Significance
Visual cycle adducts having bisretinoid structures accumulate in retinal pigment epithelial cells as lipofuscin. These light-sensitive compounds are implicated in disease mechanisms leading to visual impairment in some inherited and age-related forms of macular degeneration. The means by which these diretinal adducts impart chronic damage is not fully understood. By studying mice raised under varying levels of intraocular light and by analyzing mice treated with vitamin E, we provide evidence of chronic bisretinoid photooxidation and degradation in the eye. These processes could shed light on oxidative and light-related mechanisms involved in disease pathogenesis and provide support for therapeutic approaches that target bisretinoids.
Keywords: bisretinoid, visual cycle, retina, retinal pigment epithelium, macular degeneration
Abstract
Adducts of retinaldehyde (bisretinoids) form nonenzymatically in photoreceptor cells and accumulate in retinal pigment epithelial (RPE) cells as lipofuscin; these fluorophores are implicated in the pathogenesis of inherited and age-related macular degeneration (AMD). Here we demonstrate that bisretinoid photodegradation is ongoing in the eye. High-performance liquid chromatography (HPLC) analysis of eyes of dark-reared and cyclic light-reared wild-type mice, together with comparisons of pigmented versus albino mice, revealed a relationship between intraocular light and reduced levels of the bisretinoids A2E and A2-glycero-phosphoethanolamine (A2-GPE). Analysis of the bisretinoids A2E, A2-GPE, A2-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE), and all-trans-retinal dimer-phosphatidylethanolamine (all-trans-retinal dimer-PE) also decreases in albino Abca4−/− mice reared in cyclic light compared with darkness. In albino Abca4−/− mice receiving a diet supplemented with the antioxidant vitamin E, higher levels of RPE bisretinoid were evidenced by HPLC analysis and quantitation of fundus autofluorescence; this effect is consistent with photooxidative processes known to precede bisretinoid degradation. Amelioration of outer nuclear layer thinning indicated that vitamin E treatment protected photoreceptor cells. Conversely, in-cage exposure to short-wavelength light resulted in reduced fundus autofluorescence, decreased HPLC-quantified A2E, outer nuclear layer thinning, and increased methylglyoxal (MG)-adducted protein. MG was also released upon bisretinoid photodegradation in cells. We suggest that the lower levels of these diretinal adducts in cyclic light-reared and albino mice reflect photodegradative loss of bisretinoid. These mechanisms may underlie associations among AMD risk, oxidative mechanisms, and lifetime light exposure.
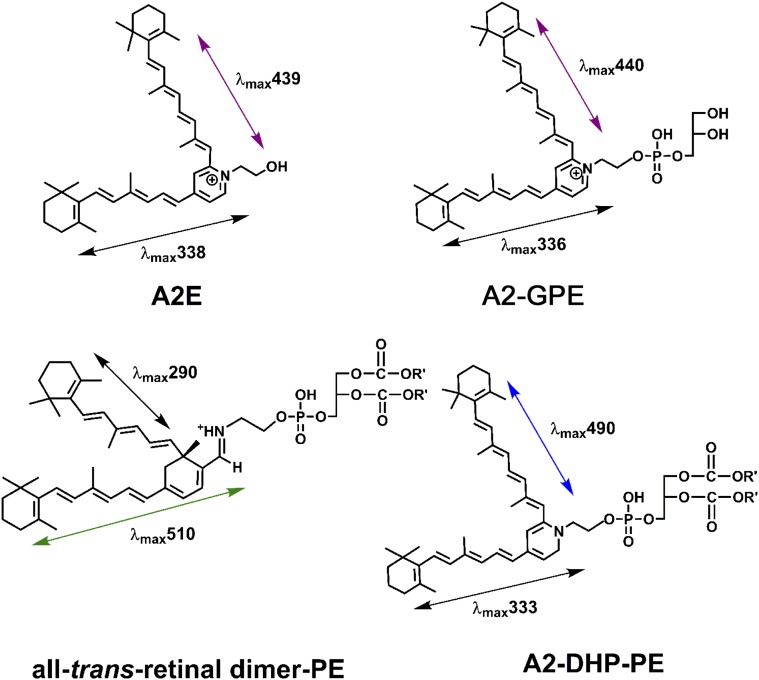
The bisretinoid fluorophores of the lipofuscin of retinal pigment epithelial (RPE) cells form nonenzymatically in photoreceptor outer segments as a consequence of indiscriminate reactions of molecules of vitamin A-aldehyde (A2, 2 vitamin A molecules) (1). These bisretinoid pigments are subsequently transferred to RPE during the process of photoreceptor outer segment shedding and phagocytosis. Various bisretinoids of RPE lipofuscin, including A2-GPE (A2-glycero-phosphoethanolamine), A2-DHP-PE (A2-dihydropyridine-phosphatidylethanolamine), all-trans-retinal dimer-PE (all-trans-retinal dimer phosphatidylethanolamine) and A2E (adduct of A2 and ethanolamine) (1–4) have been isolated and characterized structurally (Fig. S1).
Fig. S1.
Structures of some characterized bisretinoids.
An early-onset form of macular degeneration, recessive Stargardt disease (STGD1), is associated with pronounced deposition of bisretinoid lipofuscin in RPE cells; this accumulation culminates in RPE and photoreceptor cell death. The ABCA4 gene encodes the ATP-binding cassette transporter (ABCA4) in photoreceptor cells, and mutations in this gene are causal for STGD1 (5). RPE lipofuscin is also elevated in models of dominant Stargardt-like macular degeneration (6) associated with mutations in elongation of very long chain fatty acids-like 4 (ELOVL4), but the pathways involved in this perturbation have not been elucidated. Although age-related macular degeneration (AMD) stems from multiple factors (7), there may be a contribution from bisretinoid lipofuscin (1).
The ABCA4 transporter in photoreceptor cells serves in the delivery of retinaldehyde to cytosolic retinol dehydrogenases that reduce vitamin A aldehyde to the nonreactive alcohol form (8). Marked bisretinoid accumulation is replicated not only in Abca4 null mutant mice (9, 10) but also in mice deficient in retinol dehydrogenase-8 and dehydrogenase-12 (Rdh8; Rdh12) (11–13). In addition, the relationship between RPE lipofuscin accumulation and retinal disease is evidenced in the Abca4 mouse model by complement dysregulation, carbonyl-deposition, thickening of Bruch’s membrane, and a progressive loss of photoreceptor cells (11, 14–17).
Given the cellular consequences of bisretinoid build-up, efforts have been made to elucidate mechanisms by which these compounds are damaging. Although the bisretinoid fluorophores of lipofuscin do not appear to undergo lysosomal digestion, in in vitro assays, these fluorophores photodegrade. Both whole-lipofuscin mixtures (18, 19) and individual fluorophores such as A2-GPE (3), A2E (20), and all-trans-retinal dimer (21) have been shown to initiate photosensitization reactions that generate superoxide anion and singlet oxygen (20–22). Singlet oxygen, as a highly reactive form of oxygen, adds to carbon–carbon double bonds of the bisretinoid molecule, thereby photooxidizing the bisretinoid and creating unstable oxygen-containing moieties that have been detected as oxidized A2E and oxidized all-trans-retinal dimer in human and mouse retina (21, 22). It is this oxidation that causes the bisretinoids to photodegrade and release a complex mixture of aldehyde-containing fragments and the dicarbonyls methylglyoxal (MG) and glyoxal (23) that modify other molecules. Because these carbonyl-modified proteins are present in deposits (drusen) that accumulate on the basal side of RPE cells in vivo, and drusen are a major risk factor for AMD progression (24, 25), the photodegradation of bisretinoids likely constitutes a relationship to AMD.
Here we have undertaken to determine whether photodegradation of bisretinoid is ongoing in the eye. We reasoned that photodegradation could be detected, were it to occur, by comparing levels of bisretinoid in mice that were raised in cyclic light as opposed to darkness and by comparing albino versus black and agouti mice.
Results
A2E Accumulation in Albino and Pigmented Wild-Type Mice Housed in Darkness Versus Cyclic Light.
