Significance
AP endonuclease-1 (APE1)/redox effector factor-1 (Ref-1) is an essential DNA repair enzyme that has been difficult to study mechanistically because of embryonic lethality in conventional knockout animals. Thus, we generated a conditional APE1 knockout model to examine the protective role of endogenous APE1 in experimental stroke. Induced APE1 knockout in adulthood greatly exacerbated neuron and oligodendrocyte loss after mild ischemic stroke and prevented the intrinsic, long-term recovery of sensorimotor function and spatial learning and memory. APE1 knockout also aggravated ischemia-induced destruction of myelin and impairment of axon conduction in white matter. We conclude that APE1 dictates fundamental life and death decisions in both gray and white matter and plays an indispensable role in intrinsic recovery after mild ischemic injury.
Keywords: ischemia, neurodegeneration, base excision repair, oxidative DNA damage, white matter injury
Abstract
A major hallmark of oxidative DNA damage after stroke is the induction of apurinic/apyrimidinic (AP) sites and strand breaks. To mitigate cell loss after oxidative DNA damage, ischemic cells rapidly engage the base excision-repair proteins, such as the AP site-repairing enzyme AP endonuclease-1 (APE1), also named redox effector factor-1 (Ref-1). Although forced overexpression of APE1 is known to protect against oxidative stress-induced neurodegeneration, there is no concrete evidence demonstrating a role for endogenous APE1 in the long-term recovery of gray and white matter following ischemic injury. To address this gap, we generated, to our knowledge, the first APE1 conditional knockout (cKO) mouse line under control of tamoxifen-dependent Cre recombinase. Using a well-established model of transient focal cerebral ischemia (tFCI), we show that induced deletion of APE1 dramatically enlarged infarct volume and impaired the recovery of sensorimotor and cognitive deficits. APE1 cKO markedly increased postischemic neuronal and oligodendrocyte degeneration, demonstrating that endogenous APE1 preserves both gray and white matter after tFCI. Because white matter repair is instrumental in behavioral recovery after stroke, we also examined the impact of APE1 cKO on demyelination and axonal conduction and discovered that APE1 cKO aggravated myelin loss and impaired neuronal communication following tFCI. Furthermore, APE1 cKO increased AP sites and activated the prodeath signaling proteins, PUMA and PARP1, after tFCI in topographically distinct manners. Our findings provide evidence that endogenous APE1 protects against ischemic infarction in both gray and white matter and facilitates the functional recovery of the central nervous system after mild stroke injury.
Focal ischemic injury in the brain is both a spatial and temporal event, wherein the infarct zone expands gradually over several days (1, 2). However, if the stroke insult is moderate, the periinfarct regions may recover over time. The spontaneous recovery of periinfarct structures and neurological functions creates an opportunity to research the molecular mechanisms underlying endogenous neuroprotection and neural repair, which may accelerate the discovery of rational therapeutic targets for stroke treatment.
Oxidative DNA damage (ODD) is an early event following cerebral ischemia and reperfusion (3, 4), and reversal of ODD in penumbral regions is associated with eventual recovery from such insult (4, 5). Repair of ODD by the base excision-repair (BER) pathway targets oxidized DNA base lesions through a multistep process. First, base lesions are recognized by DNA glycosylases, which cleave the damaged bases and form temporary apurinic/apyrimidinic (AP) sites. The AP sites are then recognized by the critical BER enzyme, AP endonuclease-1/redox effector factor-1 (referred to here as APE1) (6, 7). APE1 repairs AP sites by cleaving the phosphodiester backbone 5′ to the AP site and thus creating a 3′-OH group and a 5′-deoxyribose phosphate residue that is the preferred substrate for DNA polymerase-β (6–8). As one of the major steps in BER, APE1 subsequently facilitates the engagement of DNA polymerase-β and DNA ligase I or III (8). Inadequate repair capacity leads to the accumulation of unrepaired AP sites (9) and is associated with inexorable progression of the ischemic injury (3). We and others have demonstrated that enhanced APE1 activity by means of transgenic overexpression or APE1 expression-inducing peptides, such as the pituitary adenylate cyclase-activating polypeptide (PACAP), confers robust neuroprotection against ischemic brain injury (10–12). Furthermore, a strong correlation exists between loss of APE1 expression in ischemic neurons and neuronal cell death after ischemia (9, 13–15). Energy failure after ischemia has been speculated to deplete APE1 expression, thereby triggering neuronal death (16). However, the role of endogenous APE1 in cellular protection from ischemic injury has not been unequivocally established, as knockout of this enzyme leads to catastrophic deficiencies in neurogenesis and is lethal embryonically (15, 17).
Concrete evidence has been lacking thus far to establish a role for endogenous APE1 in long-term functional recovery following mild neural injury. Furthermore, the mechanisms that underlie cell death as a result of the accumulation of unrepaired ODD are still poorly understood. To assess the role of endogenous APE1 in protection from ischemic injury, we generated, to our knowledge, the first APE1 conditional knockout (cKO) mouse line under the control of a tamoxifen-inducible Cre recombinase. Our findings reveal that the accumulation of ODD and the activation of prodeath programs in tamoxifen-treated APE1 cKO mice are markedly increased in neurons and oligodendrocytes after a transient cerebral ischemic insult, augmenting injury in both gray and white matter and exacerbating behavioral outcomes. These data highlight an important role for endogenous APE1 in innate protective mechanisms in gray and white matter following cerebral ischemia and reperfusion, and suggest that DNA repair through the BER pathway is an essential step for the spontaneous recovery of behavioral function following ischemic injury.
Results
Induced Knockout of APE1 Potentiates Cerebral Ischemic Injury.
Previous attempts at global knockout of the APE1 gene resulted in embryonic lethality on embryonic days (E)5 to E9 (15, 17). Thus, a conditional APE1 knockout mouse line was created by insertion of loxP sites around exons IV and V of the Apex1 gene (details are provided in SI Methods) (Fig. S1). The homozygous APE1flox/flox mice were viable, fertile, and normal in size and did not exhibit any gross physical or behavioral abnormalities. APE1flox/flox mice were then crossed with hemizygous CAGGCre-ER mice carrying tamoxifen-inducible, ubiquitously expressed Cre-recombinase (18) to generate mice of which 50% were CAGGCre-ER; APE1flox/wt (Fig. S2A). These mice were bred to APE1flox/flox mice, yielding offspring of which 25% had the genotype CAGGCre-ER; APE1flox/flox (Fig. S2C). Mice with the genotype CAGGCre-ER; APE1wt/wt served as controls (Fig. S2B) and received the same tamoxifen treatment. In the following text and figures, APE1 cKO and APE1 WT are used to refer to the CAGGCre-ER; APE1flox/flox and CAGGCre-ER; APE1wt/wt mice, respectively, both after tamoxifen treatment. To induce Cre-mediated recombination, tamoxifen was administered (75 mg/kg, i.p.) for 5 d at 8–12 wk of age, which effectively decreased APE1 protein expression in various brain regions of APE1 cKO mice (Fig. S2D). Furthermore, lysates derived from APE1 cKO brains were ineffective in repairing AP sites in an in vitro DNA repair assay (Fig. S2E). Thus, the APE1 cKO mice showed not only ablated APE1 gene expression in the brain, but also suppressed intrinsic DNA repair activity through the BER pathway.
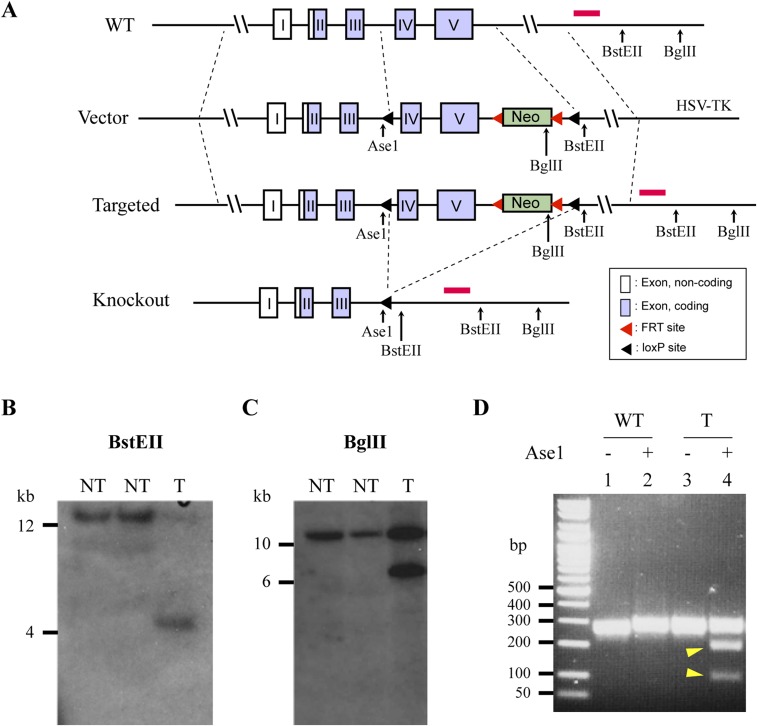
Fig. S1.
Generation of conditional APE1 knockout mice. (A) Targeting vector strategy. loxP sites were engineered flanking exons IV and V (the third and final coding exons) of the Apex1 gene to create cKO APE1 mice. Additional restriction sites were engineered into the vector to perform restriction enzyme analyses to confirm successful recombination of the loxP sites, including AseI for the 5′ loxP site, and BstEII and BglII for the 3′ loxP site and presence of the Neo gene. The inclusion of the Neo gene was to allow for selection of clones resistant to G418, and was flanked by frt sites to allow for removal from the targeted mouse strain. HSV-TK was included outside of the targeting region to eliminate cells with random integration of the targeting vector, as the expression of this gene will lead to sensitivity to gancylovir allowing these cells to be eliminated. When crossed to transgenic mice expressing the Cre protein in specific cells, the loxP sites recombine in these cells and delete the floxed region of the Apex1 gene knocking out the protein. Open boxes represent noncoding exon regions. Solid arrows represent restriction enzyme cut sites, black arrowheads represent loxP sites, red arrowheads represent Frt sites, and the short red line at the 3′ end represents the external 3′ probe. (B) Southern blot screening of ES clones digested with BstEII led to the identification of a positive clone yielding a band at the predicted size (4.7 kb) when probed with the external 3′ probe (red line in A). Both nontargeted clones (NT) and the targeted clone (T) are shown. (C) The positive clone was amplified and rescreened using BglII to confirm recombination by the 3′ loxP region, yielding a band at the predicted size (6.7 kb) when probed with an external 3′ probe (red line in A). (D) To confirm the presence of the 5′ loxP site, genomic DNA containing the region between exon III and exon IV was amplified and digested with Ase1. The resulting PCR product from the targeted clone (T) produced a single band (lane 3) and was cleaved into two smaller fragments by Ase1 (lane 4, arrows), whereas the PCR product from WT DNA (WT) produced a band (lane 1) that was not cleaved by Ase1 (lane 2) because of the lack of the inserted loxP and Ase1 sequence.
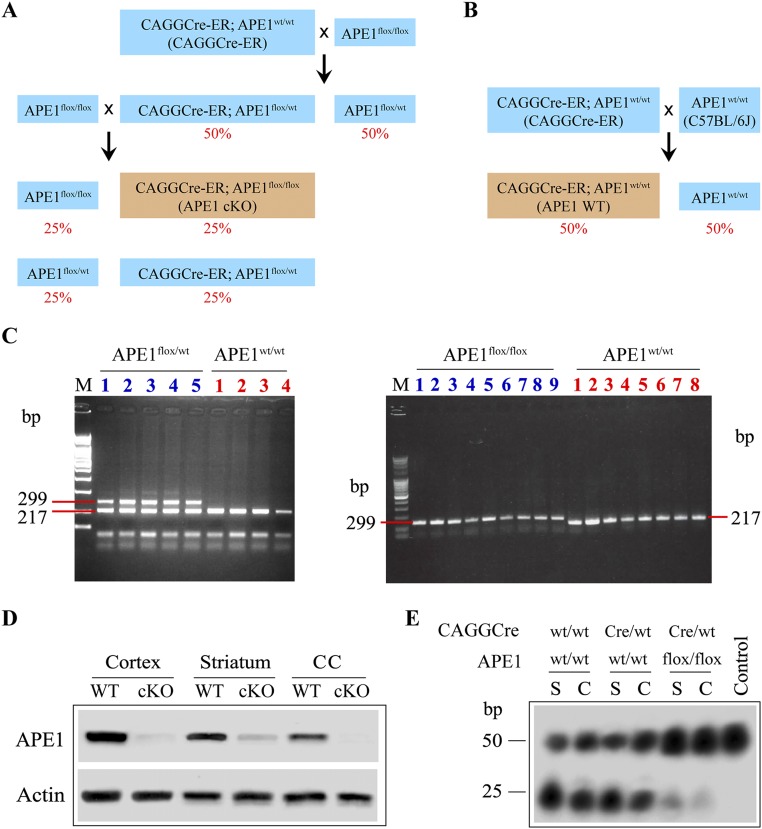
Fig. S2.
