Physiological tools provide a mechanistic basis for understanding fundamental and applied ecology of tunas, billfishes, and pelagic sharks. In this synthesis, we review several templates for the interdisciplinary interactions between physiologists and fisheries scientists and highlight three areas of successful collaborations that directly benefit pelagic fisheries management.
Keywords: Bycatch, cardiorespiratory, Fry paradigm, pelagic fishes, post-release survival
Abstract
Populations of tunas, billfishes and pelagic sharks are fished at or over capacity in many regions of the world. They are captured by directed commercial and recreational fisheries (the latter of which often promote catch and release) or as incidental catch or bycatch in commercial fisheries. Population assessments of pelagic fishes typically incorporate catch-per-unit-effort time-series data from commercial and recreational fisheries; however, there have been notable changes in target species, areas fished and depth-specific gear deployments over the years that may have affected catchability. Some regional fisheries management organizations take into account the effects of time- and area-specific changes in the behaviours of fish and fishers, as well as fishing gear, to standardize catch-per-unit-effort indices and refine population estimates. However, estimates of changes in stock size over time may be very sensitive to underlying assumptions of the effects of oceanographic conditions and prey distribution on the horizontal and vertical movement patterns and distribution of pelagic fishes. Effective management and successful conservation of pelagic fishes requires a mechanistic understanding of their physiological and behavioural responses to environmental variability, potential for interaction with commercial and recreational fishing gear, and the capture process. The interdisciplinary field of conservation physiology can provide insights into pelagic fish demography and ecology (including environmental relationships and interspecific interactions) by uniting the complementary expertise and skills of fish physiologists and fisheries scientists. The iterative testing by one discipline of hypotheses generated by the other can span the fundamental–applied science continuum, leading to the development of robust insights supporting informed management. The resulting species-specific understanding of physiological abilities and tolerances can help to improve stock assessments, develop effective bycatch-reduction strategies, predict rates of post-release mortality, and forecast the population effects of environmental change. In this synthesis, we review several examples of these interdisciplinary collaborations that currently benefit pelagic fisheries management.
Introduction
Stocks of many large, highly migratory pelagic fishes (including billfishes, tunas and pelagic sharks; Lesser Antilles Pelagic Ecosystem (LAPE) project) are being harvested at or over capacity (Worm et al., 2009), as fisheries fish through food webs to meet the protein demands for a burgeoning human population (Essington et al., 2006). The major source of fishing mortality for tunas, billfishes and pelagic sharks is either as targeted catch or bycatch in commercial pelagic longlines, gill nets and purse seines (Dulvy et al., 2008; Graves et al., 2012; Jordan et al., 2013; Graves and Horodysky, 2015; Oliver et al., 2015). Tunas, billfishes and pelagic sharks also support lucrative directed recreational fisheries that often promote catch and release. Commercially targeted populations, including tunas (Thunnus spp.; Fig. 1A) and swordfish (Xiphias gladius; Fig. 1B), are mostly fished near capacity; many of the bycatch species, such as istiophorid billfishes (Fig. 1B) and sharks (Fig. 1C), are overfished and/or experiencing overfishing, particularly in the Atlantic Ocean (Dulvy et al., 2008; Worm et al., 2013; NOAA, 2014, 2015; Punt et al., 2015).
