Significance
In evolved species, resisting the temptation of immediate rewards is a critical ability for the achievement of long-term goals. This self-control ability was found to rely on the lateral prefrontal cortex (LPFC), which also is involved in executive control processes such as working memory or task switching. Here we show that self-control capacity can be altered in healthy humans at the time scale of a workday, by performing difficult executive control tasks. This fatigue effect manifested in choice impulsivity was linked to reduced excitability of the LPFC following its intensive utilization over the day. Our findings might have implications for designing management strategies that would prevent daylong cognitive work from biasing economic decisions.
Keywords: decision-making, temporal discounting, fatigue, executive control, fMRI
Abstract
The ability to exert self-control is key to social insertion and professional success. An influential literature in psychology has developed the theory that self-control relies on a limited common resource, so that fatigue effects might carry over from one task to the next. However, the biological nature of the putative limited resource and the existence of carry-over effects have been matters of considerable controversy. Here, we targeted the activity of the lateral prefrontal cortex (LPFC) as a common substrate for cognitive control, and we prolonged the time scale of fatigue induction by an order of magnitude. Participants performed executive control tasks known to recruit the LPFC (working memory and task-switching) over more than 6 h (an approximate workday). Fatigue effects were probed regularly by measuring impulsivity in intertemporal choices, i.e., the propensity to favor immediate rewards, which has been found to increase under LPFC inhibition. Behavioral data showed that choice impulsivity increased in a group of participants who performed hard versions of executive tasks but not in control groups who performed easy versions or enjoyed some leisure time. Functional MRI data acquired at the start, middle, and end of the day confirmed that enhancement of choice impulsivity was related to a specific decrease in the activity of an LPFC region (in the left middle frontal gyrus) that was recruited by both executive and choice tasks. Our findings demonstrate a concept of focused neural fatigue that might be naturally induced in real-life situations and have important repercussions on economic decisions.
Shall I have a beer with my friends tonight, or shall I save money to buy a bike next month? This sort of intertemporal choice, between immediate and delayed rewards, is critical for strategic foraging in evolved species and has been intensively scrutinized by behavioral ecologists (1). Such choices depend not only on the options themselves (rewards and delays), but also on extraneous factors that may vary across individuals and contexts. In humans, the capacity to resist the temptation of immediate rewards at a young age is a key predictor of long-term life outcomes, as was shown with the famous marshmallow test (2). At the neural level, this capacity for self-control is associated with the recruitment of regions in the lateral prefrontal cortex (LPFC) (3–5). Conversely, LPFC inhibition through transcranial magnetic stimulation (TMS) was shown to enhance the propensity to make impulsive choices, i.e., to favor immediate rewards (6, 7). In the present study we address the question of whether alteration in LPFC activity could occur in situations encountered in everyday life and bias decisions toward impulsive behavior.
Taking inspiration from the demonstration that judicial decisions drift with time on task over a workday (8), we assessed the impact of hard cognitive work on intertemporal choice. We opted for executive control processes, namely working memory and task switching, because they typically elicit activity in LPFC regions that overlap with those implicated in intertemporal choice (9–11). When prolonged over time, executive processing can induce mental fatigue, which manifests by decreased LPFC activity (12, 13). To our knowledge, whether such decrease in LPFC activity has repercussions on economic decisions in the long run has not yet been investigated.
However, there is a literature in psychology suggesting that the capacity for self-control relies on a limited resource that is common to various cognitive processes. Therefore, prior exertion of self-control should impair the ability to perform subsequent unrelated tasks that also require self-control, a phenomenon coined “ego depletion” (14). Evidence for this phenomenon comes from numerous studies using sequential task paradigms, with a first “depleting task” affecting a second “dependent task” (15, 16). However, this literature remains controversial because replication failures have been reported occasionally (e.g., ref. 17) and probably were underestimated because of publication bias, as suggested by statistical meta-analyses (18). We conjectured that one reason for these discrepant results might be that the time scale used in depletion studies (typically around 10 min) might be too short to alter prefrontal cortex functioning significantly. Following our own failures to obtain fatigue effects at short time scales (less than 1 h), we decided to change our approach and examine the impact of executive control performance lasting for the approximate duration of a workday (i.e., more than 6 h of effective work, excluding breaks).
To reveal this impact, we inserted intertemporal choice trials between blocks of executive task trials (every minute on average). On the day before the experiment, subjects were trained on the executive tasks so that they started the experiment with a performance level exceeding 90% correct trials, and the intertemporal choice task was calibrated so that they started around indifference points (50% impulsive choices). Executive tasks were administered with two levels of difficulty in separate groups of healthy participants, to vary the intensity of LPFC activation while keeping the duration constant. We predicted that daylong performance of hard executive tasks (but not easy ones) would enhance the propensity to favor immediate rewards (i.e., to make impulsive choices). To unravel the underlying neural mechanisms, subjects were scanned using functional MRI (fMRI) at the beginning, middle, and end of the daylong experiment. Our predictions regarding brain activity were that (i) increasing difficulty of executive control would recruit the LPFC regions also involved in intertemporal choice; (ii) activity in these regions would decrease over the workday in the hard condition but not in the control condition; and (iii) this decrease in LPFC activity would mediate the effect of hard executive work on intertemporal choice.
Results
In SI Experiment S1 and Fig. S1, we report one example of our attempts to obtain fatigue effects at a short time scale. Here we report the results of the main experiment illustrated in Fig. 1. For comparison with the group that performed hard versions of executive tasks throughout the day, we implemented two kinds of control conditions in separate groups: in the first one, the easy condition, we simply made the tasks less difficult; in the second, the leisure condition, we relaxed the obligation to perform any task except in test sessions at the start, middle, and end of the day. These two control conditions were implemented to isolate both the effects of executive difficulty and the effects of being forced to perform a boring task. However, because no difference was observed between control groups, their data were pooled together in the analyses described hereafter, unless otherwise specified.
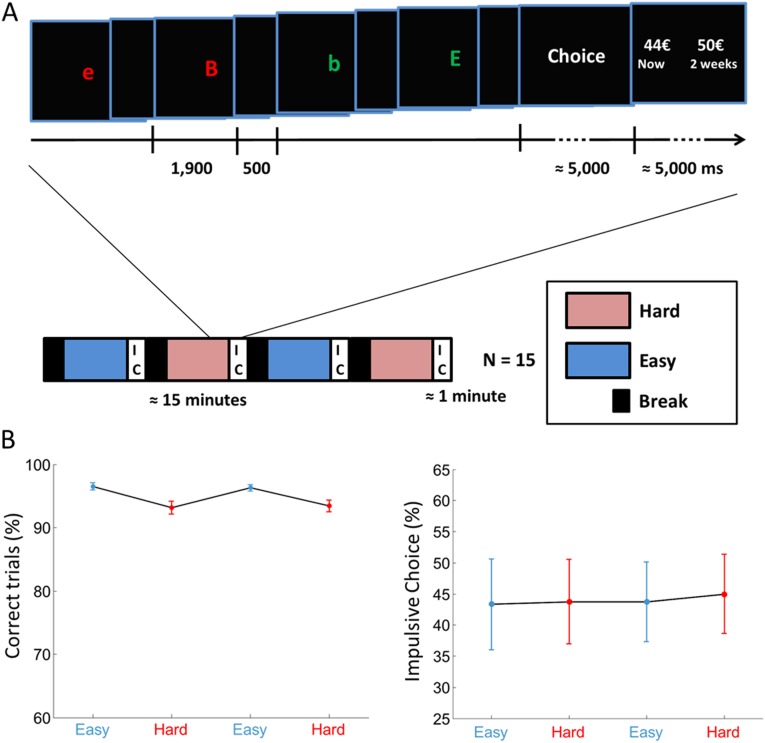
Fig. S1.
