Abstract
Life-threatening infections of odontogenic or upper airway origin may extend to potential spaces formed by fascial planes of the lower head and upper cervical area. Complications include airway obstruction, mediastinitis, necrotizing fascitis, cavernous sinus thrombosis, sepsis, thoracic empyema, Lemierre's syndrome, cerebral abscess, orbital abscess, and osteomyelitis. The incidence of these “space infections” has been greatly reduced by modern antibiotic therapy. However, serious morbidity and even fatalities continue to occur. This study reviews complications of odontogenic infections. The search done was based on PubMed and Google Scholar, and an extensive published work search was undertaken. Advanced MEDLINE search was performed using the terms “odontogenic infections,” “complications,” and “risk factors.”
Keywords: Cavernous sinus thrombosis, fascial spaces, mediastinitis, odontogenic infections
INTRODUCTION
Odontogenic infections (OIs) have been one of the most common diseases in the oral and maxillofacial region[1,2] associated with mortality rate of 10–40%.[3] With the advent of modern antibiotics, mortality rates have significantly reduced.[4,5,6] Such infections are usually self-limiting;[7,8] purulent material may occasionally burrow deep into fascial spaces. Propagation can be produced by direct continuity, by lymphatic or hematogenous dissemination and depends on the patient's local and systemic factors and on the virulence of the pathogen.[9] Multiple severe complications of OIs have been reported, such as airway obstruction, mediastinitis, necrotizing fascitis, cavernous sinus thrombosis (CST), sepsis, thoracic empyema, cerebral abscess, and osteomyelitis.
This study reviews complications of OIs. The search done was based on PubMed and Google Scholar, and an extensive published work search was undertaken. Advanced MEDLINE search was performed using the terms “odontogenic infections,” “complications,” and “risk factors.” A manual search was performed of the references of each article. All information was sorted and analyzed for suitability for inclusion and relevant articles were retained.
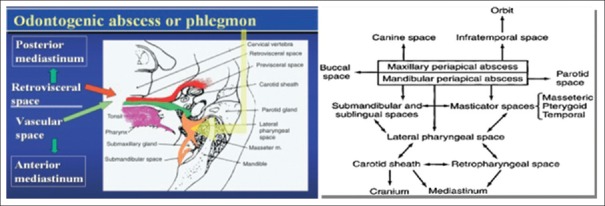
SPREAD OF ODONTOGENIC INFECTIONS
Knowledge of cervical fascial planes is essential in understanding the propagation pathways [Figure 1].
Figure 1.
Potential pathways of extension of deep fascial space infections of the head and neck
The superficial fascia extends from the head and neck to the thorax, shoulders, and axilla. Deep to the superficial fascia lies the deep cervical fascia made of three layers: Superficial, middle, and deep layer
The submandibular and sublingual spaces communicate around posterior border of mylohyoid. Edema and swelling of this space will cause superior and posterior displacement of the floor of the mouth and tongue causing airway compromise
The parapharyngeal space has the shape of a cone with its base facing the skull and communication with the brain can result in a cerebral abscess.[10] Communication occurs via various foramina such as foramen ovale, foramen lacerum, and jugular foramen
The pretracheal space lies anterior to the trachea, and it descends into the anterior mediastinum. Spread of infection along this route is not common, accounting for only 7% of cases of descending necrotizing mediastinitis[11]
The retropharyngeal space abscess can reach the mediastinum causing mediastinitis and more rarely, pericarditis. About 70% of cervical infections extend into the mediastinum via retropharyngeal space[12,13,14]
Danger space: Anterior to the vertebral bodies, the prevertebral fascia divides into the alar fascia (anterior) and the true prevertebral fascia (posterior). Between these fascial layers is a potential space called the “danger space.”[15,16]
RISK FACTORS FOR COMPLICATIONS
The major factor in the progression of OIs is impairment of host resistance by systemic disease.[5,17,18,19,20,21]
Umeda et al.[22] reviewed the systemic conditions [Table 1] and other risk factors of OIs.
Table 1.
Risk factors for complications

COMPLICATIONS OF ODONTOGENIC INFECTIONS
Odontogenic space infections may cause life-threatening complications, such as respiratory obstruction,[2,8,9,17,23,24] sepsis,[2,4,6,9,17,20,24,25,26,27,28] endocarditis,[20] pericarditis,[24] necrotizing fasciitis,[9,27,29] descending mediastinitis,[2,4,6,8,9,17,23,24,27,29] spondylitis,[20] brain abscess,[9,20,24] CST,[8,9,17,25,26] thoracic empyema,[9,27,28] pleuropulmonary suppuration,[2,23] aspiration pneumonia,[25] pneumothorax,[30] mandibular or cervical osteomyelitis,[27] abscess of the carotid sheath and jugular thrombophlebitis,[2,9,27] hematogenous dissemination to distant organs,[2,23] and coagulation abnormalities ranging from thrombocytopenia to a fulminant state of disseminated intravascular coagulation (DIC).[2,28]
SEPSIS
The sepsis syndrome is defined as the presence of confirmed or suspected infectious agents with two or more of the systemic inflammatory response syndrome criteria.[31,32]
SYSTEMIC INFLAMMATORY RESPONSE SYNDROME
The response is manifested by two or more of the following conditions:
Temperature <36.0°C and/or >38.0°C
Heart rate >90 beats per minute
Respiratory rate >20 breaths per minute or PaCO2 <32 mmHg
White blood cell count >12,000/cumm, <4000/cumm, or >10% immature (band) forms.