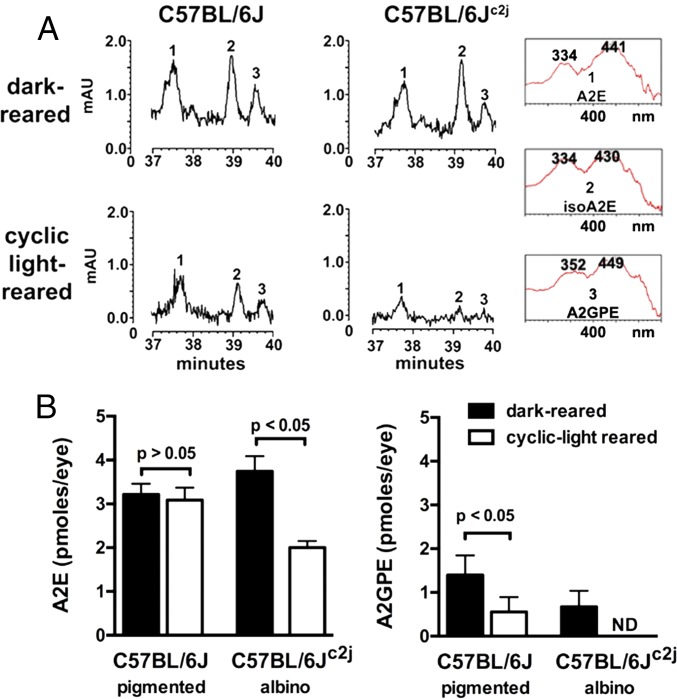
Ocular melanin serves as a neutral density filter (0.37 log units) (26). Accordingly, with the absence of melanin in the albino, light entering the eye through the iris and pars plana is substantially increased (27). Thus, pigmented C57BL/6J and albino C57BL/6Jc2j mice were housed from birth in either continuous darkness or under cyclic light; except for the mutated tyrosinase gene (c/c), these mice are genetically identical. With analysis by high-performance liquid chromatography (HPLC) and ultraperformance liquid chromatography (UPLC), the bisretinoids A2E, isoA2E, and A2-GPE were present in eyes from both cyclic light and dark-reared mice (Fig. 1 A and B and Fig. S1). Chromatographic quantitation also revealed that with housing in darkness, bisretinoid amounts were not significantly different in pigmented C57BL/6J versus albino C57BL/6Jc2j mice (P > 0.05) (Fig. 1B). Conversely, in albino C57BL/6Jc2j mice maintained under cyclic light conditions, A2E levels were reduced by 45% compared with albino C57BL/6Jc2j mice reared in the dark (P < 0.05) (Fig. 1B). In albino C57BL/6Jc2j mice, A2-GPE was not detectable.
Fig. 1.
The RPE bisretinoids A2E and A2-GPE accumulate in both light- and dark-reared mice and are present at reduced levels in light-reared versus dark-reared albino mice. (A) Reverse phase chromatographic detection of A2E and A2-GPE in dark-reared and cyclic-light reared pigmented C57BL/6J and albino C57BL/6Jc2j mice at age 6 mo. (Insets) UV-visible spectra of chromatographic peaks corresponding to A2E (all-trans-A2E), iso-A2E (13–14 cis), and A2-GPE. (B) Chromatographic quantitation. A2E and iso-A2E were quantified by HPLC, and values presented are A2E and iso-A2E summed. A2-GPE was quantified by UPLC. For each sample, six to eight eyes were pooled; means ± SEM of five or seven independent samples are plotted; P values determined by one-way ANOVA and Tukey’s multiple comparison test.
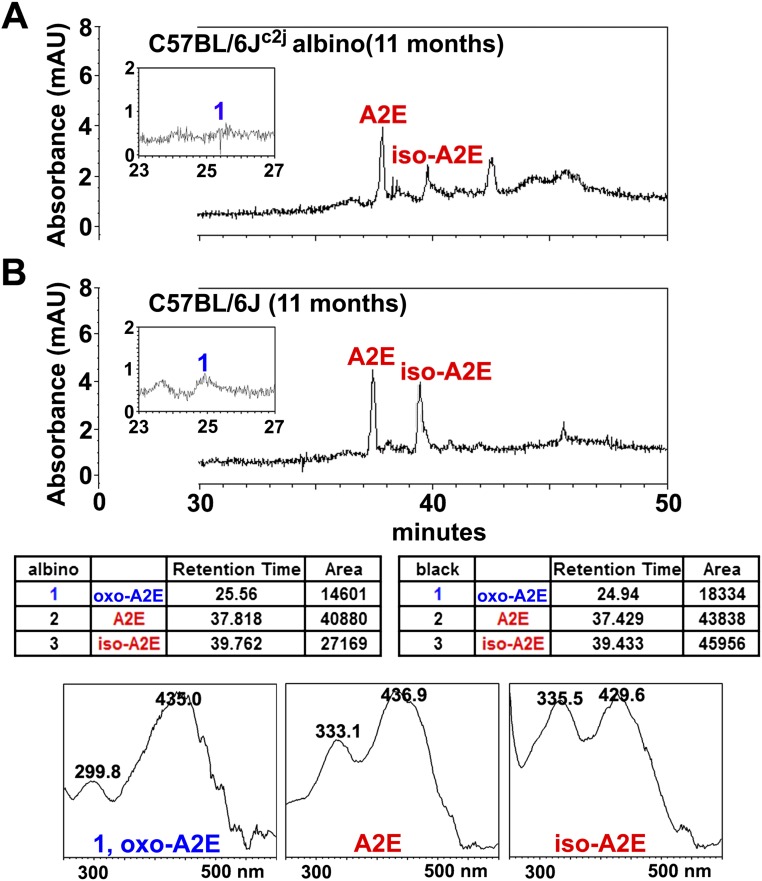
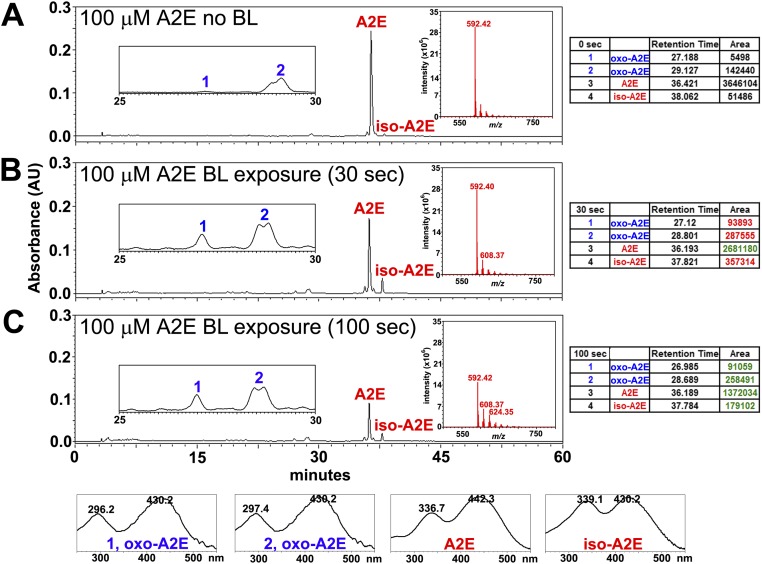
Chromatographic detection of A2E, isoA2E, and photooxidized A2E in albino C57BL/6Jc2j and black C57BL/6J mice are presented in Fig. S2. Note that in the albino mice, the sum of the integrated peak areas of A2E and isoA2E (68,049 µV⋅sec) is lower than in the black mice (89,794 µV⋅sec), but the peak area of oxidized A2E (Fig. S2, peak 1) is not correspondingly greater in the albino. As shown in Fig. S3, when synthesized samples of A2E are irradiated, the amount of remaining A2E and isoA2E is lower after 100 s irradiation (1,372,034, 179,102 µV⋅sec) than after 30 s (2,681,180, 357,314 µV⋅sec). Nevertheless, the quantity of oxidized product is not greater after the longer irradiation because oxidized A2E is lost to photodegradation. Taken together, the latter experiments provide in vivo and in vitro evidence of bisretinoid photooxidation and photodegradation and indicate that greater light exposure does not necessarily increase the amount of oxidized bisretinoid because photodegradation follows the oxidation.
Fig. S2.
Reverse-phase HPLC detection of A2E, isoA2E, and photooxidized-A2E species in albino C57BL/6Jc2j (A) and pigmented C57BL/6J (B) mice age 11 mo. In the table below, retention times and peak areas for the albino and black mice are presented. (Bottom) UV-visible absorbance spectra corresponding to peaks labeled in A and B. Oxo-A2E is more polar and elutes earlier, and the UV-visible absorbance peak is blue shifted by ∼30 nm because of oxidation on the short arm of the molecule.
Fig. S3.
HPLC and mass spectrometric detection of A2E, isoA2E (m/z 592), and photooxidized forms of A2E (oxo-A2E, m/z 608, 624) in unirradiated A2E (A), and A2E irradiated (430 nm) for 30 (B) and 100 s (C) in vitro. In tables on the right, increased peak area (relative to unirradiated) is indicated in red; decreased peak area in green. Below, UV-visible absorbance spectra correspond to peaks labeled in A–C. Peak area of A2E undergoes more pronounced reduction after a 100-s irradiation compared with 30-s irradiation, but oxidized forms of A2E are not correspondingly greater because of photodegradative consumption.
Accumulation of Bisretinoid in Dark- and Light-Reared Albino Abca4 Null Mutant Mice.