Experiment S1. (A) Experimental design. All participants (15 healthy adults) performed four 15-min sessions of an executive task (switching between discrimination tasks depending on a contextual cue), each followed immediately by a 1-min series of 16 intertemporal choices (IC). (Lower) Easy and hard versions of task switching (one versus 105 switches in a session) were always alternated in the order shown on the timeline and were separated by a 1-min break. (Upper) For the executive task a series of different letters was displayed on screen, each starting a new trial. Participants had to categorize the current letter into either vowel versus consonant or uppercase versus lowercase, depending on its color. At the end of the session participants made self-paced choices between immediate and delayed monetary rewards. (B) Behavioral results. Graphs show the sessionwise percentage of correct trials in the executive task (Left) and the percentage of impulsive choices, defined as the choice of immediate reward (Right). Dots represent means, and error bars are intersubject SEs. There was no difference in choice impulsivity between sessions or between difficulty levels.
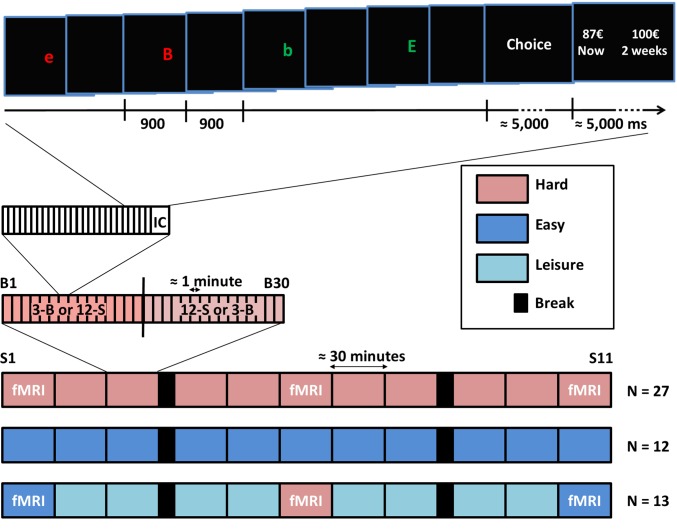
Fig. 1.
Overview of experimental procedures. Participants (58 healthy adults in total) were divided into three groups, corresponding to the three different experimental conditions shown by the horizontal bars at the bottom of the figure. Each group performed a series of 30-min sessions (S1–S11) of executive tasks intermingled with intertemporal choices (IC), for a total of about 6 h with two 10-min breaks (black rectangles) after S3 and S8. Executive tasks were 3-back (3-B) and 12-switch (12-S) in the hard condition or one-back (1-B) and one-switch (1-S) in the easy condition. A subset of participants (n = 16 in the hard group and n = 13 in the leisure group) was scanned using fMRI during S1, S6, and S11. In the leisure condition participants read or played video games between fMRI sessions. In this group executive tasks were easy in S1 and S11 but were hard in S6. Each session was divided into 30 blocks (B1 to B30), with the first 15 blocks implementing one executive task (N-back or N-switch) and the last 15 blocks implementing the other one. All tasks presented a series of different letters on screen, each starting a new trial, as shown at the top of the figure. The task to be performed was stated at the beginning of the block. In N-back tasks, participants indicated whether the current letter was the same as the one presented N trials before (irrespective of case and color). In N-switch tasks, participants categorized the current letter into either vowel versus consonant or into uppercase versus lowercase, depending on its color. N designates the number of switches (color changes) during the block. At the end of the block participants made a self-paced choice between immediate or delayed monetary rewards.
Behavioral Results.
To test for fatigue effects across sessions, we fitted three embedded models to our two dependent variables: impulsive choices (the proportion of trials in which the immediate reward was selected) and executive performance (the proportion of correct trials in N-back and N-switch tasks considered together). The first model corresponds to the null hypothesis (no fatigue effect) and included only a constant; the second model added a linear effect of session; and the third model included both linear and power effects (see Materials and Methods for equations). Group (hard versus control) was included as an additional factor so that the model space contained nine models (three possible models for the hard condition × three possible models for the control condition).
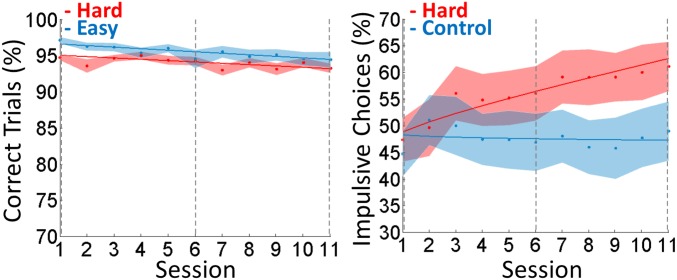
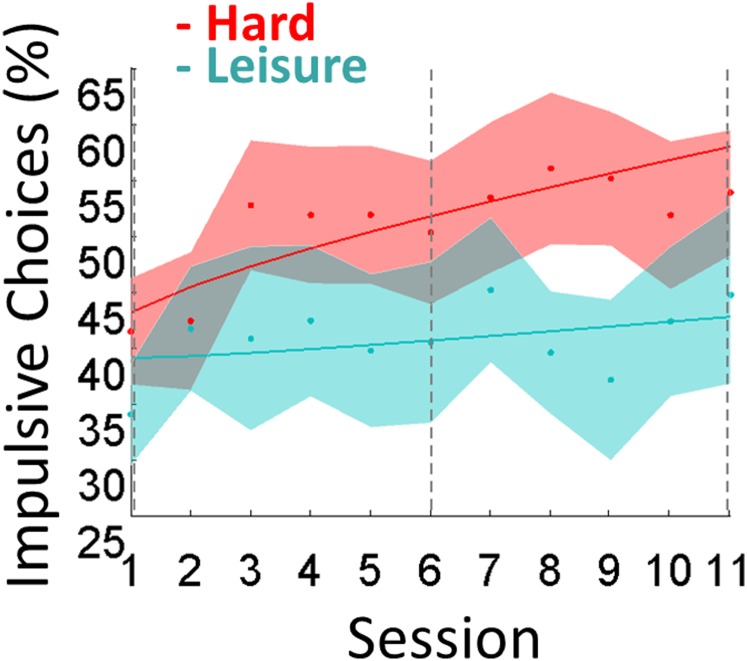
Regarding impulsive choice (Fig. 2, Right), Bayesian model selection (BMS) indicated that the family in which fatigue functions were the same in both groups was much more plausible than the others [exceedance probability (EP) = 1, expected frequency (EF) = 0.98]. The best fatigue function across groups included both linear and power effects (EP = 0.97, EF = 0.61; see fitted parameters in Table S1). Critically, the linear parameter (β) was significantly positive in the hard condition but not in the control condition, with a significant difference between the two (hard: β = 0.018 ± 0.006, Student t26 = 3.01, P = 0.003; control: β = 0.002 ± 0.007, t24 = 0.25, P = 0.41; difference: t50 = 1.80, P = 0.039, one-tailed). The distributions of change in choice impulsivity in both conditions are illustrated in Fig. S3. The increase in choice impulsivity was not linked to individuals being more or less impulsive at baseline, because it was not correlated across subjects to the log-transformed discount rate estimated during the calibration procedure (Pearson r15 = 0.011, P = 0.96). We checked that within the control condition there was no difference in this critical parameter between the easy and leisure groups (easy: β = 0.007 ± 0.013, t12 = 0.058, P = 0.95; leisure: β = 0.003 ± 0.007, t11 = 0.39, P = 0.70; difference: t23 = 0.13, P = 0.90). The power parameter (α) was significantly different from one in the hard condition but not in the control condition, but there was no significant difference between the two (hard: α = 0.78 ± 0.06, t26 = −3.38, P = 0.002; control: α = 0.90 ± 0.06, t24 = −1.58, P = 0.13; difference: t50 = 1.30, P = 0.10). By design the constant parameter (C) was near the indifference point (50%) in both conditions, with no difference between the two (hard: C = 0.44 ± 0.05, t26 = −1.06, P = 0.30; control: C = 0.48 ± 0.05, t24 = −0.51, P = 0.62; difference: t50 = −0.45, P = 0.66). Note that the increase in choice impulsivity was not related to any change in choice response time, for which we found no effect of session number in either the hard or in the control condition.
Fig. 2.
