Significance
Quantifying connectivity among geographically separated subpopulations is necessary for successful management and conservation of marine resources, and a mechanistic understanding of growth and mortality during the pelagic larval stage is essential for obtaining accurate predictions of dispersal and population replenishment. Our finding that the pelagic environment encountered by larvae of coral reef fishes significantly influences larval growth, selective mortality, and the composition of survivors has important implications for understanding population connectivity. High productivity oceanographic features enhance larval survival and potentially increase the contribution of locally spawned larvae to reef populations. Incorporation of spatially explicit larval growth and survivorship into dispersal models will improve quantification of population connectivity for marine organisms both now and in the future.
Keywords: population connectivity, selective mortality, otolith microstructure, mesoscale eddies, reef fish settlement
Abstract
Oceanographic features, such as eddies and fronts, enhance and concentrate productivity, generating high-quality patches that dispersive marine larvae may encounter in the plankton. Although broad-scale movement of larvae associated with these features can be captured in biophysical models, direct evidence of processes influencing survival within them, and subsequent effects on population replenishment, are unknown. We sequentially sampled cohorts of coral reef fishes in the plankton and nearshore juvenile habitats in the Straits of Florida and used otolith microstructure analysis to compare growth and size-at-age of larvae collected inside and outside of mesoscale eddies to those that survived to settlement. Larval habitat altered patterns of growth and selective mortality: Thalassoma bifasciatum and Cryptotomus roseus that encountered eddies in the plankton grew faster than larvae outside of eddies and likely experienced higher survival to settlement. During warm periods, T. bifasciatum residing outside of eddies in the oligotrophic Florida Current experienced high mortality and only the slowest growers survived early larval life. Such slow growth is advantageous in nutrient poor habitats when warm temperatures increase metabolic demands but is insufficient for survival beyond the larval stage because only fast-growing larvae successfully settled to reefs. Because larvae arriving to the Straits of Florida from distant sources must spend long periods of time outside of eddies, our results indicate that they have a survival disadvantage. High productivity features such as eddies not only enhance the survival of pelagic larvae, but also potentially increase the contribution of locally spawned larvae to reef populations.
The majority of benthic marine organisms with complex life histories produce minute, dispersive early life stages that, to successfully recruit to benthic adult populations, must obtain food, avoid predators, and be transported to suitable juvenile habitat. Oceanographic features, such as eddies and fronts, impose patchiness onto pelagic habitats and produce variability in the physical parameters and predator and prey fields that larvae encounter (1, 2). Mesoscale eddies have long been hypothesized to serve as important larval habitat because they potentially enhance and concentrate productivity and promote retention (3). Larvae that encounter high-productivity eddies have recently been shown to be larger and faster growing (4), and such features can physically transport older larval stages to nearshore settlement sites (5, 6). Although the broad-scale movement of larvae associated with these ubiquitous oceanographic features can be captured in high-resolution biophysical models, direct evidence of the processes influencing survival within eddies, and subsequent effects on population replenishment, are unknown.