In a survey conducted by Wong[26], 18 deaths were reported out of a total of 2790 patients. The direct causes of death were sepsis (55%), preexisting organ failure (33%), upper airway obstruction (5%), and a postanesthetic complication (5%), a risk that is not negligible. Kim et al. encountered a 64-year-old patient with a diagnosis of right temporal, infraorbital, buccal, pterygomandibular space abscess. Despite surgical and medical supportive care, the condition progressed to sepsis leading to death.[4] Fardy et al. encountered one case of a 9-year-old patient developing toxic shock syndrome secondary to dentoalveolar abscesses.[33] Despite the treatment, the patient died 2 days after admission to intensive care unit because of multisystem organ failure.
It is of the utmost importance to diagnose sepsis as early as possible.[34] The mainstays for the treatment of severe sepsis includes the following principles: (1) Early diagnosis, (2) treatment of the infection (antimicrobial therapy and surgical eradication of the inciting infectious focus),[35] (3) resuscitation and hemodynamic support (fluids and vasopressor therapy), (4) full organ support (renal replacement therapy and mechanical ventilation), (5) modulation of the inflammatory response (recombinant human activated protein C), (6) sedation and analgesia as needed, and (7) adequate nutrition. Studies[36,37] have shown that early and adequate treatment with early, goal-directed therapy after the onset of a septic episode is associated with increased favorable outcome.
Approximately 30–50% of patients presenting with a clinical picture of severe sepsis have positive blood cultures.[38] Failure to check blood cultures before the start of antimicrobial therapy will potentially influence the growth of blood-borne bacteria and prevent a culture from becoming positive later.[39]
RESPIRATORY OBSTRUCTION
This is another most concerning complication of OIs.[16,40,41] Respiratory obstruction may be due to swelling of floor of mouth, trismus, edema, and abscess formation leading to narrowing and eventually to the loss of airway. Epiglottitis, peritonsillar abscess, and retropharyngeal abscess may also lead to respiratory obstruction. In advanced cases, the patient can assume various positions to relieve partial airway obstruction. A retropharyngeal space abscess can cause the patient to assume the “sniffing position,” maneuver which straightens the upper airway.[42]
Tracheal intubation in patients with deep neck infections is challenging. The distorted airway anatomy, tissue immobility, and limited access to the mouth make orotracheal intubation with rigid laryngoscopy difficult.[41,43] Rupture of an abscess and aspiration of pus have been reported during an attempted orotracheal intubation[41] and blind nasal intubation.[44,45] Blind nasotracheal intubation should be avoided.
Tracheostomy using local anesthesia has been considered the gold standard of airway management in patients with deep neck infections.[44,46] In a group of 36 patients with Ludwig's angina, 16 underwent successful elective tracheostomy using local anesthesia; intubation attempts failed in 11 (55%) of the other 20 patients and resulted in acute airway loss that required emergency tracheostomy.[47]
The first successful fiberoptic nasotracheal intubation in a patient with Ludwig's angina was reported in 1974.[23,48] Tissue edema and immobility, a distorted airway, and copious secretions contribute to the difficulty of fiberoptic intubation. Ovassapian et al. reported the successful awake fiberoptic intubation of 25 of 26 with severe neck space infection.[49] Success rate of 95% has been reported in literature with fiberoptic intubation.
Percutaneous transtracheal and percutaneous dilatational tracheotomy have limited application in surgical management of airway.
DESCENDING NECROTIZING MEDIASTINITIS
It occurs as a complication of odontogenic or cervicofascial infections.[50,51,52,53] Of the reported cases of DNM, 60–70% originate from OIs.[54] Diagnosis of DNM mandates that the relationship between mediastinitis and oropharyngeal infection is clearly established. According to Wheatley et al., most common primary oropharyngeal infection is odontogenic (25 of 43 cases) with mandibular second or third molar abscess.[55] For the diagnosis of mediastinitis of odontogenic origin, Estrera et al.[13] proposed the following criteria: (1) Clinical manifestations of severe infection; (2) characteristic radiographic findings in the neck and chest of gas in the tissues, an air-fluid level, loss of normal cervical lordosis, and mediastinal widening; and (3) establishment of a relationship between the dental infection and the development of mediastinitis.
Review of the literature shows that although DNM is quite rare, this variety of mediastinitis is a highly lethal disease[7,56,57] with a mortality rate of 37–60% and is frequently associated with pleural and pericardial effusion, compression of the local blood vessels, persistent sepsis, and multiorgan failure.
A multidisciplinary approach is recommended for successful treatment of this life-threatening infection.