Behavioral results. Graphs show the sessionwise percentage of correct trials in executive tasks, pooling N-back and N-switch results together (Left), and the percentage of impulsive choices, defined as the choice of immediate reward (Right). Control curves in blue illustrate either the easy condition alone (for executive performance) or easy and leisure conditions pooled together (for impulsive choice). Dots represent means, and shaded areas represent intersubject SEs. Vertical dotted lines indicate sessions performed in the MRI scanner. Continuous lines show model fits (regression against fatigue function including linear and power effects). Regression slopes differ significantly between hard and control conditions for impulsive choices but not for executive performance. A similar interaction between session and condition was observed in impulsive choice when the analysis was restricted to scanned subjects only (Fig. S2).
Table S1.
Tests on fitted model parameters for choice impulsivity
| Parameters | M | SE | t | df | P |
| Constant control | 0.48 | 0.050 | −0.51 | 24 | 0.62 |
| Constant hard | 0.44 | 0.052 | −1.1 | 26 | 0.30 |
| Constant difference | −0.032 | 0.035 | −0.45 | 50 | 0.66 |
| Linear control | 0.020 | 0.070 | 0.25 | 24 | 0.41 |
| Linear hard | 0.18 | 0.060 | 3.0 | 26 | 0.0028 |
| Linear difference | 0.16 | 0.046 | 1.8 | 50 | 0.039 |
| Power control | 0.90 | 0.060 | −1.6 | 24 | 0.13 |
| Power hard | 0.79 | 0.063 | −3.4 | 26 | 0.0023 |
| Power difference | −0.12 | 0.044 | −1.3 | 50 | 0.10 |
The table gives the fitted parameters of the full fatigue model: , with Pim being the proportion of impulsive choices and S the session number. Constant, linear, and power parameters are respectively C, α, and β in the equation. Each parameter was tested against the null hypothesis (0.5 for C, 0 for β, and 1 for α) and was compared between hard and control conditions using t tests. M is overall mean and SE is intersubject SE.
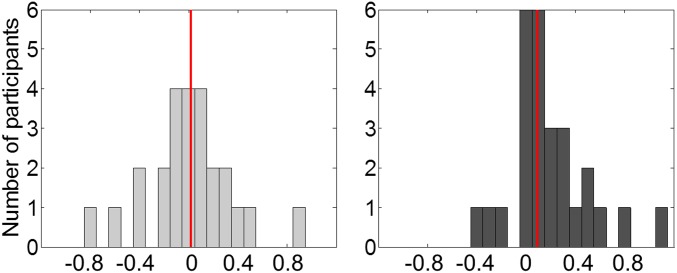
Fig. S3.
Distribution of session effect on choice impulsivity. Choice impulsivity is defined as the proportion of trials in which the immediate reward was chosen. Light- and dark-gray bars correspond to the number of participants falling in each bin of session effect (linear coefficient of the regression model) for the control and hard conditions, respectively. Vertical red lines indicate distribution medians.
Fig. S2.
Evolution of impulsive choice rate in scanned subjects. The figure is identical to the right panel of Fig. 2, except that only scanned subjects (leisure and hard condition) were included in data analysis. Graphs show the sessionwise percentage of impulsive choices, defined as the choice of an immediate reward. Vertical dotted lines indicate sessions performed in the MRI scanner. Continuous lines show model fits (regression against fatigue function including linear and power effects). The difference between regression slopes was marginally significant (P = 0.055) and was slightly higher (0.18 versus 0.16) than that observed in the pooled data presented in Fig. 2.
We conducted the same analysis on executive performance (Fig. 2, Left), although we had no specific hypothesis about how it should vary across sessions, because various opposite effects, such as increased fatigue, decreased motivation, or increased efficiency (through cognitive skill learning), have been reported (19). Family model comparison showed that the models using the same fatigue function in the hard and easy conditions were much more plausible (EP = 1, EF = 0.98) than the others. Among fatigue functions, the best was the constant model (EP = 0.95, EF = 0.61; see fitted parameters in Table S2). Note, however, that testing fitted parameters from the linear model revealed a marginally significant linear effect in both conditions, with no significant difference between the two (hard: β = −0.017 ± 0.008, t26 = −2.26, P = 0.032; easy: β = −0.012 ± 0.010, t24 = −1.89, P = 0.07; difference: 0.006 ± 0.005, t50 = −0.58, P = 0.72). However, this effect was tiny, explaining why the linear effect was not favored over the constant by BMS. The constant was largely above chance and was significantly different between the two levels of difficulty (hard: C = 0.95 ± 0.005, t26 = 84, P = 0; easy: C = 0.96 ± 0.0035, t24 = 132, P = 0; difference: −0.015 ± 0.003, t50 = −2.31, P = 0.025).
Table S2.
Tests on fitted model parameters for executive performance
| Parameters | M | SE | t | df | P |
| Constant control | 0.96 | 0.0035 | 13 × 10 | 24 | 7.8 × 10–36 |
| Constant hard | 0.95 | 0.0053 | 84 | 26 | 2.9 × 10–33 |
| Constant difference | −0.015 | 0.0032 | −2.3 | 50 | 0.025 |
| Linear control | −0.012 | 0.0061 | −1.9 | 24 | 0.071 |
| Linear hard | −0.017 | 0.0077 | −2.3 | 26 | 0.032 |
| Linear difference | 0.96 | 0.0050 | −0.58 | 50 | 0.56 |
The table gives the fitted parameters of the linear model, which is obtained by taking α to 1 in the full model. Each parameter was tested against the null hypothesis (0.5 for C, 0 for β, and 1 for α), and the hard and control conditions were compared using t tests. M is overall mean and SE is intersubject SE.
fMRI Results.
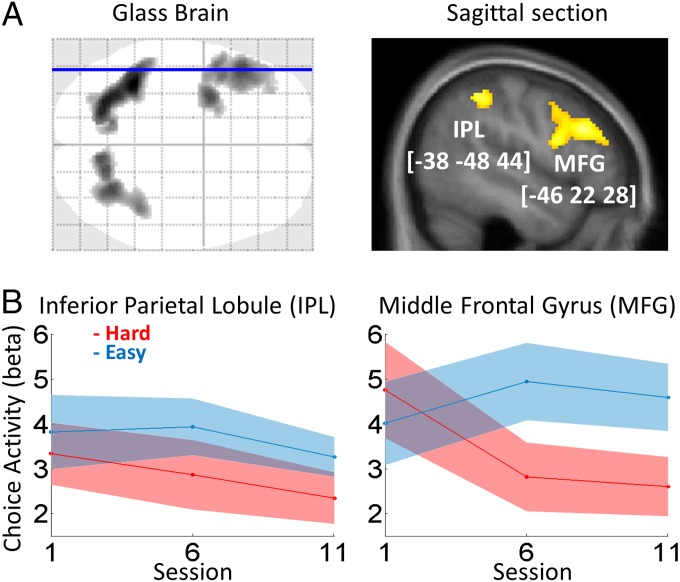
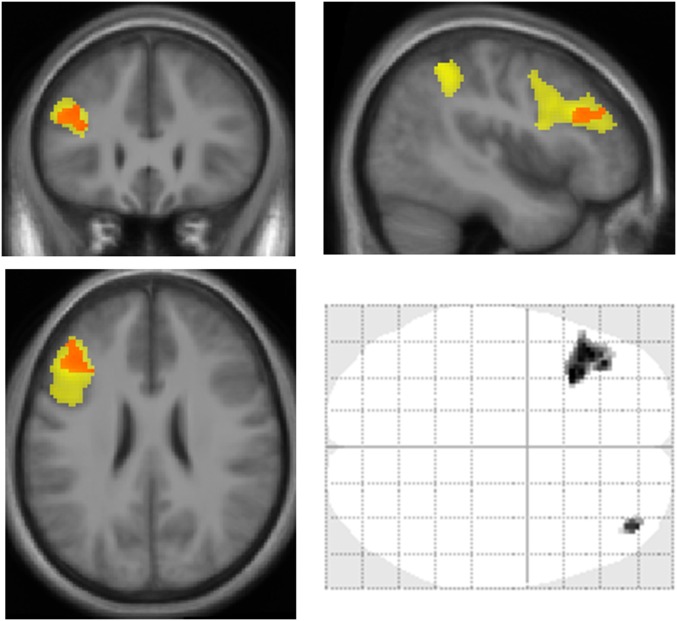
To test our first prediction that the brain regions recruited when cognitive difficulty is increased are also involved in intertemporal choice (Fig. 3A and Table S3), we performed a conjunction analysis (logical and) between difficulty (hard minus easy versions of executive tasks) and choice (contrast against implicit baseline). As expected, this conjunction yielded significant activation (P < 0.05 after clusterwise correction for multiple comparisons) in the LPFC [middle frontal gyrus (MFG), Broca’s area (BA) 46/9] as well as in the dorsal parietal cortex [inferior parietal lobule (IPL), BA 40]. The MFG and IPL clusters were significantly activated in both tasks, with a trend for higher difficulty effect in N-back than in N-switch tasks. These two regions of interest (ROIs) thus were defined using a criterion that was orthogonal from the effect of session number, which we analyze below to assess neural fatigue.