Mortality of early life stages of marine organisms is high, variable, and often nonrandom, as individuals with specific traits are eliminated from the population, and understanding the selective aspect of mortality is an essential component of predicting recruitment (7). Increased survival of larval and juvenile stages is frequently linked to fast larval growth (8–12), yet there are notable exceptions to this pattern (13, 14) and the strength and direction of selective mortality has been shown to be environmentally mediated and temporally variable (15, 16).
To better understand how patterns of mortality can be impacted by variations in larval growth and survival across oceanographic features encountered during dispersal, we sampled cohorts (i.e., individuals hatched within a 30-d window) of three coral reef fishes in successive habitats in an oceanographically dynamic region. We repeatedly sampled separate cohorts in the plankton and then once they settled to nearshore reefs and examined larval growth and patterns of selective mortality in relation to larval residence in mesoscale eddies. Examination of trait-based selective mortality inside and outside of eddies and comparison of larval traits to those of settlers on the reef enabled resolution of spatially explicit differential survival.
Results
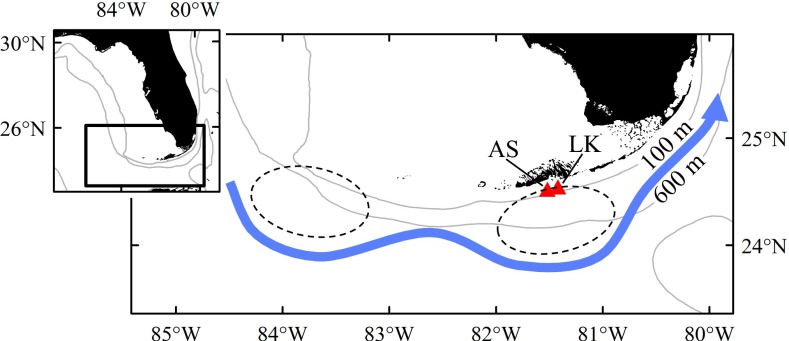
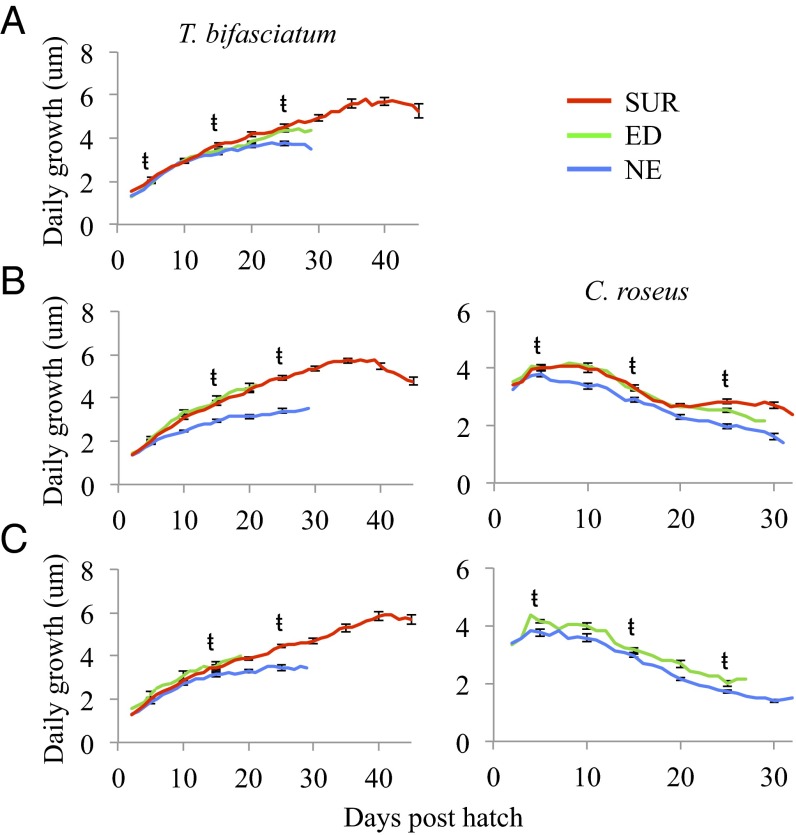
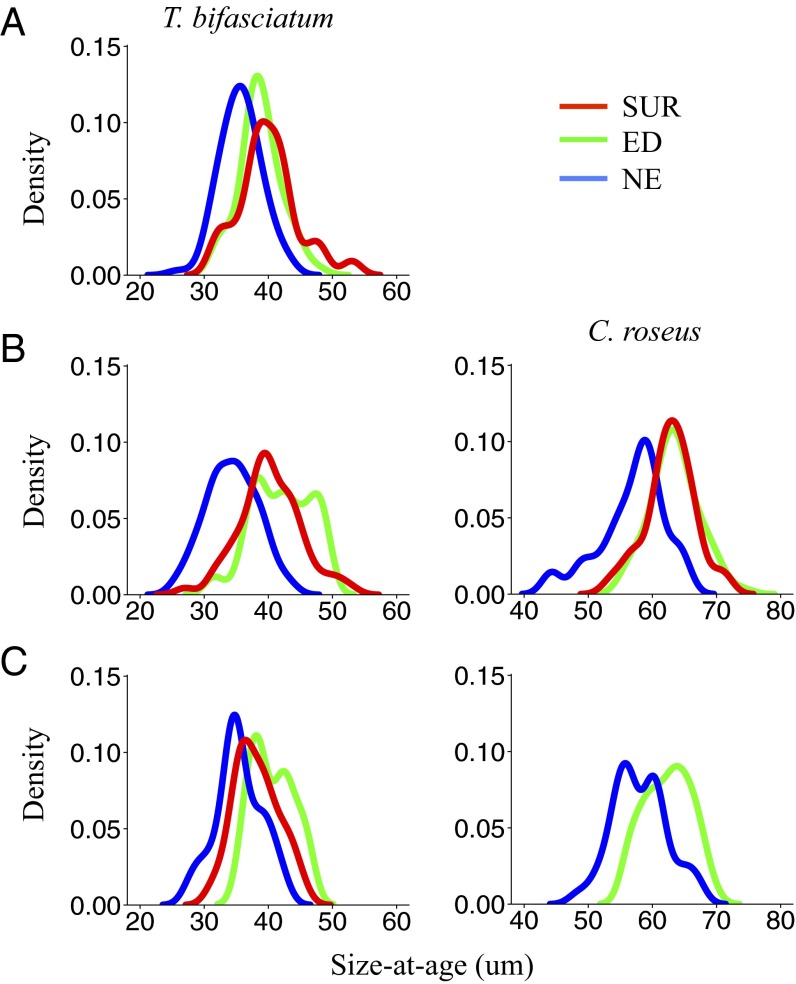
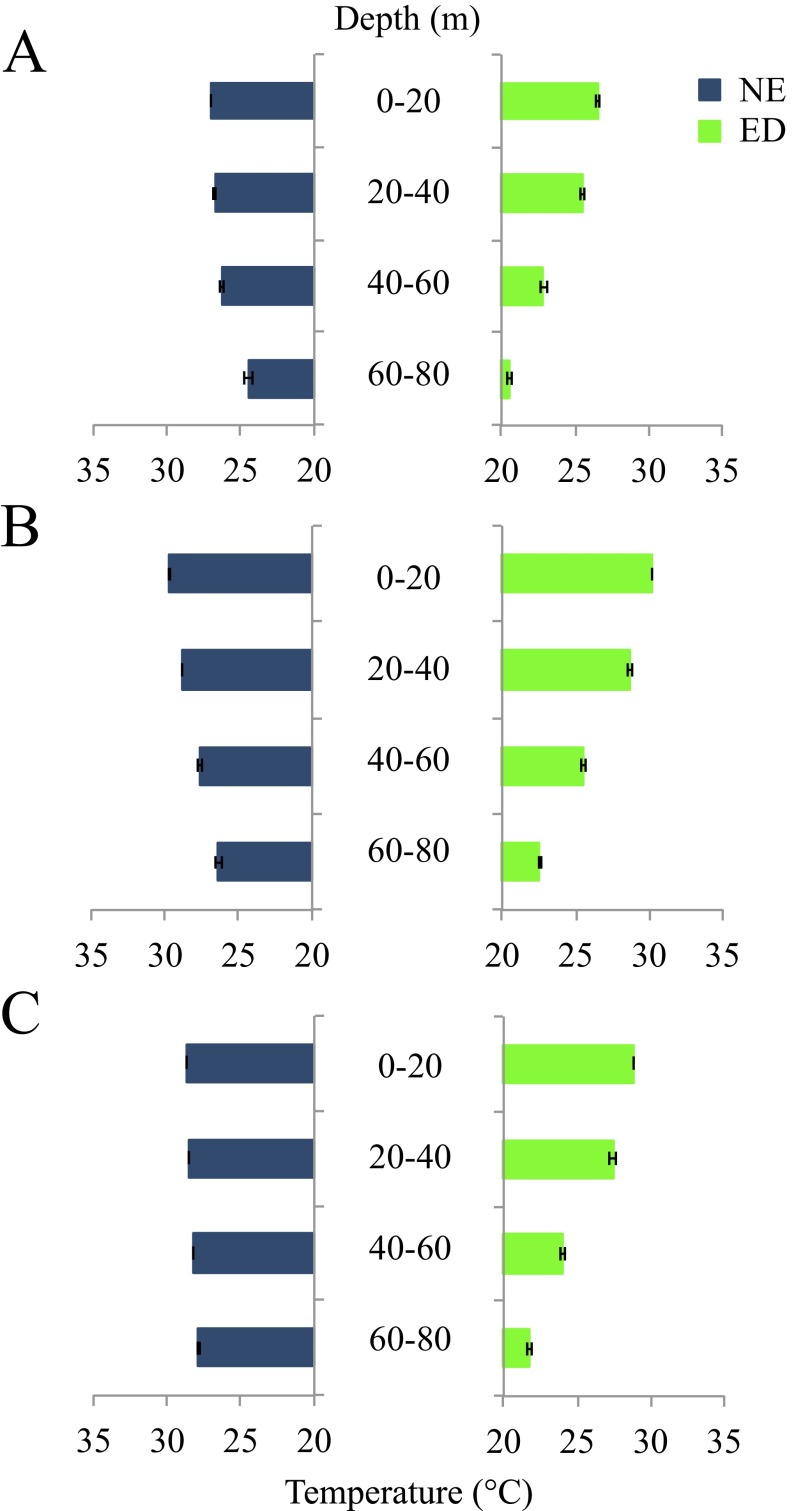
Six mesoscale eddies were sampled during three oceanographic cruises in the western Straits of Florida (Fig. 1; see ref. 4 for details) and larvae residing inside (ED) and outside (NE) of eddies were compared with survivors (SUR) collected as late-stage larvae in light traps as they settled to reefs (Cryptotomus roseus and Stegastes partitus) or as newly settled juveniles (Thalassoma bifasciatum). The pelagic environment experienced by larvae significantly affected growth and patterns of selective mortality. For two of the three reef fish species examined, larval growth differed significantly among groups: for both T. bifasciatum and C. roseus, NE larvae exhibited significantly slower growth compared with the ED and/or SUR groups in all cohorts (i.e., June 2007, August 2007, and June 2008; Table 1 and Fig. 2). ED larvae of T. bifasciatum and C. roseus exhibited faster growth and their growth trajectories closely matched those of the SUR group (Table 1 and Fig. 2). In the August 2007 cohort of C. roseus, by day 15, growth of the SUR group was significantly faster than the ED group, reflecting additional selective survival of the fastest growers within the ED group. Differences in the growth of T. bifasciatum and C. roseus manifested in significant differences in sizes such that ED and SUR fish were significantly larger at specific ages than NE fish (Table 2). By day 15, the size distributions of SUR fish more closely overlapped those of ED than NE fish for both T. bifasciatum and C. roseus (Fig. 3). These differences in larval growth and size-at-age among water masses were not due to differences in water temperature between ED and NE stations, because temperatures were similar or lower within ED stations due to upwelling (Fig. S1). For S. partitus, mean growth and corresponding sizes-at-age did not differ significantly among ED, NE, and SUR groups (Table S1).
Fig. 1.