OTHER THORACIC COMPLICATIONS
Early signs of thoracic involvement are difficult to interpret. According to Estrera et al.,[13] diffuse brawny induration, with pitting edema or crepitation at the base of the neck and the thorax are suggestive. Early diagnosis and aggressive surgical drainage and debridement of all spaces and cavities affected are required along with close monitoring.
George C. Economopoulos et al. has documented the first case of an intrathoracic vascular complication involving the descending aorta appearing as an aortopulmonary fistula, secondary to a gravitating OI of submaxillary and parapharyngeal spaces, and from there to the mediastinum and pleura, through the retrovisceral space and Sibson's fascia. Thrombophlebitis of the internal jugular vein (IJV) is dangerous, and it requires ligation to avoid septic emboli. Erosion of the internal carotid or the common carotid artery within the sheath is lethal.[10]
LEMIERRE'S SYNDROME
Lemierre's syndrome is characterized by suppurative thrombophlebitis of the IJV and is a metastatic infection.[33] In their literature search for Lemierre's syndrome, primary site of infection was oropharynx in 59.5% of cases, followed by mastoiditis (15%) and 7 cases with a primary OI (in the preantibiotic era, Lemierre's syndrome carried a mortality rate of 83%). The term postanginal sepsis is used interchangeably with Lemierre's syndrome, which was initially reported by Courmont and Cade in 1901, but best described by Andre Lemierre in 1936. Computed tomography of the neck with contrast has been shown to be the best diagnostic modality because it allows visualization of the IJV and for the diagnosis of other complications such as pulmonary emboli, abscesses, osteomyelitis, and arthritis.[58]
Magnetic resonance imaging (MRI) and Doppler ultrasonography can also be used for diagnosis. A multidisciplinary approach is necessary to treat patients with Lemierre's syndrome.
CERVICAL NECROTIZING FASCITIS
It is characterized by an extensive, severe progressive infection with dissection and necrosis of the soft tissues along the cervical planes[45] and is associated with mortality rate in between 20% and 40%.[59] Bacterial enzymes and cell wall components play an essential role in local tissue destruction, dissemination of the infection, and in systemic toxicity. Over time, the necrotic tissue begins to separate with suppuration (approximately on the 8th day). If the necrotizing process continues to spread, it involves the neighboring tissues and provokes local or systemic complications.[29,60] The literature shows that cervical necrotizing fasciitis complications as mediastinitis (100%); pericarditis (12.5%); pleural effusion (12.5%); empyema (7.5%); pericardial effusion (5%); pneumonitis (5%); cardiac tamponade (2.5%); and esophageal bleed (2.5%). Hematogenous dissemination can also occur, leading to complications such as septic shock, rheumatic disease, and cardiac problems.[61] If left untreated, the rapid dissemination of the infection can be fatal.
Certain findings on a CT scan increase the likelihood of necrotizing fascitis such as the inflammation of skin and subcutaneous fat, the involvement of more fascia than muscle, and the presence of gas gangrene in the superficial fascia. Aggressive surgical intervention with extensive debridement of necrotic tissues, fasciotomy, and exploration of all involved fascial spaces combined with circulatory and ventilatory support are mandatory.
ORBITAL ABSCESS
Organisms from an odontogenic source may gain entrance to the orbit through local tissue planes, by hematogenous spread, or via involvement of the paranasal sinuses. The orbital septum delineates these infections into pre- and post-septal disease, which is important because the latter has the potential to cause severe complications.[62]
Orbital abscesses exhibit common signs and symptoms such as chemosis, periorbital edema of the eyelid, reddening, hyperthermia, proptosis, extraocular muscle dysfunction, and decreased visual acuity. Further, pursuit of diagnosis includes advanced imaging techniques such as CT scans or MRI.[63]
Early diagnosis and appropriate medical and surgical therapy are imperative for successful outcomes of this rarely occurring disease.
Retrograde spread of infection can lead to complications such as CST, meningitis, cerebritis, brain abscess, or death.[64,65] At present, despite antimicrobial and surgical management, a substantial amount of patients with subperiosteal abscess still develop various visual sequelae.[65] Haymaker[66] in 1945 by his study on 28 cases concluded that there was direct intracranial spread in 17 cases and by hematogenous route in 11 cases following dental extraction. His series included six cases of intraorbital abscess.
CAVERNOUS SINUS THROMBOSIS
Septic CST is a rare condition.[67,68] Seven percent of all cases of thrombosis of the cavernous sinus are of dental origin. The cavernous sinuses and their connections are devoid of valves. Consequently, bidirectional spread of infection, and thrombi can occur throughout this network. Organisms may reach the cavernous sinus from the face by an anterograde route along ophthalmic veins connected to angular veins, or by a retrograde route along emissary veins connected to the pterygoid venous plexus. Contrast enhanced CT scan may reveal the primary source of infection, thickening of the superior ophthalmic vein, and irregular filling defects in the cavernous sinus.
Fewer than 40% of patients usually have full recovery, while the remainder of patients having neurological deficits such as extraocular muscle paresis, impaired visual acuity, hemiparesis, and focal seizures. Other potential long-term sequelae include hypopituitarism, the syndrome of inappropriate secretion of antidiuretic hormone, and the vascular steal syndrome (i.e., retrograde filling of the anterior circulation via the vertebral arteries). Death generally occurs within 4–7 days if the diagnosis is not made or when treatment is not instituted, usually due to meningitis, brain abscess, or generalized sepsis.