Figure 1:
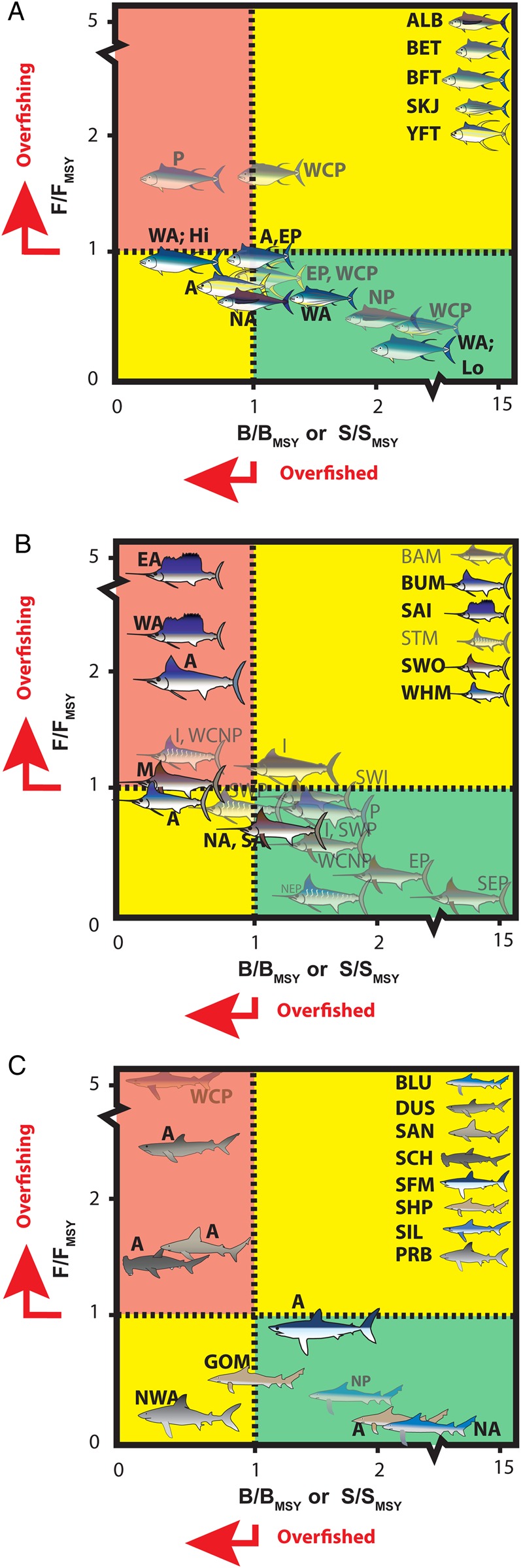
Kobe plots of the current stock status of pelagic fishes, including tunas (A), billfishes (B) and pelagic sharks (C). A species is overfished when biomass (B) or stock size (S) is less than that at maximal sustainable yield (MSY; i.e. B < BMSY), and overfishing occurs when current fishing mortality (F) is greater than that at MSY (i.e. F > FMSY). Red quadrants indicate that a species is overfished and overfishing is occurring, yellow quadrants indicate that a species is either overfished or overfishing is occurring, and species within the green quadrant are neither overfished nor experiencing overfishing. Atlantic species are presented in the foreground of figure. Regional stock abbreviations are as follows: Atlantic (A) [East Atlantic (EA), West Atlantic (WA), North Atlantic (NA), Northwest Atlantic (NW), and South Atlantic (SA)]; Gulf of Mexico (GOM); Mediterranean Sea (M); Indian Ocean (I), and Southwest Indian Ocean (SWI); and Pacific Ocean (P) [East Pacific (EP), Northeast Pacific (NEP), Southeast Pacific (SEP), Southwest Pacific (SWP), and West Central North Pacific (WCNP)]. Abbreviations for tunas (ISSF, 2013) are as follows: albacore (Thunnus alalunga; ALB), bigeye tuna (Thunnus obesus; BET), Northern bluefin tuna (Thunnus thynnus; BFT), skipjack tuna (Katsuwomis pelamis; SKJ), and yellowfin tuna (Thunnus albacares; YFT). For bluefin tuna, ‘Hi’ and ‘Lo’ refer to high- and low-recruitment scenarios, respectively. Abbreviations for billfishes (NOAA HMS SAFE, 2014; Punt et al., 2015) are as follows: black marlin (Istiompax indica; BAM), blue marlin (Makaira nigricans; BUM), sailfish (Istiophorus platypterus; SAI), striped marlin (Kajikia audax; STM), swordfish (Xiphias gladius; SWO), and white marlin (Kajikia albida; WHM). Abbreviations for pelagic sharks (Dulvy et al., 2008; NOAA HMS SAFE, 2013, 2014) are as follows: blue shark (Prionace glauca; BLU), Atlantic porbeagle (Lamna nasus; PRB), shortfin mako (Isurus oxyrhinchus; SFM), and silky shark (SIL; ICCAT, 2012; NOAA HMS SAFE, 2013, 2014). For comparison, data for several coastal sharks are included, as follows: dusky shark (Carcharhinus obscurus; DUS), sandbar shark (Carcharhinus plumbeus; SAN), scalloped hammerhead (Sphyrna lewini; SCH), and Atlantic sharpnose shark (Rhizoprionodon terraenovae; SHP; (NOAA HMS SAFE, 2013, 2014).
