8D). The anteriorly directed neurites of the monopolar SDQL neurons were significantly shorter in unc-73 mutants (Fig. S8B). These results suggest that UNC-73 and TIAM-1 generally control the directional specificity of neurite extension along the A–P axis.
The activity of TIAM-1 is not restricted to the posteriorly directed growth of bipolar neurons in the tail. The posterior neurites of BDU neurons, which are located at the anterior half of the animal, were also markedly shorter in tiam-1 mutants, but the length of their anterior neurites was not changed (Fig. S8 E and F). unc-73 mutants did not affect BDU-AN outgrowth, so other GEFs may promote this anterior growth. Overall, our data suggest that GEF-mediated directional neurite elongation is generally important for the morphological development of bipolar or multipolar neurons.
Discussion
Ever since the observation that the activity of Rho family small GTPases is locally regulated in living cells (40, 41) was made, researchers have searched for factors that control this localized activation. This issue is particularly important for understanding the development of complex neuronal structures because a wide range of extracellular guidance molecules induce highly specific growth cone behaviors through only a handful of common Rho GTPases. How is this specificity achieved? Accumulated evidence, mostly from in vitro studies, suggests that neurons use distinct GEFs to activate the same GTPases in response to different guidance cues and that the use of certain GEFs is often cell type-specific (4). Our in vivo study suggests that multiple GEF molecules can also function in the same neurons to confer directional specificity in the development of multiple neurites. Given that humans have 83 Rho family GEFs (42), we propose that specializing GEFs as signaling molecules that promote neurite growth in particular directions may be a general mechanism for the spatial control of Rho activation during neuronal development.
PLM morphology is regulated by Wnt signals, and different sensitivities to the same guidance cue may explain the use of distinct GEFs and Rac GTPases for the growth of the two opposing neurites. PLM-AN is repelled toward the anterior by Wnt proteins released at the posterior side of the cell body (21, 43). Although Rac1 is known to act downstream of Wnt signaling (44), our data suggest a link between Wnt signaling and the control of Trio and RhoG. PLM-PN grows against the gradient of the repulsive Wnt proteins, and this growth requires both the activation of intrinsic Hox proteins (45) and the attenuation of the extrinsic Wnt signals by Dishevelled (21). The specific involvement of these proteins on PLM-PN but not AN may lead to the selective employment of TIAM-1 for posterior extension.
A distinctive response to the same guidance cue in different cellular compartments has also been observed elsewhere (46, 47). Notably, semaphorin 3A repels the cortical axons of mouse pyramidal neurons but attracts their apical dendrites; this specific attraction is mediated by the asymmetric localization of soluble guanylate cyclase to the developing dendrites (48). Our findings suggest that this different responsiveness within the same cells can also be mediated by the local activation of distinct GEFs. In C. elegans PDE neurons, for example, TIAM-1 regulates growth cone extension via Rac GTPases responding to the activated netrin receptor UNC-40/DCC (33), whereas UNC-73 inhibits this extension (49). Importantly, lowering the levels of mig-2 and ced-10 causes migrating cells to be repulsed rather than attracted to Semaphorin-1 and Plexin-1 (50), suggesting that the proper activity of Rho family GTPases is critical for the response to cues.
An unexpected finding of this research is that the two pathways promoting anteriorly and posteriorly directed growth antagonize each other. Mutations in tiam-1/GEF and ced-10/Rac1 caused significant shortening of the PLM-PN and overextension of the PLM-AN (Figs. 2 and 4), both of which were also observed in egl-5 and rfip-1 mutants (egl-5 encodes a Abd-B-like Hox protein, and rfip-1 encodes a recycling endosome-associated protein) (45). Therefore, suppression of the posterior extension appeared to allow overgrowth toward the anterior in general. On the other hand, unc-73 and unc-53 mutants had not only shorter PLM-ANs but also longer PLM-PNs (Fig. 4B), suggesting that suppression of anterior growth also led to overextension toward the posterior. The phenotype of the tiam-1; unc-73 double mutants supports the mutual inhibition model, as both defects found in the single mutants were alleviated in the double mutants. These data, however, also suggest that UNC-73 and TIAM-1 regulate only the directional bias of neurite extension and are dispensable for the general neurite growth capacity of the cells.