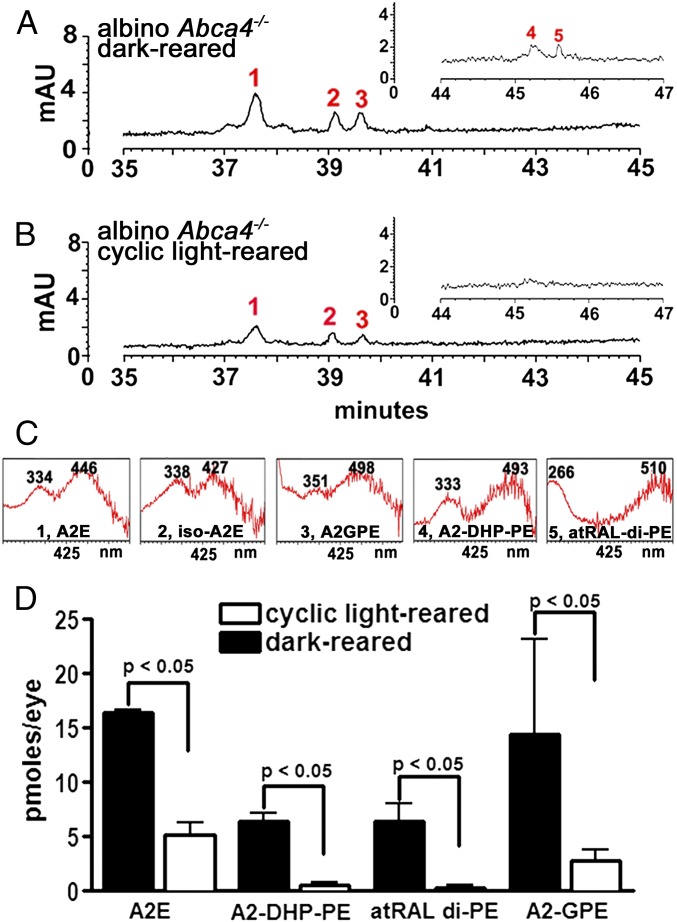
Bisretinoid fluorophores form in abundance in the retinae of Abca4−/− mice because of impaired handling of all-trans-retinal within photoreceptor outer segments (10, 28). Chromatographic detection of the bisretinoids A2E and iso-A2E (2), A2-DHP-PE (4), all-trans-retinal dimer-PE (21), and A2-GPE (3) (Fig. 2 and Fig. S1) and quantification by integrating peak areas revealed that all of these fluorophores were present in greater amounts in dark-reared albino mice compared with cyclic-light reared albino Abca4−/− mice (Fig. 2D). The decline in A2E levels in the albino cyclic light-reared Abca4−/− mice was 62% (P < 0.05), with decreases in A2-DHP-PE, all-trans-retinal dimer-PE, and A2-GPE being similar or greater. Outer nuclear layer (ONL) thinning was also less pronounced in the dark-reared mice (Fig. S4).
Fig. 2.
Comparison of bisretinoid levels in cyclic light-reared and dark-reared albino Abca4−/− mice. (A and B) Representative reverse-phase chromatograms with 430-nm monitoring to detect A2E (1) and iso-A2E (13–14 cis) (2), A2-GPE (3), A2-DHP-PE (4), and all-trans-retinal dimer-PE (atRAL dimer-PE) (5) in dark-reared (A) and cyclic light-reared (B) albino Abca4−/− mice (8 mo). (Insets) Chromatograms expanded between retention times 44–47 min. (C) UV-visible absorbance spectra of the indicated compounds. (D) Quantitation of bisretinoid compounds in albino Abca4−/− mice (6 mo) that were cyclic-light or dark-reared; picomoles of A2E and iso-A2E were summed. A2E, A2-DHP-PE, atRAL di-PE were quantified by HPLC, and A2-GPE was quantified by UPLC. Mean ± SEM of two to five samples, four to six eyes per sample; P values determined by one-way ANOVA and Sidak’s multiple comparison test.
Fig. S4.
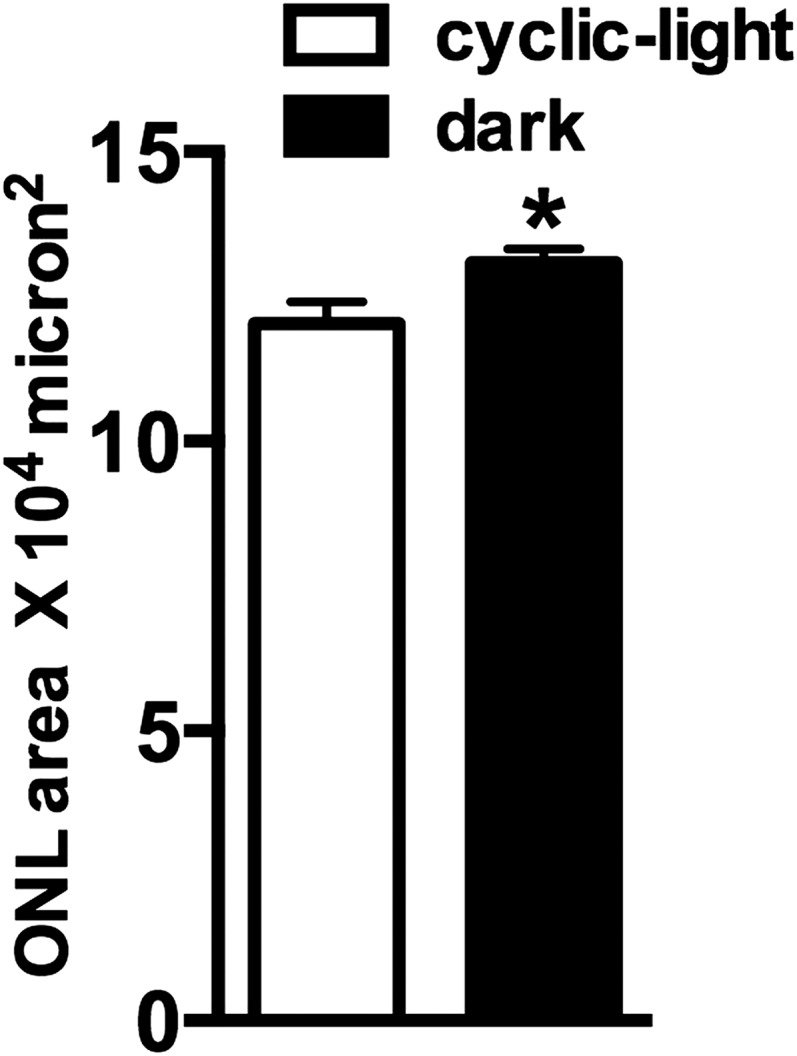
Outer nuclear layer area calculated within superior and inferior hemiretina of 8-mo-old dark-reared and cyclic-light reared albino Abca4−/− mice. Areas were determined by summing thicknesses at 0.2-mm intervals from optic nerve head to distances 1.6 mm in both superior and inferior retina. *P < 0.05, unpaired t test.
Comparing Agouti Versus Albino Mice.
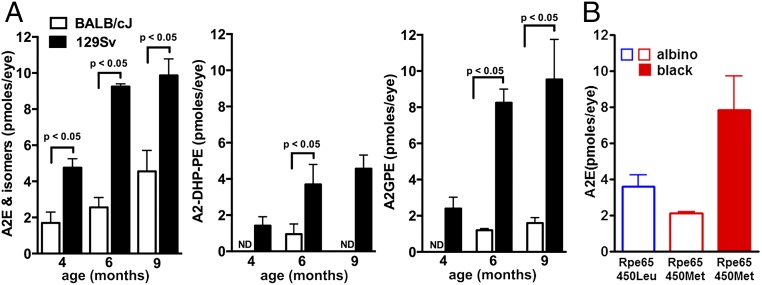
Differences were also observed between albino BALB/cJ (albino; Rpe65 Leucine450 variant) and 129/Sv mice having an agouti-coat color (Rpe65 Leucine450 variant) at 4, 6, and 9 mo of age (Fig. 3A). At 6 mo of age, A2E, A2-DHP-PE, and A2GPE were from three- to sevenfold higher in the 129/Sv mice (P < 0.05). Although A2-DHP-PE and A2-GPE were measurable in 129/Sv mice at all ages, these fluorophores were not always at detectable levels in BALB/cJ mice.
Fig. 3.
Modulation of bisretinoids levels in albino and pigmented mice under cyclic-light rearing. (A) Quantitation of A2E, A2-DHP-PE, and A2GPE in albino BALB/cJ and agouti 129/Sv wild-type mice at 4, 6, and 9 mo of age. Mice express the wild-type leucine-450 variant in Rpe65. A2E and A2-DHP-PE were quantified by HPLC, and A2E values consist of A2E and iso-A2E summed. A2-GPE was quantified by UPLC. For each sample, six eyes were pooled; means ± SEM of two to six independent samples are plotted; P values determined by one-way ANOVA and Sidak’s multiple comparison test. ND, not detected. (B) A2E levels in cyclic light-reared albino BALB/cJ (Rpe65 leucine-450), albino C57BL/6Jc2j (Rpe65 methionine-450), and pigmented C57BL/6J (Rpe65 methionine-450) mice age 9 mo. Values are means ± SEM of eight (BALB/cJ), five (C57BL/6J), and four (C57BL/6Jc2j) independent samples per group; six eyes were pooled per sample.