Management should be based on early diagnosis and prompt management with intravenous broad-spectrum antibiotics and surgical intervention.[69]
BRAIN ABSCESS
Dental cases of brain abscess have been published in the literature.[70] Haymaker's analysis of 28 fatalities resulting from central nervous system infection included 8 instances of brain abscess of odontogenic origin. Hollin and Gross reported a subdural empyema of odontogenic origin.[71,72] Direct spread tends to cause solitary abscesses, whereas hematogenous spread usually results in multiple abscesses. Anaerobic species are responsible for the majority of cases of odontogenic (78%) brain abscess.[73]
Clinically, there is usually a latent period of several days or weeks before the appearance of symptoms of intracranial involvement. Initial complaints may be mild, consisting mostly of headaches, malaise, and apathy. Neurologic signs may appear later, depending on the location of the lesion. In the case where there is suspicion of an intracranial space-occupying mass, tests such as a brain scan and carotid arteriogram are necessary. The first radiologic signs of brain abscess can be seen on CT examination 2–3 weeks after infection begins. Encapsulation of the brain abscess occurs about 6 weeks after infection, which is seen on contrast enhanced CT as a radiopaque ring surrounding a central necrotic region.[68,74]
The management includes intravenous (IV) glucocorticoids in patients with evidence of mass effect from a brain abscess. Odontogenic brain abscesses are best treated with a combination of IV ceftriaxone and IV metronidazole.
OSTEOMYELITIS
Osteomyelitis may develop in the jaws after a chronic OI or for a variety of other reasons.[75,76] In a review of 141 cases of jaw osteomyelitis in Nigeria, Adekeye, and Cornah found OIs to be the cause of 38% of mandibular and 25% of maxillary involvement.[77] Pain and tenderness, low-grade fever, draining sinus tracts, suppuration, dental loss, and sequestrum (i.e., necrotic bone fragment) formation are main clinical features. New bone and oral mucosa occasionally regenerate beneath the sequestra, probably because of activation of periosteal osteoblasts by the infectious process.[77]
Wang et al. described the first case in which recurrent vertebral osteomyelitis and psoas abscess developed in a patient with a previously unrecognized atrial septal defect and disease recurrence was ascribed to the presence of dental disease, which served as the source of infection.[78]
On radiographs, osteomyelitis appears as radiolucent (“moth-eaten”) regions representing bony destruction and avascular necrosis, with evidence of sequestrum formation and occasional pathologic fractures. Plain radiographs may not show the bony destruction in early stages of the disease. Bone scintigraphy allows earlier detection. Frank osteomyelitis is characterized on CT by erosion involving the medullary and cortical bone. Proper surgical management and antimicrobial therapy are must to avoid the complications.
THROMBOCYTOPENIA
In a case report by Kumar Verma and Rajan,[25] an adult male patient with OI, who developed life-threatening thrombocytopenia, is presented, developed due to increased platelet destruction, impairment of platelet production, or adherence of platelets to damaged endothelium. Consumptive coagulopathy such as DIC can also lead to a decrease in platelet count. The management of an OI complicated by thrombocytopenia presents a tricky situation to the surgeon.
ADULT RESPIRATORY DISTRESS SYNDROME
ARDS caused by sepsis secondary to the OI have been reported in literature. ARDS can be caused by many conditions, among which the most common are sepsis and septic shock.
ASSESSMENT OF PATIENTS WITH SEVERE ODONTOGENIC INFECTION
Assessment should focus on developing complications such as airway compromise, the spaces involved, the precise etiology of the infection, and identifying sepsis symptoms. A sublingual space infection elevates the tongue interfering with the articulation of sounds. A retropharyngeal or lateral pharyngeal space abscess can result in muffling of the voice. An impending airway collapse should be suspected if the patient is sitting in a sniffing position, drooling, and the use of accessory muscles of respiration.
Pterygomandibular space or lateral pharyngeal space infection can deviate the uvula to the opposite side. Patients with trismus and an interincisal opening of <30 mm correlates with difficulties in endotracheal intubation.[79]
Once the airway is stabilized, the next step which must be taken is to identify the source of infection and to assess spreading infection threatening vital structures, such as the eye or mediastinum.
An orthopantomogram is a useful radiograph in identifying dental sources of infection.[80] An important diagnostic tool is a posteroanterior and lateral view radiograph of the neck and chest. These can show gas in the tissues, loss of the normal cervical spine lordosis, and mediastinal widening. On a lateral soft-tissue film of the cervical airway, the soft-tissue thickness of the retropharyngeal tissues over the second cervical vertebra should be 6 mm or less. The corresponding soft-tissue thickness over the sixth cervical vertebra should be 20 mm or less.
MRI has been shown to be superior to CT in the initial evaluation of deep space infection, but may not be practical in an emergency setting.[81] Laboratory workup should include a standard septic workup, which would include a full blood count, serum glucose, electrolytes, coagulation screen, blood cultures, and an arterial blood gas.[82] Where pus is easily accessible, it should be sent for culture and sensitivity.