Map of study area depicting average location of mesoscale eddies in the Straits of Florida during sampling. Two eddies were sampled on each of three oceanographic cruises conducted in June 2007, August 2007, and June 2008. Nearshore sampling sites in the lower Florida Keys at AS (American Shoal) and LK (Looe Key) reefs where late-stage larvae and juveniles were collected are denoted by red triangles, and the approximate location of the Florida Current is shown in blue.
Table 1.
Comparisons of mean growth (otolith increment width) among ED, NE, and SUR groups for two coral reef fishes at three time points in larval life
| June 2007 | August 2007 | June 2008 | |||
| Larval age | T. bifasciatum | T. bifasciatum | C. roseus | T. bifasciatum | C. roseus |
| 4–6 dph | * | ** | ** | * | *** |
| NE, ED < SUR | NE < ED | NE < ED, SUR | NE < SUR | NE < ED | |
| F = 3.80 | F = 5.62 | F = 5.49 | F = 7.01 | F = 15.29 | |
| n = 88/86/29 | n = 58/29/61 | n = 38/119/50 | n = 25/44 | n = 39/35 | |
| 14–16 dph | *** | *** | *** | ** | ** |
| NE, ED < SUR | NE < ED, SUR | NE < ED, SUR | NE < SUR | NE < ED | |
| F = 8.16 | F = 40.76 | F = 18.31 | F = 9.37 | F = 8.77 | |
| n = 88/85/29 | n = 57/21/61 | n = 37/112/50 | n = 24/44 | n = 38/28 | |
| 24–26 dph | *** | *** | *** | *** | *** |
| NE < ED, SUR | NE < SUR | NE < ED, SUR | NE < SUR | NE < ED | |
| F = 19.23 | F = 70.18 | F = 33.14 | F = 25.86 | F = 20.55 | |
| n = 21/29/29 | n = 31/0/61 | n = 27/22/50 | n = 14/44 | n = 32/13 | |
Results from one-way ANOVAs are shown for ED, eddy; NE, non-eddy; and SUR, surviving late-stage larvae captured in light traps (C. roseus) or juveniles collected on the reef (T. bifasciatum). *P < 0.05; **P < 0.01; ***P < 0.001. Results of Tukey HSD tests are presented as an inequality statement. Note that in June 2008, tests involving T. bifasciatum did not include the ED group because of its low sample size.
Fig. 2.
Daily growth (otolith increment width) at each day after hatch for ED, eddy; NE, non-eddy; and SUR, surviving late-stage larvae captured in light traps (C. roseus) or juveniles collected on the reef (T. bifasciatum) from cohorts sampled in June 2007 (A), August 2007 (B), and June 2008 (C). Error bars (± SE) are shown every five increments for reference. ŧ = significant result (P < 0.001–0.05) in one-way ANOVAs (Table 1). Sample sizes (n) are listed in Table 1.
Table 2.
Comparisons of size-at-age (otolith radius) among ED, NE, and SUR groups for two coral reef fishes at three time points in larval life
| June 2007 | August 2007 | June 2008 | |||
| Larval age | T. bifasciatum | T. bifasciatum | C. roseus | T. bifasciatum | C. roseus |
| 5 dph | *** | ns | * | ns | * |
| NE, ED < SUR | NE < ED | NE < ED | |||
| F = 9.72 | F = 2.48 | F = 4.46 | F = 3.51 | F = 5.30 | |
| n = 88/86/29 | n = 58/29/61 | n = 38/119/50 | n = 25/44 | n = 39/35 | |
| 15 dph | * | *** | *** | ** | *** |
| NE < SUR | NE < ED, SUR | NE < ED, SUR | NE < SUR | NE < ED | |
| F = 3.24 | F = 35.97 | F = 38.33 | F = 9.62 | F = 20.82 | |
| n = 88/85/29 | n = 58/23/61 | n = 37/119/50 | n = 24/44 | n = 38/32 | |
| 25 dph | * | *** | *** | *** | *** |
| NE < SUR | NE < SUR | NE < ED, SUR | NE < SUR | NE < ED | |
| F = 4.83 | F = 85.90 | F = 41.69 | F = 24.32 | F = 46.82 | |
| n = 28/35/29 | n = 35/0/61 | n = 28/30/50 | n = 16/44 | n = 35/14 | |
Results from one-way ANOVAs are shown for ED, eddy; NE, non-eddy; and SUR, surviving late-stage larvae captured in light traps (C. roseus) or juveniles collected on the reef (T. bifasciatum). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant. Results of Tukey HSD tests are presented as an inequality statement. Note that in June 2008, tests involving T. bifasciatum did not include the ED group because of its low sample size.