Based on the localization data (Fig. 2E), we hypothesize that the ubiquitously present UNC-73 biases neurite growth toward the anterior, promoting the growth of PLM-AN and suppressing the extension of PLM-PN. The PLM-PN–specific TIAM-1 counteracts UNC-73 activities, allowing the growth of a posterior neurite. However, the TIAM-1 localization does not explain the anterior overextension induced by the loss of tiam-1 in both the wild-type and the unc-73 animals. Indirect signaling may mediate such derepression. Alternatively, an undetectably low amount of TIAM-1 in PLM-AN could limit anterior extension stimulated by UNC-73, whereas a high level of TIAM-1 in PLM-PN could override the effect of UNC-73 and drive posterior extension.
Although the mechanisms underlying the antagonism between UNC-73 and TIAM-1 are still unclear, the two pathways may signal to block each other or simply compete for limited resources (51). In either case, uncontrolled growth of one neurite would restrain the growth of the other neurite. This reciprocal regulation is reminiscent of the competitive outgrowth of neurites during axonal specification (52) or the competition between the primary axons and their branches (53) and between axons and dendrites (54). Thus, our results support the notion that communication among the multiple neurites is necessary for a coordinated development of complex neuronal shapes.
Materials and Methods
C. elegans wild-type (N2) and mutant strains were maintained at 20 °C, as previously described (55). Most strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). To create unc-73B and unc-73E null alleles, constructs that express CRISPR/Cas9 with single guide RNAs (sgRNAs) targeting exon 2 and exon 21, respectively, were injected into either wild-type or tiam-1(u914) animals, using a previously described method (28). Most of the constructs were made using the Gateway cloning system (Life Technologies); ced-10(G12V), rac-2(G12V), and mig-2(G16V) cDNA were kindly provided by Erik Lundquist. Transgenes uIs31[mec-17p::GFP] III, uIs115[mec-17p::RFP] IV, uIs152[mec-3p::RFP] IV, and uIs134[mec-17p::RFP] V were used to visualize TRN morphology, uIs129[lad-2p::GFP] was used for ALN and PLN, and uEx1005[ser-2p::RFP] was used for BDU neurons. To score the phenotype of premature termination of anteriorly directed TRN processes, the body of the animal was divided into several regions and the percentages of TRN axons terminating at each specific region were calculated. The χ2 test was used for the categorical data to find significant difference between different strains. The relative length of PLM neurites was calculated by dividing the length of the neurite by the diameter of the PLM cell body along the A–P axis. For statistical analysis, ANOVA and a post hoc Tukey–Kramer method were used to identify significant difference between the sample means in multiple comparisons. Single and double asterisks indicated P < 0.05 and P < 0.01, respectively. More details are provided in SI Materials and Methods.
SI Materials and Methods
Strains.
C. elegans wild-type (N2) and mutant strains were maintained as previously described (55). Most strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). unc-73(u908; E1212K), unc-53(u913; Q261*), klp-11(u1024; Q53*), and tiam-1(u914; R570*) were isolated by visually screening for mutants with TRN differentiation defects; TU4069 carrying uIs115[mec-17p::RFP] for the visualization of the TRNs was used as the starter strain for the screen. Mutants were outcrossed with wild-type animals, and the phenotype-causing mutations were identified by whole-genome resequencing using the service provided by the Beijing Genome Institute. Mutations were then confirmed by complementation tests with reference alleles and rescue experiments using the wild-type copy of the gene.
To create tiam-1; unc-73B, and tiam-1; unc-73E double mutants, constructs that express CRISPR/Cas9 with sgRNAs targeting 5′-ATTCTGCATGTTCTTCGTGACGG-3′ in exon 2 and 5′-AATCTTCGCCAACTCCAGCAAGG-3′ in exon 21 of unc-73, respectively, were injected into tiam-1(u914) mutants using the method of Dickinson et al. (28). Small deletions were generated in unc-73B and unc-73E isoforms (Fig. S2). unc-73B null mutations u1007 and u1031 caused sterility and were therefore maintained as heterozygotes.
Constructs and Transgenes.