Tamoxifen yields knockout of APE1 protein and inhibition of DNA repair activity in cKO mice. (A) Scheme of breeding to obtain APE1 cKO mice. Homozygous APE1flox/flox mice were crossed with hemizygous CAGGCre-ER (i.e., CAGGCre-ER; APE1wt/wt) mice to generate mice, 50% of which were CAGGCre-ER; APE1flox/wt mice. These mice were bred to APE1flox/flox mice, yielding offspring 25% of which had the genotype CAGGCre-ER; APE1flox/flox. (B) Scheme of breeding to obtain APE1 WT mice as age- and gender-matched controls to APE1 cKO mice. Hemizygous CAGGCre-ER mice were crossed with WT C57BL/6J mice (i.e., APE1wt/wt) to generate mice, 50% of which were CAGGCre-ER; APE1wt/wt mice. Tan boxes indicate genotypes used for subsequent experiments (APE1 cKO and APE1 WT). (C) PCR genotyping demonstrating generation of heterozygous mice (Left) and subsequent breeding to obtain homozygous mice containing the loxP sites on both alleles (Right). Using primers flanking the 3′ loxP site, the floxed allele runs at a weight of 299 bp, whereas the WT allele has a weight of 217 bp. (D) Successful ablation of APE1 protein expression in APE1 cKO mice treated with tamoxifen for 5 d. Tissue was harvested 3 d after the last tamoxifen injection from the cortex, striatum, and CC from both WT and APE1 cKO mice. Actin immunoblotting served as the loading control. (E) Tamoxifen-induced knockout of APE1 led to loss of DNA excision-repair activity. In vitro excision-repair activity was assessed at 3 d after last tamoxifen injection using lysates derived from the striatum (S) or cortex (C) of WT C57BL/6J mice (Cre−; APE1wt/wt), APE1 WT mice (CAGGCre-ER; APE1wt/wt), or APE1 cKO (CAGGCre-ER; APE1flox/flox). The lysates were incubated with a 32P-labeled 50-bp oligonucleotide substrate containing a synthetic AP site. APE1 activity leads to the generation of a 25-bp product. Lysates derived from the APE1 cKO mouse brain showed marked reduction in the presence of the 25-bp product. As a negative control (Control), the assay done without the protein lysates resulted in absence of the 25-bp product.
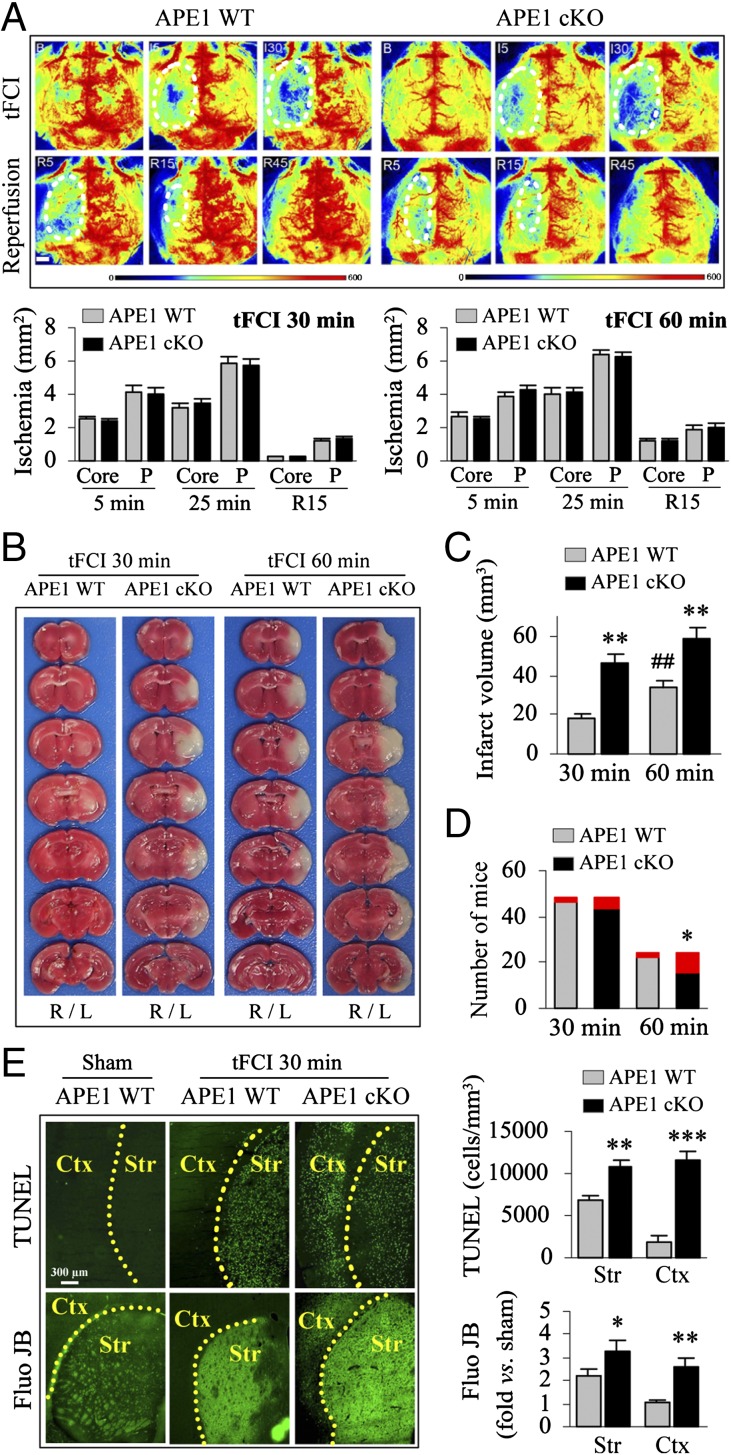
We evaluated the effect of conditional APE1 knockout on stroke outcomes to indirectly test the hypothesis that the accumulation of AP sites in the ischemic brain worsens neurological outcomes after cerebral ischemia. Transient focal cerebral ischemia (tFCI) was induced in mice for either 30 or 60 min by means of middle cerebral artery occlusion. Physiological parameters, including core temperature and blood gases, were maintained in the normal range in all groups during tFCI. Regional cerebral blood flow (CBF) was measured by 2D laser-speckle imaging during tFCI until 45 min following reperfusion onset. The ischemic core and penumbral regions during tFCI (defined as regional CBF < 20% and between 20% and 35% of preischemic baseline, respectively) were of equivalent sizes in APE1 cKO and APE1 WT brains (Fig. 1A), confirming that the initial insult was of the same magnitude in both genotypes. The ischemic infarct in APE1 WT mice was limited to the striatum 48 h after 30 min of tFCI, whereas the infarct encompassed both the striatum and the frontal-parietal cortex 48 h after 60 min of tFCI (Fig. 1 B and C). As expected, infarct volume was dramatically increased in APE1 cKO mice compared with APE1 WT mice after either 30 or 60 min of tFCI (Fig. 1 B and C). We also observed a significant increase in mortality in the APE1 cKO mice (recorded up to 28 d after 60 min of tFCI) (Fig. 1D). These findings suggest that endogenous APE1 reduces the consequences of DNA damage. To avoid potential confounds from exclusion of animals in the APE1 cKO group because of high mortality, we used the 30-min tFCI model for further experiments. Use of the milder injury also allowed us to examine whether APE1 contributes to recovery of neurological function in otherwise untreated animals.
Fig. 1.
Induced knockout of APE1 increases infarct volumes, mortality, and cell death after tFCI. tFCI was induced in tamoxifen-treated CAGGCre-ER; APE1wt/wt (APE1 WT) and CAGGCre-ER; APE1flox/flox (APE1 cKO) mice. (A) Cerebral blood flow was assessed by 2D laser-speckle imaging. Representative images show the control, the ischemic area (depicted by the dotted line) during 5 and 30 min of tFCI and after 5, 15, and 45 min of reperfusion following 60 min of tFCI. (Scale bar, 1 mm.) The bar graphs show ischemic areas at 5 and 25 min in both the ischemic core (defined as CBF < 20% of preischemia baseline) and penumbra (P) (CBF = 20–35% of preischemia baseline) during 30 or 60 min of tFCI or 15 min of reperfusion. The results reveal similar CBF changes in APE1 WT and APE1 cKO mice. n = 5 per group. (B and C) Infarct volume was assessed at 48 h after tFCI by TTC (2,3,5-triphenyltetrazolium) staining (the right and left hemispheres are labeled with R and L, respectively) and quantified as described using ImageJ under blinded conditions. Values are means ± SEM, n = 8 per group. **P ≤ 0.01 vs. the matched APE1 WT group. ##P ≤ 0.01 vs. 30 min WT group. (D) The number of animals that survived (bottom portion of bars) or died (upper red portion of bars) by 28 d after tFCI. Comparisons of animal survival rates were performed by Kaplan–Meier survival analysis. *P ≤ 0.05 vs. the matched APE1 WT group. (E) Histological assessments of cell death were performed at 48 h after tFCI (30 min) using the TUNEL stain for DNA fragmentation and Fluoro-Jade B (Fluo JB) for cell degeneration, respectively. TUNEL+ cells were counted using stereology, and Fluoro-Jade B was semiquantified by measuring the fluorescence intensity in the striatum (Str) and cortex (Ctx). Values are mean ± SEM, n = 6 per group. **P ≤ 0.01, ***P ≤ 0.001 vs. the matched APE1 WT group.
Consistent with the hypothesis that APE1 rescues cells from ischemic injury, APE1 cKO mice exhibited greater numbers of degenerating TUNEL+ and Fluoro-Jade+ profiles at 48 h after 30 min of tFCI. In APE1 WT mice, ischemia-induced cell death was prominent in the striatum but modest in the cortex; in APE1 cKO mice, ischemic cell death was significantly potentiated in both striatum and cortex, consistent with expansion of the infarct region (Fig. 1E). These results support the conclusion that APE1 attenuates the consequences of ischemic brain injury.
To determine if loss of endogenous APE1 worsens neurofunctional outcomes after cerebral ischemia, we subjected APE1 cKO and APE1 WT mice to 30 min of tFCI and performed behavioral assessments at various times after reperfusion: including the rotarod test (1–7 d), corner test (3–28 d), and Morris water maze (22–27 d). According to the rotarod and corner test results, postischemic APE1 WT mice were indistinguishable from sham control animals by 7 d after tFCI, consistent with previous observations that mild ischemia-challenged mice rapidly recover sensorimotor function. Induced deletion of APE1 significantly worsened postischemic neurobehavioral performance compared both to sham controls and postischemic APE1 WT mice throughout the testing period (Fig. 2 A and B). In addition, spatial learning and memory in the Morris water maze—which depend partially on hippocampal integrity—were significantly worse in postischemic APE1 cKO mice compared with postischemic APE1 WT mice. That is, APE1 cKO increased the amount of time within a trial that the mouse needed to find the hidden platform (impaired learning), as well as decreased the percentage of time spent swimming in the target quadrant when the platform had been removed after the learning period (impaired memory) (Fig. 2 C–E). This degree of spatial memory impairment is not typically observed in the 30-min tFCI model, as shown by the lack of difference between postischemic APE1 WT mice and sham controls (Fig. 2 C–E). No differences between postischemic groups were observed in swim speed at 27 d after tFCI (Fig. 2F), indicating that gross motor differences did not confound the interpretation of APE1 knockout effects on spatial learning and memory. The cognitive deficits observed over the extended survival period correlated with histological damage in striatum and cortex, as there was greater loss of microtubule-associated protein 2 (MAP2) staining in the APE1 cKO mice compared with APE1 WT mice at 28 d following 30 min of tFCI (Fig. S3). Taken together, these data demonstrate that loss of endogenous APE1 protein increases cellular damage and potentiates sensorimotor and cognitive impairments following cerebral ischemic insults, in support of a significant role for APE1 in the mitigation of ischemic injury.
Fig. 2.
