Significance
Pneumococcal disease represents a global health problem, especially for the young, the elderly, and the resource-limited. Disease progression begins with asymptomatic nasopharyngeal bacterial colonization before subsequent dissemination and disease (pneumonia, sepsis, and middle ear infection). Analysis of this transition from colonization to disease provided antigens that were tested in this study for directed vaccination against only the virulent subset of pneumococci. In so doing, a “smart” vaccine was sought that would address this disease broadly, effectively, and selectively.
Keywords: virulence, S. pneumoniae, pneumococcal, biofilm, vaccine
Abstract
Immunization strategies against commensal bacterial pathogens have long focused on eradicating asymptomatic carriage as well as disease, resulting in changes in the colonizing microflora with unknown future consequences. Additionally, current vaccines are not easily adaptable to sequence diversity and immune evasion. Here, we present a “smart” vaccine that leverages our current understanding of disease transition from bacterial carriage to infection with the pneumococcus serving as a model organism. Using conserved surface proteins highly expressed during virulent transition, the vaccine mounts an immune response specifically against disease-causing bacterial populations without affecting carriage. Aided by a delivery technology capable of multivalent surface display, which can be adapted easily to a changing clinical picture, results include complete protection against the development of pneumonia and sepsis during animal challenge experiments with multiple, highly variable, and clinically relevant pneumococcal isolates. The approach thus offers a unique and dynamic treatment option readily adaptable to other commensal pathogens.
Human–microbe interactions serve numerous symbiotic purposes. However, certain colonizing microorganisms have the capacity to become virulent and trigger disease. The two most common antimicrobial therapies, antibiotics and vaccines, must be reconsidered in this context because of the numerous pitfalls associated with traditional metrics of “success.”
Specifically, we suggest that treatment must be directed at a disease-progression state of a microbial population and not at the population more generally. Doing so offers the potential to optimize treatment and reduce unintended pathological consequences. In this paper, we present such an approach in the context of pneumococcal disease, culminating in a “smart” vaccine that directs an immune response to virulent cell populations while minimizing the disruption of avirulent commensal colonization.
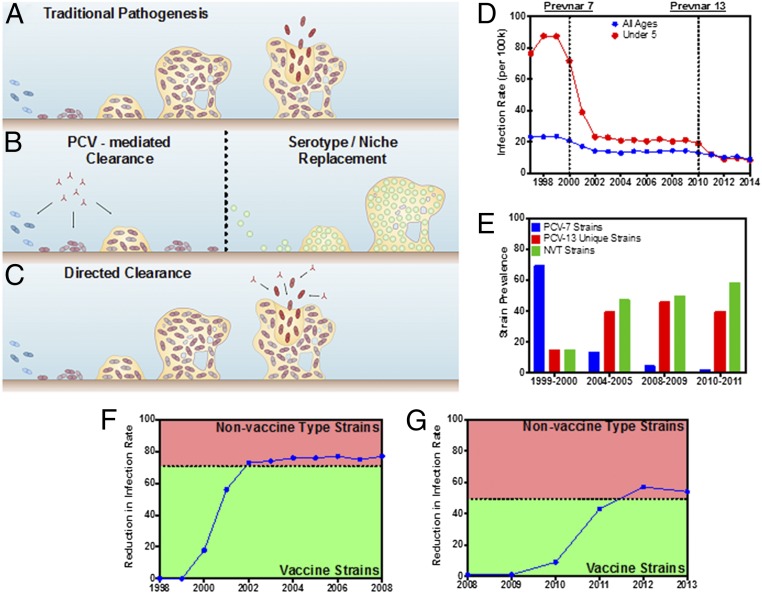
Streptococcus pneumoniae (the pneumococcus) is a regarded as a major human pathogen and is the most common cause of community-acquired pneumonia, bacterial meningitis, bacteremia, and otitis media (1). In addition, S. pneumoniae has been implicated as an important cause of sinusitis, septic arthritis, osteomyelitis, peritonitis, and endocarditis (1). Regardless of clinical manifestation, infection is always preceded by colonization of the nasopharynx, and >95% of children are colonized within the first few weeks or months of life by serotypes that are replaced sequentially as more serotypes are acquired (2–4). Interestingly, pneumococcal colonization is asymptomatic, and it is only upon external triggering (e.g., viral infection) that virulent S. pneumoniae subpopulations disseminate and cause disease (Fig. 1A) (5). The illnesses caused by this transition from carriage to disease result in a mortality rate of ∼15–20% in adults with an even higher rate in elderly patients (2–4). Pediatric cases include >20 million yearly occurrences, primarily middle ear infections, in the United States and account for the majority of emergency room admissions and associated antibiotic prescriptions, accruing billions of dollars in annual socioeconomic costs (6, 7). Invasive disease has a more devastating impact in resource-limited countries, with an estimated 1 million children (11% of all deaths below age 5 y) succumbing to pneumococcal infection annually (8–11).
Fig. 1.
S. pneumoniae pathogenesis outcomes and infectious disease statistics in the United States (1998–2013). (A) S. pneumoniae colonizes the human nasopharynx and produces a bacterial biofilm with an accompanying extracellular matrix capable of providing protection from external and host challenges. External triggers such as viral infection prompt the active release of virulent pneumococci that disseminate to secondary sites and cause disease. (B) Leading vaccination strategies [polysaccharide conjugate vaccines (PCVs), such as the Prevnar family] mediate protection against certain bacterial serotypes by promoting clearance of pneumococci before biofilm establishment. Clearing all bacteria opens the niche to colonization by nonvaccine serotypes or other bacterial species. (C) The strategy featured in this work mediates clearance of only virulent biofilm-released bacteria while maintaining the presence of the preexisting biofilm. (D) The annual infection rate per 100,000 people for the total population (blue) and children under the age of 5 y (red), 1998–2013. The first years following the introduction of Prevnar 7 and 13 are marked with dotted lines. (E) Prevalence of infectious pneumococcal strains, 1998–2013 (29). Strains are grouped into those covered by Prevnar 7 (blue), those covered by Prevnar 13 (red), and nonvaccine types (NVT; green). (F) The reduction in the annual infection rate in children under the age of 5 y from 1998–2008 relative to 1998–1999. The dashed line corresponds to the division between Prevnar 7 vaccine and nonvaccine type strains in 1999–2000 (35). (G) The reduction in the annual infection rate in children under the age of 5 y, 2008–2013. The dashed line corresponds to the division between Prevnar 13 vaccine and nonvaccine type strains in 2008–2009 (35).
As introduced above, effectively treating pneumococcal disease is difficult because of the multiple populations of S. pneumoniae with different characteristics, including cells localized to a colonizing biofilm and cells triggered for dissemination and disease. Antibiotic treatment options have become limited by the emergence of antibiotic resistance. Notably, before the 1990s, most S. pneumoniae strains demonstrated universal sensitivity to penicillin (12). Today, however, penicillin resistance varies from 5–60% in various parts of the world (13, 14). Of particular concern is the increase in multidrug-resistant S. pneumoniae strains demonstrating resistance to three or more drug classes (15–18), which creates substantial concerns about both the efficacy of current antibiotic regimens and the continual development of resistance. In addition, the formation of S. pneumoniae biofilm during colonization provides a barrier to effective antibiotic activity (19–23), thus limiting complete bacterial clearance and promoting the development of resistance (24, 25). Finally, and more importantly, even in the event of successful bacterial clearance with antibiotic treatment, there is a risk for recolonization by potentially more dangerous serotypes or alternative pathogens (e.g., Staphylococcus aureus), which are equally adept at biofilm formation and have effective mechanical tolerance of and high biological resistance to antibiotics (26–28).