Fig. 3.
Kernel density estimates of size-at-age (otolith radius) distributions at 15 dph in ED, eddy; NE, non-eddy; and SUR, surviving late-stage larvae captured in light traps (C. roseus) or juveniles collected on the reef (T. bifasciatum) from cohorts sampled in June 2007 (A), August 2007 (B), and June 2008 (C). Sample sizes (n) are listed in Table 1.
Fig. S1.
Mean temperature (± SE) at ED, eddy (green bars), and NE, non-eddy (blue bars) stations across depth bins sampled for ichthyoplankton in June 2007 (A), August 2007 (B), and June 2008 (C).
Table S1.
Comparisons of mean growth (otolith increment width) and size-at-age (otolith radius) among ED, NE, and SUR groups at two time points in larval life for S. partitus
| Trait | n | F-statistic | P value |
| 4–6 dph | 56/22/50 | 0.604 | 0.548 |
| 9–11 dph | 34/14/50 | 0.112 | 0.894 |
| 5 dph | 56/22/50 | 2.337 | 0.101 |
| 10 dph | 53/16/50 | 1.094 | 0.338 |
Results from one-way ANOVAs are shown for mean growth (4–6 and 9–11 dph) and size-at-age (5 and 10 dph). ED, eddy; NE, non-eddy; SUR, surviving late-stage larvae captured in light traps. Sample sizes (n) are reported in order for NE/ED/SUR groups.
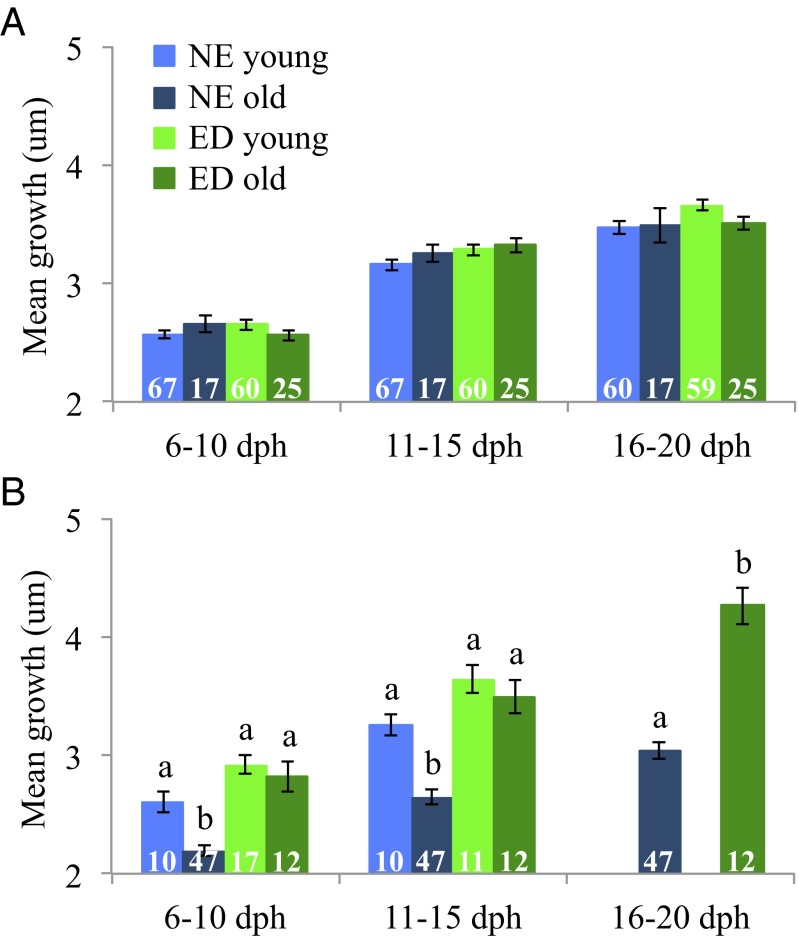
To examine whether patterns of selective mortality varied within water masses, we divided T. bifasciatum larvae from two cohorts (June and August 2007 had sufficient sample sizes) into young and old age groups. Two clear patterns emerged from these comparisons. In June 2007, there were no significant differences in early larval growth among the four groups [groups = NE young, NE old, ED young, and ED old; 6–10 d post hatch (dph): F = 1.67, P = 0.32; 11–15 dph: F = 2.03, P = 0.11; 16–20 dph: F = 2.43, P = 0.07; Fig. 4]. This pattern remained consistent in August 2007 for ED fish [i.e., ED young versus ED old: Tukey honest significant difference (HSD) test: P > 0.05], but for NE larvae, there was strong evidence of selective mortality. Older (surviving) NE larvae had significantly slower growth than younger NE larvae at 6–10 dph (F = 27.40, P < 0.001) and 11–15 dph (F = 29.01, P < 0.001; Fig. 4), indicating selective loss of faster growers from the larval group residing outside of mesoscale eddies.
Fig. 4.
Mean growth (± SE) of young and old age groups for T. bifasciatum larvae collected inside (ED) and outside (NE) of eddies from cohorts sampled in June 2007 (A) and August 2007 (B). Comparisons among groups were made by using one-way ANOVAs for each of three periods of mean growth (6–10 dph, 11–15 dph, and 16–20 dph), and significant results (P < 0.001–0.05) are denoted with lowercase letters. Sample sizes (n) for each group are denoted in white on the corresponding bar.