APE1 deficiency impairs sensorimotor and cognitive recovery after tFCI. In sham control animals, neither the sensorimotor function tests (rotarod test, corner test) nor the cognitive test (Morris water maze) revealed significant difference between the APE1 WT and APE1 cKO mice. Thus, the “sham group” in A, B, and D refers to the combination of both APE1 WT and APE1 cKO genotypes. (A) Rotarod performance was measured every other day for the first week following 30 min of tFCI. The latency to fall after ischemic injury was normalized to latency before the induction of ischemia. n = 6–8 per group. *P ≤ 0.05; **P ≤ 0.01 vs. the matched APE1 WT group; #P ≤ 0.05 vs. the sham group. (B) The corner test was performed up to 28 d following 30 min of tFCI. The number of left turns per 10 trials was recorded and plotted. The sham control group showed no turning bias or asymmetry (average of 5 left turns/10 trials); the postischemic mice showed increased turning toward the body side with unaffected mobility (left, ipsilateral to the ischemic hemisphere). The APE1 WT mice showed complete recovery by 7–10 d, whereas the APE1 cKO mice showed only partial recovery by 28 d. n = 6–8 per group. *P ≤ 0.05; **P ≤ 0.01 vs. the matched APE1 WT group; #P ≤ 0.05 vs. the sham group. (C–F) Long-term cognitive functions were assessed by the Morris water maze at 22–27 d after 30 min of tFCI. n = 8–10 per group. Shown are the mean ± SEM *P ≤ 0.05 vs. the matched APE1 WT group; ##P ≤ 0.01 vs. the sham group. (C) Representative swim path traces (24–26 d after tFCI) of mice attempting to find a hidden platform (top traces, “learning”), or searching for the platform after its removal (bottom traces, “memory”). (D) The escape latency (i.e., time needed to discover the platform) was recorded on days 22–26 after tFCI. (E) Memory was tested at 27 d after tFCI. The percentage of time spent in the target quadrant after the platform was removed was plotted to reflect memory. (F) Swimming speed was assessed at 27 d after tFCI for each genotype, to ensure that the observed differences did not reflect compromised swimming abilities.
Fig. S3.
APE1 deficiency increases neuronal tissue loss after tFCI. Brains were harvested at 28 d after 30 min tFCI and processed for MAP2 immunostaining. The representative images show MAP2 immunofluorescence in APE1 WT and APE1 cKO brains after tFCI, respectively; the dashed line depicts the MAP2− infarct area on each brain section (30-μm thick). The right and left hemispheres are labeled with R and L, respectively. (Scale bar, 1 mm.) Areas of neuronal tissue loss were measured on six equally-spaced coronal sections within the MCA territory per brain, and subsequently converted into infarct volumes. Data are mean ± SEM, n = 8 per group. **P ≤ 0.01 vs. the matched APE1 WT group.
APE1 Deficiency Potentiates the Accumulation of ODD Following Mild Ischemic Injury.
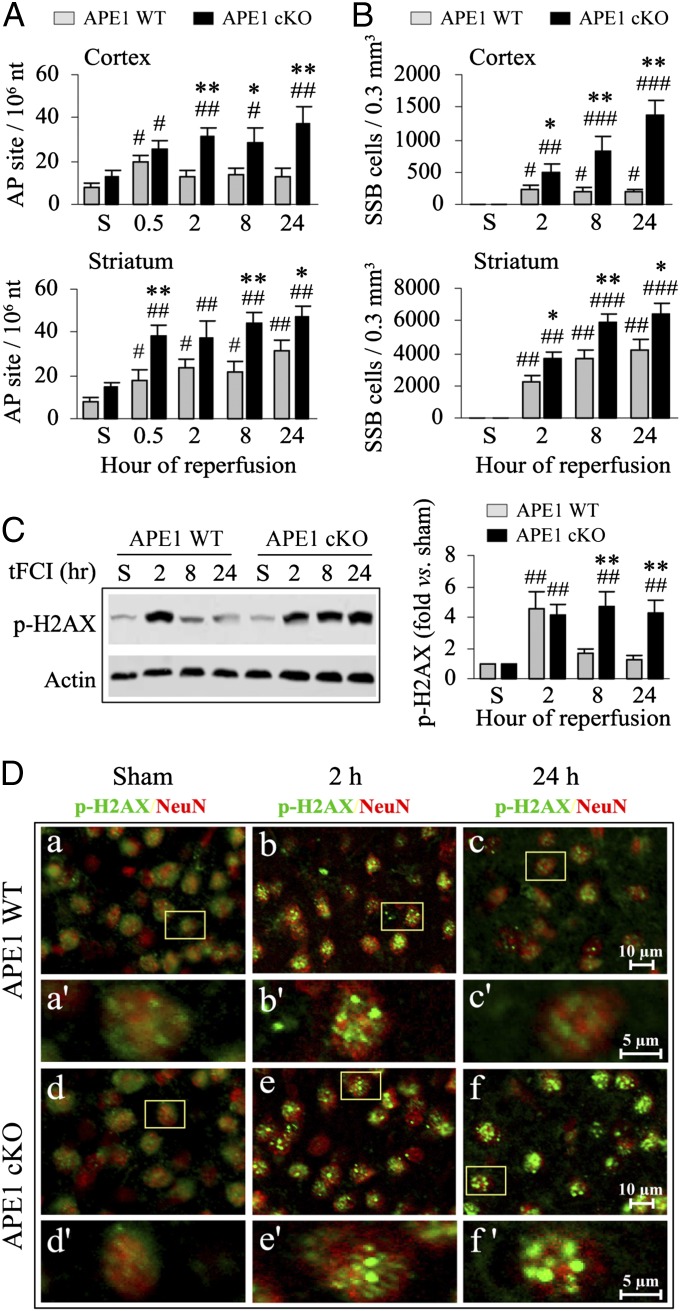
Acute ODD has long been associated with cell injury, and the accumulation of unrepaired ODD in response to cytotoxic stimuli, including ischemia, engages cell death cascades (1, 3, 19). As APE1 is an essential component of the DNA BER process, loss of APE1 activity is expected to lead to robust accumulation of ODD in cells after ischemia with reperfusion. Using three distinct measures of ODD, we verified that induced knockout of APE1 led to significant increases in ODD in the postischemic brain. Tissues collected from postischemic cortex and striatum, respectively, were quantitatively assessed for the number of AP sites and cells containing DNA single strand breaks (SSB) using the ELISA and DNA polymerase I-mediated biotin-dATP nick-translation (PANT) assay (3), respectively. In postischemic APE1 WT mice, AP sites (Fig. 3A) and SSB-containing cells (Fig. 3B) were increased early (0.5 h of reperfusion) after 30 min of tFCI in both the striatum and cortex. For at least 24 h thereafter, AP sites and SSB-containing cells remained elevated over sham controls in the striatum but subsided near to sham control levels in the cortex, reflecting an effective intrinsic recovery process in the postischemic cortex of APE1 WT mice. In contrast, AP sites and SSB-containing cells were remarkably elevated in both the postischemic striatum and cortex in APE1 cKO mice throughout the 24-h reperfusion timeframe, and ODD levels were significantly increased compared with the APE1 WT mice in both brain regions (Fig. 3 A and B). Because the increase in AP sites in the cortex is sustained instead of transient following loss of APE1, we conclude that APE1 facilitates recovery from DNA damage in gray matter.
Fig. 3.
APE1 deficiency increases the accumulation of ODD after tFCI. (A) AP site frequency was quantitatively measured in cortical and striatal extracts, respectively, from APE1 WT and APE1 cKO mice under sham condition (S) or at 0.5, 2, 8, and 24 h after 30 min of tFCI. Data are expressed as the number of AP site per 106 nucleotides. (B) SSBs were detected immunohistochemically using the PANT assay, and positively labeled cells were counted using stereology at the indicated time points after 30 min of tFCI. Values are means ± SEM, n = 6 per group. *P ≤ 0.05; **P ≤ 0.01 vs. the matched APE1 WT group; #P ≤ 0.05; ##P ≤ 0.01; ###P ≤ 0.001 vs. the sham group. (C) Levels of phospho-H2AX (marker of genomic damage) were assessed in cortical lysates by Western blotting at 2, 8, and 24 h after tFCI. n = 4 per group. **P ≤ 0.01 vs. the matched APE1 WT group; ##P ≤ 0.01 vs. the sham group. (D) Double-label immunofluorescent staining for p-H2AX (green) and the neuronal marker NeuN (red) in the cortices of sham controls and at 2 or 24 h after tFCI (a–c, APE1 WT; d–f, APE1 cKO mice). High-magnification images from the boxed regions revealed distinctly punctate patterns of p-H2AX immunofluorescence in postischemic cortical neurons (a′–c′, APE1 WT; d′–f ′, APE1 cKO mice). The frequency of punctate p-H2AX immunofluorescence subsided to control levels at 24 h after tFCI in APE1 WT brain, but remained elevated at 24 h after tFCI in APE1 cKO brains.
H2AX, a member of the histone H2A family, is a DNA damage-sensing protein that is activated by phosphorylation at Serine 139, and this phosphorylated H2AX (p-H2AX) can be detected immunohistochemically as dense foci in nucleosomes (20). The levels of p-H2AX were significantly increased in the postischemic cortex 2 h after 30 min of tFCI in the APE1 WT mice, but subsided to control levels within 8 h (Fig. 3C), again consistent with an intrinsic recovery process for ODD in the postischemic cortex. APE1 cKO mice exhibited similar p-H2AX levels in the postischemic cortex as APE1 WT mice at 2 h following 30 min of tFCI, but had significantly higher p-H2AX levels at 8 and 24 h following reperfusion, suggesting impaired recovery from ODD (Fig. 3C). Double-label immunofluorescence revealed elevated immunofluorescence of p-H2AX, exhibiting increased intensity of punctate granules in NeuN+ cortical neurons of APE1 WT mice at 2 h after 30 min of tFCI, but this pattern of punctate staining was diminished by 24 h following reperfusion (Fig. 3D). In contrast, in APE1 cKO mice there was a similarly high level of p-H2AX immunofluorescence at 2-h reperfusion, but it persisted for at least 24 h (Fig. 3D). Not surprisingly, the persistent presence of DNA damage in the cortex of APE1 cKO mice closely correlates with the regional patterns of ODD induction in the forms of AP sites and SSB (Fig. 3 A and B). These findings strongly suggest that loss of APE1 interferes with the cellular recovery process in the postischemic penumbra, likely contributing to the expansion of the infarct zone to the overlying cortex (Fig. 1 B and C). Thus, induced deletion of APE1 leads to the failure of DNA repair, and the resulting accumulation of ODD correlates with potentiation of cerebral ischemic injury.
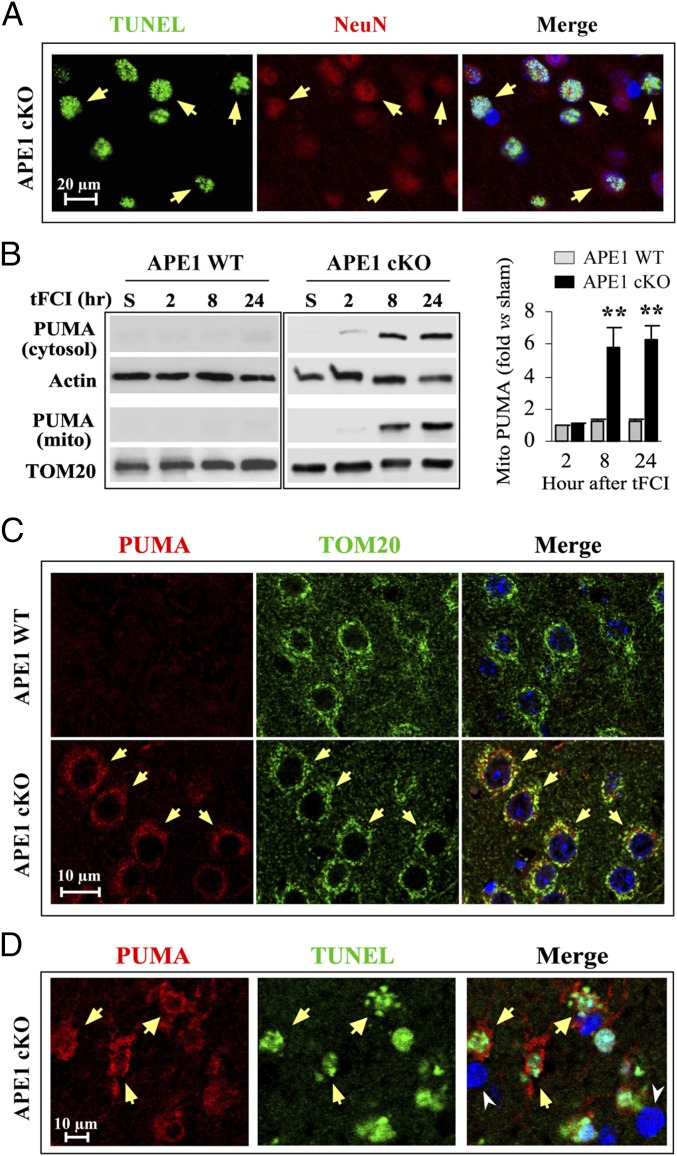
APE1 Deficiency Enhances Mitochondrial Translocation of PUMA in Apoptotic Ischemic Neurons.