Alternatively, two pneumococcal vaccine compositions are currently on the market in the United States: the Prevnar family (Pfizer) and Pneumovax (Merck). Prevnar vaccines contain capsular polysaccharides conjugated to the diphtheria CRM197 protein. The most recent composition is Prevnar 13, which is designed to encompass 13 of the most common invasive serotypes of S. pneumoniae and provides protection against 74–88% of invasive pneumococcal disease cases (29, 30). Pneumovax is a pneumococcal polysaccharide vaccine, introduced in 1977, that since 1983 has provided protection against 23 serotypes of S. pneumoniae (PPSV23) with 56–75% efficacy overall (29, 30). However, current vaccination strategies have met with incomplete success because of (i) an inability to account for and include all current and future S. pneumoniae serotypes capable of establishing nasopharyngeal residence and (ii), analogous to antibiotic treatment, the displacement of the asymptomatic vaccine-type S. pneumoniae biofilms with nonvaccine serotypes and by organisms (such as methicillin-resistant S. aureus or Haemophilus influenzae) capable of equal or greater pathologies (Fig. 1B) (31–33).
Recognizing the need to develop a new generation of pneumococcal vaccines, we present a strategy to direct protection against virulent biofilm-released S. pneumoniae while retaining their stable nasopharyngeal commensalism (Fig. 1C). Specifically, vaccine candidates (i.e., antigens) were discovered by building on the fundamental insight that S. pneumoniae colonizes the nasopharynx as a biofilm and that disease progression occurs when external triggers resulting from changes in the nasopharyngeal environment prompt escape from the asymptomatic biofilm of bacteria with a changed transcriptional profile associated with increased virulence (22, 23, 34). Although current vaccines have provided protection and expanded coverage over time (Fig. 1D), clinical data suggest the emergence of new serotypes that must be addressed in future vaccination efforts (Fig. 1 E–G) (35). Effectively, the propensity for serotype replacement, within or outside current treatment options, underscores the need to identify and use pneumococcal antigens capable of providing broad serotype coverage in a manner that will minimize asymptomatic biofilm disruption and the associated opportunities for niche replacement. One option in this regard is to target only those pneumococci triggered for virulent biofilm escape in response to changes in the nasopharyngeal environment (Fig. 1C).
Results and Discussion
Virulence-Associated Antigens Selected from and Screened Against Biofilm-Released, Clinically Conditioned S. pneumoniae.
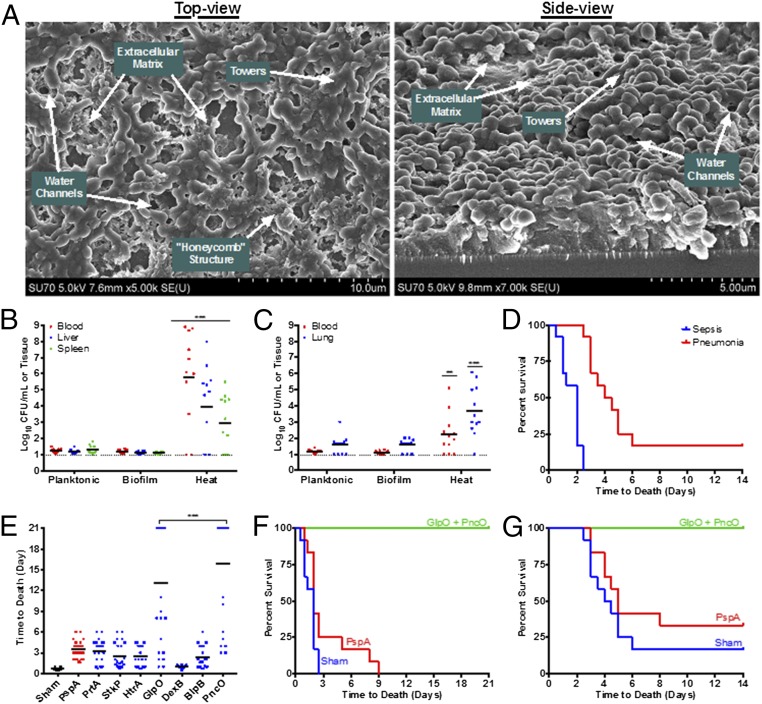
Through the combination of an in vitro biofilm model (Fig. 2A) and transcriptional analysis of the bacterial populations comprising this model (23, 36), antigens were identified that were significantly up-regulated in biofilm-released pneumococci demonstrating increased virulence. In addition to providing target antigens, the biofilm model served another key purpose in this study: Namely, S. pneumoniae is a human pathogen that, with the exception of a few strains, cannot cause invasive disease in mice. Thus, the biofilm model was used to condition a clinical isolate of S. pneumoniae (EF3030, serotype 19F) to become lethally infectious. Specifically, EF3030 cells released from biofilms by increased temperature (38.5 °C, mimicking fever) induced septicemia and death in mice and thus offered a clinically relevant surrogate model of human pneumococcal disease (Fig. 2 B–D). Broth-grown (planktonic) EF3030 pneumococci or those mechanically isolated from biofilms had no such virulence. As such, the biofilm model enables clinically conditioned S. pneumoniae strains to be subsequently assessed in mouse protection assays, allowing a substantial increase in the number of strains tested in this study.
Fig. 2.
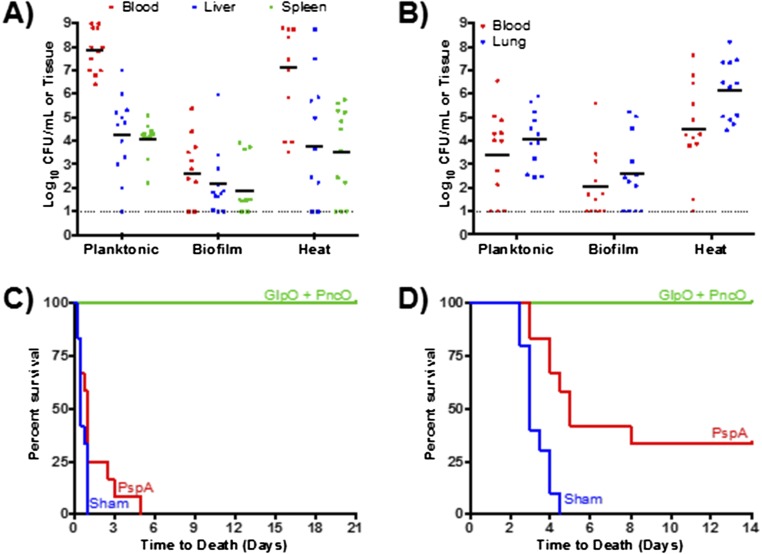
Antigen identification and S. pneumoniae conditioning through an in vitro biofilm model. (A) S. pneumoniae were seeded on epithelial cells, and the biofilm structure was investigated using SEM. Visible in these images are the extracellular matrix, water channels, tower formations, and the honeycomb structure that pneumococci form with larger biofilms. (B and C) Mouse bacterial burden was determined after i.p. injections (sepsis model) (B) or aspiration with anesthesia (pneumonia model) (C) using broth-grown (Planktonic), biofilm-associated (Biofilm), or biofilm heat-released (Heat) S. pneumoniae strain EF3030. Each dot in the graphs represents an individual mouse. The dotted line represents the limit of detection for bacterial counts. (D) Time-to-death assessment of mice inoculated with biofilm heat-released bacteria; mice that were inoculated with either planktonic or biofilm-associated bacteria did not die in any challenge model. (E–G) Mice were immunized with various antigens and challenged with biofilm heat-released EF3030 in sepsis (E and F) and pneumonia (G) models. ***P < 0.001, compared with planktonic and biofilm samples (B and C) and PspA (E).
Initial protection then was investigated by immunizing mice with a range of promising antigen targets (Table S1) selected on the basis of (i) conserved sequence homology across S. pneumoniae strains (thus offering broad-coverage potential) and, critically, (ii) specific prominence in the virulent, biofilm-released bacterial population compared with asymptomatic biofilm pneumococci. Namely, antigen targets up-regulated in biofilm-released bacteria were prepared as recombinant proteins and tested for protection relative to the well-established S. pneumoniae surface protein antigen (PspA), which is one of the best-studied protein protective vaccine candidates and also is up-regulated during virulence transition (Fig. 2E) (36–38). Under these conditions, all antigens except DexB showed protection comparable to PspA, with two antigens, GlpO (an α-glycerophosphate oxidase) and PncO (a bacteriocin ABC transporter transmembrane protein), demonstrating promising individual protection surpassing that of PspA. Complete protection and effective bacterial reduction were conferred upon immunization with both antigens (Fig. 2 F and G); thus, remaining analyses were conducted using these two antigens in combination.