Modulation of A2E Levels by the Rpe65 Leu450Met Variant.
The Rpe65 450Met variant expressed by C57BL/6J and C57BL/6Jc2j mice slows the isomerization step in the regeneration of 11-cis retinal (26, 29); these retarded kinetics are also associated with reduced A2E in albino C57BL/6Jc2j versus BALB/cJ (wild-type Rpe65 Leu450) mice (10). Accordingly, A2E levels in 9-mo-old albino C57BL/6Jc2j (450Met) mice were ∼60% of that in the BALB/cJ (450Leu) mice (Fig. 3B). A corresponding difference between the albino BALB/cJ (450Leu) and pigmented C57BL/6J (450Met) mice was not observed, likely because of more pronounced A2E photodegradation in the albino BALB/cJ mice.
Vitamin E-Treated Mice.
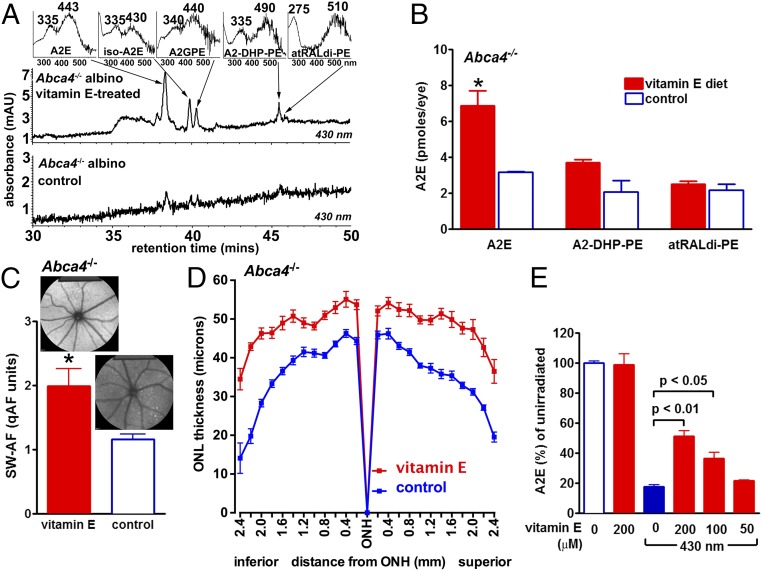
Evidence for the involvement of photooxidative processes in determining A2E levels at a given age was also obtained in experiments in which albino Abca4−/− mice received a diet supplemented with vitamin E (α-tocopherol), a potent lipid-soluble antioxidant. In studies using a singlet oxygen generator, we have previously shown that vitamin E can protect against A2E-oxidation by quenching singlet oxygen (30). In the current study, plasma levels of vitamin E measured at 8 mo of age were 6.7 ± 0.1 μg/mL (mean ± SEM) in control mice and 11.7 ± 0.5 μg/mL in vitamin E-treated mice. Mice housed in cyclic light and treated with vitamin E from 1 to 6 mo of age exhibited levels of the bisretinoids A2E, A2-DHP-PE, and all-trans-retinal dimer-PE that were greater than in control mice (Fig. 4 A and B). For example, A2E levels in the vitamin E-treated mice were 54% higher (P < 0.05). Similarly, noninvasive in vivo measurement of RPE lipofuscin by quantitative fundus autofluorescence (qAF) analysis (16), revealed higher levels of fundus autofluorescence in mice treated with vitamin E from age 1–8 mo (P < 0.05) (Fig. 4C). Importantly, the thinning of ONL that is indicative of reduced photoreceptor cell viability in albino Abca4−/− mice (15, 16, 31) was less pronounced in the vitamin E-treated mice (Fig. 4D). In vitamin E-treated albino Abca4−/− mice, ONL area in superior and inferior hemiretina was 10.0 × 104 μm2 and 10.1 × 104 μm2, respectively, compared with 7.9 × 104 and 7.7 × 104 μm2, respectively (P < 0.05, two-tailed t test) in the control Abca4−/− mice.
Fig. 4.
Vitamin E protects against bisretinoid consumption associated with photooxidation/photodegradation. (A) Chromatographic detection of the bisretinoids A2E, iso-A2E (13–14 cis), A2-GPE, A2-DHP-PE, and atRAL di-PE in vitamin E-treated versus untreated albino Abca4−/− mice raised in cyclic light. Age, 6 mo. (A, Inset) UV-visible spectra of chromatographic peaks corresponding to indicated compounds. (B) Quantitation of A2E, A2-DHP-PE, and atRAL di-PE. For each sample, six eyes were pooled; means ± SEM of two independent samples are plotted. A2E values consist of A2E and iso-A2E summed. Levels of A2E in vitamin E-treated and untreated Abca4−/− mice (age, 6 mo) are statistically different (ANOVA and Sidak’s multiple comparison test, P < 0.05). (C) Quantitative fundus autofluorescence in vitamin E-treated and untreated Abca4−/− mice (age, 8 mo). Values are means ± SEM of 8 mice; *P < 0.05, two-tailed t test. (D) Outer nuclear layer thickness measured in vitamin E-treated and untreated albino Abca4−/− mice (age, 8 mo) and plotted as distance from optic nerve head (ONH). (E) Vitamin E protects against the consumption of 430 nm-exposed A2E in an in vitro assay. Values are means ± SEM of two experiments; P values based on ANOVA and Newman-Keul multiple comparison test.
The higher levels of bisretinoid and qAF in the vitamin E-treated mice are consistent with a mechanism involving a reduction in photooxidation-associated photodegradation of bisretinoid in the presence of vitamin E. Support for the latter mechanism was also forthcoming in noncellular experiments in which we observed a concentration-dependent protection of A2E when irradiation (430 nm) was carried out in the presence of vitamin E (Fig. 4E).
Reduction of Bisretinoid and Release of Dicarbonyl by Exposure to Blue Light.
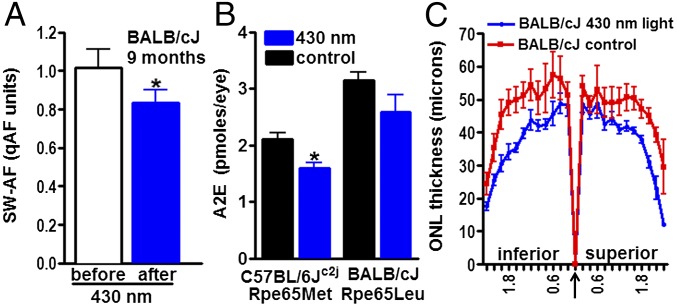
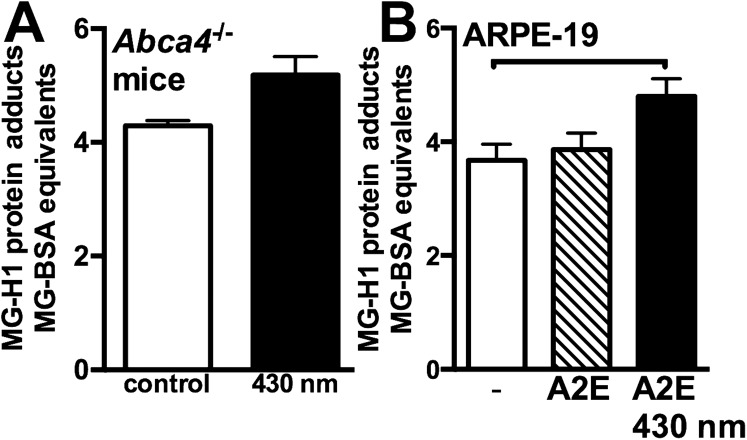
To further test for photodegradation of bisretinoid in vivo, we exposed albino C57BL/6Jc2j (8 mo) and BALB/cJ (9 mo) mice to 430 nm light, in-cage, for 2 h each day for 7 d. For the remaining hours, the mice were housed in cyclic light. qAF disclosed a reduction of 18% (P < 0.05) in the exposed BALB/cJ mice (Fig. 5A), whereas HPLC quantitation of A2E revealed a 24% (P < 0.05) and 17% reduction (P > 0.05) in C57BL/6Jc2j and BALB/cJ mice, respectively (Fig. 5B), compared with unexposed age- and strain-matched mice. In agouti Abca4−/− mice exposed to the same lighting conditions, we detected MG-derived hydroimidazolone (MG-H1), an adduct of MG with arginine, in posterior eyecups by indirect competitive ELISA (Fig. S5A). MG is released as a photodegradation product of bisretinoid (Fig. S5B). The photodegradation of bisretinoid in the BALB/cJ mice was also accompanied by a reduction in ONL thickness (Fig. 5C). ONL area in the exposed mice was 8.2 × 104 μm2 (inferior) and 8.4 × 104 μm2 (superior), as opposed to 10.4 × 104 μm2 (inferior) and 9.9 × 104 μm2 (superior) in control mice (P < 0.05, two-tailed t test). Conversely, when younger (age 2 mo) albino mice having less pronounced bisretinoid accumulation, were exposed to 430 nm light under the same conditions, no difference in ONL thickness was observed compared with in unexposed control mice (Fig. S6).