In our investigation of the effects of APE1 knockout on ischemic injury, we explored the extent of destruction of specific cell populations (i.e., neurons and oligodendrocytes) within ischemic brain regions following 30 min of tFCI. We found extensive colabeling of the neuronal marker NeuN with TUNEL in the cortex of APE1 cKO mice 24 h after tFCI (Fig. 4A). As the p53 up-regulated modulator of apoptosis (PUMA) is a proapoptotic protein whose expression is regulated by the tumor suppressor p53 as part of the DNA damage response after cerebral ischemia (21), we explored whether the exacerbation of cell death by APE1 knockout was associated with increased expression of PUMA. In sham control mice or APE1 WT mice subjected to 30 min of tFCI, PUMA was nearly undetectable by Western blot analyses in either the cytosolic or mitochondrial fraction of cerebral cortical extracts (Fig. 4B). However, in APE1 cKO mice subjected to 30 min of tFCI followed by 8 or 24 h of reperfusion, PUMA expression was significantly elevated in both cytosolic and mitochondrial fractions (Fig. 4B), which supports the notion that the accumulation of ODD because of loss of APE1 causes PUMA up-regulation in postischemic brain. The presence of PUMA in the mitochondrial fraction suggested that this protein (PUMA) is translocated into mitochondria in postischemic neurons. Indeed, double-label immunofluorescent staining exhibited a punctate pattern for PUMA that was closely associated with the mitochondrial marker TOM20 (translocase of outer membrane 20 kDa) in cortical neurons 8 h after tFCI (Fig. 4C). Moreover, the increased PUMA immunofluorescence was particularly evident in TUNEL+ cortical cells displaying condensed and fragmented nuclei 24 h after tFCI (Fig. 4D). These findings support the hypothesis that the induction of PUMA may mediate the degeneration of APE1-deficient cortical neurons after ischemia and reperfusion.
Fig. 4.
APE1 deficiency activates proapoptotic PUMA signaling in postischemic neurons. (A) Representative images demonstrating TUNEL (green, arrows) colabeling with the neuronal marker NeuN (red, arrows) in the postischemic cortex of an APE1 cKO mouse 24 h following 30 min tFCI. DAPI (blue, in merged image) staining was used to identify cell nuclei. (B) Levels of PUMA in total protein extracts and the mitochondrial fraction of cortical tissue were assessed by Western blotting after sham operation or at 2, 8, and 24 h after tFCI. β-Actin and TOM20 (a mitochondria-specific protein) served as the loading controls for whole cell protein extracts and the mitochondrial fraction, respectively. Data are presented as mean± SEM, fold-changes versus sham controls. n = 4 per group. **P ≤ 0.01 vs. the matched APE1 WT group. (C) Triple-label immunofluorescent staining for PUMA (red), TOM20 (green), and DAPI (blue, in merged images only) in the postischemic cortex at 8 h following 30 min of tFCI in APE1 WT (Upper) and APE1 cKO (Lower) mice. PUMA immunofluorescence was increased and was closely associated with TOM20 (arrows) in postischemic cortical neurons of APE1 cKO brains, but not of APE1 WT brains. (D) Close association of intense PUMA immunofluorescence (red, arrows) with TUNEL+ staining (green, arrows) in the ischemic cortex 24 h after 30 min of tFCI in an APE1 cKO brain. Cell nuclei were stained with DAPI in the merged image. Two TUNEL− cells were also immunonegative for PUMA (arrowheads).
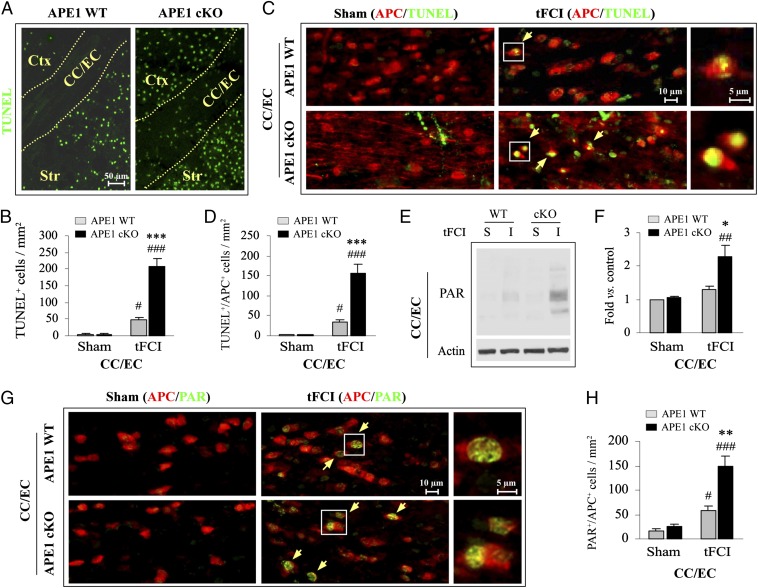
Loss of APE1 Exacerbates Oligodendrocyte Cell Death and Is Associated with Poly(ADP ribose) Polymerase 1 Activation in Postischemic Brain.
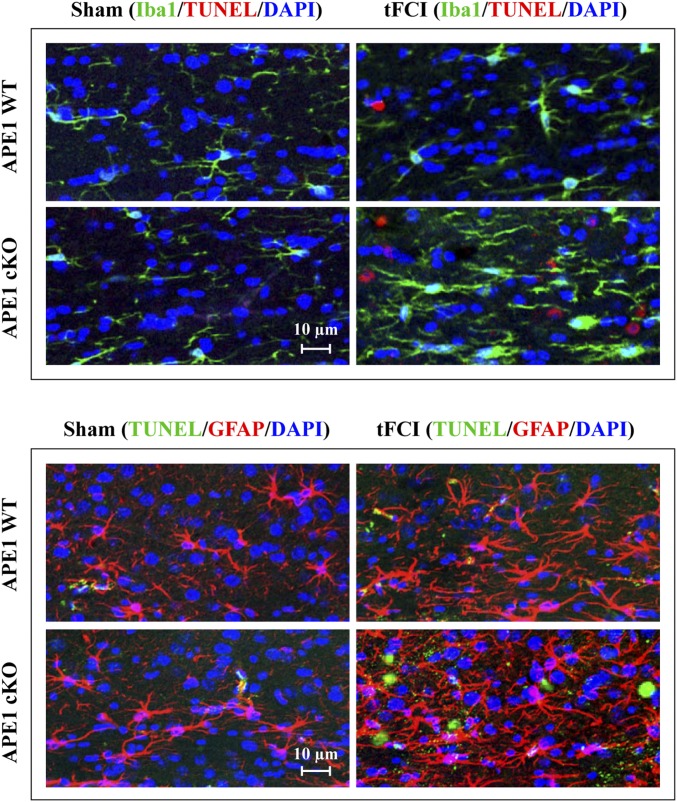
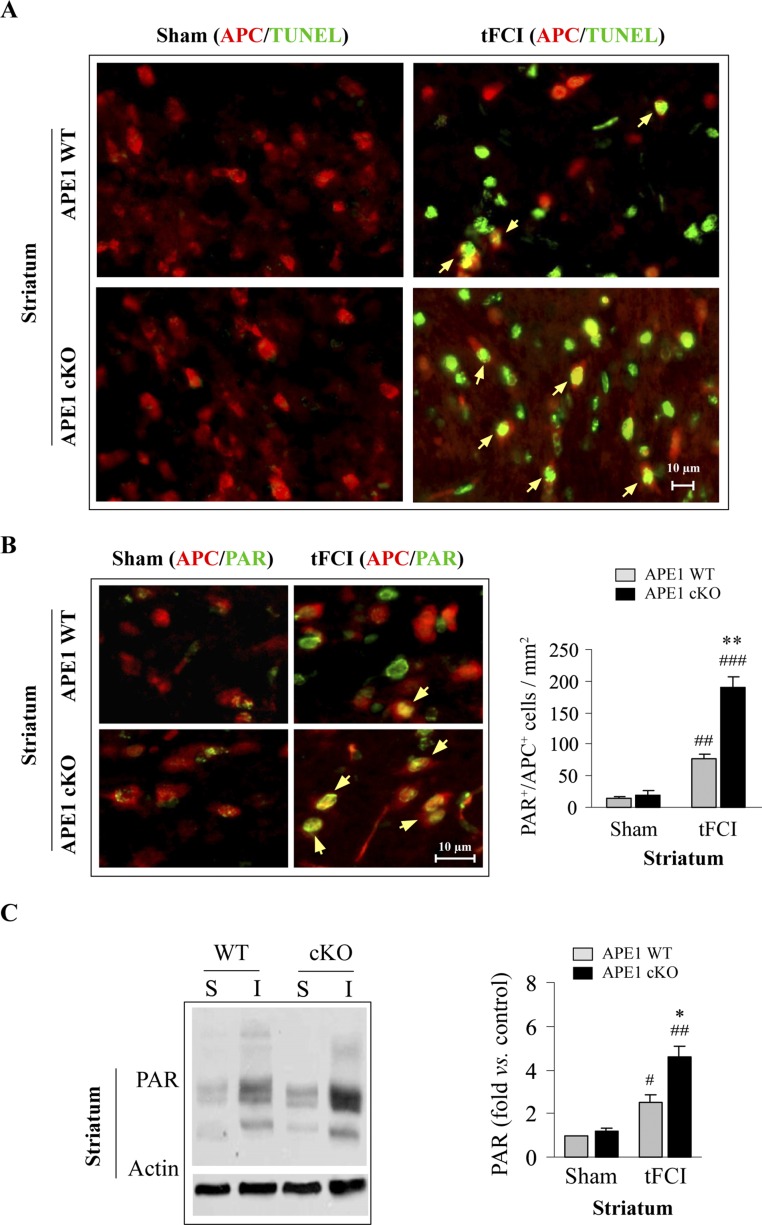
White matter injury (WMI) contributes significantly to both sensorimotor and cognitive neurological deficits after cerebral ischemia (22). However, the role of DNA damage and repair in ischemic WMI has been largely overlooked. The APE1 cKO brain exhibited a marked postischemic increase in the number of TUNEL+ cells within white matter tracts of the corpus callosum (CC) and external capsule (EC) 48 h after 30 min of tFCI compared with the APE1 WT brain (Fig. 5 A and B). To determine the cell types undergoing apoptosis, we performed double-label immunofluorescent staining for TUNEL and cell makers for microglia/macrophages (Iba1), astrocytes (glial fibrillary acidic protein, GFAP), or mature oligodendrocytes (adenomatous polyposis coli, APC). In the CC/EC of APE1 cKO mice, the majority of TUNEL+ cells expressed APC (Fig. 5 C and D). In contrast, in the CC/EC of APE1 WT postischemic mice, few APC+ cells were TUNEL+. Furthermore, microglia and astrocytes in the CC/EC were largely TUNEL− after 30 min of tFCI in both groups of animals (Fig. S4). Similarly, in the striatum (another white matter-enriched region) of postischemic APE1 cKO mice, the number of TUNEL+/APC+ cells was remarkably greater than in APE1 WT postischemic striatum (Fig. S5A). These data suggest that mature oligodendrocytes and neurons represent vulnerable cell populations in the white matter and gray matter of APE1-deficient mice, respectively.
Fig. 5.
APE1 deficiency activates proapoptotic PARP1 in postischemic oligodendrocytes. (A) TUNEL staining of brain sections at 48 h following 30 min of tFCI. Ctx, cortex; Str, striatum; CC/EC, corpus callosum and external capsule. Few TUNEL+ cells could be detected in these brain regions of the APE1 cKO or APE1 WT mice after sham surgery. (B) The number of TUNEL+ cells in the CC/EC region was quantified. n = 6 per group. ***P ≤ 0.001 vs. the matched APE1 WT group; #P ≤ 0.05; ###P ≤ 0.001 vs. the sham group. (C) Colocalization (yellow arrows) of TUNEL (green) with the mature oligodendrocytic marker APC (red) in the CC/EC in sham and 48 h following 30 min of tFCI in APE1 WT and APE1 cKO mice. (D) TUNEL+/APC+ cells were quantified from randomly selected microscopic fields in the CC/EC. n = 6 per group. ***P ≤ 0.001 vs. the matched APE1 WT group; #P ≤ 0.05; ###P ≤ 0.001 vs. the sham group. (E and F) Western blot analysis of PAR (marker of PARP1 activity, multiple bands) in extracts derived from CC/EC after sham operation (S) or 48 h after ischemia (I). PAR immunoblot results were as fold-changes over sham controls. n = 4 per group. *P ≤ 0.05 vs. the matched APE1 WT group; ##P ≤ 0.01 vs. the sham group. (G and H) Colocalization (yellow, arrows) of PAR (green) with APC (red) in the CC/EC at 48 h following 30 min of tFCI. PAR+/APC+ cells were quantified from randomly selected microscopic fields in the CC/EC. n = 6 per group. **P ≤ 0.01 vs. the matched APE1 WT group; #P ≤ 0.05; ###P ≤ 0.001 vs. the sham group.