Conclusions
Our results demonstrate that the pelagic environment significantly influences growth and shapes patterns of selective mortality in the early life stages of reef fishes. Trait-based selection varied spatially across ED and NE water masses, and temporally between summer months with contrasting temperatures. However, overall, there was consistent preferential survival of fast growers and individuals with large sizes-at-age, traits that were associated with entrainment in mesoscale eddies. Thus, larvae in eddies had a survival advantage and likely contributed disproportionately to population replenishment.
Spatial and Temporal Variation in Selective Mortality.
Dividing T. bifasciatum ED and NE fish into young and old age groups revealed both temporal and spatial variation in patterns of selective mortality. There was no significant trait-based selective loss of larvae residing in mesoscale eddies regardless of month. However, for larvae outside of eddies, patterns of selective mortality varied over time: In June, there was no significant trait-based loss, whereas in August, slow growers preferentially survived. Selective mortality favoring slow growers has been shown for T. bifasciatum larvae collected in oligotrophic waters in the Straits of Florida (14). Because most of the NE larvae in our study were collected in oligotrophic Florida Current waters, this pattern of preferential survival of slow growers is consistent with previous findings. What is particularly intriguing is the spatial and temporal variation in patterns of selective mortality. We hypothesize that in a food-poor environment such as the Florida Current, fast growth can actually be a liability because higher growth rates come with higher metabolic demands and, consequently, greater food requirements. In June, when temperatures were 2–4 °C cooler, growth rates for both ED and NE larvae were not as high as in August and, thus, there was enough prey available both inside and outside of eddies to sustain this growth. ED and NE fish grew well (similar early growth) and experienced no growth-based selective loss. By August, water temperatures pushed larval growth rates higher and prey differences between water masses became important. ED fish had access to sufficient food to sustain high growth, but in nutrient-poor waters outside of eddies, only those fish growing at relatively slower rates survived. This hypothesis merits further investigation, particularly in oligotrophic oceans, given projected climate-induced increases in larval metabolic rates (17). High productivity oceanographic features, such as eddies and fronts, may become increasingly important for larval growth and survival in the pelagic environment.
Mesoscale Eddies as Important Larval Habitat.
In addition to spatial and temporal variation in early trait-based selection during the larval stage, our results indicate consistent directional selection occurring throughout the pelagic period leading to settlement. Fast larval growth and larger sizes-at-age translated into a survival advantage suggesting that fish entrained in mesoscale eddies preferentially survived to settle to reefs, whereas slow-growing larvae outside of eddies were more likely to be selectively lost from the population. In support of this conclusion, the size-at-age distributions of survivors overlap most completely with those of the ED larvae; the distributions of NE fish only overlap a portion of the survivor distributions. It is also unlikely that the fast growers settling to nearshore reefs came from a population of larvae that were not sampled because our stations encompassed both the cross-shelf and alongshore extents of the western Straits of Florida (see figure 1 in ref. 4). Although encounter with an eddy does not guarantee larval transport to suitable habitat or survival to settlement (6, 18), our data, collected across six eddies and two summers, indicate that survival to settlement is enhanced by the increased growth and size-at-age of ED fish.
Mesoscale eddies are ubiquitous features of the global ocean, and our results are consistent with previous hypotheses that high productivity of certain eddies promotes fast larval growth and enhanced survival and that these physical features can retain, and possibly deliver, larvae to suitable settlement habitat (3). Previous studies have indirectly implicated mesoscale eddies in providing a survival advantage for fish larvae. For example, eddies have been correlated with high survival of pelagic larvae (19) and the arrival of large pulses of late-stage larvae to coral reefs (5, 6). In addition, enhanced growth and condition of larvae sampled from eddies suggest that these habitats are advantageous (4, 20). By repeatedly sampling cohorts over time from the plankton to the reef, our results provide the first evidence to our knowledge of the link between mesoscale eddies and survival to settlement.
Importantly, our results demonstrate that mesoscale eddies have a similar effect on two different fish species and over multiple cohorts. However, it is also worth noting that there was no difference in growth or size for S. partitus larvae collected between the two environments (i.e., ED and NE). This result was consistent with previous work examining recent growth in S. partitus (4) and may be due to three possible scenarios: (i) Despite a ∼30-d pelagic larval period, S. partitus larvae were only collected in the plankton up to ∼10 dph, and this time period may have been insufficient for differences between water masses to emerge; (ii) S. partitus may have a relatively invariant growth pattern, whereby if sufficient prey are not available, individuals cannot subsist at lower growth rates (consistent with a relatively invariant pelagic larval duration); or (iii) S. partitus prey items were not limited outside of the eddy so species-specific diets (21) may have resulted in species-specific responses depending on the composition of prey across water masses.
Differential Survival to Settlement.
Slow-growing larvae outside of mesoscale eddies had a survival disadvantage and, thus, were less likely to contribute significantly to the survivors settling to reef habitats. Most of the NE fish in our analyses were sampled from the oligotrophic Florida Current and could have been eliminated from the population before settlement through one or more mechanisms: (i) starvation in oligotrophic offshore waters, which likely changes in intensity seasonally as metabolic rates vary with water temperature; (ii) predation as smaller, slow-growing larvae migrate across the shelf to the reef; or (iii) advection away from suitable settlement habitat by the rapidly flowing Florida Current. Whereas some NE fish in the Florida Current may be advected away from suitable settlement habitat, settlement to reefs occurs regularly in the absence of eddies (6), demonstrating that larvae can transit from the Florida Current to nearshore reefs. Thus, advection is likely not the only mechanism by which NE larvae are eliminated from the population. These small, slow growing larvae have higher probability of starvation and predation mortality, leading to a lower chance of successful settlement. Although reduced survival to settlement for larvae outside of eddies is the most parsimonious interpretation of the data, extreme selection on the NE group late in the larval period and subsequent survival to settlement cannot be entirely ruled out. However, such extreme selection would be the result of strong predation losses, resulting in proportionally lower numbers of survivors from this group.