A 1.9-kb mec-17 promoter was cloned from wild-type genomic DNA into the Gateway pDONR221 P4-P1r vector and was used to drive TRN-specific expression. The cDNAs of the A isoform of unc-53, B and E isoforms of unc-73, and tiam-1 were cloned into Gateway pDONR221. The resulting entry vectors, together with the pENTR-mec-17-promoter, the pENTR-unc-54-3′ UTR, and destination vector pDEST-R4-R3, were used in the LR reaction to create the final expression vectors. To create translational GFP reporters, pENTR-GFP-unc-54 3′ UTR, which contains both GFP coding and unc-54 3′ UTR sequences in the pDONR221 P2r-P3 vector, was used in the LR reaction. The Gateway cloning method by Life Technologies can be found at www.invitrogen.com/site/us/en/home/Products-and-Services/Applications/Cloning/Gateway-Cloning.html. These constructs were injected into worms to form extrachromosomal arrays to test their effects.
The cDNA of wild-type ced-10 and constitutively active mutant ced-10(G12V), rac-2(G12V), and mig-2(G16V) cDNA were kindly provided by Erik Lundquist (University of Kansas) and subcloned into pDONR221. The resulting entry vectors were used to form mec-17 promoter-driven expression vectors. mec-17p::ced-10(G12V) was integrated into the genome by gamma-irradiation to form transgene uIs166. To knock down rac-2 as previously described (36), the genomic region covering the third and fourth exons of rac-2 was cloned into pDONR221 P2r-P3; the reverse complementary sequence from the antisense strand was cloned into pDONR221. These entry vectors were used together with pENTR-mec-17p to form mec-17p::rac-2/3-RNAi that expressed hairpin RNA against rac-2 and rac-3 in the touch receptor neurons. rac-3 is 98% identical to rac-2 but appears to be a nonfunctional pseudogene because RAC-3 lacks five conserved residues found in Rac proteins (PASFE, amino acids 87–91 in CED-10) and a retrotransposon splits the third exon of rac-3 in the middle (36).
Transgenes uIs31[mec-17p::GFP] III, uIs115[mec-17p::RFP] IV, uIs152[mec-3p::RFP] IV, and uIs134[mec-17p::RFP] V were used to visualize TRN morphology. qyEx115[tiam-1p::GFP] was used to confirm the expression of tiam-1 in TRNs. uEx1019[Pmec-17::tiam-1::GFP; rol-6(D)] and uEx1044[mec-18p::unc-73::GFP] were used to monitor the localization of TIAM-1 and UNC-73 in TRNs, respectively. Transgene uIs129[lad-2p::GFP], which is generated by integrated otEx331 into the genome, was used to visualize the morphology of ALN and PLN neurons. uEx1005[ser-2p::RFP] was used to visualize BDU neurons.
Phenotype Scoring and Statistical Analysis.
We scored the length of the anterior TRN neurites in relation to several landmarks (e.g., the position of the TRN cell bodies, the pharynx, and the vulva) in mutant and wild-type animals (Fig. 1C). The positions of these landmarks did not notably change in the mutants that we tested. The percentages of TRN axons terminating at each specific region were calculated, and the aggregate data from three independent trials were shown. The χ2 test was used for the categorical data to find significant difference between different strains, and the Holm–Bonferroni method was used to adjust the P values.
The relative length of the PLM posteriorly directed neurite was calculated by measuring the length of the process and dividing the length by the diameter of PLM cell body along the A–P axis. At least 30 gravid adults were measured. ANOVA and a post hoc Tukey–Kramer method was used to identify significant difference between the sample means in multiple comparisons that used more than five sets of data. When the group had no more than five sets of data, Student’s t test was used to identify significant difference, and the Holm–Bonferroni method was used to correct the P values. Single and double asterisks indicate P < 0.05 and P < 0.01, respectively.
We also examined the morphology of at least 50 cultured ALM and PLM neurons for each strain that we examined. The cells had been extracted from embryos and cultured on peanut lectin-coated glass slides for 14 h as previously described (56).
To create transgenic animals and test the effects of transgenes, DNA constructs (5 ng/μL for each expression vector) were injected into the animals to establish stable lines carrying the extrachromosomal array. At least three independent lines were tested for TRN morphogenesis. In some cases, the transgene was integrated into the genome using gamma irradiation, and at least three integrant lines were outcrossed and then subjected to various tests.
Acknowledgments
We thank Dan Dickinson, Erik Lundquist, and Yushu Chen for sharing reagents. This work was supported by NIH Grant GM30997 (to M.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607179113/-/DCSupplemental.