Fig. S4.
Astrocytes and microglia are relatively resistant to cell death in the white matter after mild ischemic injury. Double-label staining of TUNEL with the astrocyte marker GFAP (Upper) or the microglial/macrophage marker Iba1 (Lower) in the CC/EC of APE1 WT and APE1 cKO mice, respectively, at 48 h following 30 min tFCI. Few TUNEL+ cells are also GFAP+ or Iba1+, suggesting that, compared with oligodendrocytes, astrocytes and microglia/macrophages in the white matter are less vulnerable to ischemia-induced cell death. Images are representatives of five mice per group with similar results.
Fig. S5.
APE1 deficiency exacerbates striatal cell death after tFCI and is associated with PARP1 activation. (A) Colocalization (yellow arrows) of TUNEL (green) with the mature oligodendrocytic marker APC (red) in the striatum at 48 h following 30 min of tFCI. Note that the number of TUNEL/APC double-positive cells was greater in APE1 cKO than in APE1 WT postischemic striatum. (B) Colocalization (yellow arrows) of PAR (green) with APC (red) in the striatum at 48 h following 30 min tFCI. PAR/APC double-positive cells were quantified from randomly selected microscopic fields. n = 6 per group. (C) Western blot analysis of PAR in protein extracts derived from the striatum after sham operation (S) or 48 h after ischemia (I). Immunoblot results for PAR were semiquantified. n = 4 per group. #P ≤ 0.05; ##P ≤ 0.01; ###P ≤ 0.001 vs. sham controls with the same genotype; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs. the matched APE1 WT group.
DNA damage may activate the protein poly(ADP ribose) polymerase 1 (PARP1), which catalyzes the formation of poly(ADP ribose) polymers (PAR) using NAD+ as substrate, thus eliciting intracellular NAD+ and ATP depletion (21). PAR is a death signal in neurons after ischemia and reperfusion (23). Therefore, we assessed PAR immunoreactivity—evidence of PARP1 activity—in CC/EC protein extracts after tFCI. As expected, APE1 cKO mice had significantly higher levels of PAR in the CC/EC compared with APE1 WT mice (Fig. 5 E and F). Similar results were observed in the striatum (Fig. S5C). As shown by double-label immunofluorescence, PAR was largely localized to nuclei of APC+ cells in the CC/EC (Fig. 5G) at 48 h after tFCI, whereas PAR immunofluorescence was elevated in both APC+ and APC− cells in the postischemic striatum (Fig. S5B). Nevertheless, the number of PAR+/APC+ cells was markedly greater in both the CC/EC (Fig. 5H) and striatum (Fig. S5B) of APE1 cKO mice after tFCI when compared with APE1 WT mice. Taken together, these findings suggest that compromised DNA repair in APE1 cKO mice leads to the activation of the prodeath PARP1 protein in mature oligodendrocytes after tFCI, which could promote ischemic WMI.
APE1 Deficiency Exacerbates Myelin Loss and White Matter Dysfunction After Mild Ischemic Injury.
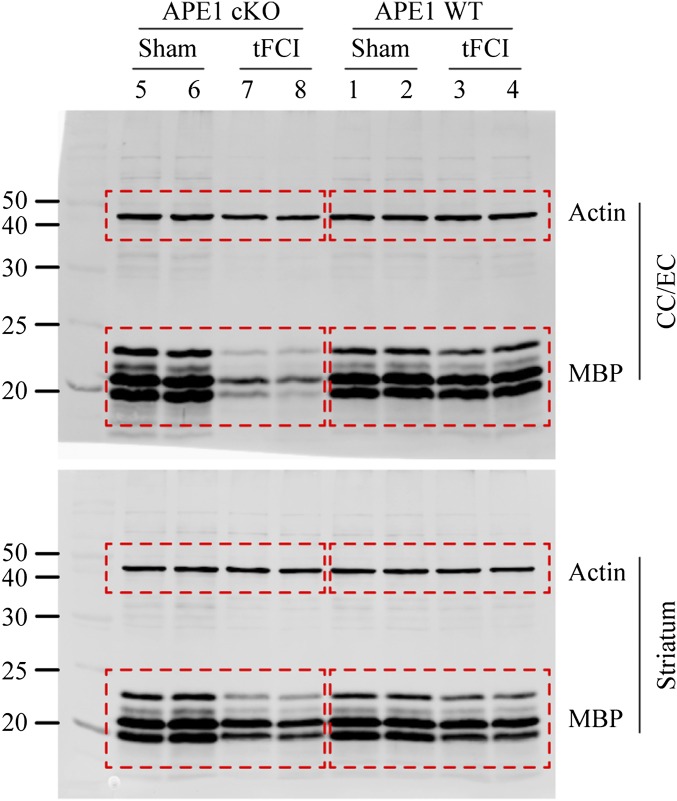
Functional assessments of white matter are becoming increasingly prevalent in stroke studies, as histological assessments of gray matter do not always coincide with changes in behavioral functions. Using histological indicators of white matter integrity, we examined the impact of APE1 knockout on WMI 28 d after 30 min of tFCI. For these studies we relied on immunohistochemical staining with the SMI-32 antibody, which recognizes the nonphosphorylated epitope of neurofilament H, a robust marker of demyelination (24). Under sham control conditions, faint staining with SMI-32 was visible in the CC/EC region and striatum (Fig. 6A). In APE1 WT mice, striatal SMI-32 immunoreactivity was enhanced after tFCI, resulting in an increased ratio of SMI-32 to myelin basic protein (MBP) (Fig. 6A). In contrast, no changes were detected in the CC/EC of these animals, suggesting that this region lay outside the zone of permanent WMI following mild ischemic injury. In APE1 cKO mice, a dramatic increase in SMI-32 immunoreactivity was evident in both the CC/EC and fascicles of the striatal internal capsule after tFCI, suggesting an expansion of the zone of permanent WMI (Fig. 6A). A simultaneous reduction in the intensity of MBP was observed in the postischemic brain of APE1 cKO mice, most notably along the fiber tracts of the CC/EC (Fig. 6A), indicating a loss of white matter myelination. Accordingly, the ratio of SMI-32 to MBP was markedly increased in the CC/EC and striatum of APE1 cKO mice compared with the APE1 WT mice after tFCI (Fig. 6A). Western blots on tissue lysates from the CC/EC and striatum showed a dramatic reduction in MBP expression in both regions after tFCI in APE1 cKO mice compared with APE1 WT mice (Fig. 6B and Fig. S6).
Fig. 6.
APE1 deficiency elicits long-term loss of white matter integrity after tFCI. (A) Double-label immunofluorescent staining for MBP and dephosphorylated neurofilament protein (SMI-32) in the ipsilesional CC/EC and striatum at 28 d after 30 min of tFCI or sham operation. tFCI decreased MBP labeling and increased SMI-32 labeling in both CC/EC and striatum; APE1 cKO mice were much more affected than APE1 WT mice. The graphs at the bottom illustrate the ratio of SMI-32 to MBP, indicating the degree of demyelination in the CC/EC and striatum. Values are mean ± SEM, n = 6 per group. **P ≤ 0.01; ***P ≤ 0.001 vs. the matched APE1 WT group; ##P ≤ 0.01; ###P ≤ 0.001 vs. sham group. (B) Western blot analysis showing multiple MBP bands in protein extracts from the CC/EC or striatum at 28 d after 30 min tFCI or sham operation. The graphs (Right) illustrate fold-changes in MBP protein levels in the CC/EC and striatum after tFCI relative to the sham group. Values were summed for all bands in each lane and are presented as mean ± SEM, n = 4–5 per group. **P ≤ 0.01; ***P ≤ 0.001 vs. the matched APE1 WT group; #P ≤ 0.05; ###P ≤ 0.001 vs. sham group. (C) Functional evaluation of white matter integrity by CAPs at 28 d after 30 min tFCI. The stimulating and recording electrodes were positioned in the CC of coronal brain slices to measure evoked CAPs (a dashed line indicates the approximate location of ischemic lesion). Representative traces of the evoked CAPs in the CC after subthreshold stimuli (labeled as 0), and 1- and 3-mA stimuli are shown. Signal conduction along nerve fibers, as measured by the amplitude of the N1 component of the CAPs in response to increasing stimulus strength (up to 5 mA) were analyzed in sham controls and after tFCI. Data are mean ± SEM, n = 6–8 per group. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs. the matched APE1 WT group.
Fig. S6.
Full-length Western blots of MBP staining. Shown are the original full-length blots from which Fig. 6B was generated. Actin immunoblotting served as the loading control. Multiple bands were identified by the anti-MBP antibody, possibly due to the existence of different MBP isoforms in the mouse brains (47). Dashed line boxes indicate regions that are presented in Fig. 6B.
The immunohistochemical detection of white matter markers described above demonstrates that the loss of APE1 enhances demyelination after ischemia. Therefore, we sought to determine if increased demyelination of the white matter tracts led to a loss in the functional integrity of white matter. Using electrophysiological assessments of compound action potentials in the CC/EC from coronal brain slices harvested 28 d after 30 min of tFCI, we found a significant reduction in the peak amplitude in APE1 cKO mice (Fig. 6C), reflecting impairments in conduction along myelinated axons. This effect was not evident in sham controls of the APE1 cKO mice or the APE1 WT mice after tFCI. Taken together, the data suggest that APE1 protects against the development of postischemic infarction and is critical for the structural preservation of gray and white matter and the recovery of behavioral function after mild ischemic injury.
SI Methods
Generation of APE1flox/flox Mice for Conditional Knockout of APE1.
APE1flox/flox mutant mice were generated for conditional knockout of APE1 using previously described methodology (33), with modifications because of the unavailability of the Strain 129 mouse sequence in the upstream region of the 5′ recombination arm. The Apex1 gene contains five exons, the first of which is noncoding, with the major ATG initiation sequence located midway through the second exon (34). A 14-kb targeting vector was constructed by PCR amplification of genomic DNA containing 7 kb isolated from the C57BL/6J genome on the 5′ arm and 7 kb isolated from the Strain 129 genome. The vector was designed such that exons IV and V (the third and fourth coding exons) of Apex1 were flanked by loxP sites (“floxed”). The vector also contained numerous other elements to allow for selection and confirmation of correct targeting, including the following: (i) a Neo-resistance gene was inserted into the 3′ region downstream of gene, flanked by short flippase recognition target (FRT) sites, for selection of embryonic stem (ES) cells resistant to neomycin (G418); (ii) the HSV-thymidine kinase (HSV-TK) gene was included at the 3′ end of the recombination regions to allow elimination of ES cells that had recombined beyond the targeted region by making them sensitive to ganciclovir; (iii) a novel Ase1 restriction enzyme site was constructed into the end of the 5′ loxP site, a novel BstEII site was included downstream of the 3′ loxP site, and a novel BglII site was included at the 3′ end of the Neo-resistance gene located upstream of the 3′ loxP site (Fig. S1A). These new restriction sites allowed for targeting confirmation. A probe external to the targeting region on the 3′ end was used for screening. The construct was then linearized, electroporated into mouse R1 ES cells (35), and the resulting cells were subject to positive/negative drug selection by exposure to G418 (265 μg/mL) and ganciclovir (2 μM). Drug-resistant clones were digested using BstEII and screened by Southern blot analysis using a 3′ probe external to the targeting region (Fig. S1B), then further expanded and confirmed positive using the BglII site engineered at the 3′ loxP site (Fig. S1C). The clones were then further confirmed positive on the 5′ end by PCR amplifying the region between exons III and IV. Only the targeted clone containing the 5′ loxP site has the inserted novel Ase1 restriction site overlapping with the first two nucleotides of the loxP sequence, so a restriction digest analysis was used on the resulting PCR products to demonstrate the presence of the Ase1 site (Fig. S1D). Of two targeted ES cell lines injected into blastocysts, one produced male chimeras, which were then bred with C57BL/6J female mice (The Jackson Laboratory) to obtain ES cell-derived offspring as determined by the presence of the agouti coat color (F1). The resulting agouti mice were confirmed heterozygous for the floxed Apex1 gene by Southern blot, and then bred to mice transgenically expressing the flippase (flp) gene (C57BL/6J background; The Jackson Laboratory, stock #003800) to remove the Neo cassette, followed by further breeding to breed out the flp gene. These pups were assessed by PCR to confirm the presence of the floxed alleles. In addition, restriction analyses using the inserted Ase1 sequence at the 5′ loxP site on PCR products was performed to ensure genetic stability of the targeted region. After back-crossing, we sequenced the region of the Apex1 gene in the APE1flox/flox mice to definitively identify the loxP sites at the appropriate locations. All mice sequenced (3) were positive for both loxP sites flanking exons IV and V, and all mice were subjected PCR analyses to confirm the presence of the targeted alleles. Mutant mice progeny were backcrossed to the C57BL/6J background for at least six generations before use, resulting in a homozygous APE1flox/flox line with minimal potential influence of genetic heterogeneity on the susceptibility of animals to cerebral ischemia. APE1flox/flox mice were then bred to hemizygous CAGGCre-ER mice (C57BL/6J background; The Jackson Laboratory, stock #004682) to produce mice that carried the hemizygous CAGGCre-ER transgene and were heterozygous for the floxed Apex1 allele (CAGGCre-ER; APE1flox/wt). Those mice were then crossed to APE1flox/flox mice, resulting in mice that were APE1flox/flox mice and were hemizygous for the CAGGCre-ER transgene (CAGGCre-ER; APE1flox/flox). These mice were used and referred to as tamoxifen-inducible APE1 conditional knockout (APE1 cKO) mice. APE1 cKO mice did not show obvious phenotype before tamoxifen administration. When exposed to tamoxifen before induction of ischemia, the Cre-induced loss of APE1 can be expected to occur in all cells. Mice with genotype CAGGCre-ER; APE1wt/wt were used as WT controls (APE1 WT). All mice carrying the CAGGCre-ER transgene were bred to maintain hemizygosity, as homozygous CAGGCre-ER mice were reported to be not fertile or viable (The Jackson Laboratory, stock #004682 genetics datasheet).