Table S1.
Antigen cloning summary
| Antigen gene | Primers | Source | Restriction sites | Plasmid | Name |
| dexB | F: TAAGCACATATGCAAGAAAAATGGTGGCATAATGCCGTAG | D39 | NdeI/XhoI | pET21c | pCJ05 |
| R: TAAGCACTCGAGTTCCACACAGAAAGCATCCCA | |||||
| htrA | F: TAAGCACCATGGGGAAACATCTAAAAACATTTTACAA | D39 | NcoI/XhoI | pET20b | pCJ06 |
| R: TAAGCACTCGAGAGATTCTAAATCACCTGAAC | |||||
| glpO | F: TAAGCAGAGCTCGAATTTTCAAAAAAAACACGTGAATTGTC | D39 | SacI/XhoI | pET21c | pCJ07 |
| R:TAAGCACTCGAGATTTTTTAATTCTGCTAAATCGTTGTTAG | |||||
| stkP | F:TAAGCACATATGATCCAAATCGGCAAGATTTT | D39 | NdeI/NotI | pET21c | pCJ08 |
| R: TAAGCAGCGGCCGCAGGAGTAGCTGAAGTTGTTTTA | |||||
| blpB | F: TAAGCACCATGGGGAATCCTAATCTTTTTAGAAG | D39 | NcoI/XhoI | pET20b | pCJ09 |
| R: TAAGCACTCGAGATCAGAATGGGTTAAAATTTTA | |||||
| pncO | F:TAAGCACATATGAAAAAGTATCAACTTCTATT | EF3030 | NdeI/XhoI | pET21c | pCJ10 |
| R: TAAGCACTCGAGCCCCAAGACCCTATGTAGAAAA | |||||
| prtA | F: TAAGCAGAGCTCAAAAAAAGCACAGTATTGTC | D39 | SacI/XhoI | pET21c | pCJ12 |
| R:TAAGCACTCGAGATCTTGATTTTTTTTCTTCAAT |
Antigen Delivery Using Co-PoP Liposomal Surface Display.
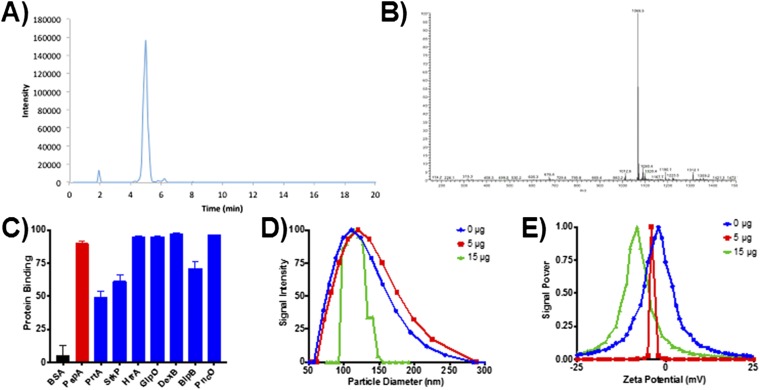
The codelivery of GlpO and PncO, both recombinantly produced with 6× histidine tags, was facilitated using a cobalt porphyrin-phospholipid (Co-PoP) liposomal carrier capable of surface-orienting and delivering multiple his-tagged peptide-based antigens (Fig. S1) (39). The liposomal device thus offers a unique vaccine formulation based on a simple and stable antigen–carrier complex without the need for advanced chemical conjugation. Furthermore, the technology is well aligned with the antigen discovery and production techniques offered by the aforementioned biofilm model and well-established recombinant protein production. Therefore, we adopted the technology here in the delivery of GlpO and PncO. Looking forward, the Co-PoP delivery platform offers even more potential in the way of unprecedented valency of discovered antigens via the surface localization and presentation of hundreds of additional protein or peptide products.
Fig. S1.
Characterization of Co-PoP liposomal delivery device. (A and B) HPLC analysis of Co-PoP (A) and the resulting mass spectrum (B). (C) Liposome-binding analysis for all proteins used in the study. (D and E) Particle diameter (D) and zeta potential (E) of liposomes formulated with 0, 5, and 15 μg of PspA.
Directed Response and Extended Coverage Provided by Combined Virulent Antigens.
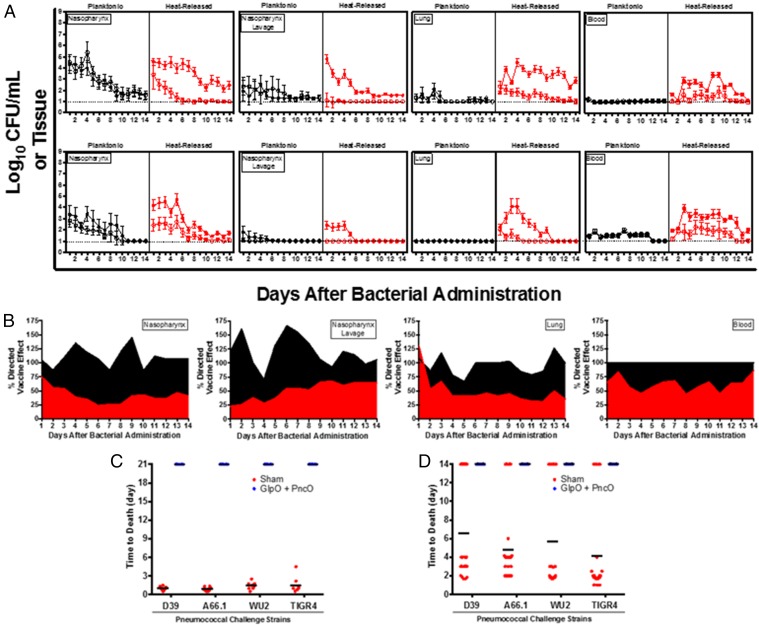
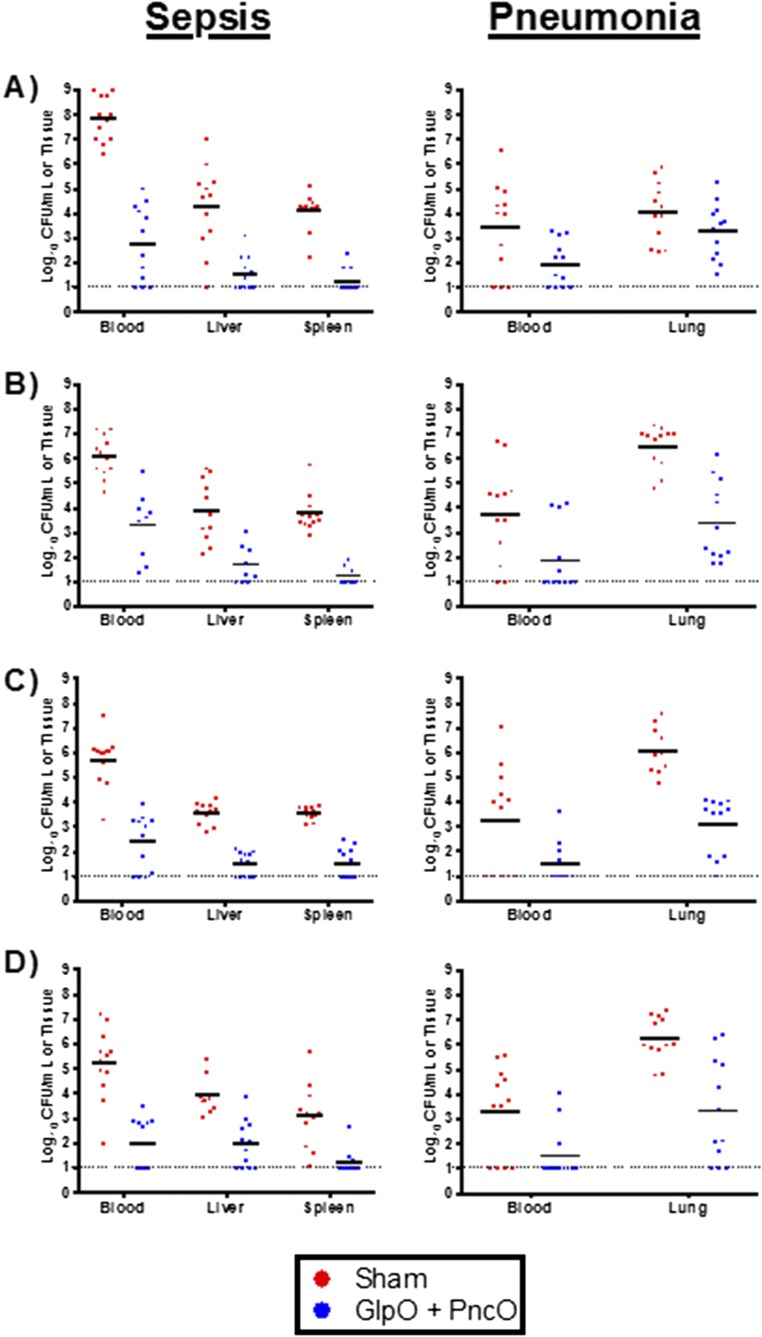
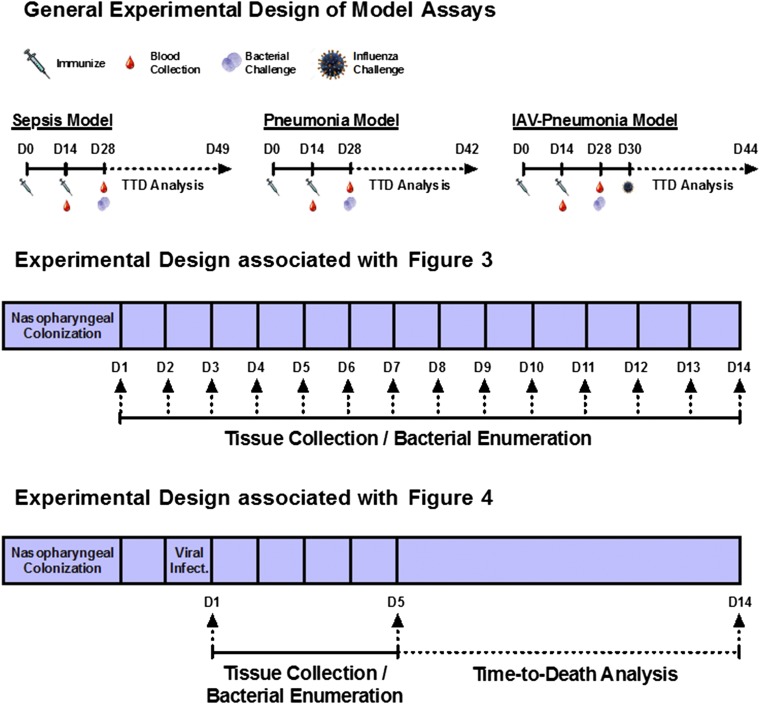
The directed nature of the antigens was tested in a series of experiments presented in Fig. 3A. Across different anatomical locations representative of bacterial colonization (nasopharynx) and displacement (nasopharynx lavage), disseminating pneumonia (lung), and invasive septicemia (blood), vaccinated and nonvaccinated mice were challenged with planktonic and in vitro biofilm-released S. pneumoniae, and bacterial clearance was monitored over time. In the absence of an external stimulus (e.g., viral infection), mice will remain colonized with planktonic D39 or EF3030 for 1–3 wk without infection of the lower respiratory tract or the development of bacteremia. Biofilm-released bacteria demonstrate a colonization pattern similar to