The finding that settlers are composed disproportionately of fish that were within recirculating mesoscale eddies as larvae has important implications for the relative success of long distance dispersal versus retention events. Larvae from distant upstream sources likely arrive to the Straits of Florida via the Florida Current. Given transit times (i.e., days to weeks), these larvae must spend a substantial portion of their early life in these oligotrophic waters, outside of mesoscale eddies. In contrast, locally spawned larvae need not spend extensive time in nutrient-poor waters before becoming entrained in an eddy. Although biophysical models show that some larvae may arrive to Florida reefs from distant upstream sources via the Florida Current (e.g., ref. 22), our results suggest that those exhibiting slow growth do not survive the journey. Although some NE fish may have contributed to the SUR population, based on trait distributions, it is likely that ED fish make up a significantly larger proportion of the fast-growing survivors. Additional selection for fast larval growth occurring during the juvenile stage (i.e., carry-over; ref. 9) would further decrease the contribution of NE fish to survivor populations. Therefore, entrainment in an eddy not only can facilitate physical retention of locally spawned larvae (as hypothesized by Lee et al., ref. 23), but via increased larval growth and survival, may also substantially enhance the contribution of locally spawned larvae to reef populations.
Implications for Management and Conservation.
Marine environments are spatially complex and patchy, and our results demonstrate that larval entrainment in oceanographic features, such as eddies and fronts, which enhance and concentrate productivity, may be critical for population replenishment. These findings contribute to a growing body of literature highlighting the importance of the pelagic environment not only to the transport of early life stages, but also to their growth and survival. For example, water temperature directly affects traits such as pelagic larval duration through its regulation of developmental processes (17) and changes the strength and direction of selective mortality (16). Retention of larvae in nearshore versus offshore waters also significantly alters larval quality and postsettlement survival (24, 25). Similarly, larval encounter with particular water masses significantly influences larval growth (4) and subsequent juvenile condition through carry-over processes (26). These data move us closer to the goal of identifying predictable patterns in larval growth and survival in association with oceanographic features and incorporating such data into biophysical models.
As exploitation of populations and a changing climate continue to alter marine ecosystems, knowledge of population connectivity is critical to successful management and conservation (27). Our ability to quantify patterns of population connectivity continues to improve (28–32), and biophysical and individual-based modeling has become particularly important for examining connectivity across large spatial and temporal scales and for quantifying future population dynamics (e.g., refs. 33 and 34). Recent advances in the field have led to the incorporation of metapopulation models and climate change scenarios into simulations of larval dispersal (e.g., refs. 35 and 36), yet an ability to relate larval survival to quantifiable environmental features will allow for the further refinement of models by providing a mechanistic basis for simulating population connectivity. These refined models, in turn, will facilitate the placement and evaluation of marine protected areas to account for and capitalize on variability in the pelagic environment. Importantly, with a more mechanistic understanding of dispersal and connectivity, we will be better prepared to predict responses of marine populations to a changing climate.
Materials and Methods
Field Collections.
Our study was conducted in the Straits of Florida where the Florida Current, a western boundary current, is the dominant feature (Fig. 1). The formation and propagation of cyclonic mesoscale eddies along the front of the Florida Current is well documented, and upwelling at the cores of these eddies has been linked to increases in both primary and secondary productivity (23, 37). To repeatedly sample fish cohorts in different habitats through time, we used ichthyoplankton tows to collect larvae with a broad size and age range from the plankton, light traps to collect late-stage larvae as they moved onshore to settle, and diver surveys to collect juveniles that recently settled to shallow reefs. Ichthyoplankton sampling was conducted during three 15–16-d cruises in the summers of 2007 (May 29–June 13; July 30–August 13) and 2008 (June 17–July 1). Seven stations were sampled along six cross-shelf transects spanning the western Straits of Florida where mesoscale eddies form coherent structures and have relatively long residence times (Fig. 1). Ichthyoplankton tows were conducted with a modified Multiple Opening Closing Net and Environmental Sensing System (MOCNESS; ref. 4) that sampled discrete 20-m-depth bins down to 80 m by using paired nets (4 m2 and 1 m2) fitted with 1-mm and 150-μm mesh, respectively. The MOCNESS was equipped with a flowmeter to determine the volume sampled during each net tow, as well as sensors to collect temperature, salinity, and fluorescence data. A ship-mounted 76.8-kHz RD Instruments Acoustic Doppler Current Profiler sampled the water column, and two Lagrangian drifters (Technocean) drogued at 15 m were deployed during each cruise. All ichthyoplankton tows were conducted during daylight hours, excluding dawn and dusk.
During and after the cruises (i.e., June–September 2007; July–September 2008), late-stage larvae and juveniles were sampled from American Shoal (AS) and Looe Key (LK) reefs in the lower Florida Keys (Fig. 1). Four replicate light traps were deployed at each reef 1 m below the surface and 50 m apart. Traps fished from sunset to sunrise during 15-d periods encompassing both the new and third-quarter lunar phases, when most coral reef fishes settle. One week into light-trap sampling, juvenile collections were initiated by SCUBA divers using quinaldine and hand nets.
Based on our ability to morphologically identify larvae to species and obtain adequate sample sizes for examining cohorts through time, we chose three common reef fish species for otolith analysis: T. bifasciatum (bluehead wrasse), C. roseus (bluelip parrotfish), and S. partitus (bicolor damselfish).
Trait Measurements with Otolith Analysis.
We followed standard techniques for analyzing otolith microstructure on a subset of larvae from each species to obtain daily growth rates and sizes-at-age (e.g., ref. 14). The abundance and size distribution of the subset of larvae used for otolith analysis was proportional to that of all larvae in each sample. Standard length or notochord length was measured to the nearest 0.01 mm for each larva by using a Leica MZ12 dissecting microscope, a Cool Snap-Pro monochrome digital camera, and Image-Pro Plus 7.0 image analysis software (Media Cybernetics). Sagittal (T. bifasciatum and C. roseus) or lapillar (S. partitus) otoliths were dissected from each individual and stored in immersion oil for ∼7–14 d to facilitate reading. All otoliths from a given species were analyzed by a single reader. Otoliths were read along the longest axis at 400× magnification through a Leica DMLB microscope and with the aid of the digital camera and Image-Pro Plus software. All otoliths were read at least twice, and if the reads differed by ≤5%, one read was randomly chosen for analysis. If reads differed by >5%, a third read was conducted and compared with the first two reads. If any comparison differed by ≤5%, one read from that comparison was randomly chosen for analysis; otoliths where all reads differed by >5% were removed from any further analyses (14).
Data Analysis.