Mice were housed in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle. Food and water were available ad libitum. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (36). All efforts were made to minimize animal suffering and the number of animals used.
tFCI Model.
tFCI was induced in adult male mice (8–10 wk old, 25–30 g) by intraluminal occlusion of the left middle cerebral artery (MCA), as described previously (31). Briefly, mice were anesthetized with 3% (vol/vol) isoflurane in 67%:30% (vol/vol) N2O/O2, using lack of tail pinch response as an indicator of sufficient depth of anesthesia. Anesthesia was maintained under anesthesia via a nose cone blowing 1.5% (vol/vol) isoflurane. An 8-0 monofilament with silicon-coated tip was introduced into the common carotid artery, advanced to the origin of the MCA, and left in place for either 30 or 60 min. Mice were placed on a temperature-controlled heating pad during the surgery to maintain rectal temperature at 37.0 ± 0.5 °C. Mean arterial blood pressure was monitored during surgery by a tail cuff, and arterial blood gas was analyzed using the EPOC veterinary blood gas and electrolyte analyzer (Heska Corporation) 15 min after MCA occlusion. CBF was measured using laser-Doppler flowmetry (regional CBF) or using 2D laser-speckle techniques (cortical CBF) as described below. Failure to reduce CBF to at least 25% of baseline level led to subject exclusion. Mortality was less than 10%. Sham-operated animals underwent the same anesthesia and surgical procedures, with the exception of the MCA occlusion.
Stroke Therapy Academic Industry Roundtable (STAIR) guidelines for preclinical evaluation of stroke therapeutics (37) were strictly followed throughout the experiments. For example, animals were randomly assigned to experimental groups with the use of a lottery-drawing box. We verified that blood pH, gases, glucose levels, and CBF were not altered by genotype. Furthermore, surgeries and all outcome assessments were performed by investigators blinded to mouse genotype and experimental group assignments.
Two-Dimensional Laser-Speckle Imaging.
Cortical CBF was monitored using the laser-speckle technique in two cohorts of mice (30 min or 60 min tFCI, n = 5 per group) according to the manufacturer’s instructions and as described previously (38). Briefly, a charge-coupled device camera (PeriCam PSI System; Perimed) was placed above the head, and the exposed intact skull surface was illuminated by a laser diode (785 nm) to allow laser penetration into the brain. Two-dimensional microcirculation images were obtained starting 15 min before tFCI and continued throughout the ischemic period until 15 min after the onset of reperfusion. The area of the ischemic core (0–20% residual CBF) or the penumbra (20–35% residual CBF) region (39, 40) was measured from laser-speckle images.
Immunohistochemistry and Image Analysis.
At multiple reperfusion time points after tFCI, surviving animals were deeply anesthetized and transcardially perfused with 0.9% NaCl followed by 4% (wt/vol) paraformaldehyde in PBS. Brains were harvested, cryoprotected in 30% (wt/vol) sucrose in PBS, frozen, and serial 30-μm-thick sections were cut serial on a cryostat (CM1900, Leica). The tissue was then blocked with 10% (vol/vol) donkey serum in PBS for 1 h, followed by overnight incubation (4 °C) with the following primary antibodies: rabbit anti-MAP2 (1:200; Santa Cruz Biotechnology), rabbit anti-Iba1 (1:1,000; Wako), rabbit anti-GFAP (1:500; EMD Millipore), rabbit anti-NeuN (1:500; EMD Millipore), rabbit anti–p-H2AX (Ser139; 1:300; EMD Millipore), rabbit anti-PUMA (1:200; Abcam), rabbit anti TOM20 (1:250; Santa Cruz Biotechnology), rabbit anti-APC [mature oligodendrocyte marker (41); 1:400; EMD Millipore], rabbit anti- PAR polymer (1:250; Trevigen), mouse anti-nonphosphorylated neurofilaments (SMI-32; Abcam), and rabbit anti MBP (myelin marker; Abcam). Sections were washed and then incubated for 1 h at room temperature with donkey secondary antibodies conjugated with DyLight 488 or Cy3 (1:1,000; Jackson ImmunoResearch Laboratories). Nonspecific staining was assessed by incubating alternate sections from each experimental condition in all solutions except the primary antibodies. Sections were then counterstained for nuclear morphology with DAPI (Thermo Scientific) for 2 min at room temperature, mounted, and coverslipped with Fluoromount-G (Southern Biotech). Fluorescence images were captured with an Olympus Fluoview FV1000 confocal microscope using FV10-ASW 2.0 software (Olympus America).
Tissue loss was measured on six MAP2-stained sections that were equally spaced through the MCA territory using ImageJ. Tissue atrophy was calculated as the volume of the contralateral hemisphere minus the ipsilateral hemisphere.
The MBP/SMI-32-immunostained CC/EC area was contrasted between poststroke animals and sham animals using confocal microscopy and analyzed with ImageJ software. A region of interest (ROI) was drawn over the ipsilateral CC/EC by a blinded investigator and similar ROIs were drawn in sham animals. Three sections were analyzed for each brain and six ROIs were randomly selected from each section. White matter damage was expressed as the ratio of SMI-32 to MBP immunostaining relative to sham animals.
Stereology.
TUNEL-stained neurons or neurons containing SSBs, both indicators of with DNA damage, were stereologically quantified by a blinded observer with the Bioquant Image Analysis program (Bioquant), as described previously (42), with modifications. Briefly, images were captured from the striatum or cortex with a CCD camera and grid squares of 50 × 50 µm were generated. The counting frame was 25 × 25 µm with a focal depth of 25 µm and 7.5-µm guard zones. Coronal sections (thickness of 40 µm) were cut continuously between the levels of 1.5 and −1.0 mm to bregma, and every tenth section was counted. An average of nine dissectors per section was used. The total number (n) of neurons was equal to the quotient of the total number of neurons counted and the product of the fractions for sampling section frequency (SSF, fraction of sections counted), area section frequency (ASF, sampling area/area between dissectors), and thickness section frequency (TSF, dissector depth/section thickness), or n = neurons counted/(SSF × ASF × TSF). For the present study, SSF = one-quarter sections, ASF = 25 × 25 µm/50 × 50 µm, and TSF = 25 µm/40 µm.
TUNEL+/APC+, PAR+/APC+ cells were counted in both the cortex and striatum at two coronal levels (0.2 mm and −0.5 mm to bregma). The mean was calculated from three fields in the cortex or striatum of each section and expressed as mean number of cells per square millimeter.
Measurement of Infarct Volume.
Forty-eight hours after tFCI, brains were harvested, and sliced into coronal sections, each 1-mm thick. Forebrain sections were stained with 3% (wt/vol) 2,3,5-triphenyltetrazolium (TTC) in saline for 20 min and then fixed with 4% (wt/vol) paraformaldehyde in PBS, pH 7.4. Infarct volume was determined using MCID image analysis software (31) by an observer blinded to experimental group assignment.
Fluoro-Jade B Staining.
Degenerating neurons were detected by Fluoro-Jade B (EMD Millipore) as described previously (43). Briefly, brains were harvested two days after MCAO and processed into paraffin blocks. Sections were cut (6-μm thick) and mounted onto slides, immersed in 100% alcohol for 3 min, followed by 70% (vol/vol) alcohol for 1 min, and then washed with distilled water. After a 15-min incubation in 0.06% potassium permanganate, sections were washed with distilled water and then stained with 0.001% Fluoro-Jade B in 0.09% acetic acid for 20 min. Fluorescent intensity was measured in three consecutive coronal sections per brain in the striatum and cortex, respectively, by a blinded observer. Data are expressed as fold-changes over sham controls.
Neurobehavioral Tests.
Animals subjected to the stroke model or sham surgery described above (i.e., male mice subjected to stroke or sham controls at 8–10 wk of age) were used for neurobehavioral assessments at the indicated time points.
Rotarod test.
The rotarod test was performed to assess poststroke motor functions, as described previously (39). Briefly, animals were placed on a rotating drum with a speed accelerating from 2.5 to 25 rpm within 5 min. The latency to fall (i.e., how long the mouse was able to stay on the drum) was recorded. Pretesting began 3 d before surgery and consisted of two trials each day. On the day of surgery, five trials were performed on each mouse and the mean of the last three of these trials was used as the presurgery baseline value. At 1, 3, 5, and 7 d after tFCI or sham surgery, mice were tested for five trials on each day, spaced by intervals of 15 min. Data were calculated based on mean of the last three of these trials, and expressed as the mean latency to fall on each testing day.
Corner test.
The corner test was performed as described previously (44). Briefly, control mice will not display a preference in turns (left or right) when presented with a corner, whereas stroke-injured mice turn preferentially toward the lesioned (left) side, because the motor impairment is contralateral (i.e., on the right side). Performance was expressed as the number of left turns out of 10 trials for each test.
Morris water maze test.
The Morris water maze test was carried out 22–27 d after tFCI to evaluate long-term cognitive functions, as we described previously (45). Briefly, a square platform (11 cm × 11 cm) was submerged in a pool (diameter: 109 cm) of opaque water. Mice were pretrained for 3 d prior to tFCI or sham surgery (3 trials per day). To assess learning, mice were placed into the pool from one of the four locations and allowed 90 s to locate the hidden platform. At the end of each trial, the mouse was placed on the platform or allowed to stay on the platform for 30 s with prominent spatial cues displayed around the room. The time required for the animal to find the platform (escape latency) was recorded for each trial. Four trials were performed on each day for 5 consecutive days (days 22–26 after surgery). On day 27, the platform was removed and a single, 60-s probe trial was performed. The time spent in the target quadrant where the platform was previously located was recorded to assess memory. Swim speed was also recorded to ensure differences in latency to escape were not due to gross changes in locomotion.
AP Site Measurements.
The number of AP sites in nuclear DNA extracts was measured, as described previously (10). Nuclear DNA was extracted from the cortex and striatum after tFCI or sham operation. Purified DNA (OD 260 nm/280 nm > 1.8) was dissolved to a concentration of 100 µg/mL in Tris-EDTA (TE) buffer. Using a biotin-labeled reagent specific for the aldehyde group in the ring-open form of an AP site (designated Aldehye Reactive Probe, or ARP; Dojindo Molecular Technologies), 10 µL of DNA solution was incubated with 10 µL of ARP solution (5 mM) for 1 h at 37 °C. DNA was then ethanol-precipitated, and the pellet was suspended in TE to determine DNA concentration. ARP-labeled DNA was measured with an ELISA-like assay in a microplate, which was irradiated overnight with a 40W, unfiltered UV lamp with a wavelength of 254 nm before the assay. ARP-labeled DNA (30 ng in 60 µL) and 90 µL of DNA binding solution were added to each well. The plate was covered and left in the dark at 37 °C overnight. The next day, 100 µL of ABC solution (Vector Laboratories) was added for 1 h at 37 °C, followed by K-Blue substrate solution (Neogen Corporation) at room temperature for 1 h. Optical density measurements were then recorded at 650 nm. The number of AP sites was calculated by a blinded observer using a linear calibration curve with ARP-DNA standard solutions. Data were expressed as the number of AP sites per 106 total nucleotides.