The heavy exploitation of large pelagic fishes by commercial fisheries necessitates an accurate understanding of the status of their stocks (Uozumi, 2003; Braccini, 2015; Punt et al., 2015). Stock assessment models of pelagic fishes typically incorporate catch-per-unit-effort (CPUE) time-series data from commercial fisheries, but there have been notable changes in targeted species, areas fished and depth-specific gear deployments over the years (Lynch et al., 2011). For example, in the 1970s pelagic longline gear deployments shifted from shallow sets primarily targeting yellowfin tuna (Thunnus albacares) to deeper sets primarily targeting swordfish and bigeye tuna (Thunnus obesus; Lynch et al., 2012); how these shifts in fishers' behaviours altered the catchability and estimated abundances of target and bycatch species is less clear (Horodysky et al., 2007). Attempts to standardize CPUE time series for changes in target species, gear, and spatial behaviour have typically incorporated generalized linear models for Atlantic Ocean species or populations and led to the development of habitat-based standardization (HBS) approaches for population assessments of those in the Pacific Ocean (Lynch et al., 2011). In a generalized linear model, environmental data are considered indirectly via the inclusion of variables related to longline gear configurations as fixed effects that serve as proxies for habitats. In contrast, the HBS approach directly incorporates data on oceanographic conditions, fisheries gear behaviour and the ecophysiology and behaviour of pelagic fishes (Hinton and Nakano, 1996; Lynch et al., 2012). Finally, although they are not strictly stock assessment models, the recent development of spatial ecosystem, species distribution and seascape spatial distribution models also show great potential to refine spatial understanding of fish behaviour, movements, and distribution (Lehodey et al., 2008, 2015; Kearney and Porter, 2009; Alvarez-Berastegui et al., 2014; Everson et al., 2015).
Collectively, modern assessment and spatial approaches that incorporate environmental variation into pelagic fish stock assessments improve population and distribution estimates. We posit, as have others previously (e.g. Barkley et al., 1978; Brill, 1994; Brill and Lutcavage, 2001), that such approaches require a thorough mechanistic understanding of the relationship of fisheries resources to environmental variation (including perturbations of anthropogenic origin) over space and time. Likewise, a better understanding of the lethal and sublethal effects of interactions with gear and handling procedures prior to release is required to improve estimates of total fisheries mortality and the applicability of model outputs. The discipline of conservation physiology can provide these fundamental mechanistic links, because physiology is the transfer function that links specific environmental conditions to behaviour and fitness (Weissburg and Browman, 2005; Jusup et al., 2011; Horodysky et al., 2015). However, a rigorous mechanistic understanding of individual physiological response to a specific environmental parameter conducted in a reductionist laboratory setting is a grand challenge for pelagic fishes. Large, rare-event, high-oxygen-demand pelagic species are expensive and difficult to obtain, and in some cases impossible to maintain, in captivity (Box 1). Through interdisciplinary collaboration, these hurdles are being overcome.
Box 1: Unique attributes of pelagic fishes, and the challenge in studying them.
Large, highly migratory pelagic fishes, herein defined as tunas (Thunnidae), billfishes (Istiophoridae), and pelagic sharks (including Alopidae, Lamnidae, and some Carcharhinidae; Dulvy et al., 2008), inhabit the vast geographical expanse of world's open oceans. During movements and migrations throughout their extremely broad ranges, these fishes interact with tremendous acute and chronic variation in the water column's physicochemical parameters. Collectively, these fishes contain a suite of morphological and physiological adaptations for life in perpetual motion that has expanded their vertical and horizontal niches, including the following: streamlining of body and fins; regional or whole-body endothermy; large gill surface areas; blood with a higher affinity for O2; and modifications to cardiac calcium-cycling processes (Brill and Lutcavage, 2001; Bernal et al., 2009). Additionally, controlling for phylogeny, these fishes have some of the highest energetic demands and fastest growth rates among fishes (Brill and Bushnell, 1991; Brill, 1996).