that of planktonic bacteria but also have the propensity to disseminate into secondary anatomical sites and cause disease. Thus, planktonic EF3030 and D39 cells provided a clearance baseline to compare bacterial loads using biofilm-released pneumococci challenge. Clearance of biofilm-released bacteria was mediated only in vaccinated mice; the bacterial load was increased significantly and was lethal in nonvaccinated mice. Interestingly, the rate of clearance of planktonic bacteria was unchanged despite vaccination. A comparison of bacterial burden across the planktonic and biofilm-released EF3030 clinical isolate is provided in Fig. 3B, emphasizing a directed vaccination strategy using GlpO and PncO.
Fig. 3.
Directed clearance of biofilm-released bacteria and protection against mouse-passaged challenge strains. (A) Bacterial burden at various anatomical sites was determined daily in unimmunized (filled circles) and GlpO + PncO immunized (open circles) mice. Mice were inoculated intranasally without anesthesia with planktonic or biofilm heat-released EF3030 (Upper) or D39 (Lower) bacteria. (B) Comparative analysis of the planktonic (black) and biofilm-released (red) EF3030 clinical isolate data from A. A value of 100% represents no difference between immunized and unimmunized subjects; values below 100% indicate a directed response. A full statistical comparison is presented in Table S5. (C and D) Protective capabilities of GlpO + PncO immunization were evaluated further in sepsis (C) and pneumonia (D) models with established mouse-passaged pneumococcal bacteria. Dotted lines represent the limit of detection for bacterial counts.
Table S5.
Statistical comparison of the percent of directed vaccine effect for anatomical locations indicated in Fig. 3B
| Day | Mean diff. | 95% CI of diff. | Significant |
| Nasopharynx | |||
| 1 | 30 | 24.02–35.98 | Yes |
| 2 | 31 | 25.02–36.98 | Yes |
| 3 | 56 | 50.02–61.98 | Yes |
| 4 | 96 | 90.02–102 | Yes |
| 5 | 84 | 78.02–89.98 | Yes |
| 6 | 83 | 77.02–88.98 | Yes |
| 7 | 61 | 55.02–66.98 | Yes |
| 8 | 94 | 88.02–99.98 | Yes |
| 9 | 104 | 98.02–110 | Yes |
| 10 | 43 | 37.02–48.98 | Yes |
| 11 | 75 | 69.02–80.98 | Yes |
| 12 | 71 | 65.02–76.98 | Yes |
| 13 | 60 | 54.02–65.98 | Yes |
| 14 | 66 | 60.02–71.98 | Yes |
| Nasopharynx lavage | |||
| 1 | 98 | 92.02–104 | Yes |
| 2 | 135 | 129–141 | Yes |
| 3 | 64 | 58.02–69.98 | Yes |
| 4 | 43 | 37.02–48.98 | Yes |
| 5 | 96 | 90.02–102 | Yes |
| 6 | 112 | 106–118 | Yes |
| 7 | 100 | 94.02–106 | Yes |
| 8 | 83 | 77.02–88.98 | Yes |
| 9 | 40 | 34.02–45.98 | Yes |
| 10 | 25 | 19.02–30.98 | Yes |
| 11 | 61 | 55.02–66.98 | Yes |
| 12 | 50 | 44.02–55.98 | Yes |
| 13 | 33 | 27.02–38.98 | Yes |
| 14 | 41 | 35.02–46.98 | Yes |
| Lung | |||
| 1 | −22 | −27.98 to −16.02 | Yes |
| 2 | 33 | 27.02–38.98 | Yes |
| 3 | 52 | 46.02–57.98 | Yes |
| 4 | 37 | 31.02–42.98 | Yes |
| 5 | 26 | 20.02–31.98 | Yes |
| 6 | 59 | 53.02–64.98 | Yes |
| 7 | 54 | 48.02–59.98 | Yes |
| 8 | 59 | 53.02–64.98 | Yes |
| 9 | 58 | 52.02–63.98 | Yes |
| 10 | 49 | 43.02–54.98 | Yes |
| 11 | 47 | 41.02–52.98 | Yes |
| 12 | 54 | 48.02–59.98 | Yes |
| 13 | 77 | 71.02–82.98 | Yes |
| 14 | 65 | 59.02–70.98 | Yes |
CI, confidence interval; Mean Diff., mean difference between planktonic and biofilm-released values. All adjusted P values <0.0001.