Detection of DNA SSBs in Brain Sections.
The DNA PANT assay was performed on coronal brain sections after tFCI or sham operation, as described previously (3), with slight modifications. In brief, after fixation with 10% (vol/vol) formalin for 10 min, sections were washed three times in PBS and permeabilized with 1% Triton X-100 for 20 min. The sections were then incubated in a moist-air chamber at 37 °C for 90 min with PANT reaction mixture containing 5 mM MgCl2, 10 mM 2-mercaptoethanol, 20 μg/mL BSA, dGTP, dCTP, and dTTP at 30 μM each, 29 μM biotinylated dATP, 1 μM dATP, and 40 U/mL Escherichia coli DNA polymerase I Large Fragment (Sigma-Aldrich) in PBS (pH 7.4). The reaction was terminated by three PBS washes of 5 min each. The biotin-16-dATP–labeled cells were detected by incubation of the sections for 15 min in 10 μg/mL of Rhodamine Avidin D (Vector Laboratories) followed by three PBS washes of 20 min each. Fluorescence was observed under excitation of 550 nm and emission of 575 nm.
DNA Repair Assay for APE1 Activity.
The oligonucleotide incision assay was performed on nuclear extracts as described previously (10). This DNA repair assay used a 5′-end [γ32P]ATP-labeled 50-mer oligonucleotide with a synthetic AP site at position 26 (5′-TCG GTA CCC GGG GAT CCT CTA GAG TOG ACC TGC AGG CAT GCA AGC TTG GC-3′; O = AP site). The oligonucleotide was 5′-end-labeled with T4 polynucleotide kinase and [γ32P]ATP. The mixture was passed through a G-25 spin column to remove free, unlabeled ATP. Labeled oligonucleotide was then annealed to the complementary oligonucleotide in 100 mM KCl, 10 mM Tris (pH 7.8), and 1 mM EDTA (EDTA) by heating to 55 °C, followed by slow cooling to room temperature. Of the resulting 32P-labeled DNA duplex, 300 fmol was diluted in 40 mM Hepes-KOH (pH 7.6), 75 mM KCl, 2 mM DTT, 1 mM EDTA, 0.1 mg/mL BSA, 2 mM CaCl2, 20 µM zinc acetate, and 10% (vol/vol) glycerol, and combined with the indicated amounts of nuclear protein extracts. After incubating 15 min at 32 °C, the reaction was terminated and DNA was ethanol-precipitated and resuspended in formamide dye [90% (vol/vol) formamide, 0.002% bromophenol blue, 0.002% xylene cyanol]. Samples were then heated to 80 °C for 2 min and subjected to electrophoresis on a denaturing 20% (wt/vol) polyacrylamide gel that contained 7 mM urea. The presence of a 25-bp product was indicative of AP endonuclease-1 activity and was quantified by densitometry by a blinded observer.
Preparation of Subcellular Fractions.
All procedures for protein fractionation were carried out at 4 °C, using the method reported previously (46). Brain tissue was minced and homogenized using a Dounce homogenizer in 1× M-SHE buffer containing 0.21 M mannitol, 0.07 M sucrose, 10 mM Hepes-KOH (pH 7.4), 1 mM EDTA, 0.15 mM spermine, and 0.75 mM spermidine. Freshly prepared protease inhibitors were then added (1 μg/mL each of leupeptin, aprotinin, and pepstatin; 1 mM PMSF; and 1 mM DTT). Cells were lysed for 30 min on ice, and then unbroken cells and nuclei were pelleted at 1,200 × g. The supernatant was centrifuged at 10,000 × g for 15 min to pellet the mitochondria, and the resulting supernatant from that spin (cytosolic fraction) was removed while taking care to avoid the pellet. To further isolate mitochondrial protein, the pellet was resuspended in a solution containing 3% (wt/vol) Ficoll 400, 0.12 M mannitol, 0.03 M sucrose, and 25 M EDTA (pH 7.4), and gently loaded into 6% (wt/vol) Ficoll 400 solution to produce a discontinuous density gradient. After centrifugation at 10,400 × g for 25 min, the sediment was resuspended in a lysis buffer containing 10 mM Hepes (pH 7.4), 142.5 mM KCl, 5 mM MgCl2, 1 mM EGTA, 0.5% Nonidet P-40, 0.5 mM PMSF, 10 g/mL aprotinin, and 1 g/mL each of leupeptin, chymostatin, antipain, and pepstatin, followed by sonication. The lysate was centrifuged at 130,000 × g for 1 h and stored at −80 °C in aliquots.
Western Blotting.
Protein isolation from brain was performed as we described previously (46). Western blotting was performed using the standard SDS/PAGE method and enhanced chemiluminescence detection reagents (GE Healthcare Biosciences). Immunoreactivity was semiquantitatively measured by gel densitometric scanning and analyzed with ImageJ. For some experiments, the membrane was incubated with fluorescent-labeled secondary antibodies (1:20,000) in a darkroom for 60 min at room temperature. The membrane was then washed with PBS with Tween-20 (PBST) and scanned in appropriate channels using Odyssey CLX Infrared Imaging system (LI-COR Biosciences) for image development. Antibodies against the following proteins were used: rabbit anti-p-H2AX (1:1,000; EMD Millipore), rabbit anti-PUMA (1:1,000; Abcam), rabbit anti-TOM20 (1:500; Santa Cruz Biotechnology), rabbit anti-poly(ADP ribose) (PAR) polymer (1:1,000; Trevigen), rabbit anti-APE1 (1:2,000; Abcam), rabbit anti-MBP (1:2,000; Abcam), and mouse anti–β-actin antibody (1:2,000; Sigma-Aldrich).
CAP Measurements.
CAPs in the CC and EC were measured as described previously (32). Coronal brain slices (350-µm thick, bregma −1.06 mm) were placed in pregassed [95% O2/5% (vol/vol) CO2] artificial cerebrospinal fluid (aCSF; NaCl 126 mM, KCl 2.5 mM, NaH2PO4 1 mM, CaCl2 2.5 mM, NaHCO3 26 mM, MgCl2 1.3 mM, glucose 10 M; pH 7.4) for 1 h at room temperature, and then perfused with aCSF (22 °C) at a constant rate (3–4 mL/min). A bipolar tungsten stimulating electrode (intertip distance: 100 µm) was positioned across the corpus callosum about 0.9-mm lateral to the midline. A glass extracellular recording pipette (5–8 MΩ tip resistance when filled with aCSF) was placed in the external capsule, 0.24–0.96 mm from the stimulating electrode in 0.24-mm increments. The recording made at 0.48 mm from the stimulating electrode was reported in this manuscript. Both electrodes were placed 50–100 μm below the surface of the slice, with adjustments to optimize the signal. The CAP was amplified (× 1 k) and recorded using Axoclamp 700B (Molecular Devices), and analyzed using the pCLAMP 10 software (Molecular Devices). Input–output curves were generated by varying the intensity of the stimuli from 0 mA to 5 mA with 1 mA increments (100-μs duration, delivered at 0.05 Hz). The responses from two consecutive brain slices for each mouse were averaged, and six to eight mice were recorded for each experimental group (Fig. 6). Myelinated fiber-response amplitude was defined as the voltage difference from the first peak to the first trough (N1).
Discussion
To our knowledge, the present study provides the first direct evidence that endogenous APE1 naturally blunts the progression of gray and white matter injury in experimental stroke and facilitates poststroke functional recovery. Four major findings from this study contribute to our understanding of the role of DNA repair in the intrinsic recovery process after cerebral ischemia and reperfusion. First, the loss of APE1 largely prevents the intrinsic repair of AP sites in gray matter and promotes the accumulation of ODD in the form of AP sites and strand breaks in the postischemic brain. Second, loss of APE1 dramatically exacerbates ischemic cell death of neurons and oligodendrocytes, probably through activation of distinct cell death programs, such as PARP1- and PUMA-dependent signaling pathways. Third, the loss of APE1 augments postischemic demyelination and white matter dysfunction. Fourth, the loss of APE1 inhibits the endogenous recovery of sensorimotor function and spatial learning and memory under mild injury conditions.
Historically, the role of endogenous APE1 has been difficult to assess, particularly in targeted structures, such as the brain or in specific cellular subtypes. The conventional global knockout of APE1 causes early embryonic lethality (15, 17), which both underscores its essential role in cell survival and creates an impediment to studying its function in adult animals. Several techniques have been used to generate APE deletion models, including RNA interference and the generation of APE1 knockout cells that survive because of the coexpression of human APE1 under a conditional promoter (14, 25). However, these models only have limited applicability in animal studies. By creating a conditional APE1 knockout mouse that allows for disruption of the APE1 gene in adulthood, we have demonstrated here that APE1 unequivocally contributes to endogenous neuroprotection against ischemic injury in vivo, validating it as a potential target in neurotherapeutic strategies. A robust exacerbation of ischemic injury was evident in APE1 cKO mice, not only promoting cell death in gray and white matter, but also extending to behavioral outcomes. Although the data contribute to our understanding of the role of DNA repair in cellular recovery from cerebral ischemia, the use of this APE1 cKO mouse line could easily be extended to other models, including genesis of chemotherapy-resistant tumors in different cellular subtypes, traumatic CNS injury, aging and neurodegenerative diseases, mitochondrial DNA repair, and so forth.
Our data consistently demonstrate that APE1 knockout mice exhibit worse outcomes following transient cerebral ischemia. This observation could be interpreted as arising from two distinct mechanisms that are not mutually exclusive. First, given that the mortality rate after moderate (60 min) ischemia was higher in APE1 knockout mice, the loss of APE1 before the insult could potentiate cellular injury by creating a baseline level of unrepaired ODD that rises above a lethality threshold upon ischemic injury. This stressful baseline environment might be considered an “aging-like” phenotype, wherein accumulation of ODD sensitizes the system to subsequent oxidative insults, such as ischemia. Alternatively, ischemic injury and ODD might induce APE1 activity immediately following reperfusion, especially in those cells that are not severely injured or are located in the penumbra. The loss of APE1 would then represent a failure in postischemic recovery that may be independent of a predisposition, as implied in the first scenario. In either scenario, the repair of ODD would be essential to eventual structural and functional recovery. Both alternatives are consistent with a prolonged accumulation of AP sites and DNA strand breaks in ischemic APE1 knockout animals. Further studies to distinguish between the two alternatives are warranted.
The molecular mechanisms underlying WMI after stroke and the roles of APE1/DNA repair in this process have only recently begun to be explored. White matter is remarkably sensitive to ischemic injury (26), and white matter repair may be critical to improving functional recovery following cerebral ischemia. Our data demonstrate that APE1 promotes the survival of oligodendrocytes and both the structural and functional integrity of white matter after a transient ischemic episode. These data support the concept that white matter mounts a programmed response to ischemic injury and that APE1 is a major component of a white matter self-defense and self-repair program. Future studies targeting APE1 knockout specifically to white matter cell populations, such as the myelin-producing oligodendrocytes, may help verify the role of white matter injury and repair in postischemic outcomes and potentially lead to new therapeutic strategies that encompass both gray and white matter.
Our data suggest that the loss of APE1 leads to the activation of two distinct prodeath signaling pathways, PUMA and PARP1, which may underlie pathological events following ODD accumulation. The potentiation of ischemic WMI by compromised DNA repair (or the lack of adequate recovery) appears to be primarily associated with PARP1 activation. Whether the activation of PARP1 in white matter reflects a secondary or bystander cell death process in response to neuronal injury is still unknown, but can be addressed by further studies using cell-specific approaches. The neuronal activation of PUMA in the ischemic cortex represents a proapoptotic process that demands energy (27), thus indicating that the molecular mechanisms underpinning the ischemic response by neurons may be intrinsically different from in the white matter because of distinct energy reserves or capacities. In contrast to PUMA, PARP1 activation is generally associated with necrosis and catastrophic depletion of energy stores.
