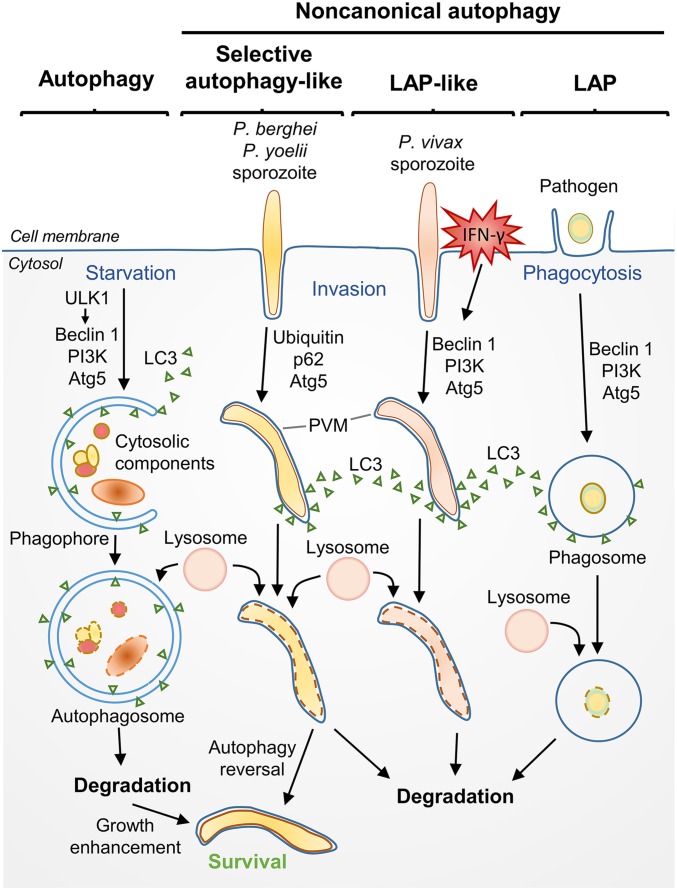
The first few days of exposure to Plasmodium sporozoites following a mosquito bite is a critical stage of malaria infection. From the bite site, sporozoites quickly migrate through the bloodstream and invade hepatocytes. Hepatocyte invasion occurs via invagination of the host cell plasma membrane, which parasites use to form a parasitophorous vacuole membrane (PVM) within the cell for their development. Single sporozoites within the PVM grow rapidly and replicate into thousands of merozoites. These are released into the blood stream and go on to invade red blood cells, initiating the cyclic, self-perpetuating blood stage of infection responsible for the clinical symptoms associated with malaria. Relatively few parasites reside within the host during the early liver stage compared with the latter blood stage; therefore, the hepatocyte stage of infection is particularly important and presents an attractive therapeutic target. However, our knowledge of Plasmodium sporozoite−hepatocyte interactions is very limited, and largely relies on rodent studies. The study of human Plasmodium sporozoites during the liver stage is extremely difficult, particularly in the case of Plasmodium vivax, which cannot be continuously cultured in vitro. Despite these experimental difficulties, in PNAS, Boonhok et al. (1) use a membrane feeding system with freshly isolated blood from P. vivax patients to infect mosquitoes and obtain sporozoites for study in a specially designed human hepatocyte cell line. With this, Boonhok et al. (1) provide an essential piece of information on the P. vivax and hepatocyte interaction by demonstrating that liver cells stimulated with interferon gamma (IFN-γ) can mount an intracellular immune response against P. vivax sporozoites that largely depends on a novel form of noncanonical autophagy. This mechanism is shown to be similar to microtubule-associated protein light chain 3 (LC3)-associated phagocytosis (LAP), a process in which LC3 is deposited directly on the pathogen-containing vacuole, leading to a formation of single-membrane phagosome with final lysosomal fusion and degradation of the pathogen (Fig. 1).
Fig. 1.
Canonical macroautophagy (referred to as autophagy) and noncanonical autophagy of pathogens. In autophagy, starvation triggers ULK1 to participate in the formation of a double membrane cup known as a phagophore. Beclin 1, PI3K, and Atg5 are involved in targeting LC3 and cytoplasmic components to the phagophore and formation of the autophagosome, which then fuses with lysosomes, facilitating degradation of the engulfed components. In noncanonical autophagy, autophagic proteins are deposited directly onto the pathogen-containing vacuole. In LAP, phagocytosis is followed by deposition of LC3 onto the phagosomal membrane, a process dependent on Beclin 1, PI3K, and Atg5, but not requiring ULK1. In human hepatocytes stimulated with IFN-γ, Boonhok et al. (1) demonstrate that a LAP-like process occurs when LC3 is deposited onto the PVM of sporozoites, followed by lysosomal fusion and degradation. This differs from a previously described autophagic pathway, with similarities to selective autophagy, described for P. berghei and P. yoelii sporozoites (5, 6). In this pathway, ubiquitin and p62 localize with the PVM in unstimulated hepatocytes, whereas PI3K and Beclin 1 do not. The majority of P. berghei sporozoites are able to reverse this autophagy pathway, and ultimately benefit from ongoing canonical autophagy, presumably through nutrient acquisition from the hepatocyte (5).