Fig. 5.
Exposure of mice to short-wavelength light. Mice were illuminated with 430 nm (1 mW) light for 2 h/day on 7 consecutive days. (A) Quantitative fundus autofluorescence was used to measure RPE lipofuscin noninvasively in BALB/cJ mice (9 mo) before and after exposure to the light paradigm; *P < 0.05, two-tailed t test. (B) HPLC quantitation of A2E in eyes of exposed and control (nonexposed) C57BL/6Jc2j mice (8 mo; Rpe65 450Met) and BALB/cJ mice (9 mo; Rpe65 Leu450). For each sample, six eyes were pooled; means ± SEM of five (control C57BL/6Jc2j) or two independent samples are plotted. *P < 0.05, two-tailed t test. (C) Outer nuclear layer thicknesses in exposed and control (nonexposed) BALB/cJ mice (9 mo) are plotted as distance from optic nerve head (arrow).
Fig. S5.
Quantitation of MG-derived hydroimidazolone (MG-H1) adducts as products of bisretinoid photodegradation. (A) MG-adducts in posterior eyecups of agouti Abca4−/− mice were measured by competitive indirect ELISA. MG can also form from intermediates of glycolysis and fatty acid metabolism. Thus, we selected for bisretinoid photodegradation as the source of MG release by exposing mice to blue light (430 nm), as described in SI Materials and Methods. Control mice were not exposed to blue light. Mean ± SE of two samples assayed in duplicate; four mice/sample. (B) Bisretinoid photodegradation is a source of MG. MG-adducts were measured in ARPE-19 cells that had accumulated A2E and were exposed to 430 nm light (P < 0.05). Quantitation by competitive indirect ELISA. Mean ± SE of five to six samples per group; horizontal bar, P < 0.05, one-way ANOVA and Tukey’s multiple comparison test.
Fig. S6.
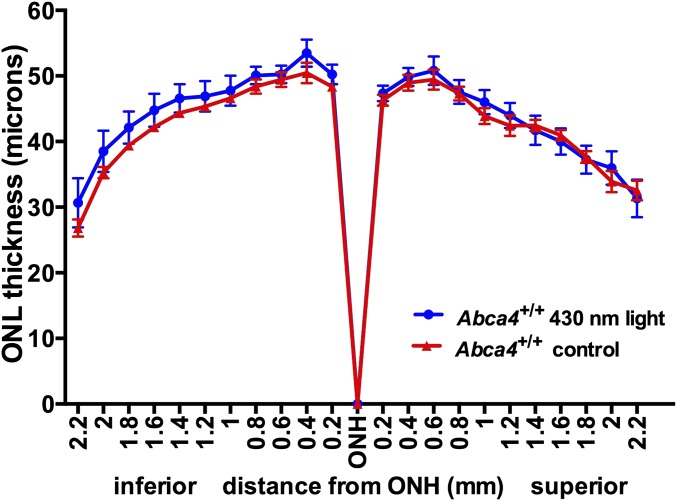
Exposure of young mice to short-wavelength light. Two-month-old albino Abca4+/+ wild-type mice were illuminated with 430 nm (1 mW) light for 2 h/day on 7 consecutive days. Outer nuclear layer thicknesses in exposed and control (nonexposed) mice are plotted as distance from optic nerve head (ONH).
Comparison of A2E and 11-Cis-Retinal in Light-Reared Albino and Pigmented Mice.
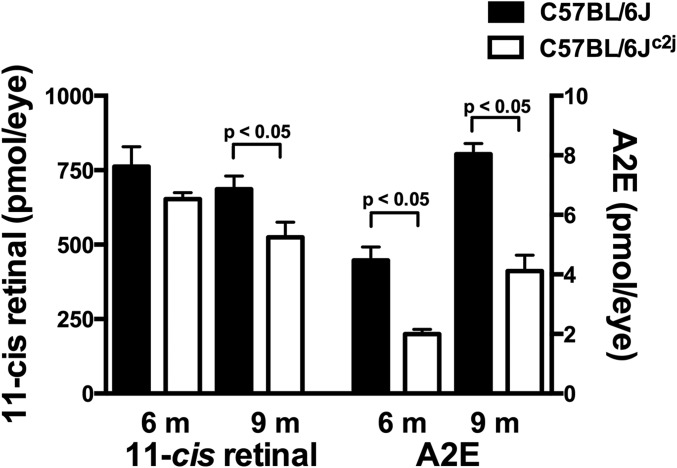
An additional issue relevant to these experiments is that to adjust for increased photon catch, albino mice have reduced rhodopsin content (photostasis) (32). Thus, we measured 11-cis-retinal and A2E in the same eye samples obtained from C57BL/6J and C57BL/6Jc2j mice. In the albino mice, 11-cis-retinal was 14% and 23% lower, at 6 and 9 mo, respectively, whereas the decrease in A2E in the albino was greater (6 mo: 55% lower; 9 mo: 59% lower) (Fig. S7).
Fig. S7.
Quantitation of A2E and 11-cis-retinal and in cyclic light-reared C57BL/6J (pigmented) and C57BL/6Jc2j (albino) mice. A2E and 11-cis-retinal were measured in the same sets of pooled samples at 6 and 9 mo. Values are means ± SEM, 5–11 independent samples per group; P < 0.05 determined by one-way ANOVA and Sidak’s multiple comparison test.
A2E Synthesis from Cis and Trans Isomers of Retinaldehyde.
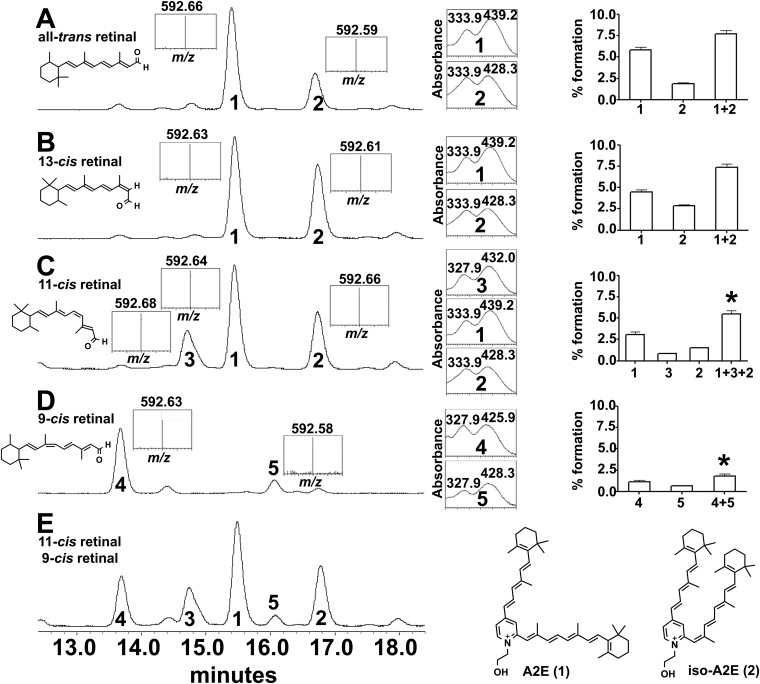
Because the retinaldehyde double-bond isomers contributing to bisretinoid formation in the dark (Fig. 1) are uncertain, we examined trans- and cis-isomers of retinaldehyde for their ability to form the all-trans- and cis-isomers of A2E (2) when reacted with ethanolamine. A2E and isoA2E were both generated when the starting material was all-trans-, 13-cis-, or 11-cis-retinal (Fig. S8 A–C). 11-cis-A2E was also generated when 11-cis-retinal reacted with ethanolamine. A2E and isoA2E did not form when 9-cis-retinal was the precursor (Fig. S8D); of the two products (4, 5) that were generated, one was likely 9-cis-A2E. Total conversion from 11-cis-retinal and 9-cis-retinal to bisretinoid was lower than conversion from all-trans-retinal (P < 0.05) (Fig. S8 C and D, Right).
Fig. S8.
UPLC-MS analysis of synthetic A2E isomers formed from isomers of retinaldehyde. Reaction with all-trans retinal (A), 13-cis retinal (B), 11-cis retinal (C), 9-cis retinal (D), and 11-cis retinal and 9-cis retinal (E). The chromatograms were monitored and quantified at absorbance 430 nm. Bisretinoid products were characterized by absorbance spectra (nm; to the right of the chromatograms) and ESI(+) mass spectrometry (m/z, Insets). Retention times of bisretinoid products were: 15.5 min (product 1, A2E; m/z 592, λmax 439 and 334 nm); 16.8 min (product 2, 13-cis-A2E (iso-A2E); m/z 592, λmax 428 and 334 nm); 14.7 min (product 3; 11-cis-A2E; m/z 592, λmax 432 and 328 nm). Either product 4 (13.7 min) or product 5 (16.1 min) is 9-cis-A2E. Values plotted (Far Right) are percentage bisretinoid formed relative to amount of starting retinaldehyde; the products are also summed (+). Means ± SEM, three replicates; *P < 0.05 compared with sums of the products generated with all-trans-retinal (1+2), one-way ANOVA and Tukey’s multiple comparisons test.