Fig. 3.
fMRI results. (A) Conjunction between choice-related activity (against baseline) and effect of difficulty (hard versus easy version of executive tasks, i.e., 3-back and 12-switch versus one-back and one-switch). Statistical maps show significant activation in a dorsal parieto-prefrontal network including the MFG and IPL. The voxelwise threshold was P < 0.001 uncorrected, and the clusterwise threshold was P < 0.05 FWE corrected (corresponding to a cluster size of 315 voxels). The sagittal section corresponds to the blue line on the glass brain. It shows functional activations superimposed on anatomical scans averaged across subjects. The x, y, z coordinates refer to the MNI space. (B) Variations of neural activity across experimental sessions. Graphs show means and intersubject SEs of regression estimates (β) extracted from the ROIs, which were obtained from group-level activations shown on the maps above. Regression estimates correspond to neural activity observed during choices with respect to the baseline. Red curves correspond to the group that performed hard versions of executive tasks throughout the experiment. Blue curves correspond to the leisure group, which performed easy tasks in the first and last sessions and hard tasks in the intermediate session (and played video games or read between fMRI sessions). A significant interaction between group and session was observed in the MFG but not in the IPL.
Table S3.
Conjunction between difficulty and choice contrasts
| Anatomical label | Peak T | Peak uncorrected, P | Cluster FWE, P | No. of voxels | Peak MNI coordinates | ||
| x | y | z | |||||
| Inferior parietal lobule | 5.84 | 0.000000031 | 0.0000028 | 1,535 | −38 | −48 | 44 |
| Middle frontal gyrus | 4.90 | 0.0000018 | 0.0000017 | 1,720 | −46 | 22 | 28 |
| Precuneus | 4.88 | 0.0000018 | 0.000021 | 1,162 | 30 | −62 | 40 |
All clusters that survived P < 0.05 after FWE correction for multiple comparisons (minimum size of 319 voxels) are listed. The voxelwise threshold was P < 0.001, uncorrected.
To test our second prediction, that neural activity in common regions should parallel the fatigue effects observed in behavior (Fig. 3B), we searched for interactions between session number and condition (hard versus control). In the MFG, choice-related activity decreased in the hard condition but not in the control condition, with a significant difference between the two effects of session number (hard: β = −1.07 ± 0.40, t15 = −2.68, P = 0.017; easy: β = 0.29 ± 0.48; t12 = 0.60, P = 0.56; difference: −1.36 ± 0.31, t27 = −2.19, P = 0.038). In the IPL, choice-related activity did not decrease in either the hard or easy condition, with no significant difference between session effects (hard: β = −0.49 ± 0.39, t15 = −1.27, P = 0.22; control: β = −0.28 ± 0.47; t12 = −0.59, P = 0.56; difference: −0.22 ± 0.30 t27 = −0.36, P = 0.72).
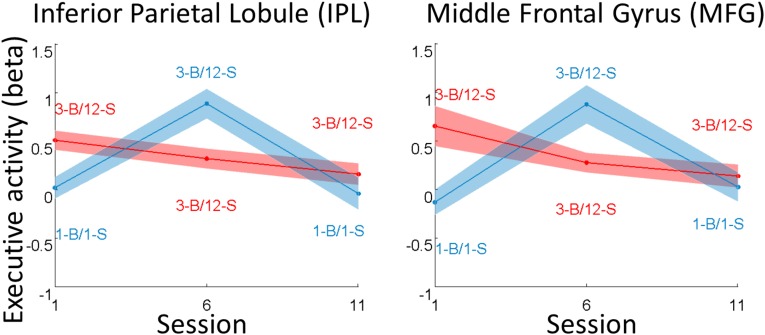
We searched for other brain regions that would decrease their activity across sessions with an exploratory whole-brain analysis using a liberal threshold (P < 0.01 uncorrected at the voxel level). The only significant cluster was located in the MFG (Fig. S4 and Table S4) and overlapped with the cluster activated in the conjunction between choice and difficulty. Note that our general linear model (GLM) was able to dissociate between choice- and task-related activities (Fig. S5), which followed distinct patterns across sessions in the control condition (only task-related activity increased in the intermediate session, because of the increase in task difficulty).
Fig. S4.
Whole-brain statistical maps of decreasing activity with session number. Clusters surviving a liberal voxelwise threshold of P < 0.01 uncorrected, with a minimum of 33 voxels corresponding to smoothing Gaussian kernel appear in red on brain sections and in black on the glass brain (Lower Left). The yellow patch illustrates the MFG cluster activated in the conjunction between difficulty and choice contrasts (as in Fig. 3A).
Table S4.
Decrease in choice-related activity with session number
| Anatomical label | Peak T | Peak uncorrected, P | Cluster uncorrected, P | No. of voxels | Peak MNI coordinates | ||
| x | y | z | |||||
| Middle frontal gyrus | 3.2 | 0.0016 | 0.055 | 281 | −32 | 20 | 20 |
| Middle frontal gyrus | 3.0 | 0.0026 | 0.45 | 40 | 40 | 52 | 4 |
All voxels that survived P < 0.01 uncorrected, with a minimum number of 33 voxels in a cluster (corresponding to the smoothing Gaussian kernel) are listed.
Fig. S5.
Variations of task-related activity across sessions. Graphs show means and intersubject SEs of regression estimates (β) extracted from the ROIs, which were obtained from group-level activations shown in Fig. 3. Regression estimates correspond to neural activity observed during executive tasks with respect to the baseline. Red curves correspond to the group that performed hard versions of executive tasks throughout the experiment. Blue curves correspond to the leisure group, which performed easy tasks in the first and last sessions, hard tasks in the intermediate session, and played video games or read between fMRI sessions. When the first and last sessions are compared, the only significant difference was observed in the MFG (for the hard condition).
The preceding analyses were done on β estimates of categorical regressors that modeled choice- or task-related activity against an implicit baseline. Parametric regressors were also included to account for quantities that varied across choice trials (Materials and Methods), namely delay, immediate reward, choice (patient versus impulsive), and response time (as a proxy for choice difficulty). None of these parametric regressors yielded any significant activation at corrected threshold. However, it must be remembered that all choice options were taken near the indifference points, so there was little variation in discounted value across trials. We did not find any activity significantly related to the parametric regressor that accounted for the effect of block number within sessions. This result is consistent with our working hypothesis that cognitive fatigue is negligible at a short time scale.
Brain–Behavior Relationships.
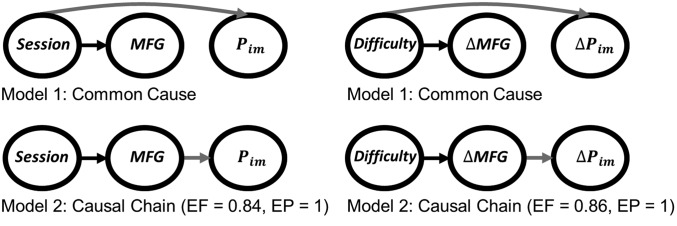
Finally, we examined whether the decrease in MFG choice-related activity mediates the impact of session number on choice impulsivity. To do so, we compared a causal chain model, in which session number impacts MFG activity, which in turn impacts choice impulsivity, and a common cause model, in which changes in both MFG activity and choice impulsivity are driven by session number (Fig. 4, Left). BMS designated the causal chain model as being significantly more plausible, given both neural and behavioral data in the hard condition (EF = 0.84, EP = 1). The causal chain model also is more plausible (EF = 0.86, EP = 1) if the control group is included in the comparison (Fig. 4, Right), indicating that the effect of cognitive difficulty on how choice impulsivity drifts over the workday is mediated by its effect on MFG activity.