CONCLUSION
Owing to the widespread availability of preventive dental care and the development of effective antibiotics for the treatment of orofacial infection, the incidence of serious OIs has decreased dramatically over the past 50 years. However, they can still carry the potential for lethal complications, especially in the immunocompromised patient. However, attention to airway maintenance, appropriate antibiotic therapy, and judicious surgical intervention enable the health care professions to continue their remarkable progress in treating these once-dreaded infections. Recognition of the classic signs of severe OIs by the general practitioner and expeditious referral to a higher level of care benefits the patient and may be life-saving.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Iwasaki Y, Nagata K, Nakanishi M, Natuhara A, Harada H, Kubota Y, et al. Spiral CT findings in septic pulmonary emboli. Eur J Radiol. 2001;37:190–4. doi: 10.1016/s0720-048x(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 2.Zamiri B, Hashemi SB, Hashemi SH, Rafiee Z, Ehsani S. Prevalence of odontogenic deep head and neck spaces infection and its correlation with length of hospital stay. Shiraz Univ Dent J. 2012;13:29–35. [Google Scholar]
- 3.Britt JC, Josephson GD, Gross CW. Ludwig's angina in the pediatric population: Report of a case and review of the literature. Int J Pediatr Otorhinolaryngol. 2000;52:79–87. doi: 10.1016/s0165-5876(99)00295-5. [DOI] [PubMed] [Google Scholar]
- 4.Kim MS, Kim SG, Moon SY, Oh JS, Park JU, Jeong MA, et al. Sepsis developed from an odontogenic infection: Case report. J Korean Assoc Maxillofac Plast Reconstr Surg. 2011;33:445–8. [Google Scholar]
- 5.Zhang C, Tang Y, Zheng M, Yang J, Zhu G, Zhou H, et al. Maxillofacial space infection experience in West China: A retrospective study of 212 cases. Int J Infect Dis. 2010;14:e414–7. doi: 10.1016/j.ijid.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Amponsah E, Donkor P. Life-threatening oro-facial infections. Ghana Med J. 2007;41:33–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitoun IM, Dhanarajani PJ. Cervical cellulitis and mediastinitis caused by odontogenic infections: Report of two cases and review of literature. J Oral Maxillofac Surg. 1995;53:203–8. doi: 10.1016/0278-2391(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 8.Ryan P, McMahon G. Severe dental infections in the emergency department. Eur J Emerg Med. 2012;19:208–13. doi: 10.1097/MEJ.0b013e32834ddb68. [DOI] [PubMed] [Google Scholar]
- 9.Arias-Chamorro B, Contreras-Morillo M, Acosta-Moyano A, Ruiz-Delgado F, Bermudo-Añino L, Valiente-Álvarez A. Multiple odontogenic abscesses. Thoracic and abdomino-perineal extension in an immuno competent patient. Med Oral Patol Oral Cir Bucal. 2011;16:e772–5. doi: 10.4317/medoral.16852. [DOI] [PubMed] [Google Scholar]
- 10.Economopoulos GC, Michalis A, Palatianos GM, Sarris GE. Management of catheter-related injuries to the coronary sinus. Ann Thorac Surg. 2003;76:112–6. doi: 10.1016/s0003-4975(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 11.Singhal P, Kejriwal N, Lin Z, Tsutsui R, Ullal R. Optimal surgical management of descending necrotising mediastinitis: Our experience and review of literature. Heart Lung Circ. 2008;17:124–8. doi: 10.1016/j.hlc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Alsoub H, Chacko KC. Descending necrotising mediastinitis. Postgrad Med J. 1995;71:98–101. doi: 10.1136/pgmj.71.832.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estrera AS, Landay MJ, Grisham JM, Sinn DP, Platt MR. Descending necrotizing mediastinitis. Surg Gynecol Obstet. 1983;157:545–52. [PubMed] [Google Scholar]
- 14.Moncada R, Warpeha R, Pickleman J, Spak M, Cardoso M, Berkow A, et al. Mediastinitis from odontogenic and deep cervical infection. Anatomic pathways of propagation. Chest. 1978;73:497–500. doi: 10.1378/chest.73.4.497. [DOI] [PubMed] [Google Scholar]
- 15.Moriwaki Y, Sugiyama M, Matsuda G, Date K, Karube N, Uchida K, et al. Approach for drainage of descending necrotizing mediastinitis on the basis of the extending progression from deep neck infection to mediastinitis. J Trauma. 2002;53:112–6. doi: 10.1097/00005373-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Branstetter BF, 4th, Weissman JL. Infection of the facial area, oral cavity, oropharynx, and retropharynx. Neuroimaging Clin N Am. 2003;13:393–410. doi: 10.1016/s1052-5149(03)00034-0. ix. [DOI] [PubMed] [Google Scholar]