A parsimonious explanation of the observed regional differences between the striatum and cortex is differences in the degree of blood flow interruption during the stroke event itself. In this context, induction of APE1 is well established in sublethal injury models (5, 28). Therefore, regions that lie proximal versus distal to the ischemic core may rely differentially on APE1. Other topographical differences between the striatum and cortex, such as unique efferent and afferent projections, heterogeneity in glial subpopulations, and differential expression of neurotransmitters and prosurvival proteins, might contribute somewhat to the regionally selective responses to ischemic injury in the APE1 knockout mouse. Further investigations using cell and region-specific targeting of APE1 knockout could help address these questions and better define mechanisms associated with specific cell subtypes (neurons, astrocytes, oligodendrocytes, endothelial cells, and so forth) and with topographically selective vulnerabilities. The new conditional APE1 knockout model described here offers a novel platform on which to rigorously examine these important variables in greater mechanistic depth.
Although best characterized for its DNA repair function, APE1 also contains a highly sensitive redox region that is dependent on a cysteine residue within its N-terminal region (29). Several studies implicate this region in influencing the activity of a number of transcription factors, such as AP-1, Myb, NF-κB, HIF-1α, ATF/CREB, p53, and so forth (reviewed in ref. 28), and indicate that the redox function of APE1 acts independently of DNA repair activity. Our current model explored a relatively acute timeframe following cerebral ischemia and correlated the loss of APE1 with accumulation of ODD. However, it is possible that deleterious outcomes following a transient ischemic insult in the APE1 knockout mouse may be influenced by the lack of the redox action of APE1 in addition to its role in DNA repair. Comparison of the effects of gene knock-in of APE1 containing point mutations of the redox-active site versus of the DNA repair-competent site would be a logical progression for future studies. It should be noted here that overexpression of exogenous APE1 with a mutated DNA repair site but an intact redox site does abolish its protective effects against ischemia (10), indicating that the repair function is indeed critical for protection against ischemic injury.
We have provided evidence that cKO of APE1 in the adult mice exacerbates ischemic injury in both gray and white matter, perhaps via topographically distinct activation of prodeath signaling pathways. To our knowledge these data provide the first animal model of targeted APE1 knockout and support the engagement of multiple distinct cell death mechanisms following cerebral ischemia. The defensive role of endogenous APE1 in stroke is consistent with human studies showing that single nucleotide polymorphisms in the APE1 gene are associated with greater risk for cerebral ischemic infarction (30). Further studies assessing the role of DNA repair in specific cell types in the brain and under different injury conditions may accelerate the development of successful therapeutic interventions for stroke victims.
Methods
Generation of Conditional APE1 Knockout Mice.
APE1flox/flox mice were generated on a C57BL/6J background as described in SI Methods. To obtain conditional APE1 knockout mice, homozygous APE1flox/flox mice were crossed with hemizygous CAGGCre-ER mice (The Jackson Laboratory), in which the Cre-mediated recombination is controlled by tamoxifen and is ubiquitous in all tissues (18). CAGGCre-ER; APE1flox/flox mice were obtained after at least two generations of crossing (Fig. S2A) and transformed into conditional APE1 knockout (APE1 cKO) animals following tamoxifen injections (75 mg/kg, i.p., once a day for 5 d). Mice with the genotype of CAGGCre-ER; APEwt/wt were used as controls (APE1 WT) (Fig. S2B) and received the same tamoxifen treatment regimen in all studies. Induced knockout of APE1 was confirmed by Western blotting (Fig. S2D).
Transient Focal Cerebral Ischemia Model.
Twenty-four hours after the last tamoxifen injection, tFCI was induced in adult male mice (8–10 wk old, 25–30 g) by intraluminal occlusion of the left middle cerebral artery, as described previously (31). Surgeries and all outcome assessments were performed by investigators blinded to mouse genotype and experimental group assignments. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and performed to NIH guidelines.
Neurofunctional Assessments.
Neurobehavioral tests were carried out 3 d before and 1–28 d after tFCI. Sensorimotor deficits were evaluated by the rotarod and corner tests. Cognitive deficits were evaluated by the Morris water maze test.
Assessment of Gray and WMI.
Details of histological assessments for brain injury are further described in SI Methods. Neuronal (i.e., gray matter) injury was assessed by MAP2 immunostaining (28 d following tFCI) and by double-label immunofluorescent staining for NeuN and the DNA damage marker p-H2AX (2 h and 24 h following tFCI) or TUNEL (24 h following tFCI). WMI induced by tFCI was examined at the histological and functional levels. Free-floating coronal brain sections (30 µm) were processed for immunostaining with MBP and SMI-32 antibodies. White matter damage was quantitated as the ratio of SMI-32 to MBP immunostaining relative to sham animals. The functional integrity of white matter was assessed by measuring compound action potentials (CAPs) in the CC and EC of coronal brain slices (350-µm thick), as described previously (32).
Assessment of ODD.
DNA SSB were detected on fixed coronal brain sections using DNA PANT, as described previously (3). PANT+ cells were stereologically quantified by a blinded investigator. Contents of AP sites were measured with the biotin-labeled aldehyde reactive probe in a colorimetric assay (10).
Statistical Analyses.
Data are presented as mean ± SEM. Differences between means from two groups were analyzed by the Student’s t test (two-tailed). Differences in means from multiple groups were analyzed using one- or two-way ANOVA followed by the Bonferroni/Dunn post hoc correction. Comparisons of animal survival rates were performed by Kaplan–Meier survival analysis. A P value less than 0.05 was deemed statistically significant.
Acknowledgments
We thank Ed Mallick for technical assistance in generating the apurinic/apyrimidinic endonuclease-1 conditional knockout mouse line. This research was supported by National Institutes of Health Grants NS036736, NS045048, and NS089534 (to J.C.), NS45287 (to M.V.L.B.), and AA10422 (to G.E.H); a US Department of Veterans Affairs Senior Research Career Scientist Award (to J.C.); Chinese Natural Science Foundation Grants 81020108021, 81171149, and 81371306 (to Y.G.), and 81228008 (to J.C.); and the High-end Distinguished Professorship GDW20133100069 from the State Administration of Foreign Experts Affairs of China (to M.V.L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606226113/-/DCSupplemental.
References
- 1.Nagayama T, et al. Activation of poly(ADP-ribose) polymerase in the rat hippocampus may contribute to cellular recovery following sublethal transient global ischemia. J Neurochem. 2000;74(4):1636–1645. doi: 10.1046/j.1471-4159.2000.0741636.x. [DOI] [PubMed] [Google Scholar]
- 2.Schwamm LH, et al. Time course of lesion development in patients with acute stroke: Serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29(11):2268–2276. doi: 10.1161/01.str.29.11.2268. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, et al. Early detection of DNA strand breaks in the brain after transient focal ischemia: Implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69(1):232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 4.Lan J, et al. Inducible repair of oxidative DNA lesions in the rat brain after transient focal ischemia and reperfusion. J Cereb Blood Flow Metab. 2003;23(11):1324–1339. doi: 10.1097/01.WCB.0000091540.60196.F2. [DOI] [PubMed] [Google Scholar]
- 5.Li W, et al. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2006;26(2):181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- 6.Ljungquist S, Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. I. Purification and general properties. J Biol Chem. 1974;249(5):1530–1535. [PubMed] [Google Scholar]
- 7.Ljungquist S, Andersson A, Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. II. Further studies on the substrate specificity. J Biol Chem. 1974;249(5):1536–1540. [PubMed] [Google Scholar]
- 8.Srivastava DK, et al. Mammalian abasic site base excision repair. Identification of the reaction sequence and rate-determining steps. J Biol Chem. 1998;273(33):21203–21209. doi: 10.1074/jbc.273.33.21203. [DOI] [PubMed] [Google Scholar]
- 9.Kawase M, Fujimura M, Morita-Fujimura Y, Chan PH. Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats: Implication of the failure of DNA repair in neuronal apoptosis. Stroke. 1999;30(2):441–448, discussion 449. doi: 10.1161/01.str.30.2.441. [DOI] [PubMed] [Google Scholar]
- 10.Leak RK, et al. Apurinic/apyrimidinic endonuclease 1 upregulation reduces oxidative DNA damage and protects hippocampal neurons from ischemic injury. Antioxid Redox Signal. 2015;22(2):135–148. doi: 10.1089/ars.2013.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HW, Cho KJ, Park SC, Kim HJ, Kim GW. The adenoviral vector-mediated increase in apurinic/apyrimidinic endonuclease inhibits the induction of neuronal cell death after transient ischemic stroke in mice. Brain Res. 2009;1274:1–10. doi: 10.1016/j.brainres.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Stetler RA, et al. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc Natl Acad Sci USA. 2010;107(7):3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita-Fujimura Y, Fujimura M, Kawase M, Chan PH. Early decrease in apurinic/apyrimidinic endonuclease is followed by DNA fragmentation after cold injury-induced brain trauma in mice. Neuroscience. 1999;93(4):1465–1473. doi: 10.1016/s0306-4522(99)00231-6. [DOI] [PubMed] [Google Scholar]
- 14.Vasko MR, Guo C, Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair (Amst) 2005;4(3):367–379. doi: 10.1016/j.dnarep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig DL, et al. A murine AP-endonuclease gene-targeted deficiency with post-implantation embryonic progression and ionizing radiation sensitivity. Mutat Res. 1998;409(1):17–29. doi: 10.1016/s0921-8777(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Englander EW. Nuclear depletion of apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1) is an indicator of energy disruption in neurons. Free Radic Biol Med. 2012;53(9):1782–1790. doi: 10.1016/j.freeradbiomed.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc Natl Acad Sci USA. 1996;93(17):8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 19.Guillet M, Boiteux S. Endogenous DNA abasic sites cause cell death in the absence of Apn1, Apn2 and Rad1/Rad10 in Saccharomyces cerevisiae. EMBO J. 2002;21(11):2833–2841. doi: 10.1093/emboj/21.11.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paull TT, et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10(15):886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 21.Li P, et al. Mechanistic insight into DNA damage and repair in ischemic stroke: Exploiting the base excision repair pathway as a model of neuroprotection. Antioxid Redox Signal. 2011;14(10):1905–1918. doi: 10.1089/ars.2010.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissela B, et al. Clinical prediction of functional outcome after ischemic stroke: The surprising importance of periventricular white matter disease and race. Stroke. 2009;40(2):530–536. doi: 10.1161/STROKEAHA.108.521906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrabi SA, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA. 2006;103(48):18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 25.Izumi T, et al. Two essential but distinct functions of the mammalian abasic endonuclease. Proc Natl Acad Sci USA. 2005;102(16):5739–5743. doi: 10.1073/pnas.0500986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27(9):1641–1646, discussion 1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 27.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 28.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11(3):601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naganuma T, et al. Haplotype-based case-control study between human apurinic/apyrimidinic endonuclease 1/redox effector factor-1 gene and cerebral infarction. Clin Biochem. 2009;42(15):1493–1499. doi: 10.1016/j.clinbiochem.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Stetler RA, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28(49):13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA. 2015;112(9):2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson C, et al. New insight into the role of the beta3 subunit of the GABAA-R in development, behavior, body weight regulation, and anesthesia revealed by conditional gene knockout. BMC Neurosci. 2007;8:85. doi: 10.1186/1471-2202-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiyama K, et al. Cloning, sequence analysis, and chromosomal assignment of the mouse Apex gene. Genomics. 1995;26(1):63–69. doi: 10.1016/0888-7543(95)80083-x. [DOI] [PubMed] [Google Scholar]
- 35.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD), DHHS Publ No (NIH) 85-23.
- 37.Fisher M, et al. STAIR Group Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, et al. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013;44(12):3509–3515. doi: 10.1161/STROKEAHA.113.002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gan Y, et al. Transgenic overexpression of peroxiredoxin-2 attenuates ischemic neuronal injury via suppression of a redox-sensitive pro-death signaling pathway. Antioxid Redox Signal. 2012;17(5):719–732. doi: 10.1089/ars.2011.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leak RK, et al. HSP27 protects the blood-brain barrier against ischemia-induced loss of integrity. CNS Neurol Disord Drug Targets. 2013;12(3):325–337. doi: 10.2174/1871527311312030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fancy SP, et al. Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nat Neurosci. 2014;17(4):506–512. doi: 10.1038/nn.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao G, et al. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27(35):9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang F, et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43(5):1390–1397. doi: 10.1161/STROKEAHA.111.647420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117(2):207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 45.Wang G, et al. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway:scriptaid protects against TBI via AKT. Neurotherapeutics. 2013;10(1):124–142. doi: 10.1007/s13311-012-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao G, et al. Intracellular Bax translocation after transient cerebral ischemia: Implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab. 2001;21(4):321–333. doi: 10.1097/00004647-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Karthigasan J, Garvey JS, Ramamurthy GV, Kirschner DA. Immunolocalization of 17 and 21.5 kDa MBP isoforms in compact myelin and radial component. J Neurocytol. 1996;25(1):1–7. doi: 10.1007/BF02284781. [DOI] [PubMed] [Google Scholar]