Fig. 4.
Brain–behavior relationships. BMS was used to compare common cause models (Upper), in which brain and behavior are independently driven by experimental manipulations, and causal chain models (Lower), in which the behavioral effects are mediated by neural effects. Causal chain models appeared much more plausible, meaning that in the hard condition the effect of session number on choice impulsivity was mediated by MFG activity (Left) and that the effect of cognitive difficulty (hard versus control condition) on the change in choice impulsivity across sessions was mediated by the change in MFG activity (Right). The gray arrows emphasize differences between models. BMS results are expressed as EF and EP.
Discussion
Our findings suggest that exerting intense cognitive control over several hours might reduce the excitability of LPFC areas, which in turn would impair the capacity to resist the temptation of immediate rewards in intertemporal choices. Below we discuss the behavioral, neuroimaging, and theoretical aspects of these findings.
Choice impulsivity increased over the experiment when participants were required to perform an intense cognitive control task (3-back and 12-switch) but not when the same task was administered at a lower level of difficulty (one-back and one-switch) or when the participants engaged in activities (reading or gaming) that might require some control but were not imposed. Because we found no difference between the two control conditions (easy and leisure), we conclude that the decision bias relates to the intensity of cognitive control and not to the obligation to execute a boring task. On the one hand, this result fulfills the predictions of the ego depletion theory (14, 16), except that the time scale is significantly longer (by one order of magnitude) than usually considered. On the other hand, it was preceded by many failed attempts to observe the same phenomenon at a shorter time scale (see the example in SI Experiment S1), which add to the number of published and unpublished negative results (17, 18, 20, 21). In the main experiment, choice impulsivity did not increase significantly at the scale of blocks (within sessions), and a significant difference from chance level appeared only after eight sessions (4.5 h). Therefore choice impulsivity did not result from the need to split cognitive resources between executive and choice tasks, in which case it would have occurred from the beginning, as seen in dual-task paradigms (22). Thus, although our design departs from sequential-task paradigms, with the dependent task intermingled with the depleting tasks, our result nonetheless may be qualified as a carry-over effect, because fatigue affected economic choices only after sufficient time was spent on hard executive tasks.
It has been shown that the ego depletion phenomenon critically depends on whether participants believe that willpower is a limited resource (23, 24). However, beliefs in a limited strength model would affect performance (or persistence) only in executive tasks; there is no reason it should favor one option over the other in economic choice, unless subjects guess the aim of the experiment. For this reason, we carefully debriefed our participants and excluded from the analyses the only participant who guessed what the effect of interest was. Note that the participants were not aware of other participants following the same procedure with a different level of difficulty, so each participant could imagine a priori that we expected an increase in choice impulsivity; however, except for the one excluded subject, none did so. Our dependent task (intertemporal choice) has the interesting property that there is no correct or incorrect answer, as was explained to participants, who are simply asked to state their preference, knowing that one of their choices would actually be implemented. This property might explain why fatigue effects were much more striking in choice impulsivity than in executive performance, in terms of both effect size and P value (P < 0.003 versus P < 0.032 in the hard condition). One might assume that some compensatory brain mechanism was recruited (25) in order to maintain executive performance (around 95% correct on average, even after 6 h), and hence the related financial payoff. By contrast, economic choices might have drifted because they were not a measure of performance but only an expression of preference, such that no compensatory mechanism needed to be implemented.
However, the analysis of fMRI data showed no brain region with increased activity across experimental sessions. The only effect of session number was a decrease in activity that was specific to the brain region involved in both hard executive control and intertemporal choice, namely the MFG. This region is typically activated, with a predominance on the left side when using verbal stimuli, by tasks assessing working memory such as 3-back, which involves updating and maintaining representations in the absence of stimuli so as to control the behavioral response (10), and in tasks assessing contextual control, which involves adjusting the behavioral response to a mapping rule that is not directly specified by the stimulus (9). The left MFG also emerged as the main intersection between working memory and delay-discounting networks in a recent meta-analysis (11). Our MFG cluster [Montreal Neurological Institute (MNI) peak coordinates: −46, 22, 28] overlaps with the target (center: −36, 30, 27) of a previous study that applied TMS to the MFG and also observed an enhanced propensity to choose immediate rewards (6). However, we do not draw general conclusions about laterality, because there also was decreased activity in the right MFG in our experiment and because TMS to the right LPFC also biased choices toward immediate rewards in another study (7). Interestingly, only the MFG exhibited the expected pattern, i.e., activation in the conjunction between choice and difficulty and a decrease with session number specifically in the hard condition. In particular, the cluster in the IPL that also was activated in the conjunction did not show any fatigue effect. Thus, it appears that within the prefronto-parietal network classically involved in working memory and contextual control, only the MFG was susceptible to fatigue effects on economic choices.
Perhaps surprisingly, the MFG cluster was not activated by the contrast between patient and impulsive choice, contrary to observations in some previous studies (5, 26). This result might come from a limitation of our design. With the aim of maximizing sensitivity to fatigue effects, we kept choice options around indifference points, so that subjects started by making random choices, leaving room for an increase or decrease in choice impulsivity. Because choices were too noisy, it was impossible to fit a discounting model and provide a computational characterization of fatigue effects. However, our null result might help sort out different hypotheses that were suggested regarding the role of the LPFC in intertemporal choice (3, 26–28). More specifically, the increase in choice impulsivity could arise from a failure to inhibit the selection of immediate rewards (6) or from a change in the relative valuation of choice options (7). At indifference points, the first hypothesis, that an increase in impulsivity arises from a failure to inhibit the selection of immediate rewards, implies that the LPFC should be more active when patient choices are made. In the second hypothesis, the influence of the LPFC is limited to making the two option values similar but not in determining which option is eventually selected, so the same LPFC activity could occur in both impulsive and patient choices. Another consequence of the MFG being equally active in patient and impulsive choices is that the fMRI result cannot be considered as a trivial reflection of the behavioral result (i.e., increased frequency of impulsive choice).
The link between MFG activity and impulsive choice nonetheless was established across subjects through model comparison, which favored the hypothesis that MFG activity mediated the effect of fatigue on choice behavior, rather than the possibility that MFG activity and choice impulsivity were independently driven by a common cause, i.e., the fatigue linked to the time spent on hard executive tasks. In other words, MFG activity was a better predictor of choice impulsivity than experimental factors such as group (hard versus control) or session number (beginning to end). Thus, participants who were more susceptible to reducing their MFG activity during choice also were more prone to enhancing their impulsivity level, presumably because the value of immediate rewards was not sufficiently down-regulated or because the value of delayed rewards was not sufficiently emphasized. This finding does not mean that choices were processed in a more automatic manner in later sessions, because there was no change in choice response time.
Although we identified a neural mechanism underlying the effect of cognitive fatigue on choice impulsivity, our experiment could not provide explanations for why LPFC activity should decrease. Note that here we use the term “fatigue” for convenience, but we have not established that the neural and behavioral manifestations correspond to what participants would report as mental fatigue, and we remain open to different interpretations. A first explanation would be that LPFC activity is related to a limited resource that is depleted by its utilization (14). The main problem with this theory is specifying what the resource is at the biological level. Blood glucose has been proposed as a possible resource, with some supporting evidence (29, 30), but it was later shown than the beneficial effects of glucose ingestion on self-control were difficult to replicate and probably were more psychological than biological (24, 31–33). In any case, this hypothesis would not explain why only the MFG (and not, for instance, the IPL) is susceptible to fatigue effect. An alternative explanation would be that the decrease in LPFC represents a functional adaptation to the cost of executive control, which might be subjectively perceived as mental effort (34–36). This notion seems plausible, because left LPFC activity has been correlated with self-reported measures of cognitive costs and to cost-based decisions (37) and because the avoidance of cognitive cost was correlated across individuals with impulsivity in intertemporal choice (38). The issue in this hypothesis is explaining the origin of the cost. An interesting possibility is opportunity cost, associated with the notion that cognitive control capacity is limited and shared among many tasks, so that engaging cognitive modules in a specific task incurs the cost of losing the potential benefits of engaging the same modules in other tasks (34). However, why this opportunity cost should increase over time, i.e., why in our experiment the cost of engaging cognitive resources in intertemporal choice should increase across sessions, is unclear. To reconcile the depletion and adaptation hypotheses, one might speculate that the cost increases precisely because some biological alteration makes the LPFC harder to activate.