Eukaryotic cells continuously degrade and recycle their components through an intracellular engulfment process known as macroautophagy, herein referred to as autophagy (also named canonical autophagy). A unique double-membrane vesicle known as the autophagosome (characterized by the ubiquitin-like protein LC3 conjugation system) is formed during this process, which eventually fuses with lysosomes for the final digestion of components for their nutrient content (Fig. 1). Although autophagy is critical for maintaining cellular homeostasis, particularly during periods of starvation and stress, it is now also recognized to play a key role in intracellular immunity (2, 3). Autophagy can help to destroy pathogens through selective autophagy (also known as xenophagy), which involves formation of the autophagosome around the pathogen followed by lysosomal fusion and pathogen degradation. Additionally, several noncanonical autophagy pathways have been elucidated that involve autophagy proteins but do not result in autophagosome formation, such as LAP (4). On the other hand, autophagy can also be hijacked to deliver nutrients to support the growth of invading pathogens. In malaria, both autophagic clearance and autophagy-mediated growth have recently been shown to play an important role in the development of rodent Plasmodium sporozoites in the liver (5, 6). In the basal state, both Plasmodium berghei and Plasmodium yoelii parasites hijack the hepatocyte cell autophagy pathway to gain nutrients to support their rapid growth (5, 6). Importantly, autophagy inhibition in these models results in decreased parasite size and lower parasite burden. However, a subset of P. berghei sporozoites are degraded by a mechanism similar to selective autophagy, involving ubiquination, deposition of p62 and LC3 on the PVM, and lysosomal colocalization, leading to the hypothesis that parasites may initially contest with host cell autophagy, with surviving parasites able to overcome the host cell defense mechanisms and bend them to their own devices (5) (Fig. 1). Boonhok et al. (1), however, took a slightly different approach toward examining the role of autophagy in P. vivax, in that the response of cells stimulated with IFN-γ was examined rather than those in the basal state. As such, a direct comparison between these studies cannot be made, and it remains to be elucidated if basal autophagy can be beneficial to the growth of P. vivax sporozoites as seen in rodent malaria infection.
Parasite success during the liver stage, in fact, largely depends on the ability of sporozoites to avoid host immune system. Within hepatocytes, Plasmodium sporozoites must subvert an array of extracellular and intracellular immune defenses deployed by the host. The cytokine IFN-γ has long been thought to be critical in this process. Mice depleted of IFN-γ cannot protect themselves against liver stage parasites (7), whereas IFN-γ treatment protects against both human and mouse Plasmodium species (7). However, the mechanisms by which IFN-γ acts to suppress parasite growth are unclear. Using their in vitro human hepatocyte infection system, Boonhok et al. (1) demonstrate that IFN-γ treatment of hepatocytes results in clearance of ∼50% of intracellular P. vivax sporozoites without host cell death. Importantly, this demonstrates that IFN-γ can trigger an intracellular defense mechanism against P. vivax parasites that is independent of immune effector cells. This is in line with previous observations showing that CD8 T-cell-mediated parasite clearance can occur in a contact-independent mechanism via excretion of IFN-γ (7). In contrast to previous studies in mouse hepatocytes, which report that IFN-γ stimulated clearance of P. berghei parasites is dependent on nitric oxide (NO) (8), Boonhok et al. (1) show that P. vivax clearance is NO-independent. This highlights the differences between human and mouse immune responses and emphasizes the importance of human studies.
To examine the potential role and mechanisms of autophagy, Boonhok et al. (1) used chemical and siRNA inhibition of the autophagy-associated proteins Beclin-1, phosphoinositide 3-kinase (PI3K), autophagy protein 5 (Atg5), and unc-51 like autophagy activating kinase 1 (ULK1). Both canonical and noncanonical autophagy require Beclin-1, PI3K, and Atg5, whereas ULK1 is only required for the canonical autophagy pathway. Boonhok et al. (1) demonstrate that inhibition of Beclin-1, PI3K, or Atg5 results in ∼50% less IFN-γ-triggered parasite clearance, whereas inhibition of ULK1 had no effect, suggesting that the clearance mechanism largely depends on a noncanonical autophagy pathway, similar to LAP, with the exception that sporozoites are not phagocytized but rather invade the hepatocyte. They further validated this finding through microscopy studies showing LC3 deposition on the parasitophorous vacuole, and colocalization of parasites with Lysotracker red (LTR) stained acidic vesicles in response to IFN-γ stimulation. Notably, these results are in line with a study of the rodent parasite P. yoelii, which demonstrated that LTR-positive vesicles colocalized with sporozoites that failed to egress from their traversal vacuoles within hepatocytes (9).