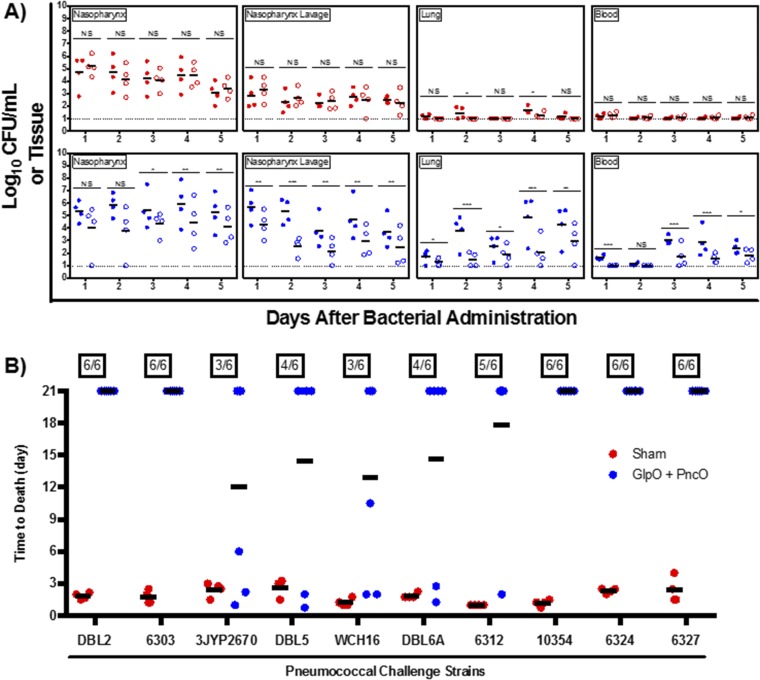
However, the potential of S. pneumoniae for antigenic drift emphasizes the need for any new antigens to be general and effective across a wide range of challenge strains (Tables S2 and S3). To this end, the antigens were tested in mice infected with a range of S. pneumoniae strains chosen for the notable difficulty of protecting against these strains and for the variability in serotype and genetic background among the strains. Complete protection was provided for a panel of strains across both sepsis and pneumonia challenge models (Fig. 3 B and C and Figs. S2 and S3). Ten additional strains were tested in protection experiments, in which the average time to death in treated mice ranged from 12–21 d as opposed to <3 d for controls (Fig. S4). Importantly, like EF3030, several of the S. pneumoniae strains tested required virulent conditioning using the in vitro biofilm model, thus emphasizing the importance of this tool in both antigen discovery and broad challenge assessment. These challenge assays included strains of serotype 12, 15B, and 27 that are not covered by current vaccines and suggest that (i) this vaccine composition can offer protection against strains currently circulating and causing disease in the population and (ii) the methodology of in vitro biofilm release can be used to produce additional mouse-virulent bacterial populations (beyond those tested here) for future vaccine protection screening. The combined results emphasize a degree of coverage not previously reported when using a protein-based antigen with the added potential to identify and test new antigens continually in response to disease variation over time. Finally, broad protection is supported by a sequence-conservation analysis of the new antigens across S. pneumoniae serotypes (Table S4). The results therefore support the potential for widespread protection and resistance to antigenic drift.
Table S2.
S. pneumoniae strains used in the current study
| Strain | Capsule type (serotype) | Virulence pattern | Included in current vaccines | Protection in this study, % |
| D39 | 2 | 1 | Yes | 100 |
| D39, heat-released | 2 | 4 | Yes | 100 |
| DBL2 | 2 | 2 | Yes | 100 |
| A66.1 | 3 | 1 | Yes | 100 |
| WU2 | 3 | 1 | Yes | 100 |
| ATCC6303 | 3 | 2 | Yes | 100 |
| 3JYP2670 | 3 | 2 | Yes | 50 |
| TIGR4 | 4 | 2 | Yes | 100 |
| DBL5 | 5 | 2 | Yes | 66 |
| WCH16, heat-released | 6A | 4 | Yes | 50 |
| DBL6A | 6B | 2 | Yes | 66 |
| ATCC-6312, heat-released | 12F | 4 | No | 83 |
| ATCC-10354, heat-released | 15B | 4 | No | 100 |
| EF3030 | 19F | 3 | Yes | 100 |
| EF3030, heat-released | 19F | 4 | Yes | 100 |
| ATCC-6324, heat-released | 24 | 4 | No | 100 |
| ATCC-6327, heat-released | 27 | 4 | No | 100 |
Table S3.
Virulence pattern description
| Virulence pattern classification | Carriage | Lung infection | Sepsis | Comments |
| 1 | — | ++ | +++ | Bacteria carries poorly and transitions to the blood wherever placed |
| 2 | ± | ++ | ++ | Bacteria causes strong infection wherever placed; occasionally will transition to the blood and cause death |
| 3 | ++ | +++ | ± | Bacteria carries well and infects locally but is unlikely to kill; rarely transitions to the blood and causes death |
| 4 | ++ | +++ | ++ | Bacteria carries well and causes strong infection wherever placed and can transition to the blood |
Fig. S2.
Characterization of biofilm-released D39 bacterial virulence and vaccine protective capabilities. (A and B) Bacterial burden was determined for pneumococcal populations after i.p. injections (sepsis model) (A) or intranasal aspiration after anesthesia (pneumonia model) (B). Mice were inoculated with broth-grown (Planktonic), biofilm-associated (Biofilm), or biofilm heat-released (Heat) D39. Each dot in the graphs represents an individual mouse; an “x” represents a mouse that became moribund and was killed before the end of the experiment. The dotted line represents the limit of detection for bacterial counts. (C and D) Time-to-death assessment of mice inoculated with heat-released bacteria from the sepsis (C) and pneumonia (D) models after immunization with PspA or GlpO + PncO.
Fig. S3.
Bacterial burden associated with protection against mouse-passaged pneumococcal challenge strains. Mice were immunized with GlpO + PncO and challenged with planktonic D39 (A), A66.1 (B), WU2 (C), and TIGR4 (D) cells in either a sepsis or pneumonia model. Dotted lines represent the limit of detection for bacterial counts.
Fig. S4.
Characteristics of directed clearance and protection against an expanded set of strains. (A) Directed clearance of biofilm-released bacteria in passively immunized mice. Bacterial burden at various anatomical sites was determined daily in unimmunized (filled circles) and GlpO + PncO passively immunized (open circles) mice. Mice were inoculated intranasally without anesthesia with planktonic (red) or biofilm heat-released (blue) EF3030. Dotted lines represent the limit of detection for bacterial counts. (B) Protective capabilities of GlpO + PncO immunization were evaluated further in the sepsis model using an expanded set of mouse pneumococcal strains. *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant.
Table S4.
PncO and GlpO antigen description and analysis
| Gene | Size, bp | Function | Virulent gene expression (log2) relative to | Average log2 fold change | Surface accessible | Strain conservation, % homology | |||
| Planktonic | Biofilm | Full protein | Surface-accessible regions | Surface-accessible epitopes | |||||
| pncO | 690 | Bacteriocin ABC transporter transmembrane protein | 8.5 | 4.8 | 6.6 | Yes | 95 | 93 | 97 |
| glpO | 1,827 | α-Glycerophosphate oxidase | 9 | 5.9 | 7.4 | Yes | 99 | 98 | 98 |
Gene expression values were determined by Pettigrew et al. (36), and surface accessibility was established using the InterPro database (www.ebi.ac.uk/interpro/). Strain conservation was calculated by accounting for gap and mismatches in the gene sequences of 30 S. pneumoniae strains with complete genomes available in BLAST (blast.ncbi.nlm.nih.gov/Blast.cgi). Epitope regions were predicted with the BepiPred linear epitope prediction method using IEDB Analysis Resource (tools.immuneepitope.org/bcell/).
Extension to an in Vivo Model of Virulence Progression.