In conclusion, our findings provide a neural mechanism explaining why daylong intense cognitive work might increase impulsivity in economic decisions. We believe that such findings have applications in the domain of management, because the number and duration of work breaks could be adapted to avoid any dramatic LPFC dysfunction. If workers have control over their time schedule, they might spontaneously decide to take a break on the basis of some cost signal related to LPFC activity, as was shown with insular activity in the case of physical effort (39). Otherwise, LPFC dysfunction following overly intense cognitive work, with insufficient breaks, at longer time scales (weeks or months) might induce pathological conditions such as burnout syndromes.
Materials and Methods
Please see SI Materials and Methods for details on participants, behavioral tasks, fMRI data acquisition, and BMS.
General Design.
The study was approved by the ethics committee (Comité de Protection des Personnes) of the Pitié-Salpêtrière Hospital. All participants gave informed consent before partaking in the study. Participants came to the laboratory twice on two consecutive days: the first for training sessions on the executive tasks and calibration in the choice task and the second for the proper experiment. Correct performance in executive task was incentivized (1 cent per trial), and intertemporal choice was real, in that the chosen option in one pseudorandomly selected trial was actually implemented. During the experiment day each group performed 11 sessions of executive tasks, together lasting around 6 h. Each session lasted 30 min divided into a 13-min series of N-switch blocks and a 17-min series of N-back blocks. The task to be performed was indicated by a 5-s instruction screen presented at the beginning of each block. The length of blocks varied randomly between 16 and 32 trials (24 on average, duration = 43 s) for N-switch tasks and between 22 and 30 trials (26 on average, duration = 47 s) for N-back tasks. The order of N-switch and N-back tasks was counterbalanced across subjects. Every minute on average (at the end of blocks), another 5-s instruction screen told participants that they would have to make an intertemporal choice, giving a total of 30 choices per session. The options proposed in intertemporal choices were based on the results of a calibration session conducted on the training day.
fMRI data were acquired in a subset of 35 subjects (18 from the hard group and 16 from the leisure group) while they performed the executive tasks and the intermingled choice task. There were three fMRI sessions, at the beginning, middle, and end of the 6-h experiment (corresponding to sessions 1, 6, and 11). Participants in the easy and hard conditions performed only the easy or hard versions of the executive tasks, respectively. Participants in the leisure condition performed the tasks only during fMRI sessions; for this group the tasks were easy in the first and last sessions and were hard in the intermediate session. This approach provided a within-subject contrast, isolating the effects of cognitive difficulty (by subtracting the easy task-related activity from the hard task-related activity), so as to dissociate choice-related from task-related activity (only the latter should be influenced by the change in difficulty). During the other sessions the participants in the leisure group first entertained themselves by reading or playing video games and then performed a series of 30 intertemporal choices.
Behavioral Data Analysis.
Two dependent variables were analyzed: impulsive choices (the proportion of trials in which the immediate reward was selected) and executive performance (the proportion of correct trials in N-back and N-switch tasks pooled together). We did not fit a delay discounting model to choices because options were too close to indifference points, so that rewards and delays had little weight on decisions. Instead we fitted models of fatigue effects to sessionwise impulsivity levels, an approach that also could be used to account for executive performance. Because we had no a priori notions about the shape of fatigue functions, we tried three embedded models, from the null hypothesis (no session effect) to nonlinear variations: model 1, Pim = C (no effect); model 2, Pim = C + β·Session (linear effect); model 3, Pim = C + β·Sessionα (linear and power effects), with Pim being the proportion of impulsive choices, C denoting a constant, β a linear regression weight, Session the session number (from 1 to 11), and α a power parameter. Different sets of parameters were used to fit the hard and control conditions. They were inverted by minimizing free energy, using a variational Bayes approach under the Laplace approximation (40, 41), as implemented in the VBA Matlab toolbox (42), available at mbb-team.github.io/VBA-toolbox/). We normalized the dependent and explanatory variables (both proportion of impulsive choice and session number varied between 0 and 1), so that we could use means corresponding to the null hypothesis (0.5 for C, 0 for β and 1 for α) and SDs equal to 1 as priors on free parameters. Note that as a consequence β should be multiplied by 11 to be directly interpreted as the weight of one session number.
We first compared model families to determine whether the same fatigue function was used in hard and control conditions. We then compared the three fatigue models within the chosen family. The fitted parameters of the winning model were tested against the null hypothesis and were compared between groups using two-tailed t tests (unless otherwise specified), depending on whether we had a priori hypothesis about the direction of effects. Note that models are embedded so as model 3 reduces to model 2 if α is not different from 1 and to model 1 if β is not different from 0.
fMRI Data Analysis.
To identify the regions involved in both executive tasks and intertemporal choices, we regressed the following GLM to subject-level preprocessed fMRI time series, each session being modeled separately (with a specific constant). A first categorical regressor was included to model blocks of executive task trials with a boxcar function. It was parametrically modulated by the block number within a session (to capture fatigue effects across blocks). A second categorical regressor was included to model choice trial onsets with a stick function. It was modulated by four parametric regressors including log-transformed delay (in months), immediate reward (in euros), choice (1 for patient, −1 for impulsive, 0 for no response), and response time. These parametric regressors were meant to capture the specificities of each particular trial, whereas the categorical regressor captured common processes involved in performing an intertemporal choice. All regressors of interest were convolved with a canonical hemodynamic response function. The GLM also included subject-specific realignment parameters to correct for motion artifacts, adding six regressors of noninterest.
Linear contrasts of regression estimates (β) were computed at the subject level and were taken to group-level random effect analysis. Subject-level contrasts were categorical regressors against implicit baseline: choice-related activity, hard task-related activity, and easy task-related activity. The two last sessions of the hard condition were not included because they were expected to be affected by cognitive fatigue. A conjunction analysis (logical and) was conducted at the group level between the difficulty contrast (1 on hard and −1 on easy task-related regressors) and the choice contrast (1 on choice-related regressors). Unless otherwise specified, activation maps were thresholded at both the voxel level (P < 0.001, uncorrected) and the cluster level (P < 0.05 after familywise error (FWE) correction for multiple comparison, corresponding to a minimum of 319 voxels).
ROIs were defined using the MarsBaR toolbox (43) as spheres of 8-mm diameter, centered on group-level activation peaks in the left MFG and left IPL clusters. Regression estimates (β) of subject-level GLM were extracted separately from the two ROI for the different sessions. These estimates of choice- and task-related activity then were regressed against session number, using robust regression tool implemented in Matlab. To test for fatigue effects, the slopes of this regression were compared with zero and between conditions using two-tailed t tests. We did not search for nonlinear fatigue effects because we only had three data points (first, middle, and last sessions) for choice- and hard task-related activity and two data points (first and last sessions) for easy task-related activity.
Brain–Behavior Relationships.
To support the idea that choice-related MFG activity mediated the effect of fatigue on choice impulsivity, we compared between the two following models in the hard condition: Pim = C1 + β1·Session and Pim = C2 + β2·MFG, with C1 and C2 being two different constants and Session 1, 2, or 3 for the first, middle, and last sessions. Indeed the critical difference between the common cause and causal chain models is whether to predict choices from session number or from MFG activity. To include the control group in the model selection and test whether the difficulty effect on choice was mediated by the difficulty effect on MFG, we also compared Pim = β1·Group with Pim = β2. MFG, with representing the change from first to last session and Group being an indicator of cognitive difficulty (1 for hard and 0 for leisure). Models were inverted and compared following the same BMS procedure used for behavioral data analysis.