References
- 1.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb Perspect Biol. 2011;3(6):a001727. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011;3(3):a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouquet C, Nothias F. Molecular mechanisms of axonal growth. Adv Exp Med Biol. 2007;621:1–16. doi: 10.1007/978-0-387-76715-4_1. [DOI] [PubMed] [Google Scholar]
- 4.Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2(2):a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 6.Estrach S, et al. The human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr Biol. 2002;12(4):307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 7.Leeuwen FN, et al. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139(3):797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakeda-Suzuki S, et al. Rac function and regulation during Drosophila development. Nature. 2002;416(6879):438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- 9.Lundquist EA. Rac proteins and the control of axon development. Curr Opin Neurobiol. 2003;13(3):384–390. doi: 10.1016/s0959-4388(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 10.Ng J, et al. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416(6879):442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- 11.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19(1):1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 12.Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. 2014;8:338. doi: 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25(2):307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 14.Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19(19):8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gualdoni S, Albertinazzi C, Corbetta S, Valtorta F, de Curtis I. Normal levels of Rac1 are important for dendritic but not axonal development in hippocampal neurons. Biol Cell. 2007;99(8):455–464. doi: 10.1042/BC20060119. [DOI] [PubMed] [Google Scholar]
- 16.Luo L, et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379(6568):837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 17.Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol. 1981;82(2):358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 18.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8(1):43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 19.Bounoutas A, et al. Microtubule depolymerization in Caenorhabditis elegans touch receptor neurons reduces gene expression through a p38 MAPK pathway. Proc Natl Acad Sci USA. 2011;108(10):3982–3987. doi: 10.1073/pnas.1101360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalfie M, Thomson JN. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J Cell Biol. 1979;82(1):278–289. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng C, Diaz-Cuadros M, Chalfie M. Dishevelled attenuates the repelling activity of Wnt signaling during neurite outgrowth in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2015;112(43):13243–13248. doi: 10.1073/pnas.1518686112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du H, Chalfie M. Genes regulating touch cell development in Caenorhabditis elegans. Genetics. 2001;158(1):197–207. doi: 10.1093/genetics/158.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.deBakker CD, et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol. 2004;14(24):2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci. 2007;10(2):169–176. doi: 10.1038/nn1834. [DOI] [PubMed] [Google Scholar]
- 25.Alexander M, et al. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development. 2009;136(6):911–922. doi: 10.1242/dev.030759. [DOI] [PubMed] [Google Scholar]
- 26.Hu S, Pawson T, Steven RM. UNC-73/trio RhoGEF-2 activity modulates Caenorhabditis elegans motility through changes in neurotransmitter signaling upstream of the GSA-1/Galphas pathway. Genetics. 2011;189(1):137–151. doi: 10.1534/genetics.111.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer AG, Orita S, Malone CJ, Han M. A RHO GTPase-mediated pathway is required during P cell migration in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2001;98(23):13132–13137. doi: 10.1073/pnas.241504098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat Methods. 2013;10(10):1028–1034. doi: 10.1038/nmeth.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steven R, et al. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92(6):785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 30.van Haren J, et al. Dynamic microtubules catalyze formation of navigator-TRIO complexes to regulate neurite extension. Curr Biol. 2014;24(15):1778–1785. doi: 10.1016/j.cub.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Hekimi S, Kershaw D. Axonal guidance defects in a Caenorhabditis elegans mutant reveal cell-extrinsic determinants of neuronal morphology. J Neurosci. 1993;13(10):4254–4271. doi: 10.1523/JNEUROSCI.13-10-04254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen EB, Branda CS, Stern MJ. Genetic enhancers of sem-5 define components of the gonad-independent guidance mechanism controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev Biol. 1997;182(1):88–100. doi: 10.1006/dbio.1996.8473. [DOI] [PubMed] [Google Scholar]