Because a LAP-like process has now been described for P. vivax sporozoite invasion of hepatocytes, it will be of immediate interest to determine if a similar process occurs in P. falciparum. Similarly, determining the potential role of autophagy in combating hypnozoite persistence in the liver is of critical importance, and may provide therapeutic opportunities to prevent long-term relapse of P. vivax malaria. Toward this goal, a recently developed humanized mouse model of P. vivax hypnozoite persistence will be invaluable (10). The mechanisms by which ∼50% of parasites are able to avoid IFN-γ-mediated clearance, and the autophagy-independent mechanisms of IFN-γ-mediated clearance of parasites, remain to be determined and should be the subject of future study. Finally, it would be of particular interest to examine the role of autophagy during hepatocyte infection by irradiated or genetically attenuated sporozoites, as this would provide clues to how this vaccination strategy has been so successful in inducing CD8 T-cell responses and preventing malaria infection in mice and man (11–13).
Overall, although originally described as a cellular recycling mechanism, autophagy now has an established role as an important intracellular immune defense mechanism. Intracellular autophagy recognition can occur via a myriad of mechanisms that are not fully understood. These include recognition of pathogen containing vacuoles, cytosolic pathogens that have escaped their vacuole, and damaged pathogen vacuoles, with recognition often involving initial binding of autophagy proteins ubiquitin and p62 (reviewed in detail in refs. 2, 3, and 14). Pathogen neutralization can then occur by autophagosome engulfment or fusion with pathogen vacuoles, followed by lysosomal fusion, direct lysosomal fusion to pathogen vacuoles mediated by autophagy proteins, or lysosome-independent pathogen vacuole membrane destruction mediated by autophagy proteins, such as described for Toxoplasma gondii (15, 16). Boonhok et al. (1) now provide the first evidence, to our knowledge, of a unique noncanonical autophagic pathway for the clearance of the human pathogen, P. vivax, providing important information about the host−pathogen interaction in malaria.
It seems as though we are only just beginning to uncover what is likely a vast array of intracellular defense pathways mediated by autophagy and/or autophagy-related processes. Further research into these pathways will no doubt provide critical insights into malaria disease pathogenesis and may ultimately provide new drug targets for malaria elimination.
Acknowledgments
The authors’ research is supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan (KAKENHI-Kiban B) and the Uehara Memorial Foundation and Yamada Science Foundation.
Footnotes
The authors declare no conflict of interest.
See companion article on page E3519.
References
- 1.Boonhok R, et al. LAP-like process as an immune mechanism downstream of IFN-γ in control of the human malaria Plasmodium vivax liver stage. Proc Natl Acad Sci USA. 2016;113:E3519–E3528. doi: 10.1073/pnas.1525606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16(10):1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 4.Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450(7173):1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 5.Prado M, et al. Long-term live imaging reveals cytosolic immune responses of host hepatocytes against Plasmodium infection and parasite escape mechanisms. Autophagy. 2015;11(9):1561–1579. doi: 10.1080/15548627.2015.1067361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thieleke-Matos C, et al. Host cell autophagy contributes to Plasmodium liver development. Cell Microbiol. 2016;18(3):437–450. doi: 10.1111/cmi.12524. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira A, et al. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 8.Mellouk S, Green SJ, Nacy CA, Hoffman SL. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991;146(11):3971–3976. [PubMed] [Google Scholar]
- 9.Risco-Castillo V, et al. Malaria sporozoites traverse host cells within transient vacuoles. Cell Host Microbe. 2015;18(5):593–603. doi: 10.1016/j.chom.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Mikolajczak SA, et al. Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe. 2015;17(4):526–535. doi: 10.1016/j.chom.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman SL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 13.Spring M, et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013;31(43):4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Rubinsztein DC, Bento CF, Deretic V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J Exp Med. 2015;212(7):979–990. doi: 10.1084/jem.20150956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohshima J, et al. Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. 2014;192(7):3328–3335. doi: 10.4049/jimmunol.1302822. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, et al. The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity. 2014;40(6):924–935. doi: 10.1016/j.immuni.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]