SI Materials and Methods
Participants.
In total, 58 healthy adults participated in the study. They were divided into three groups corresponding to the three experimental conditions (Fig. 1): n = 29 (16 females, mean age = 24.4 ± 0.55 y) in the hard condition, n = 12 (eight females, mean age = 24.1 ± 0.72 y) in the easy condition, and n = 16 (nine females, mean age = 24.1 ± 0.68 y) in the leisure condition. All participants gave informed consent before partaking in the study. They were screened for exclusion criteria: left-handedness, age under 18 or above 39 y, regular use of drugs or medication, history of psychiatric or neurological illness, and contraindications to MRI scanning (pregnancy, claustrophobia, metallic implants). In addition to receiving a compensation of 50€ for their time, participants gained up to 50€ depending on their performance in the executive tasks and a variable amount randomly chosen from the options they selected in the choice task. The study was approved by the ethics committee (Comité de Protection des Personnes) of the Pitié-Salpêtrière Hospital.
Two participants in the hard group were excluded from all analyses because of technical issues. Three participants in the leisure group also were excluded, one because he guessed the aim of the experiment at debriefing and the other two because they did not push any button during fMRI sessions. Thus, behavioral analyses were conducted in n = 27 subjects for the hard condition, and n = 25 subjects in the control condition (results in the easy and leisure groups were pooled together, because they showed no difference in executive performance or choice impulsivity; see Results in the main text).
Executive Tasks.
All tasks were programmed on a personal computer using the Cogent 2000 (Wellcome Department of Imaging Neuroscience) library of Matlab functions for stimuli presentation.
Participants were told that each correct trial, regardless of reaction time, would be rewarded 1 cent (except for training sessions), yielding a total of about 50€ if they made no mistakes. On the day before the experiment, participants read the instructions and were trained to perform all versions of the tasks until they reached a performance criterion (four consecutive blocs above 90%) or until they reached a maximal duration of 3 h.
In both the N-back and N-switch tasks, letters appeared successively at the center of the screen. They could be vowels (e, a, i, o, u, y) or consonants (b, c, g, k, m, p), written in upper- or lowercase and colored either red or green. On every trial the letter was displayed for 900 ms, corresponding to the time window during which participants could give their response, followed by a blank screen lasting for 900 ms.
N-back.
For the N-back task, participants were instructed to indicate when the current letter was the same as the letter presented N trials before. The “yes” and “no” responses were given by pressing the left or right arrow on the keyboard (key-response associations being counterbalanced across participants). Difficulty was manipulated by changing N from one (easy version) to three (hard version). The sequence of letters was pseudorandomized so that the correct response was yes in one-third of the trials and was no in two-thirds of the trials; half of the no trials were traps (two- or three-back repeats in the one-back version and one- or two-back repeats in the 3-back version). Color and case varied but had to be ignored in this task.
N-switch.
For the N-switch task, color served as a contextual cue telling participants whether to perform a vowel/consonant or an upper/lowercase discrimination task. For example, a subject had to indicate a consonant (using the left arrow) or a vowel (using the right arrow) when the letter was green or indicate whether the letter was uppercase (using the left arrow) or lowercase (using the right arrow) when it was red. Colors, discrimination tasks, and response keys were fully counterbalanced across participants. Letters were pseudorandomly distributed over trials to balance the frequency of each task (vowel/consonant or upper/lowercase discrimination) and the side of correct response (left or right). The difficulty was imposed by the frequency of switches (color changes) from one switch per block in the easy version to 12 switches per block (40% of trials) in the hard version.
Choice Task.
On the day before the experiment, participants were instructed in and familiarized with the intertemporal choice task (using fictive options) and then performed a calibration session. They were told that one of the choices made either during the calibration or during test sessions would be randomly selected and implemented. This implementation was actually done, except that randomization was biased to exclude delays longer than 1 y. The amount of money participants could receive varied between 1€ and 100€, which was quite significant relative to the fixed payoff (50€). By fitting a standard hyperbolic discounting model to calibration choice data, we checked that there was no difference between groups in initial intertemporal preference, which was defined as the log-transformed discount factor, corresponding to the constant k in the following hyperbolic discounting function: value = reward/(1+k·delay). It was not significantly different between the hard and control conditions (Khard = −1.90 ± 0.23, Kcontrol = −2.24 ± 0.20, t51 = 1.13, P = 0.26).
Choice task trials were intermingled with executive task trials (one per minute on average), except for sessions outside the MRI scanner in the leisure group. There were 30 choices per session, i.e., 330 choices in the entire experiment. In each trial, participants had a maximum of 5 s to state their preference between a small immediate reward (with a variable amount) and a 100€ delayed reward (with a variable delay). The location (left or right) of the immediate and delayed options on the screen was counterbalanced across trials. There were 10 possible delays (3 d, 1 wk, 2 wk, 3 wk, 1 mo, 3 mo, 6 mo, 1 y, 5 y, and 10 y), which were presented in a randomized order. The immediate rewards were derived from subject-specific indifference points, which describe how a 100€ reward is discounted with delay. These indifference points were obtained using a bisection procedure (with 11 steps for each delay) that was implemented in the calibration session. In each session of the experiment, three immediate rewards were presented for each of the 10 delays: one around the indifferent point, one above, and one below. The two options of a choice therefore were close in (discounted) value, maximizing the sensitivity to potential fatigue effects, as it was previously implemented for TMS studies (7). Between sessions, the amounts proposed as immediate rewards were randomly varied by ±1€ to avoid repeating choices and hence automatic responding. We checked that randomization did not promote an increase in immediate rewards across sessions, which would have artificially favored impulsive choices.
MRI Data Acquisition.
T2*-weighted echo planar images (EPIs) were acquired with blood oxygenation level-dependent (BOLD) contrast on a 3.0-T magnetic resonance scanner (Siemens Trio). A tilted-plane acquisition sequence was used to optimize sensitivity to BOLD signal in the orbitofrontal cortex (44). To cover the whole brain with sufficient temporal resolution (TR = 2.01 s), we used the following parameters: 37 slices, 2.5-mm thickness, 1-mm interslice gap. Structural T1-weighted images were coregistered to the mean EPI, segmented and normalized to the standard T1 template, and then averaged across subjects for anatomical localization of group-level functional activation. EPI images were analyzed in a block-related manner, using general linear model analysis, implemented in the statistical parametric mapping (SPM8) environment (Wellcome Trust Center for NeuroImaging). The first five volumes of each session were discarded to allow for T1 equilibration effects. Preprocessing consisted of spatial realignment, normalization using the same transformation as anatomical images, and spatial smoothing using a Gaussian kernel with an FWHM of 8 mm.
BMS.
The VBA algorithm not only inverts nonlinear models with an efficient and robust parameter estimation but also estimates the model evidence, which represents a trade-off between accuracy (goodness of fit) and complexity (degrees of freedom). The log-evidences estimated for each subject and model were submitted to a group-level random-effect analysis, which assumes that models could differ between subjects and that they have a fixed (unknown) distribution in the population. The BMS procedure estimates both the expected frequency and the exceedance probability (43). The EF quantifies the posterior probability, i.e., the probability that the model generated the data for any randomly selected subject. This quantity must be compared with chance level (one divided by the number of models or families in the search space). EP quantifies the belief that the particular model is more likely than all the other models of the set, i.e., the confidence that in the model has the highest expected frequency.
SI Experiment S1
Aim.
Using the dual-task paradigm, we tested whether performing tasks requiring high executive control (task switching) would bias subsequent intertemporal choices toward immediate rewards at a short time scale.
Methods.
Seventeen participants (three females, mean age 26.4 ± 1.9 y old) performed four sessions of task switching (Fig. S1). Each session included 372 trials and lasted for around 15 min. The stimuli and tasks were the same as those used in the experiment described in the main text. Two hard sessions (105 switches) were alternated with two easy sessions (one switch only) with a 1-min break between sessions. The experiment always started with an easy session to establish a baseline for choice impulsivity. After each task session, participants made a series of 16 intertemporal choices that took about 1 min on average. As in the main experiment, the delayed reward was kept constant (50€), but delays were varied (1 wk, 1 mo, 6 mo, or 1 y). Immediate rewards were derived from indifference curves and varied slightly (by 1€) between sessions to avoid repetitions.