The ecophysiology of pelagic fishes is thus a fruitful platform to relate form to function and the environment; however, it remains a frontier field because of the logistical challenges of working with these fishes. Pelagic fishes are generally very patchily distributed in the vast ocean; therefore, obtaining samples is a difficult, time-consuming and very expensive proposition. This is especially true if live animals (or living tissues from them) are required, because the large body sizes and high oxygen demands of these fishes make them extremely challenging to safely control, possess and successfully maintain at sea. Many of these species are thus also exceedingly difficult to transport to, and maintain in, captivity. There are only a few research facilities in the world with the proximity to pelagic habitats and the logistical resources to maintain these fishes; even then, billfishes and many species of pelagic sharks have eluded successful captive husbandry.
Where there are challenges, however, lie opportunities. New technologies inspire new directions. With the continuing evolution of electronic tags, laptop and hand-held computers and tablets, and portable blood analysers, interdisciplinary physiological studies are increasingly moving between the laboratory and ship, each erecting and assessing hypotheses generated in the other. Conservation physiology of pelagic fishes thus represents a rapidly expanding interdisciplinary growth area for the fields of physiology, conservation, and fisheries science, replete with profound socioeconomic and management implications.
Interdisciplinary collaborations between the mechanistically driven physiological sciences, the pattern-oriented behavioural sciences, and the quantitatively driven applied fisheries sciences will greatly advance the synoptic understanding of the environment–pelagic fish–ecosystem interface. An earlier synthesis addressed interactions between mechanistic physiology and field-based and quantitative ecological sciences in the service of the interdisciplinary field of marine and freshwater fisheries science (Horodysky et al., 2015). Here, we examine how physiological studies of large, highly migratory pelagic fishes have improved their management and conservation via interdisciplinary collaborations that are directed at: (i) fish–environment relationships; (ii) bycatch reduction; and (iii) post-release survival.
Relationships of pelagic fishes to the environment: paradigms
How pelagic fishes relate to their environment bears clear implications for the generation of accurate population assessments and the resultant management and policy decisions. Pelagic fishes sample the environment with sensory receptors tuned to solutes, gases, temperature, bulk flow, electrical and magnetic fields, light, and acoustic vibrations (e.g. Kapoor and Hara, 2001; Sloman et al., 2006; Hara and Zielinski, 2007). The distributions and functional characteristics of these receptors are shaped by intense selective pressures according to species-specific life histories and ecologies (e.g. Ladich et al., 2006; Horodysky et al., 2010; Kaijura et al., 2010; Kalinoski et al., 2014). Environmental signals amplify to individual behaviour (via physiological abilities and tolerances), from the individual to the population (via behavioural iteration across individuals), and ultimately, from populations to ecosystems (via ecological iteration across species; Weissburg and Browman, 2005; Seebacher and Franklin, 2012). Disruptions from optimal conditions lead to departures from homeostasis, decreasing fitness by negatively affecting survivorship, growth and/or reproduction. Fishes respond to such deviations via complex interactive biochemical, neurological, endocrine and behavioural feedbacks (sensu Ricklefs and Wikelski, 2002). The interplay between the sensory, neural, and motor systems renders environmental conditions actionable at the organismal level.
Fry (1947) developed a classic paradigm for the fish–environment interface along different scales of biological organization by elucidating how environmental variation affects individual metabolic scope (defined as the difference between standard and maximal metabolic rate, within which all bioenergetic requirements must be met). Recent applications of aerobic scope modelling unite physiological experiments with spatial and quantitative modelling approaches to predict the effects of environmental change, and demonstrate great potential for improving mechanistic prediction of pelagic fish movements (e.g. Lehodey et al., 2013; Del Raye and Weng, 2015). Fry's paradigm forms a template for the interdisciplinary integration of pelagic fish physiology and fisheries science (Claireaux and Lefrancois, 2007) and can be used to assess the relationships between aerobic performance and fitness (Pörtner, 2010), spatial ecology (Del Raye and Weng, 2015) or be combined with more detailed quantitative models to assess bioenergetic effects on fitness (Jørgensen and Holt, 2013; Cooke et al., 2014a).