- 17.Jundt JS, Gutta R. Characteristics and cost impact of severe odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:558–66. doi: 10.1016/j.oooo.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 18.Seppänen L, Lemberg KK, Lauhio A, Lindqvist C, Rautemaa R. Is dental treatment of an infected tooth a risk factor for locally invasive spread of infection? J Oral Maxillofac Surg. 2011;69:986–93. doi: 10.1016/j.joms.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Byers J, Lowe T, Goodall CA. Acute cervico-facial infection in Scotland 2010: Patterns of presentation, patient demographics and recording of systemic involvement. Br J Oral Maxillofac Surg. 2012;50:626–30. doi: 10.1016/j.bjoms.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Seppänen L, Lauhio A, Lindqvist C, Suuronen R, Rautemaa R. Analysis of systemic and local odontogenic infection complications requiring hospital care. J Infect. 2008;57:116–22. doi: 10.1016/j.jinf.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Uluibau IC, Jaunay T, Goss AN. Severe odontogenic infections. Aust Dent J. 2005;50(4 Suppl 2):S74–81. doi: 10.1111/j.1834-7819.2005.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 22.Umeda M, Minamikawa T, Komatsubara H, Shibuya Y, Yokoo S, Komori T. Necrotizing fasciitis caused by dental infection: A retrospective analysis of 9 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:283–90. doi: 10.1067/moe.2003.85. [DOI] [PubMed] [Google Scholar]
- 23.Huovinen P, Cars O. Control of antimicrobial resistance: Time for action. The essentials of control are already well known. BMJ. 1998;317:613–4. doi: 10.1136/bmj.317.7159.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds SC, Chow AW. Life-threatening infections of the peripharyngeal and deep fascial spaces of the head and neck. Infect Dis Clin North Am. 2007;21:557–76, viii. doi: 10.1016/j.idc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Kumar Verma D, Rajan R. A case of thrombocytopenia due to odontogenic infection. J Dent Res Dent Clin Dent Prospects. 2011;5:144–7. doi: 10.5681/joddd.2011.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong TY. A nationwide survey of deaths from oral and maxillofacial infections: The Taiwanese experience. J Oral Maxillofac Surg. 1999;57:1297–9. doi: 10.1016/s0278-2391(99)90863-7. [DOI] [PubMed] [Google Scholar]
- 27.Spitalnic SJ, Sucov A. Ludwig's angina: Case report and review. J Emerg Med. 1995;13:499–503. doi: 10.1016/0736-4679(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 28.Parker MI, Khateery SM. A retrospective analysis of orofacial infections requiring hospitalization. Saudi Dent J. 2001;13:96–100. [Google Scholar]
- 29.Jiménez Y, Bagán JV, Murillo J, Poveda R. Odontogenic infections complications, systemic manifestations. Med Oral Patol Oral Cir Bucal. 2004;9:139–47. [PubMed] [Google Scholar]
- 30.Barsamian JG, Scheffer RB. Spontaneous pneumothorax: An unusual occurrence in a patient with Ludwig's angina. J Oral Maxillofac Surg. 1987;45:161–8. doi: 10.1016/0278-2391(87)90407-1. [DOI] [PubMed] [Google Scholar]
- 31.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 32.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 33.Fardy CH, Findlay G, Owen G, Shortland G. Toxic shock syndrome secondary to a dental abscess. Int J Oral Maxillofac Surg. 1999;28:60–1. [PubMed] [Google Scholar]
- 34.Blot S, Vandewoude K. Early detection of systemic infections. Acta Clin Belg. 2004;59:20–3. doi: 10.1179/acb.2004.003. [DOI] [PubMed] [Google Scholar]
- 35.Dominique MV, Stijin IB, John MD. Update on the management of infection in patients with severe sepsis. Dimens Crit Care Nurs. 2008;27:244–48. doi: 10.1097/01.DCC.0000338868.31917.0e. [DOI] [PubMed] [Google Scholar]
- 36.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 37.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–22. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 38.Crowe M, Ispahani P, Humphreys H, Kelley T, Winter R. Bacteraemia in the adult intensive care unit of a teaching hospital in Nottingham, UK, 1985-1996. Eur J Clin Microbiol Infect Dis. 1998;17:377–84. doi: 10.1007/BF01691564. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein MP, Reller LB, Murphy JR, Lichtenstein KA. The clinical significance of positive blood cultures: A comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983;5:35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- 40.Potter JK, Herford AS, Ellis E., 3rd Tracheotomy versus endotracheal intubation for airway management in deep neck space infections. J Oral Maxillofac Surg. 2002;60:349–54. doi: 10.1053/joms.2002.31218. [DOI] [PubMed] [Google Scholar]