Discussion
Levels of RPE bisretinoid that are measured appear to be the result of the opposing activities of synthesis and photodegradation (20–22). By assessing bisretinoid levels in albino versus pigmented mice, and in mice housed in darkness compared with cyclic light, we observed that the levels of the lipofuscin fluorophores in cyclic light-reared albino mice were less than in cyclic light-reared pigmented mice and in mice reared in darkness. With dark-rearing, the presence or absence of ocular melanin had no effect on the levels of RPE bisretinoid lipofuscin. These findings, together with our previous work (20–23), are indicative of light-driven mechanisms involving bisretinoid photosensitization and oxidation followed by photodegradative consumption of bisretinoid. It is possible that bisretinoid formation under cyclic light is more pronounced than is apparent here; for instance, photodegradative loss could mask a greater propensity for lipofuscin formation under cyclic light. On the practical side, investigators quantifying bisretinoids in mouse models should anticipate measurement variability caused, at least partially, by variations in ambient light intensities experienced by mice in the vivarium.
Here we also observed that in mice treated with the antioxidant vitamin E, photodegradation of bisretinoid was suppressed, resulting in increased bisretinoid and sparing of photoreceptor cells. Vitamin E intercepts bisretinoid oxidation by scavenging reactive oxygen species generated by bisretinoid photosensitization (23). These results show that bisretinoid photodegradation is damaging and that bisretinoid levels can be modulated by mechanisms not involving a change in synthesis.
Photodegradation products of bisretinoids such as A2E constitute a mixture of aldehyde-bearing fragments, together with the dicarbonyls MG and glyoxal (GO) (23), that ravage cellular and extracellular molecules. Quantitation of MG-adducts by ELISA revealed that in albino light-reared Abca4−/− mice accumulating bisretinoid at elevated levels, MG-adducts were elevated threefold compared with wild-type mice (17). Using immunocytochemical approaches that detected MG-carbonyl-modified proteins, labeling in the location of Bruch’s membrane was more pronounced in Abca4−/− versus Abca4+/+ mice and in older versus younger mice. The photodegradation products released from bisretinoids cross-link protein and suppress matrix metalloproteinase activity (17). Proteins modified by these reactive species are also constituents of drusen (24, 25), the sub-RPE deposits associated with AMD (33). A limitation of this work is that, because of practical considerations, the study did not include measurement of MG-adducts in dark-reared versus light-reared mice.
We have previously shown that Abca4−/− mice burdened with increased bisretinoid are more susceptible to retinal light damage than are wild-type mice (31). Older mice carrying greater levels of bisretinoid are also more susceptible than younger mice, and light damage was not observed in Rpe65rd12 mice that carry little or no RPE bisretinoid. These findings are consistent with reports in the literature indicating that light damage can occur independent of phototransduction (34) and that photooxidative mechanisms are involved (35, 36). Depending on the conditions, light damage can also originate within photoreceptor cells or RPE, although the greatest damage is reported for RPE (36).
In the present study, we demonstrated by in vitro synthesis assays that the bisretinoid A2E can form when 11-cis-retinal, all-trans-retinal, and 9-cis-retinal are used as starting material, although steric hindrance introduced by the 9-cis-retinaldehyde configuration reduces the synthetic efficiency of the latter. In disease marked by 11-cis-retinal deficiency, 9-cis-retinal can form iso-rhodopsin (37) and generate small light-evoked rod responses (38). The propensity for 9-cis-retinal to form cis-A2E likely explains the detection of some RPE autofluorescence in Rpe65−/− mice (39).
Our findings in dark-reared mice and the data of Boyer et al. support the notion that bisretinoid can form from 11-cis-retinal in vivo (40). The relative contributions of 11-cis- and all-trans-retinal to bisretinoid formation under lighted conditions are not known, but in both cases, only a small portion of retinoid escapes the visual cycle to form bisretinoid. With respect to bisretinoid formation in Abca4−/− mice, it is significant to note that ABCA4 can transport both 11-cis-retinal and all-trans-retinal as a reversible Schiff base with PE (40).
The phagocytosis of shed photoreceptor cell disk membrane continues in an entrained fashion in rats raised in total darkness, although the numbers of phagosomes may or may not be be altered (41). Nevertheless, bisretinoid formation in darkness or in cyclic light is unlikely to depend on phagocytosis, as the bisretinoid constituents form in photoreceptor cells before disk shedding and phagocytosis, and even in the absence of RPE phagocytosis (42–45). We can exclude reduced numbers of photoreceptor cells as an explanation for the decrease in lipofuscin, as in 12-mo-old C57BL/6Jc2j mice, thinning of ONL is not observed (46). In addition, photostasis (32) cannot account for bisretinoid differences in albino versus pigmented mice.
The use of the albino mouse in these studies presumably allowed us to observe photodegradative consumption of A2E during a relatively short period, whereas the dense ocular melanin of black mice likely enabled the differences between C57BL/6J and C57BL/6Jc2j mice to emerge. Nevertheless, photooxidation and photodegradation of RPE bisretinoids can be observed in humans and nonhuman primates. For instance, photobleaching of RPE lipofuscin likely explains aberrations in fundus autofluorescence images (488 nm excitation) observed after retinal translocation in humans (47), and the diminished RPE fluorescence observed after in vivo fluorescence imaging by adaptive optics scanning laser ophthalmoscopy in nonhuman primates (36). At higher light levels, but still within approved safety standards, this photobleaching is accompanied by permanent RPE cell damage, indicating that bisretinoid photodegradation is not benign.
Although epidemiological evidence of an association between sunlight exposure and AMD has been inconsistent over the years (48–50), recent studies have provided supported for such a link (51–53). The adverse consequences of lipofuscin bisretinoid photodegradation could be a pathway that explains this relationship. Similarly, the ability of the antioxidant vitamins E and C and minerals to reduce AMD progression (54) may be understood, at least in part, from the perspective of antioxidant suppression of lipofuscin photooxidation/degradation.
Because the photodegradative processes examined here could over time be responsible for cumulative damage to retina and Bruch’s membrane, the present findings have implications with respect to development of therapeutics that address bisretinoid lipofuscin deposition in recessive Stargardt disease and AMD (55–60) and justify efforts to protect the retina of STGD1 patients by reducing light exposure through the use of a black contact lens (61).
Materials and Methods
Mouse Models.
BALB/cJ (albino, Rpe65 Leu450), C57BL/6J (black, Rpe65 450Met variant), C57BL/6Jc2j (B6(Cg)-Try < c-2J>/J, albino, Rpe65 450Met variant), and 129S1/SvImJ (agouti, Rpe65 Leu450) mice were purchased or bred and housed (see details in SI Materials and Methods). In some experiments, mice were exposed in-cage to 430 (± 30 nm) light from a halogen source (1 mW; measured with Newport Optical Power Meter Model 840) for 2 h each day for 7 d before euthanasia. Animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University and complied with guidelines set forth by the Association for Research in Vision and Ophthalmology (ARVO) Animal Statement for the Use of Animals in Ophthalmic and Vision Research.
Quantitative Fundus Autofluorescence.
Images were acquired and analyzed as previously described (16).
Vitamin E Treatment.
Albino Abca4−/− (Rpe65 450Leu) mice were treated from age 1 mo to 6 or 8 mo with Adjusted Vitamin E Diet (960) (Harlan Teklad), which included 960 mg (IU)/kg vitamin E (as dl-alpha tocopheryl acetate) or a standard diet (vitamin E, 99IU/kg; control). Plasma vitamin E levels were measured (62).
Quantitative HPLC and UPLC.
Mouse eyecups (5–12 eyes per sample, as indicated) were homogenized, extracted in chloroform/methanol, concentrated, and analyzed by HPLC and UPLC, as previously described (3, 4) (see details in SI Materials and Methods).
In Vitro Photooxidation Assay.
Synthesized A2E (2) (100 μM) with vitamin E (50–200 μM) in PBS and was irradiated (430 ± 30 nm; 30 s), extracted, and quantified.
Measurement of Outer Nuclear Layer Thickness.
Three histological sections through the optic nerve head were analyzed (31). ONL area was calculated as the sum of the ONL thicknesses in superior and inferior retina (0.2–2.0 mm) multiplied by the measurement interval of 200 microns.
Statistical Analyses.
Prism 6 (GraphPad Software) was used.
SI Materials and Methods
Mouse Models.