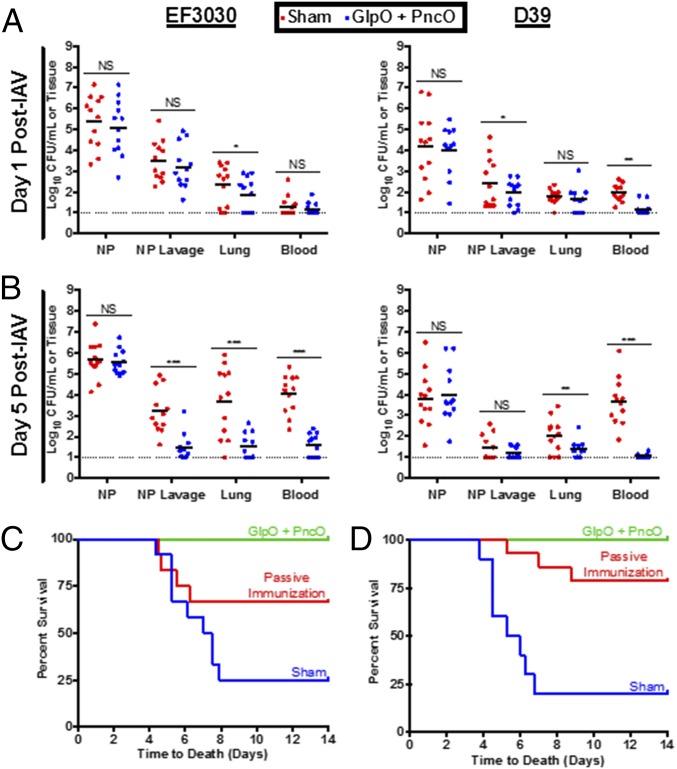
In a final set of experiments presented in Fig. 4, we explored protection in an in vivo model that mimics the clinical progression of pneumococcal disease onset. Epidemiological evidence suggests that pneumococcal disease is strongly associated with a concomitant infection with upper respiratory tract viruses, such as influenza A virus (IAV) (40, 41). Mice were infected intranasally with IAV 48 h after colonization with S. pneumoniae, a protocol designed to mediate the release of virulent pneumococci from colonizing biofilms for subsequent dissemination to the lungs and blood. Mice vaccinated with GlpO and PncO displayed a limited spread of D39 and EF3030 S. pneumoniae strains in this clinically relevant model, with the reduced onset of dissemination of virulent organisms indicated on day 1 post viral infection and significantly pronounced reduction in the lung and the blood on day 5 (Fig. 4 A and B). Of major importance, the nasopharyngeal burden in immunized and nonimmunized animals remained unchanged, suggesting that harmless and potentially beneficial commensal colonization was unaffected. This result further supports a paradigm shift in protection against accidental pathogens or those commensals that colonize asymptomatically but have the capacity to cause disease in response to inflammation or other external triggers.
Fig. 4.
Bacterial dissemination and time-to-death assessment of mice stably colonized with pneumococci and triggered with IAV. (A and B) Bacterial burden of EF3030 or D39 in unimmunized or GlpO + PncO immunized mice was measured at 1 (A) or 5 (B) d postinfection with IAV. The dotted line represents the limit of detection for bacterial counts. (C and D) Protective capabilities of traditionally or passively GlpO + PncO immunized mice against in vivo IAV-mediated bacterial release of EF3030 (C) or D39 (D). *P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant.
When using this same in vivo model of IAV-induced pneumococcal disease, full protection against septicemia and death was provided by the combination of GlpO and PncO using traditional immunization, whereas partial protection was accomplished via adoptive/passive vaccination strategies (Fig. 4 C and D and Fig. S4). The latter result indicates an important role of the humoral immune system arm in the mechanism driving the results obtained throughout this study and suggests that an additional T-cell response also contributes. Interestingly, these observations are in line with the recognition that priming of TH17 cells is an important factor mediating the clearance of pathogens at mucosal surfaces (42). However, a more mechanistic analysis of individual and combined antigens is required to characterize better the immunological underpinnings of the results presented. We also recognize the potential for immune response cross-talk, in that analogous antigens might be targeted in other commensal organisms. For the primary antigens tested in this study, GlpO is present in other streptococcal species as well as in enterococci, lactococci, and lactobacilli. However, PncO appears to be restricted to pneumococci, based upon homology analysis. Similar to the pneumococci emphasis of this study, we would expect that cross-reactivity with other members of the host microbial flora would be restricted to those populations that have up-regulated versions of the antigens tested here. However, a more complete analysis of microbial content postvaccination would be required to elucidate the impact on global microflora variation. This analysis would be a fruitful and interesting topic of future research.
Conclusions
In summary, the availability of tools derived from a better understanding of the mechanisms involved in pneumococcal biofilm establishment and transition to disease and of effective delivery technology has enabled a directed and potent strategy to induce an antibody-mediated immune response against a disease-causing subset of a commensal microbial population. Specifically, antigens associated with biofilm-released, virulent pneumococci have demonstrated unequivocal protection against challenge and reduction in the bacterial burden of a range of clinically relevant S. pneumoniae strains under varying disease conditions. The results offer a response to a globally relevant disease and to growing concerns associated with current treatment options, including risks posed by emerging serotype- and niche-replacement pathogens. Importantly, the tools enabling the results of this work can be applied to identify new antigens continually and simultaneously to address the diversity and antigenic drift potential of S. pneumoniae and other pathogens that exhibit similar virulence progression.
Materials and Methods
Ethics Statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (43). The protocols were approved by the Institutional Animal Care and Use Committee at the University at Buffalo, The State University of New York. All bacterial inoculations and treatments were performed under conditions designed to minimize any potential suffering of the animals.
Vaccine Formulation and Immunization.
Antigen Co-PoP liposomal carrier vectors were generated as described previously (39). Each injection dose contained 25 µg of monophosphoryl lipid A (MPLA) in liposomes comprising 1,2-dioleoyl-sn-glycero-3-phosphocholine:cholesterol:MPLA:Co-PoP at a molar ratio of 50:30:5:5. After liposomes were dissolved in chloroform in a test tube, the solvent was evaporated, and the film was further dried under vacuum overnight. Liposomes then were rehydrated with PBS and sonicated. The binding ability of recombinant antigens was evaluated by incubating 25 μg of protein with 20 μg of liposomes in 200 μL PBS within a well of a 96-well plate. Fluorescence in the FRET channel (excitation: 430 nm, emission: 525 nm) was measured periodically with a fluorescence microplate reader (Tecan Infinite II). Data were normalized to the FRET signal for protein without addition of liposomes. Once binding was confirmed, antigens were incubated at 4 °C with Co-PoP liposomes overnight before animal injections. Dynamic light scattering was used to evaluate the particle diameter and zeta potential of liposomes containing three concentrations of PspA (0, 5, and 15 μg).
Outbred 6-wk-old female CD-1 mice (Harlan Laboratories) were used in immunization experiments. Mice were immunized by s.c. injection (200 μL). All samples contained PBS as the background solution, and final antigen (Table S1) doses ranged from 5–15 μg. The sham vaccination control was the Co-PoP delivery device in PBS. When combined, PncO and GlpO (Table S4) were administered at 15 μg each. After 14 d, mice were boosted with the same formulations. At day 14 and day 28, serum samples were collected from the mice by retro-orbital bleeding. For passive immunizations, respective sera were diluted 10 times and administered via i.p. injection (200 μL).
Bacterial Preparation and Biofilm Release.
The bacterial strains used in this study are listed in Tables S2 and S3 and were initially grown on Todd–Hewitt agar plates supplemented with 0.5% yeast extract and 5% (vol/vol) sheep blood and were incubated overnight at 37 °C. Single colonies were used to inoculate 5 mL of Todd–Hewitt broth containing 0.5% yeast extract and were incubated at 37 °C to an OD600 of 0.6. At this point, mouse-passaged strains of S. pneumoniae (which display a virulent phenotype) were used for challenge studies after one washing with and resuspension in PBS (Fig. S5).
Fig. S5.
Schedule of immunization and bacterial challenge.
Other S. pneumoniae strains are clinical isolates that do not demonstrate a virulent phenotype in mice unless conditioned using an in vitro biofilm release model. Specifically, NCI-H292 epithelial cells were cultured in RPMI-1640 medium in T75 flasks at 37 °C and 5% CO2. After reaching 100% confluency, H292 cells were prefixed in 4% (wt/vol) buffered paraformaldehyde at 34 °C for 48 h followed by three washes with PBS. Pneumococci grown in chemically defined bacterial growth medium (CDM) (JRH Biosciences) then were seeded onto fixed H292 cells, and the medium was changed every 12 h. Formed biofilms were exposed to heat (38.5 °C) for 4 h, and released cells were collected by centrifugation, washed and resuspended in PBS, and quantified by OD600 measurement. Biofilm-associated cells were disrupted by gentle pipetting, collected by centrifugation, washed and resuspended in PBS, and quantified by OD600 measurement.