- 41.Heindel DJ. Deep neck abscesses in adults: Management of a difficult airway. Anesth Analg. 1987;66:774–6. [PubMed] [Google Scholar]
- 42.Flynn TR. Oral-facial emergencies. Emerg Med Clin North Am. 2000;18:577–600. [Google Scholar]
- 43.Neff SP, Merry AF, Anderson B. Airway management in Ludwig's angina. Anaesth Intensive Care. 1999;27:659–61. doi: 10.1177/0310057X9902700323. [DOI] [PubMed] [Google Scholar]
- 44.Candamourty R, Venkatachalam S, Babu MR, Kumar GS. Ludwig's angina – An emergency: A case report with literature review. J Nat Sci Biol Med. 2012;3:206–8. doi: 10.4103/0976-9668.101932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colmenero Ruiz C, Labajo AD, Yañez Vilas I, Paniagua J. Thoracic complications of deeply situated serous neck infections. J Craniomaxillofac Surg. 1993;21:76–81. doi: 10.1016/s1010-5182(05)80151-9. [DOI] [PubMed] [Google Scholar]
- 46.Irani BS, Martin-Hirsch D, Lannigan F. Infection of the neck spaces: A present day complication. J Laryngol Otol. 1992;106:455–8. doi: 10.1017/s0022215100119826. [DOI] [PubMed] [Google Scholar]
- 47.Parhiscar A, Har-El G. Deep neck abscess: A retrospective review of 210 cases. Ann Otol Rhinol Laryngol. 2001;110:1051–4. doi: 10.1177/000348940111001111. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz HC, Bauer RA, Davis NJ, Guralnick WC. Ludwig's angina: Use of fiberoptic laryngoscopy to avoid tracheostomy. J Oral Surg. 1974;32:608–11. [PubMed] [Google Scholar]
- 49.Ovassapian A, Tuncbilek M, Weitzel EK, Joshi CW. Airway management in adult patients with deep neck infections: A case series and review of the literature. Anesth Analg. 2005;100:585–9. doi: 10.1213/01.ANE.0000141526.32741.CF. [DOI] [PubMed] [Google Scholar]
- 50.Sandner A, Börgermann J, Kösling S, Silber RE, Bloching MB. Descending necrotizing mediastinitis: Early detection and radical surgery are crucial. J Oral Maxillofac Surg. 2007;65:794–800. doi: 10.1016/j.joms.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 51.Huang SM, Wu RC, Chi T. Rare type of deep neck infection: Two cases of descending necrotizing mediastinitis. Med J. 2009;21:348–51. [Google Scholar]
- 52.Chen KC, Chen JS, Kuo SW, Huang PM, Hsu HH, Lee JM, et al. Descending necrotizing mediastinitis: A 10-year surgical experience in a single institution. J Thorac Cardiovasc Surg. 2008;136:191–8. doi: 10.1016/j.jtcvs.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Papalia E, Rena O, Oliaro A, Cavallo A, Giobbe R, Casadio C, et al. Descending necrotizing mediastinitis: Surgical management. Eur J Cardiothorac Surg. 2001;20:739–42. doi: 10.1016/s1010-7940(01)00790-4. [DOI] [PubMed] [Google Scholar]
- 54.Sakamoto H, Aoki T, Kise Y, Watanabe D, Sasaki J. Descending necrotizing mediastinitis due to odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:412–9. doi: 10.1016/s1079-2104(00)70121-1. [DOI] [PubMed] [Google Scholar]
- 55.Wheatley MJ, Stirling MC, Kirsh MM, Gago O, Orringer MB. Descending necrotizing mediastinitis: Transcervical drainage is not enough. Ann Thorac Surg. 1990;49:780–4. doi: 10.1016/0003-4975(90)90022-x. [DOI] [PubMed] [Google Scholar]
- 56.Bulut M, Balci V, Akköse S, Armagan E. Fatal descending necrotising mediastinitis. Emerg Med J. 2004;21:122–3. doi: 10.1136/emj.2003.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levine TM, Wurster CF, Krespi YP. Mediastinitis occurring as a complication of odontogenic infections. Laryngoscope. 1986;96:747–50. [PubMed] [Google Scholar]
- 58.Kuppalli K, Livorsi D, Talati NJ, Osborn M. Lemierre's syndrome due to Fusobacterium necrophorum. Lancet Infect Dis. 2012;12:808–15. doi: 10.1016/S1473-3099(12)70089-0. [DOI] [PubMed] [Google Scholar]
- 59.Storoe W, Haug RH, Lillich TT. The changing face of odontogenic infections. J Oral Maxillofac Surg. 2001;59:739–48. doi: 10.1053/joms.2001.24285. [DOI] [PubMed] [Google Scholar]
- 60.Roberson JB, Harper JL, Jauch EC. Mortality associated with cervicofacial necrotizing fasciitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:264–7. doi: 10.1016/s1079-2104(96)80350-7. [DOI] [PubMed] [Google Scholar]
- 61.Jiménez Y, Bagán JV, Murillo J, Poveda R. Odontogenic infections. Complications. Systemic manifestations. Med Oral Patol Oral Cir Bucal. 2004;(Suppl No 9):143–7. 139-43. [PubMed] [Google Scholar]