BALB/cJ (albino, Rpe65 Leu450), C57BL/6J (black, Rpe65 450Met), C57BL/6Jc2j (B6(Cg)-Try < c-2J>/J, albino, Rpe65 450Met), and 129S1/SvImJ (agouti, Rpe65 Leu450) mice were purchased from Jackson Laboratories. Albino Abca4−/− (10) (albino, Rpe65 Leu450) were bred in-house. Timed pregnant C57BL/6J and C57BL/6Jc2j mice were housed either in constant darkness or on a 12-h light/12-h dark cycle (cyclic light) within ventilated light-controlled chambers. The mouse litters were raised under the same uninterrupted dark or cyclic-light conditions. Cyclic-light was provided by a strip of white light-emitting diodes (LED) mounted on the ceiling of each shelf of the cabinet (40–60 lx; LL-RIBFLEX-W from LED Liquidators, Inc.). For the mice maintained in constant darkness, red LED lighting (HLMP-AD060-P0000, λmax 639 ± 9 nm; < 1 lx; ∼2 min daily) was used for husbandry. For other experiments, C57BL/6J, C57BL/6Jc2j, 129S1/SvImJ, and BALB/cJ mice were purchased as young adults and then aged to 4 and 6 mo by housing in the institutional vivarium, or were retired breeders (aged 8–9 mo) that were purchased and not housed in the institutional vivarium. In some experiments, mice were exposed in-cage to 430 (± 30 nm) light from a halogen source (1 mW; measured with Newport Optical Power Meter Model 840) for 2 h each day for 7 d before euthanasia.
Quantitative HPLC and UPLC Analysis.
Mouse eyecups (5–12 eyes per sample, as indicated) were homogenized, extracted in chloroform/methanol, concentrated, and analyzed by HPLC (Alliance System, Waters Corp.), as previously described (3, 4). Absorbance peaks were identified using synthesized authentic compounds, and molar quantities per eye were calculated by comparison with synthesized standards. A2E and isoA2E were measured separately and summed.
For UPLC quantitation, murine eyecup samples were redissolved in ethanol after extraction and injected (10 μL) into a Waters Acquity UPLC-MS system (Waters), using an Acquity BEH Phenyl Column (1.7 µm, 2.1 × 100 mm; Waters). Eluents were water-acetonitrile (1:1) with 0.1% formic acid (A) and isopropanol-acetonitrile (9:1) with 0.1% formic acid (B). Separation was achieved using the following elution gradients: 0–50 min, 100–55%; 50–110 min, 55–35%. Flow rate was 0.2 mL/min. UV absorbance peaks were identified by comparison with external standards of synthesize and purified A2GPE. Identification was confirmed by mass to charge ratio (m/z). Molar quantities per eye were calculated on the basis of standard solutions with concentrations determined spectrophotometrically. Peak areas were calculated using Waters Empower Software and results were analyzed in Excel (Microsoft).
Analysis of Retinoids and Bisretinoid in Light-Reared Albino and Pigmented Mice.
C57BL/6J or C57BL/6Jc2j mice were dark-adapted for 24 h before euthanasia. Under dim red light, mouse eyecups (eight eyes/sample) were placed in 1.6 mL PBS with 2 M hydroxylamine and homogenized for 30 min at room temperature. Ethanolic all-trans retinal was added as an internal standard. The mixture was extracted with chloroform and dried under argon, and the residue was redissolved in ethanol (for retinoids) or chloroform/methanol (1:1 for bisretinoids) for UPLC-MS analysis. Samples were analyzed using an Acquity UPLC coupled with electrospray ionization mass spectrometry (UPLC-ESI/MS; Waters Corp.) with a reverse-phase Acquity UPLC CSH C18 column (2.1 × 100 mm, 1.7 μm; Waters Corp.) and an acetonitrile and water gradient with 0.1% trifluoroacetic acid (60–80%, 0.3 mL/min in 60 min). UV absorbance peaks were identified by comparison with external standards of retinal oximes of 11-cis-retinal and synthesized A2E (2). Identification was confirmed by mass to charge ratio (m/z). Molar quantities per eye were calculated from peak areas, using standard curves with concentrations determined spectrophotometrically, using published extinction coefficients.
Analysis of A2E/Bisretinoids Formation from Vitamin A Aldehyde Isomers in Vitro.
All-trans, 13-cis, 9-cis-retinals (0.5 mg) (Sigma-Aldrich Corp.) and 11-cis-retinal (0.5 mg) (Toronto Research Chemicals) were dissolved in ethanol (100 μL); ethanolamine (0.05 μL, 1 equivalent) and acetic acid (0.1 μL, 2 equivalents) were then added in 10 μL ethanol (2). The mixtures were incubated at 37 °C for 3 d (2) and then analyzed by UPLC-ESI/MS, using the Acquity C18 column described earlier. The mobile phases were water with 0.1% trifluoroacetic acid (A) and acetonitrile with 0.1% trifluoroacetic acid (B). Elution was performed with a gradient of 30–20% mobile phase A from 0 to 30 min, with a flow rate of 0.3 mL/min. The chromatography was monitored by absorbance (220–600 nm). The mass spectrometry was performed under ESI positive mode (m/z 150–2,000). Peak areas were converted to picomoles, using published extinction coefficients for A2E and iso-A2E (2). Total bisretinoid produced from each retinaldehyde was determined by summing the products and plotting as percentage formation relative to starting retinaldehyde.
Detection of MG-Protein Adducts by ELISA.
Posterior eyecups were placed in lysis buffer (Cell Signaling) with protease inhibitors (complete protease inhibitor mixture tables; Roche Applied Science), and after sonication and centrifugation, the protein concentration in the supernatant was measured (BCA protein assay; Pierce), and MG-derived hydroimidazolone (MG-H1) protein adducts were quantified by competitive ELISA, using an HRP-conjugated secondary antibody (OxiSelect; Cell Biolabs). MG content was determined by comparison with an MG-BSA standard curve, a four-parameter fit algorithm, and absorbance at 450 nm (y) as a function of log concentration (x) (17). For quantitation of MG-H1 protein adducts in ARPE-19 cells that had accumulated A2E and were exposed to 430 nm light (17), lysates were prepared and ELISA was used as described earlier.
SI Results
Because the retinaldehyde double-bond isomer contributing to bisretinoid formation in the dark (Fig. 1) is uncertain, we examined trans- and cis-isomers of retinaldehyde for their ability to form the trans- and cis-isomers of A2E (2) when reacted with ethanolamine. As shown in Fig. S8, reaction of all-trans-retinal with ethanolamine generated two isomers: the more abundant (retention time, rt, 15.5 min) had an absorbance spectrum exhibiting two peaks (λmax 439 and 334 nm) and was attributable to all-trans-A2E (2) (Fig. S8A, product 1). The second, more hydrophobic isomer (Fig. S8A, product 2; rt, 16.8 min) exhibited a biphasic absorbance spectrum (λmax 428 and 334 nm) and is known from previous work to be iso-A2E, with a cis double bond at the C13–C14 position (2). A2E and iso-A2E were also observed at the same rt when 13-cis-retinal (Fig. S8B) and 11-cis-retinal (Fig. S8C) were reacted with ethanolamine. In the case of the 11-cis-retinal reaction, however, an additional smaller peak (Fig. S8C, product 3) was detected at rt 14.7 min. Given that the absorbance maxima (λ max 432 and 328 nm) were blue-shifted relative to A2E, as is the case for other cis-isomers, we assumed that this isomer (Fig. S8C, product 3) also contained a cis-double bond at the C11 position. The formation of both all-trans-A2E and 11-cis-A2E would be expected, as 11-cis-retinal is energetically unfavorable under ambient conditions and isomerizes to the all-trans form. With 9-cis-retinal as starting material neither all-trans-A2E nor iso-A2E were observed. Instead, two new isomers were observed (Fig. S8D, products 4 and 5), both exhibiting blue-shifted absorbances relative to all-trans-A2E. Because 9-cis-retinal is relatively stable, one of these isomers is likely 9-cis-A2E.
We calculated the percentage formation of each A2E isomer relative to the starting levels of retinaldehyde (Fig. S8, boxes on right). All-trans and 13-cis-retinaldehyde showed similar percentage conversion to total bisretinoids (one-way ANOVA and Tukey’s multiple comparisons test, P > 0.05), whereas the conversion from 11-cis-retinal was slightly lower than for all-trans and 13-cis-retinals (P < 0.05). In contrast, the 9-cis-retinal isomer afforded considerably less total bisretinoid (products 4 and 5) than with all-trans-retinal and the other cis-retinals (P < 0.05). The lower conversion rate of 9-cis-retinal could reflect steric hindrance. Finally, the yield of all-trans-A2E (product 1) was higher from all-trans-retinal than from 11-cis-retinal (P < 0.05), but was similar when either all-trans-retinal or 13-cis-retinal were the starting materials (P > 0.05).