Challenge Models.
To induce sepsis or pneumonia, mice were administered i.p. or intranasally (with isoflurane), respectively, with 1 × 104 to 1 × 106 cfu of pneumococci strains (Tables S2 and S3). To induce colonization, mice were administered 1 × 106 cfu bacteria intranasally without isoflurane. To mimic influenza-induced pneumonia, pneumococci colonization (with biofilm-grown EF3030 or D39) was followed 48 h later by intranasal inoculation with 40 pfu of IAV in 50 µL of PBS. Mouse-adapted A/PR/8/34 (H1N1) (ATCC VR-95) was used, and viral titers were determined by plaque assays. Mice were monitored every 4 h for signs of morbidity (huddling, ruffled fur, lethargy, and abdominal surface temperature). Mice found to be moribund were killed via CO2 asphyxiation and cervical dislocation. When IAV addition was replaced with background PBS inoculation, mice remained colonized by S. pneumoniae strains D39 and EF3030 for 1–3 wk without lethargy, huddling, and ruffled fur (as observed with viral inoculation), infection of the lower respiratory tract, or the development of bacteremia.
All remaining experimental details are described in SI Materials and Methods.
SI Materials and Methods
Reagents.
Bacterial and cell-culture media and reagents were purchased from Fisher Chemical and Sigma-Aldrich. CDM was obtained from JRH Biosciences. Sheep blood was purchased from Hemostat Laboratories. All remaining reagents were purchased from Sigma-Aldrich.
Recombinant Antigen Preparation.
All antigen genes were cloned from the genomic DNA of S. pneumoniae strains using the primers summarized in Table S1. Each PCR product then was inserted into pET expression vectors as outlined in Table S1 using flanking restriction sites designed within the primers. Constructs were verified by colony PCR and restriction digest analysis and then were chemically transformed into BL21(DE3) with resulting single-colony transformants cultured in 3 mL of lysogeny broth (LB) at 37 °C with shaking before 15% (vol/vol) glycerol stock storage at −80 °C. Expression was initially confirmed by 3- to 5-mL LB cultures started from glycerol stocks and cultured at 37 °C with shaking until an OD600 of 0.4 was achieved. Plasmid selection antibiotics were included in cultures as needed. Then cultures were induced with isopropyl β-d-1-thiogalactopyranoside (IPTG) (1 mM) for 1 h. Upon collection via centrifugation, cells were washed twice with PBS and resuspended in 25 mM Tris⋅HCl before boiling with loading dye and expression analysis/confirmation via SDS/PAGE.
Glycerol stocks were then used to start overnight 3-mL LB cultures incubated at 37 °C with shaking and used to inoculate [1% (vol/vol)] 1 L of terrific broth; cultures then were grown to a concentration of 0.4–0.6 OD600 under the same culture conditions. IPTG induction (100 µM) occurred at this point, before continued culture overnight at 22 °C. After clarification by centrifugation, cells were resuspended in buffer A [50 mM Na2PO4, 500 mM NaCl, and 10% (vol/vol) glycerol] and were lysed via French press. The resulting lysate was clarified by centrifugation, and the resulting lysate supernatant was clarified further via ultracentrifugation at 142,000 × g (relative centrifugal force) for 45 min. The lysate collected was confirmed by SDS/PAGE at this stage before column purification.
Antigen protein products then were purified by passing the lysate supernatant through a fast protein LC column (GE Healthcare HisTrap HP, 1 × 1 mL) using 2% (vol/vol) buffer B [50 mM Na2PO4, 500 mM NaCl, 10% (vol/vol) glycerol, and 250 mM imidazole] and 98% (vol/vol) buffer A as the mobile phase. The protein was eluted with 100% buffer B, and a UV absorbance of 280 nm was used to detect the fractions containing protein in the elution. Those fractions were pooled, and protein content characterized by SDS/PAGE and quantified by the Pierce Micro BCA Protein Assay assessment.
SEM.
Biofilms grown in vitro for 48 h were fixed using 2.5% (vol/vol) glutaraldehyde, 0.075% ruthenium red, and 0.075 M lysine acetate in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h at 22 °C. This procedure has been shown to retain carbohydrate structures and improve the preservation of biofilm morphology (44). Samples were washed three times without shaking for 15 min at 22 °C in 0.075% ruthenium red in 0.2 M sodium cacodylate buffer and then were dehydrated with a graded series of ethanol solutions [10, 30, 50, 75, 95, and 100% (vol/vol)] at 22 °C, for 15 min at each step. Samples were exchanged into 100% hexamethyldisilazane and allowed to air dry before being mounted onto stubs, carbon coated, and analyzed using an SU70 scanning electron microscope at an acceleration voltage of 5.0 kV (available through the South Campus Instrumentation Center, University at Buffalo, The State University of New York, Buffalo, NY).
Tissue Bacterial Count.
At predefined time points (24 and 48 h postinfection for i.p. and intranasal challenges, respectively) or upon becoming moribund, mice were killed. A combination of nasopharynx tissue, nasopharyngeal lavage fluid, lung, liver, spleen, and blood samples was collected, and the bacterial burden was determined as described previously (45). Briefly, tissue and organ homogenate, lavage fluid, and blood were homogenized (Tissue-Tearor, Biospec Products, Inc., setting 10 for 30 s or until homogenized completely) to ensure dissociation of bacterial aggregates and then were serially diluted on tryptic soy and 5% (vol/vol) blood agar plates before enumeration.
Disease Incidence Analysis.
The data presented in Fig. 1 D, F, and G were obtained from the Centers for Disease Control and Prevention’s Active Bacterial Core Surveillance (ABC) for Streptococcus pneumoniae (www.cdc.gov/abcs/reports-findings/surv-reports.html). The infection rate for infectious pneumococcal disease was obtained from the annual reports from 1998–2014 for both children under age 5 y and the total population. The data presented in Fig. 1E were obtained from table 2 in ref. 35, which provided information regarding pneumococcal strain prevalence from 1998–2011; the strain distribution for children under age 5 y was organized into three different groups: PCV-7, PCV-13, and nonvaccine-type strains. Fig. 1F represents the efficacy of the PCV-7 vaccine by plotting the percent reduction in infection rate following the introduction of the vaccine. The baseline infection rate was determined by averaging the rates from 1998–1999. Similarly, the efficacy of the PCV-13 vaccine (Fig. 1G) was demonstrated by plotting the percent reduction in infection rate using the 2008–2009 infection rates as a baseline. The dashed lines in Fig. 1 F and G correspond to the prevalence of the PCV-7 and PCV-13 strains, respectively (35), 1 y before the introduction of the vaccines.
Statistical Analysis.
Column comparisons were analyzed for statistical significance using a two-tailed Student t test for unpaired data. Multivariance analysis was done using one-way ANOVA that was corrected using the Bartlett variance test and for multiple comparisons using the Bonferroni multiple-comparison test. For both tests, a P value of 0.05 was considered significant. For statistical comparison of the directed clearance data of Fig. 3A, fractional differences between average log10 bacterial load values obtained from immunized and unimmunized subjects were calculated, and 1 minus this value was plotted as a percentage (i.e., [1− (unimmunized value − immunized value)/unimmunized value]×100) for subjects challenged with planktonic and biofilm-released pneumococci (Fig. 3B). For this comparison, a two-way ANOVA analysis was performed using the Sidak test to correct for multiple comparisons using statistical hypothesis testing. Statistical analysis was performed using GraphPad Prism software (version 6.0h.283; GraphPad Software Inc.). All data resulted from a minimum of three samples; animal experiments used 6–12 animals per group.
Acknowledgments
We thank Randall Smith (University at Buffalo, The State University of New York) and David Briles (University of Alabama at Birmingham) for assistance with recombinant protein purification and provision of S. pneumoniae strains, respectively. This work was supported by Swedish Medical Research Council (VR) Award 2015-03074 (to A.P.H.), NIH Awards AI122964 and DP5OD017898 (to J.F.L.), and NIH Awards AI088485 and AI117309 (to B.A.P.). C.H.J. is the recipient of a University at Buffalo, The State University of New York Schomburg Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603007113/-/DCSupplemental.