Mesoscale eddies were located by using a suite of oceanographic data (see ref. 4 for details). Larvae collected at stations well inside of an eddy were classified as ED fish and those collected well outside of an eddy as NE fish. To ensure that larvae belonged to a single cohort (i.e., hatched within a limited time window), hatch date was calculated for each individual by subtracting the age at collection from the collection date. Then all hatch dates falling outside of a 30-d hatch window were removed from further analyses. Otolith increment width and otolith radius were used as proxies for daily growth and size-at-age, respectively; the relationship between otolith and somatic growth was confirmed by a significant relationship between otolith size-on-age residuals and standard length-on-age residuals (38) (linear regression: T. bifasciatum: F1,657 = 777.60, P < 0.001, R2 = 0.54; C. roseus: F1,369 = 346.56, P < 0.001, R2 = 0.48; S. partitus: F1,182 = 82.75, P < 0.001, R2 = 0.31). In contrast to previous work comparing only recent (last 3 d before collection) growth of larvae (4), here we examined larval growth (i.e., otolith increment width) and size-at-age (i.e., otolith radius) across the entire larval period of ED and NE larvae. Further, to examine the impact of water mass on survival to settlement and, thus, population replenishment, we compared larval traits between offshore larvae (i.e., ED and NE) and late-stage larvae collected in light traps (C. roseus and S. partitus) or juveniles collected on the reef (T. bifasciatum), representing SUR that successfully settled nearshore. Sample sizes allowed the examination of cohorts for all three species in August 2007 and an additional cohort of T. bifasciatum in June 2007. In June 2008, there were adequate sample sizes of T. bifasciatum for NE and SUR groups but only a few individuals for the ED group. We plotted growth trajectories for all three groups for illustrative purposes, but statistical tests compared only NE and SUR groups. Similarly, for C. roseus in June 2008, sample sizes of the SUR group were insufficient so only the ED and NE groups were compared. Data were available only for a single cohort of S. partitus.
We tested growth and size among groups at two to three points in larval life. To reduce noise in the data, we averaged growth over 3-d intervals (T. bifasciatum and C. roseus: 4–6, 14–16, and 24–26 dph; S. partitus: 4–6 and 9–11 dph). Size-at-age is a cumulative trait and, thus, was compared among groups at specific time points (T. bifasciatum and C. roseus: 5, 15, and 25 dph; S. partitus: 5 d and 10 dph). Data were log or square root transformed when necessary to conform to assumptions of normality and homoscedasticity. For a few cases, transformation was ineffective and data remained nonnormal or heteroscedastic; however, because ANOVA has been shown to be robust to deviations from assumptions when sample sizes are sufficiently large, and considering our sample sizes, we proceeded with ANOVAs (39). Mean otolith growth and size-at-age were compared among groups by using one-way ANOVAs (SYSTAT 11), followed by Tukey post hoc pairwise comparisons.
Because patterns of mortality have the potential to vary among water masses, we examined patterns of selective mortality within ED and NE groups of T. bifasciatum (for two cohorts where sample sizes were sufficient). ED and NE fish were divided into young (June 2007: 18–26 dph; August 2007: 12–19 dph) and old (June 2007: 27–36 dph; August 2007: 20–36 dph) age groups. We then compared mean growth among all four groups (NE young, NE old, ED young, ED old) at 6–10 dph, 11–15 dph, and 16–20 dph by using one-way ANOVAs and Tukey post hoc pairwise comparisons (SYSTAT 11).
Biological data are available from the Biological and Chemical Oceanography Data Management Office (www.bco-dmo.org/dataset/529658) and physical data from the National Oceanographic Data Center (data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.nodc:0066847).
Acknowledgments
We thank the scientific party; R/V Walton Smith crew; and the nearshore team, J. Boulay, L. Havel, E. D’Alessandro, S. Bignami, L. Jones, T. Rankin, K. Huebert, and T. Murphy, for their contributions to the field sampling. T. Murphy, L. Parsons, J. Downing, K. Doering, and K. Ternus helped process the samples, J. Llopiz provided guidance with larval identification and data processing, and statistical analyses were enhanced by discussions with E. Goldstein. Earlier drafts benefited from comments of A. Bakun, G. Hitchcock, and S. Sogard. This study was supported by National Science Foundation (NSF) OCE Grant 0550732. K.S. also was supported during this work by a NSF “Science Made Sensible” fellowship, and during manuscript preparation, S.S. received support from National Oceanic and Atmospheric Administration Award NA11NOS4780045 and NSF Grant OCE 1419987.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Biological data are available from the Biological and Chemical Oceanography Data Management Office (www.bco-dmo.org/dataset/529658) and physical data from the National Oceanographic Data Center (data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.nodc:0066847).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601606113/-/DCSupplemental.