Modern quantitative approaches, including the metabolic theory of ecology, dynamic energy budget, species distribution models, and HBS-based assessments, may also provide both mechanistic and quantitative explanations of the organism–environment interaction by modelling energy fluxes and growth potential as a function of environmental conditions (Maury, 2010; Jusup et al., 2011; Nisbet et al., 2012). They can also be used to assess and predict current and future patterns of distribution and abundance (Hinton and Nakano, 1996; Humston et al., 2000; Del Raye and Weng, 2015; Everson et al., 2015; Lehodey et al., 2015). Collectively, these approaches share a common challenge, namely how to parameterize models with robust data that encapsulate the relevant climatic, temporal, ontogenetic, and intraspecific variation (Cooke et al., 2014b). But simply correlating field data with catch data to infer environment–fish relationships is circular reasoning. As discussed by Brill (1994) and Brill and Lutcavage (2001) for tuna fisheries, this is especially true when field-based catch or abundance proxies are used to determine the effects of environmental conditions on catch or abundance. Rather than field correlations alone, laboratory (Blank et al., 2004) and/or shipboard investigations (Fritsches et al., 2005; Galli et al., 2009), combined with field surveys and electronic tagging (e.g. Holland et al., 1990, 1992; Josse et al., 1998; Brill et al., 1999; Bertrand et al., 2003; Hussey et al., 2015), can improve mechanistic understanding of the dynamic temporal and spatial nature of the fish–environment interface that can be comprehended by stakeholders and effectively applied by resource managers.
The desire for simplicity and dimension reduction in pelagic fish habitat modelling has led to mechanistic missteps that make little sense from the perspective of the fishes being modelled (Brill and Lutcavage, 2001). This is likely to result from the fundamental disconnect between how humans and fishes sample the environment. For various reasons, scientists studying pelagic fishes (often via fishery-dependent means) measure variables of spatiotemporal relevance to humans (geography, depth, and time); these may be of little relevance to fish. In contrast, individual fish can only experience their immediate microhabitat (Helmuth, 2009), stratifying by the physicochemical variables they can detect in their immediate surroundings given their sensory mechanisms (temperature, oxygen, salinity, light and day length, substrate, and prey/predator abundance).
A mechanistic understanding of habitat selection by pelagic fishes therefore considers the following tenets: (i) individuals experience only their immediate surrounding environment (delimited by their multimodal sensory integration abilities in the current physicochemical conditions); and (ii) individuals can only truly prefer an environmental variable they can sense and where there is a direct relationship between receptor and/or afferent nerve activity and the physical variable (Horodysky et al., 2015). For these reasons, species-specific depth ‘preference’ mentioned in tagging studies and population assessments of large pelagic fishes (e.g. Block et al., 2001; Ward and Myers, 2005; Evans et al., 2008) is a mechanistically nonsensical concept; fishes do not have an absolute sense of depth per se (or its correlate, for that matter) and thus cannot prefer it (Bernal et al., 2009). ‘Depth preference’ is thus more likely to be a result of the interactions of light, temperature, and oxygen conditions from the perspective of a pelagic fish, and may or may not be a useful covariate for modelling fish vertical movements. Strongly positive covariation with the real mechanistic driver of behaviour would result in little bias when using depth as a proxy. However, if depth either does not correlate or has an inverse (or unknown) relationship with a mechanistic driver, model outputs may be completely inaccurate. Regardless, we posit that the term ‘depth preference’ should be avoided altogether in the literature.
A corollary concern which involves the application of HBS standardizations of CPUE data for pelagic fish stock assessments is their underlying assumption that feeding motivation (and thus catchability) is proportional to time spent at depth (Graves et al., 2003). This assumption superimposes an unknown and unmeasured behavioural driver on a non-mechanistic covariate to parameterize a population assessment. Feeding motivation integrates prey availability to the predator, energetics, homeostasis, predation risk, and energy expenditure. What if pelagic fishes are more motivated to feed at cooler, dimmer depths rather than in the surface waters where most species spend the majority of time? Habitat-based standardization applications that make this assumption risk mischaracterizing catchability and decoupling catch from abundance (Brill and Lutcavage, 2001; Horodysky et al., 2007). Reliable demographic estimates of pelagic fish populations require a rigorous understanding of which (and when) environmental and biological parameters are true drivers that affect the fitness, performance and survival of pelagic fishes, and which (and when) they are noise, (Helmuth, 2009). Such mechanistic understanding has been provided via the study of pelagic fish physiology.