- 33.Demarco RS, Struckhoff EC, Lundquist EA. The Rac GTP exchange factor TIAM-1 acts with CDC-42 and the guidance receptor UNC-40/DCC in neuronal protrusion and axon guidance. PLoS Genet. 2012;8(4):e1002665. doi: 10.1371/journal.pgen.1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heiman MG, Shaham S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell. 2009;137(2):344–355. doi: 10.1016/j.cell.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubiseski TJ, Culotti J, Pawson T. Functional analysis of the Caenorhabditis elegans UNC-73B PH domain demonstrates a role in activation of the Rac GTPase in vitro and axon guidance in vivo. Mol Cell Biol. 2003;23(19):6823–6835. doi: 10.1128/MCB.23.19.6823-6835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundquist EA, Reddien PW, Hartwieg E, Horvitz HR, Bargmann CI. Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development. 2001;128(22):4475–4488. doi: 10.1242/dev.128.22.4475. [DOI] [PubMed] [Google Scholar]
- 37.Struckhoff EC, Lundquist EA. The actin-binding protein UNC-115 is an effector of Rac signaling during axon pathfinding in C. elegans. Development. 2003;130(4):693–704. doi: 10.1242/dev.00300. [DOI] [PubMed] [Google Scholar]
- 38.Kishore RS, Sundaram MV. ced-10 Rac and mig-2 function redundantly and act with unc-73 trio to control the orientation of vulval cell divisions and migrations in Caenorhabditis elegans. Dev Biol. 2002;241(2):339–348. doi: 10.1006/dbio.2001.0513. [DOI] [PubMed] [Google Scholar]
- 39.Feig LA. Tools of the trade: Use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1(2):E25–E27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- 40.Kraynov VS, et al. Localized Rac activation dynamics visualized in living cells. Science. 2000;290(5490):333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 41.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440(7087):1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 42.Bernards A. Ras superfamily and interacting proteins database. Methods Enzymol. 2006;407:1–9. doi: 10.1016/S0076-6879(05)07001-1. [DOI] [PubMed] [Google Scholar]
- 43.Hilliard MA, Bargmann CI. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell. 2006;10(3):379–390. doi: 10.1016/j.devcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 45.Zheng C, Diaz-Cuadros M, Chalfie M. Hox genes promote neuronal subtype diversification through posterior induction in Caenorhabditis elegans. Neuron. 2015;88(3):514–527. doi: 10.1016/j.neuron.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyuksyutova AI, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302(5652):1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 47.Battisti AC, Fantetti KN, Moyers BA, Fekete DM. A subset of chicken statoacoustic ganglion neurites are repelled by Slit1 and Slit2. Hear Res. 2014;310:1–12. doi: 10.1016/j.heares.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404(6778):567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 49.Norris AD, Sundararajan L, Morgan DE, Roberts ZJ, Lundquist EA. The UNC-6/Netrin receptors UNC-40/DCC and UNC-5 inhibit growth cone filopodial protrusion via UNC-73/Trio, Rac-like GTPases and UNC-33/CRMP. Development. 2014;141(22):4395–4405. doi: 10.1242/dev.110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalpé G, Zhang LW, Zheng H, Culotti JG. Conversion of cell movement responses to Semaphorin-1 and Plexin-1 from attraction to repulsion by lowered levels of specific RAC GTPases in C. elegans. Development. 2004;131(9):2073–2088. doi: 10.1242/dev.01063. [DOI] [PubMed] [Google Scholar]
- 51.Goldberg JL. How does an axon grow? Genes Dev. 2003;17(8):941–958. doi: 10.1101/gad.1062303. [DOI] [PubMed] [Google Scholar]
- 52.Hutchins BI. Competitive outgrowth of neural processes arising from long-distance cAMP signaling. Sci Signal. 2010;3(118):jc1. doi: 10.1126/scisignal.3118jc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruthel G, Hollenbeck PJ. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J Neurosci. 2000;20(6):2266–2274. doi: 10.1523/JNEUROSCI.20-06-02266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, et al. Bimodal control of dendritic and axonal growth by the dual leucine zipper kinase pathway. PLoS Biol. 2013;11(6):e1001572. doi: 10.1371/journal.pbio.1001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topalidou I, Chalfie M. Shared gene expression in distinct neurons expressing common selector genes. Proc Natl Acad Sci USA. 2011;108(48):19258–19263. doi: 10.1073/pnas.1111684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng C, Jin FQ, Chalfie M. Hox proteins act as transcriptional guarantors to ensure terminal differentiation. Cell Reports. 2015;13(7):1343–1352. doi: 10.1016/j.celrep.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]