References
- 1.Hausdorff WP, Hoet B, Adegbola RA. Predicting the impact of new pneumococcal conjugate vaccines: Serotype composition is not enough. Expert Rev Vaccines. 2015;14(3):413–428. doi: 10.1586/14760584.2015.965160. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert D, De Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 3.Gray BM, Converse GM, 3rd, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: Acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142(6):923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 4.Huebner RE, Dagan R, Porath N, Wasas AD, Klugman KP. Lack of utility of serotyping multiple colonies for detection of simultaneous nasopharyngeal carriage of different pneumococcal serotypes. Pediatr Infect Dis J. 2000;19(10):1017–1020. doi: 10.1097/00006454-200010000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Heron MP, Smith BL. Deaths: Leading causes for 2003. National vital statistics reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2007;55(10):1–92. [PubMed] [Google Scholar]
- 6.Bakaletz LO. Bacterial biofilms in the upper airway - evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev. 2012;13(3):154–159. doi: 10.1016/j.prrv.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froom J, et al. Antimicrobials for acute otitis media? A review from the International Primary Care Network. BMJ. 1997;315(7100):98–102. doi: 10.1136/bmj.315.7100.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sniadack DH, et al. Potential interventions for the prevention of childhood pneumonia: Geographic and temporal differences in serotype and serogroup distribution of sterile site pneumococcal isolates from children--implications for vaccine strategies. Pediatr Infect Dis J. 1995;14(6):503–510. [PubMed] [Google Scholar]
- 9.O’Brien KL, et al. Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 10.Klein JO. Otitis media. Clin Infect Dis. 1994;19(5):823–833. doi: 10.1093/clinids/19.5.823. [DOI] [PubMed] [Google Scholar]
- 11.Leowski J. Mortality from acute respiratory infections in children under 5 years of age: Global estimates. World Health Stat Q. 1986;39(2):138–144. [PubMed] [Google Scholar]
- 12.Dowson CG, Coffey TJ, Spratt BG. Origin and molecular epidemiology of penicillin-binding-protein-mediated resistance to beta-lactam antibiotics. Trends Microbiol. 1994;2(10):361–366. doi: 10.1016/0966-842x(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan SL, et al. Multicenter surveillance of Streptococcus pneumoniae isolates from middle ear and mastoid cultures in the 13-valent pneumococcal conjugate vaccine era. Clin Infect Dis. 2015;60(9):1339–1345. doi: 10.1093/cid/civ067. [DOI] [PubMed] [Google Scholar]
- 14.Kempf M, et al. Decline in antibiotic resistance and changes in the serotype distribution of Streptococcus pneumoniae isolates from children with acute otitis media; a 2001-2011 survey by the French Pneumococcal Network. Clin Microbiol Infect. 2015;21(1):35–42. doi: 10.1016/j.cmi.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Mayanskiy N, et al. Bacterial etiology of acute otitis media and characterization of pneumococcal serotypes and genotypes among children in Moscow, Russia. Pediatr Infect Dis J. 2015;34(3):255–260. doi: 10.1097/INF.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 16.Safari D, et al. Serotype distribution and antibiotic susceptibility of Streptococcus pneumoniae strains carried by children infected with human immunodeficiency virus. PLoS One. 2014;9(10):e110526. doi: 10.1371/journal.pone.0110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somech I, et al. Distribution, dynamics and antibiotic resistance patterns of Streptococcus pneumoniae serotypes causing acute otitis media in children in southern Israel during the 10 year-period before the introduction of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2011;29(25):4202–4209. doi: 10.1016/j.vaccine.2011.03.103. [DOI] [PubMed] [Google Scholar]
- 18.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298(15):1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 19.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 20.Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chole RA, Faddis BT. Anatomical evidence of microbial biofilms in tonsillar tissues: A possible mechanism to explain chronicity. Arch Otolaryngol Head Neck Surg. 2003;129(6):634–636. doi: 10.1001/archotol.129.6.634. [DOI] [PubMed] [Google Scholar]
- 22.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio. 2013;4(4):e00438-13. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks LR, Parameswaran GI, Hakansson AP. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect Immun. 2012;80(8):2744–2760. doi: 10.1128/IAI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen R, et al. One dose ceftriaxone vs. ten days of amoxicillin/clavulanate therapy for acute otitis media: Clinical efficacy and change in nasopharyngeal flora. Pediatr Infect Dis J. 1999;18(5):403–409. doi: 10.1097/00006454-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Dagan R, et al. Dynamics of pneumococcal nasopharyngeal colonization during the first days of antibiotic treatment in pediatric patients. Pediatr Infect Dis J. 1998;17(10):880–885. doi: 10.1097/00006454-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spijkerman J, et al. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One. 2012;7(6):e39730. doi: 10.1371/journal.pone.0039730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blyth CC, et al. ANZIC Influenza Investigators COSI Microbiological Investigators The impact of bacterial and viral co-infection in severe influenza. Influenza Other Respi Viruses. 2013;7(2):168–176. doi: 10.1111/j.1750-2659.2012.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alicino C, Barberis I, Orsi A, Durando P. Pneumococcal vaccination strategies in adult population: Perspectives with the pneumococcal 13 - valent polysaccharide conjugate vaccine. Minerva Med. 2014;105(1):89–97. [PubMed] [Google Scholar]
- 30.Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on prevention of pneumonia. Pediatr Infect Dis J. 2014;33(Suppl 2):S140–S151. doi: 10.1097/INF.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor S, et al. Impact of pneumococcal conjugate vaccination on otitis media: A systematic review. Clin Infect Dis. 2012;54(12):1765–1773. doi: 10.1093/cid/cis292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldman C, Anderson R. Review: Current and new generation pneumococcal vaccines. J Infect. 2014;69(4):309–325. doi: 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Madhi SA, et al. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae--and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization--in HIV-Infected and HIV-uninfected children. J Infect Dis. 2007;196(11):1662–1666. doi: 10.1086/522164. [DOI] [PubMed] [Google Scholar]
- 34.Mahdi LK, Wang H, Van der Hoek MB, Paton JC, Ogunniyi AD. Identification of a novel pneumococcal vaccine antigen preferentially expressed during meningitis in mice. J Clin Invest. 2012;122(6):2208–2220. doi: 10.1172/JCI45850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter SS, et al. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.) Emerg Infect Dis. 2013;19(7):1074–1083. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettigrew MM, et al. Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza A virus infection. Infect Immun. 2014;82(11):4607–4619. doi: 10.1128/IAI.02225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche H, Håkansson A, Hollingshead SK, Briles DE. Regions of PspA/EF3296 best able to elicit protection against Streptococcus pneumoniae in a murine infection model. Infect Immun. 2003;71(3):1033–1041. doi: 10.1128/IAI.71.3.1033-1041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roche H, Ren B, McDaniel LS, Håkansson A, Briles DE. Relative roles of genetic background and variation in PspA in the ability of antibodies to PspA to protect against capsular type 3 and 4 strains of Streptococcus pneumoniae. Infect Immun. 2003;71(8):4498–4505. doi: 10.1128/IAI.71.8.4498-4505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao S, et al. Functionalization of cobalt porphyrin-phospholipid bilayers with his-tagged ligands and antigens. Nat Chem. 2015;7(5):438–446. doi: 10.1038/nchem.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettigrew MM, et al. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49(11):3750–3755. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chonmaitree T, Howie VM, Truant AL. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics. 1986;77(5):698–702. [PubMed] [Google Scholar]
- 42.Moffitt KL, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9(2):158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (NIH, Bethesda), DHHS Publ No (NIH) 85-23.
- 44.Hammerschmidt S, et al. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun. 2005;73(8):4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyx RE, Roche-Hakansson H, Hakansson AP. Role of dihydrolipoamide dehydrogenase in regulation of raffinose transport in Streptococcus pneumoniae. J Bacteriol. 2011;193(14):3512–3524. doi: 10.1128/JB.01410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]