- 62.Bullock JD, Fleishman JA. The spread of odontogenic infections to the orbit: Diagnosis and management. J Oral Maxillofac Surg. 1985;43:749–55. doi: 10.1016/0278-2391(85)90328-3. [DOI] [PubMed] [Google Scholar]
- 63.Youssef OH, Stefanyszyn MA, Bilyk JR. Odontogenic orbital cellulitis. Ophthal Plast Reconstr Surg. 2008;24:29–35. doi: 10.1097/IOP.0b013e318160c950. [DOI] [PubMed] [Google Scholar]
- 64.Koch F, Breil P, Marroquín BB, Gawehn J, Kunkel M. Abscess of the orbit arising 48 h after root canal treatment of a maxillary first molar. Int Endod J. 2006;39:657–64. doi: 10.1111/j.1365-2591.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 65.Allan BP, Egbert MA, Myall RW. Orbital abscess of odontogenic origin. Case report and review of the literature. Int J Oral Maxillofac Surg. 1991;20:268–70. doi: 10.1016/s0901-5027(05)80150-x. [DOI] [PubMed] [Google Scholar]
- 66.Haymaker W. Fatal infections of the central nervous system and meninges after tooth extraction with analysis of 28 cases. Am J Orthod. 1945;31:117. [Google Scholar]
- 67.Kiddee W, Preechawai P, Hirunpat S. Bilateral Septic Cavernous Sinus Thrombosis Following the Masticator and Parapharyngeal Space Infection from the Odontogenic Origin: A Case Report. World Ophthalmology Congress (WOC); 28 June-2 July; Hong Kong, China. 2008 [PubMed] [Google Scholar]
- 68.Cannon ML, Antonio BL, McCloskey JJ, Hines MH, Tobin JR, Shetty AK. Cavernous sinus thrombosis complicating sinusitis. Pediatr Crit Care Med. 2004;5:86–8. doi: 10.1097/01.PCC.0000102385.95708.3B. [DOI] [PubMed] [Google Scholar]
- 69.Kiddee W, Preechawai P, Hirunpat S. Bilateral septic cavernous sinus thrombosis following the masticator and parapharyngeal space infection from the odontogenic origin: A case report. J Med Assoc Thai. 2010;93:1107–11. [PubMed] [Google Scholar]
- 70.Li X, Tronstad L, Olsen I. Brain abscesses caused by oral infection. Endod Dent Traumatol. 1999;15:95–101. doi: 10.1111/j.1600-9657.1999.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 71.Baddour HM, Durst NL, Tilson HB. Frontal lobe abscess of dental origin. Report of a case. Oral Surg Oral Med Oral Pathol. 1979;47:303–6. doi: 10.1016/0030-4220(79)90250-0. [DOI] [PubMed] [Google Scholar]
- 72.Hollin SA, Gross SW. Subdural empyema of odontogenic origin. J Mt Sinai Hosp N Y. 1964;31:540–4. [PubMed] [Google Scholar]
- 73.Sethi DS, Stanley RE. Deep neck abscesses – Changing trends. J Laryngol Otol. 1994;108:138–43. doi: 10.1017/s0022215100126106. [DOI] [PubMed] [Google Scholar]
- 74.Mylonas AI, Tzerbos FH, Mihalaki M, Rologis D, Boutsikakis I. Cerebral abscess of odontogenic origin. J Craniomaxillofac Surg. 2007;35:63–7. doi: 10.1016/j.jcms.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Bernier S, Clermont S, Maranda G, Turcotte JY. Osteomyelitis of the jaws. J Can Dent Assoc. 1995;61:441. [PubMed] [Google Scholar]
- 76.Donohue WB, Abelardo LM. Osteomyelitis of the jaw. Can Med Assoc J. 1970;103:748–50. [PMC free article] [PubMed] [Google Scholar]
- 77.Adekeye EO, Cornah J. Osteomyelitis of the jaws: A review of 141 cases. Br J Oral Maxillofac Surg. 1985;23:24–35. doi: 10.1016/0266-4356(85)90075-0. [DOI] [PubMed] [Google Scholar]
- 78.Wang TD, Chen YC, Huang PJ. Recurrent vertebral osteomyelitis and psoas abscess caused by Streptococcus constellatus and Fusobacterium nucleatum in a patient with atrial septal defect and an occult dental infection. Scand J Infect Dis. 1996;28:309–10. doi: 10.3109/00365549609027179. [DOI] [PubMed] [Google Scholar]
- 79.Killey HC, Kay LW, Seward GR, Harris M, McGowan DA, editors. 4th ed. Oxford, Great Britain: Reed Educational and Professional Publishing Limited; 1989. Orofacial infections. An Outline of Oral Surgery. Part 2; pp. 310–30. [Google Scholar]
- 80.Stalfors J, Adielsson A, Ebenfelt A, Nethander G, Westin T. Deep neck space infections remain a surgical challenge. A study of 72 patients. Acta Otolaryngol. 2004;124:1191–6. doi: 10.1080/00016480410017864. [DOI] [PubMed] [Google Scholar]
- 81.Muñoz A, Castillo M, Melchor MA, Gutiérrez R. Acute neck infections: Prospective comparison between CT and MRI in 47 patients. J Comput Assist Tomogr. 2001;25:733–41. doi: 10.1097/00004728-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 82.Marioni G, Rinaldi R, Staffieri C, Marchese-Ragona R, Saia G, Stramare R, et al. Deep neck infection with dental origin: Analysis of 85 consecutive cases (2000-2006) Acta Otolaryngol. 2008;128:201–6. doi: 10.1080/00016480701387157. [DOI] [PubMed] [Google Scholar]