References
- 1.McGillicudy DJ, Jr, et al. Influence of mesoscale eddies on new production in the Sargasso Sea. Nature. 1998;394(6690):263–266. [Google Scholar]
- 2.Galarza JA, et al. The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc Natl Acad Sci USA. 2009;106(5):1473–1478. doi: 10.1073/pnas.0806804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakun A. 1996. Patterns in the Ocean: Ocean Processes and Marine Population Dynamics. (University of California Sea Grant and Centro de Investigaciones Biológicas de Noroeste, La Paz, Mexico)
- 4.Shulzitski K, et al. Close encounters with eddies: Oceanographic features increase growth of larval reef fishes during their journey to the reef. Biol Lett. 2015;11(1):20140746. doi: 10.1098/rsbl.2014.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sponaugle S, Lee T, Kourafalou V, Pinkard D. Florida Current frontal eddies and the settlement of coral reef fishes. Limnol Oceanogr. 2005;50(4):1033–1048. [Google Scholar]
- 6.D’Alessandro E, Sponaugle S, Lee T. Patterns and processes of larval fish supply to the coral reefs of the upper Florida Keys. Mar Ecol Prog Ser. 2007;331:85–100. [Google Scholar]
- 7.Johnson DW, Grorud-Colvert K, Sponaugle S, Semmens BX. Phenotypic variation and selective mortality as major drivers of recruitment variability in fishes. Ecol Lett. 2014;17(6):743–755. doi: 10.1111/ele.12273. [DOI] [PubMed] [Google Scholar]
- 8.Hare JA, Cowen RK. Size, growth, development, and survival of the planktonic larvae of Pomatomus saltatrix (Pisces: Pomatomidae) Ecology. 1997;78(8):2415–2431. [Google Scholar]
- 9.Searcy SP, Sponaugle S. Selective mortality during the larval-juvenile transition in two coral reef fishes. Ecology. 2001;82(9):2452–2470. [Google Scholar]
- 10.Takasuka A, Aoki I, Mitani I. Evidence of growth-selective predation on larval Japanese anchovy Engraulis japonicas in Sagami Bay. Mar Ecol Prog Ser. 2003;252:223–238. [Google Scholar]
- 11.Vigliola L, et al. Genetic identity determines risk of post-settlement mortality of a marine fish. Ecology. 2007;88(5):1263–1277. doi: 10.1890/06-0066. [DOI] [PubMed] [Google Scholar]
- 12.Perez KO, Munch SB. Extreme selection on size in the early lives of fish. Evolution. 2010;64(8):2450–2457. doi: 10.1111/j.1558-5646.2010.00994.x. [DOI] [PubMed] [Google Scholar]
- 13.Munch SB, Conover DO. Rapid growth results in increased susceptibility to predation in Menidia menidia. Evolution. 2003;57(9):2119–2127. doi: 10.1111/j.0014-3820.2003.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 14.Sponaugle S, Boulay JN, Rankin TL. Growth- and size-selective mortality in pelagic larvae of a common reef fish. Aquat Biol. 2011;13(3):263–273. [Google Scholar]
- 15.Gagliano M, McCormick MI, Meekan MG. Survival against the odds: Ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish. Proc Biol Sci. 2007;274(1618):1575–1582. doi: 10.1098/rspb.2007.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grorud-Colvert K, Sponaugle S. Variability in water temperature affects trait-mediated survival of a newly settled coral reef fish. Oecologia. 2011;165(3):675–686. doi: 10.1007/s00442-010-1748-4. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor MI, et al. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA. 2007;104(4):1266–1271. doi: 10.1073/pnas.0603422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams DK, et al. Surface-generated mesoscale eddies transport deep-sea products from hydrothermal vents. Science. 2011;332(6029):580–583. doi: 10.1126/science.1201066. [DOI] [PubMed] [Google Scholar]
- 19.Logerwell EA, Smith PE. Mesoscale eddies and survival of late stage Pacific sardine (Sardinops sagax) larvae. Fish Oceanogr. 2001;10(1):13–25. [Google Scholar]
- 20.Nakata H, Kimura S, Okazaki Y, Kasai A. Implications of meso-scale eddies caused by frontal disturbances of the Kuroshio Current for anchovy recruitment. ICES J Mar Sci. 2000;57(1):143–151. [Google Scholar]
- 21.Llopiz JK, Cowen RK. Variability in the trophic role of coral reef fish larvae in the oceanic plankton. Mar Ecol Prog Ser. 2009;381:259–272. [Google Scholar]
- 22.Sponaugle S, Paris C, Walter KD, Kourafalou V, D’Alessandro E. Observed and modeled larval settlement of a reef fish to the Florida Keys. Mar Ecol Prog Ser. 2012;453:201–212. [Google Scholar]
- 23.Lee TN, Clarke ME, Williams E, Szmant AF, Berger T. Evolution of the Tortugas gyre and its influence on recruitment in the Florida Keys. Bull Mar Sci. 1994;54(3):621–646. [Google Scholar]
- 24.Hamilton SL, Regetz J, Warner RR. Postsettlement survival linked to larval life in a marine fish. Proc Natl Acad Sci USA. 2008;105(5):1561–1566. doi: 10.1073/pnas.0707676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shima JS, Swearer SE. Larval quality is shaped by matrix effects: Implications for connectivity in a marine metapopulation. Ecology. 2009;90(5):1255–1267. doi: 10.1890/08-0029.1. [DOI] [PubMed] [Google Scholar]
- 26.Sponaugle S, Pinkard D. Impact of variable pelagic environments on natural larval growth and recruitment of the reef fish Thalassoma bifasciatum. J Fish Biol. 2004;64(1):34–54. [Google Scholar]
- 27.Gaines SD, White C, Carr MH, Palumbi SR. Designing marine reserve networks for both conservation and fisheries management. Proc Natl Acad Sci USA. 2010;107(43):18286–18293. doi: 10.1073/pnas.0906473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones GP, Milicich MJ, Emslie MJ, Lunow C. Self-recruitment in a coral reef fish population. Nature. 1999;402(6763):802–804. [Google Scholar]
- 29.Swearer SE, Caselle JE, Lea DW, Warner RR. Larval retention and recruitment in an island population of a coral-reef fish. Nature. 1999;402(6763):799–802. [Google Scholar]
- 30.Almany GR, Berumen ML, Thorrold SR, Planes S, Jones GP. Local replenishment of coral reef fish populations in a marine reserve. Science. 2007;316(5825):742–744. doi: 10.1126/science.1140597. [DOI] [PubMed] [Google Scholar]
- 31.Cowen RK, Sponaugle S. Larval dispersal and marine population connectivity. Annu Rev Mar Sci. 2009;1:443–466. doi: 10.1146/annurev.marine.010908.163757. [DOI] [PubMed] [Google Scholar]
- 32.Planes S, Jones GP, Thorrold SR. Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci USA. 2009;106(14):5693–5697. doi: 10.1073/pnas.0808007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowen RK, Paris CB, Srinivasan A. Scaling of connectivity in marine populations. Science. 2006;311(5760):522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- 34.Werner FE, Cowen RK, Paris CB. Coupled biological and physical models: Present capabilities and necessary developments for future studies of population connectivity. Oceanography (Wash DC) 2007;20(3):54–69. [Google Scholar]
- 35.Watson JR, et al. Identifying critical regions in small-world marine metapopulations. Proc Natl Acad Sci USA. 2011;108(43):E907–E913. doi: 10.1073/pnas.1111461108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrello M, Mouillot D, Somot S, Thuiller W, Manel S. Additive effects of climate change on connectivity between marine protected areas and larval supply to fished areas. Divers Distrib. 2015;21(2):139–150. [Google Scholar]
- 37.Hitchcock GL, et al. Property fields in a Tortugas Eddy in the southern Straits of Florida. Deep Sea Res Part I Oceanogr Res Pap. 2005;52(12):2195–2213. [Google Scholar]
- 38.Thorrold SR, Hare JA. Otolith applications in reef fish ecology. In: Sale PF, editor. Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem. Academic; San Diego: 2002. [Google Scholar]
- 39.Underwood AJ. 1997. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance (Cambridge Univ Press, Cambridge, UK), pp 522.