Acknowledgments
Dr. Zhao Liu contributed to some experiments. This work was supported by grants from the National Eye Institute (EY12951 and P30EY019007), Foundation Fighting Blindness, and Research to Prevent Blindness to the Department of Ophthalmology, Columbia University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.S.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524774113/-/DCSupplemental.
References
- 1.Sparrow JR, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31(2):121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA. 1998;95(25):14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto K, Yoon KD, Ueda K, Hashimoto M, Sparrow JR. A novel bisretinoid of retina is an adduct on glycerophosphoethanolamine. Invest Ophthalmol Vis Sci. 2011;52(12):9084–9090. doi: 10.1167/iovs.11-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J Biol Chem. 2009;284(30):20155–20166. doi: 10.1074/jbc.M109.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allikmets R, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 6.Vasireddy V, et al. Elovl4 5-bp deletion knock-in mouse model for Stargardt-like macular degeneration demonstrates accumulation of ELOVL4 and lipofuscin. Exp Eye Res. 2009;89(6):905–912. doi: 10.1016/j.exer.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassmann F, Fauser S, Weber BH. The genetics of age-related macular degeneration (AMD) - Novel targets for designing treatment options? Eur J Pharm Biopharm. 2015;95(Pt B):194–202. doi: 10.1016/j.ejpb.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274(12):8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 9.Weng J, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98(1):13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim SR, et al. Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E. Proc Natl Acad Sci USA. 2004;101(32):11668–11672. doi: 10.1073/pnas.0403499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283(39):26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda A, Golczak M, Maeda T, Palczewski K. Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest Ophthalmol Vis Sci. 2009;50(11):5435–5443. doi: 10.1167/iovs.09-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrispell JD, et al. Rdh12 activity and effects on retinoid processing in the murine retina. J Biol Chem. 2009;284(32):21468–21477. doi: 10.1074/jbc.M109.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radu RA, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008;49(9):3821–3829. doi: 10.1167/iovs.07-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, Nagasaki T, Sparrow JR. Photoreceptor cell degeneration in Abcr (-/-) mice. Adv Exp Med Biol. 2010;664:533–539. doi: 10.1007/978-1-4419-1399-9_61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparrow JR, et al. Quantitative fundus autofluorescence in mice: Correlation with HPLC quantitation of RPE lipofuscin and measurement of retina outer nuclear layer thickness. Invest Ophthalmol Vis Sci. 2013;54(4):2812–2820. doi: 10.1167/iovs.12-11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Ueda K, Zhao J, Sparrow JR. Correlations between photodegradation of bisretinoid constituents of retina and dicarbonyl-adduct deposition. J Biol Chem. 2015;290(45):27215–27227. doi: 10.1074/jbc.M115.680363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rózanowska M, et al. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270(32):18825–18830. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard ER, Atherton SJ, Eldred G, Dillon J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol. 1995;61(5):448–453. doi: 10.1111/j.1751-1097.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Shabat S, et al. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed Engl. 2002;41(5):814–817. doi: 10.1002/1521-3773(20020301)41:5<814::aid-anie814>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Kim SR, et al. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc Natl Acad Sci USA. 2007;104(49):19273–19278. doi: 10.1073/pnas.0708714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J Biol Chem. 2005;280(48):39732–39739. doi: 10.1074/jbc.M504933200. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc Natl Acad Sci USA. 2010;107(16):7275–7280. doi: 10.1073/pnas.0913112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handa JT, et al. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci. 1999;40(3):775–779. [PubMed] [Google Scholar]
- 25.Crabb JW, et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nusinowitz S, et al. Electroretinographic evidence for altered phototransduction gain and slowed recovery from photobleaches in albino mice with a MET450 variant in RPE65. Exp Eye Res. 2003;77(5):627–638. doi: 10.1016/s0014-4835(03)00217-3. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg TJTP, IJspeert JK, de Waard PWT. Dependence of intraocular straylight on pigmentation and light transmission through the ocular wall. Vision Res. 1991;31(7-8):1361–1367. doi: 10.1016/0042-6989(91)90057-c. [DOI] [PubMed] [Google Scholar]
- 28.Mata NL, et al. Delayed dark-adaptation and lipofuscin accumulation in abcr+/- mice: Implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42(8):1685–1690. [PubMed] [Google Scholar]
- 29.Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21(1):53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparrow JR, et al. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J Biol Chem. 2003;278(20):18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Ueda K, Nagasaki T, Sparrow JR. Light damage in Abca4 and Rpe65rd12 mice. Invest Ophthalmol Vis Sci. 2014;55(3):1910–1918. doi: 10.1167/iovs.14-13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penn JS, Williams TP. Photostasis: Regulation of daily photon-catch by rat retinas in response to various cyclic illuminances. Exp Eye Res. 1986;43(6):915–928. doi: 10.1016/0014-4835(86)90070-9. [DOI] [PubMed] [Google Scholar]
- 33.Bird AC, et al. The International ARM Epidemiological Study Group An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5):367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 34.Hao W, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32(2):254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- 35.Organisciak DT, Vaughan DK. Retinal light damage: Mechanisms and protection. Prog Retin Eye Res. 2010;29(2):113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter JJ, et al. The susceptibility of the retina to photochemical damage from visible light. Prog Retin Eye Res. 2012;31(1):28–42. doi: 10.1016/j.preteyeres.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda T, et al. QLT091001, a 9-cis-retinal analog, is well-tolerated by retinas of mice with impaired visual cycles. Invest Ophthalmol Vis Sci. 2013;54(1):455–466. doi: 10.1167/iovs.12-11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan J, Rohrer B, Moiseyev G, Ma JX, Crouch RK. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc Natl Acad Sci USA. 2003;100(23):13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyer NP, et al. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: Their origin is 11-cis-retinal. J Biol Chem. 2012;287(26):22276–22286. doi: 10.1074/jbc.M111.329235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quazi F, Molday RS. ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal. Proc Natl Acad Sci USA. 2014;111(13):5024–5029. doi: 10.1073/pnas.1400780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terman JS, Remé CE, Terman M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605(2):256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- 42.Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Cruz PM, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9(4):645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275(38):29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Shabat S, et al. Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J Biol Chem. 2002;277(9):7183–7190. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- 46.Bravo-Nuevo A, Walsh N, Stone J. Photoreceptor degeneration and loss of retinal function in the C57BL/6-C2J mouse. Invest Ophthalmol Vis Sci. 2004;45(6):2005–2012. doi: 10.1167/iovs.03-0842. [DOI] [PubMed] [Google Scholar]
- 47.Sparrow JR, Duncker T. Fundus autofluorescence and RPE lipofuscin in age-related macular degeneration. J Clin Med. 2014;3(4):1302–1321. doi: 10.3390/jcm3041302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomany SC, Cruickshanks KJ, Klein R, Klein BEK, Knudtson MD. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol. 2004;122(5):750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- 49.McCarty CA, et al. Risk factors for age-related maculopathy: The Visual Impairment Project. Arch Ophthalmol. 2001;119(10):1455–1462. doi: 10.1001/archopht.119.10.1455. [DOI] [PubMed] [Google Scholar]
- 50.Hyman LG, Lilienfeld AM, Ferris FL, 3rd, Fine SL. Senile macular degeneration: A case-control study. Am J Epidemiol. 1983;118(2):213–227. doi: 10.1093/oxfordjournals.aje.a113629. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher AE, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008;126(10):1396–1403. doi: 10.1001/archopht.126.10.1396. [DOI] [PubMed] [Google Scholar]
- 52.Schick T, et al. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina. 2015;36(4):787–790. doi: 10.1097/IAE.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 53.Sui GY, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97(4):389–394. doi: 10.1136/bjophthalmol-2012-302281. [DOI] [PubMed] [Google Scholar]
- 54.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maiti P, et al. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2006;45(3):852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 56.Maeda A, et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2011;8(2):170–178. doi: 10.1038/nchembio.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radu RA, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: A potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46(12):4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Zhou J, Fishkin N, Rittmann BE, Sparrow JR. Enzymatic degradation of A2E, a retinal pigment epithelial lipofuscin bisretinoid. J Am Chem Soc. 2011;133(4):849–857. doi: 10.1021/ja107195u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nociari MM, et al. Beta cyclodextrins bind, stabilize, and remove lipofuscin bisretinoids from retinal pigment epithelium. Proc Natl Acad Sci USA. 2014;111(14):E1402–E1408. doi: 10.1073/pnas.1400530111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobri N, et al. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Invest Ophthalmol Vis Sci. 2013;54(1):85–95. doi: 10.1167/iovs.12-10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teussink MM, et al. The effect of light deprivation in patients with Stargardt disease. Am J Ophthalmol. 2015;159(5):964–972. doi: 10.1016/j.ajo.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 62.De Bandt M, et al. Vitamin E uncouples joint destruction and clinical inflammation in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2002;46(2):522–532. doi: 10.1002/art.10085. [DOI] [PubMed] [Google Scholar]