Results.
Three participants were excluded because they did not perform the executive task correctly (accuracy was at chance level). We expected that choice impulsivity would increase with difficulty (hard versus easy sessions), possibly in interaction with session number (last versus early sessions). To our surprise (Fig. S1), repeated two-way ANOVA did not show any main effect (difficulty: F1,14 = 0.26, P = 0.61; session number: F1,14 = 0.1, P = 0.76) or any interaction between executive difficulty and session number (F1,14 = 0.04, P = 0.84). Regarding performance, we observed the expected effect of difficulty (F1,14 = 16.11, P = 0.0013) but no effect of session number (F1,14 = 0, P = 0.96) or any interaction (F1,14 = 1.08, P = 0.32).
Conclusion.
The time scale that is typically used in ego depletion paradigms (15 min of executive control) was not sufficient to induce any fatigue effect capable of biasing economic decision-making, although the same brain regions in the LPFC are known to be involved in task switching (or contextual control) and intertemporal choice (9, 45).
Acknowledgments
This study was funded by a starting grant for the European Research Council (ERC-BioMotiv). B.B. received a PhD fellowship from the Direction Générale de l’Armement. This work also received support from the program ‘‘Investissements d’avenir’’ Grant ANR-10-IAIHU-06.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.W.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520527113/-/DCSupplemental.
References
- 1.Stephens DW. Decision ecology: Foraging and the ecology of animal decision making. Cogn Affect Behav Neurosci. 2008;8(4):475–484. doi: 10.3758/CABN.8.4.475. [DOI] [PubMed] [Google Scholar]
- 2.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 3.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 4.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 5.Lebreton M, et al. A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biol. 2013;11(10):e1001684. doi: 10.1371/journal.pbio.1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figner B, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13(5):538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 7.Essex BG, Clinton SA, Wonderley LR, Zald DH. The impact of the posterior parietal and dorsolateral prefrontal cortices on the optimization of long-term versus immediate value. J Neurosci. 2012;32(44):15403–15413. doi: 10.1523/JNEUROSCI.6106-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danziger S, Levav J, Avnaim-Pesso L. Extraneous factors in judicial decisions. Proc Natl Acad Sci USA. 2011;108(17):6889–6892. doi: 10.1073/pnas.1018033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 10.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesley MJ, Bickel WK. Remember the future II: Meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson J, Larsson A, Reuter-Lorenz PA. Imaging fatigue of interference control reveals the neural basis of executive resource depletion. J Cogn Neurosci. 2013;25(3):338–351. doi: 10.1162/jocn_a_00321. [DOI] [PubMed] [Google Scholar]
- 13.Esposito F, Otto T, Zijlstra FR, Goebel R. Spatially distributed effects of mental exhaustion on resting-state FMRI networks. PLoS One. 2014;9(4):e94222. doi: 10.1371/journal.pone.0094222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? J Pers Soc Psychol. 1998;74(5):1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- 15.Hagger MS, Wood C, Stiff C, Chatzisarantis NL. Ego depletion and the strength model of self-control: A meta-analysis. Psychol Bull. 2010;136(4):495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- 16.Baumeister RF. Self-regulation, ego depletion, and inhibition. Neuropsychologia. 2014;65:313–319. doi: 10.1016/j.neuropsychologia.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Carter EC, McCullough ME. Is ego depletion too incredible? Evidence for the overestimation of the depletion effect. Behav Brain Sci. 2013;36(6):683–684, discussion 707–726. doi: 10.1017/S0140525X13000952. [DOI] [PubMed] [Google Scholar]
- 18.Carter EC, McCullough ME. Publication bias and the limited strength model of self-control: Has the evidence for ego depletion been overestimated? Front Psychol. 2014;5:823. doi: 10.3389/fpsyg.2014.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerman PL. Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications. American Psychological Association; Washington, DC: 2011. [Google Scholar]
- 20.Murtagh AM, Todd SA. Self-regulation: A challenge to the strength model. J Artic Support Null Hypothesis. 2004;3(1):19–51. [Google Scholar]
- 21.Xu X, et al. Failure to replicate depletion of self-control. PLoS One. 2014;9(10):e109950. doi: 10.1371/journal.pone.0109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pashler H. Dual-task interference in simple tasks: Data and theory. Psychol Bull. 1994;116(2):220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 23.Job V, Dweck CS, Walton GM. Ego depletion--is it all in your head? implicit theories about willpower affect self-regulation. Psychol Sci. 2010;21(11):1686–1693. doi: 10.1177/0956797610384745. [DOI] [PubMed] [Google Scholar]
- 24.Job V, Walton GM, Bernecker K, Dweck CS. Beliefs about willpower determine the impact of glucose on self-control. Proc Natl Acad Sci USA. 2013;110(37):14837–14842. doi: 10.1073/pnas.1313475110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hockey GR. Compensatory control in the regulation of human performance under stress and high workload; a cognitive-energetical framework. Biol Psychol. 1997;45(1-3):73–93. doi: 10.1016/s0301-0511(96)05223-4. [DOI] [PubMed] [Google Scholar]
- 26.Hare TA, Hakimi S, Rangel A. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Front Neurosci. 2014;8:50. doi: 10.3389/fnins.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45(1):143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol Bull. 2000;126(2):247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- 30.Gailliot MT, et al. Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. J Pers Soc Psychol. 2007;92(2):325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- 31.Molden DC, et al. Motivational versus metabolic effects of carbohydrates on self-control. Psychol Sci. 2012;23(10):1137–1144. doi: 10.1177/0956797612439069. [DOI] [PubMed] [Google Scholar]
- 32.Lange F, Eggert F. Sweet delusion. Glucose drinks fail to counteract ego depletion. Appetite. 2014;75:54–63. doi: 10.1016/j.appet.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Kurzban R. Does the brain consume additional glucose during self-control tasks? Evol Psychol. 2010;8(2):244–259. [PubMed] [Google Scholar]
- 34.Hockey R. The Psychology of Fatigue: Work, Effort and Control. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 35.Kurzban R, Duckworth A, Kable JW, Myers J. An opportunity cost model of subjective effort and task performance. Behav Brain Sci. 2013;36(6):661–679. doi: 10.1017/S0140525X12003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inzlicht M, Schmeichel BJ, Macrae CN. Why self-control seems (but may not be) limited. Trends Cogn Sci. 2014;18(3):127–133. doi: 10.1016/j.tics.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 37.McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci USA. 2010;107(17):7922–7926. doi: 10.1073/pnas.0910662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kool W, McGuire JT, Wang GJ, Botvinick MM. Neural and behavioral evidence for an intrinsic cost of self-control. PLoS One. 2013;8(8):e72626. doi: 10.1371/journal.pone.0072626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyniel F, Sergent C, Rigoux L, Daunizeau J, Pessiglione M. Neurocomputational account of how the human brain decides when to have a break. Proc Natl Acad Sci USA. 2013;110(7):2641–2646. doi: 10.1073/pnas.1211925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friston K, Mattout J, Trujillo-Barreto N, Ashburner J, Penny W. Variational free energy and the Laplace approximation. Neuroimage. 2007;34(1):220–234. doi: 10.1016/j.neuroimage.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Daunizeau J, Friston KJ, Kiebel SJ. Variational Bayesian identification and prediction of stochastic nonlinear dynamic causal models. Physica D. 2009;238(21):2089–2118. doi: 10.1016/j.physd.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daunizeau J, Adam V, Rigoux L. VBA: A probabilistic treatment of nonlinear models for neurobiological and behavioural data. PLOS Comput Biol. 2014;10(1):e1003441. doi: 10.1371/journal.pcbi.1003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16:S497. [Google Scholar]
- 44.Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46(4):1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: Understanding variability. Trends Cogn Sci. 2011;15(5):227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]