Mechanistic interdisciplinary investigations link a species' physiology and ecology
Behaviours are often directed by the need to maintain physiological homeostasis in the face of environmental variation, coupling the physiology of a species to its ecology. Several physicochemical variables have received attention as mechanistic drivers of the aerobic scope and behaviour of pelagic fishes. In this section, we describe interdisciplinary collaborations between fish physiologists and pelagic fisheries scientists that have combined laboratory and field-based approaches to examine how pelagic fishes interact with driving variables. Powerful examples of this union include the explanation of the vertical movement patterns of tunas and other pelagic fishes via the effects of temperature on cardiovascular performance (e.g. Brill et al., 1998, 1999; Brill and Bushnell, 2001; Blank et al., 2004; Galli et al., 2009, 2011; Shiels et al., 2015), the expansion of vertical habitat enabled by the sensory thermophysiology (Brill et al., 2005; Fritsches et al., 2005) and the compression of realized niches in pelagic fishes based on dissolved oxygen (Brill, 1994; Prince and Goodyear, 2006; Prince et al., 2010).
Temperature controls biochemical reactions and metabolic rates (Fry, 1947) and is arguably the best understood and most influential environmental variable driving pelagic fish behaviour and distribution (Brill, 1994; Dickson, 1995; Braun et al., 2015). There has been much study of the thermal physiology and metabolic rates of tunas (reviewed by Graham and Dickson, 2001; Korsmeyer and Dewar, 2001). Inferences from these studies have been extended by analogy to billfishes and some pelagic elasmobranchs. Owing to a suite of anatomical and physiological adaptations, the aerobic scope, standard metabolic rate, and maximal aerobic metabolic rate of tunas are each three to five times greater than those of active teleosts and are dependent on environmental temperatures (Brill and Bushnell, 1991; Dewar and Graham, 1994; Brill, 1996; Korsmeyer and Dewar, 2001). The vertical and horizontal extent of habitat, combined with wide geographical distribution of highly migratory pelagic species, exposes them to a wide range of ambient temperatures. Recent studies have expanded this synthesis further by examining the effect of increased global temperatures and climate change on pelagic fish reproduction and distribution (Polovina, 2007; Muhling et al., 2011; Bell et al., 2013; Lehodey et al., 2013, 2015).
In order to exploit vertical and horizontal thermal gradients better across large and potentially shifting ranges, a phylogenetically diverse group of pelagic fishes has evolved regional endothermy (Carey, 1982; Carey and Teal, 1966, 1969; Carey et al., 1981; Wegner et al., 2015). In tunas, and in sharks of the families Lamnidae and Alopiidae, vascular countercurrent heat exchangers conserve metabolically produced heat and maintain elevated temperatures in internalized red (i.e. slow-twitch aerobic) swimming muscles and (in some species) also in the viscera, eyes and brain (Carey and Teal, 1966; Linthicum and Carey, 1972; Block et al., 1993; Block and Finnerty, 1994; Graham and Dickson, 2001; Patterson et al., 2011). In contrast, billfishes have evolved cranial, but not swimming muscle, endothermy (Carey, 1982; Block and Finnerty, 1994). Regional endothermy affects numerous biological characteristics of relevance to fisheries, including high somatic and gonadal growth rates (Dickson, 1995; Brill, 1996). However, energetic benefits of cold tolerance and elevated metabolic rates are context dependent; they are likely to be advantageous when quality prey is abundant, but not when such prey is scarce (Madigan et al., 2015). Collectively, regional endothermy of pelagic fishes enables geographical and vertical niche expansion and increased access to prey (e.g. Block et al., 1993; Dickson, 1995; Lowe et al., 2000; Schaefer and Fuller, 2010; Madigan et al., 2015).
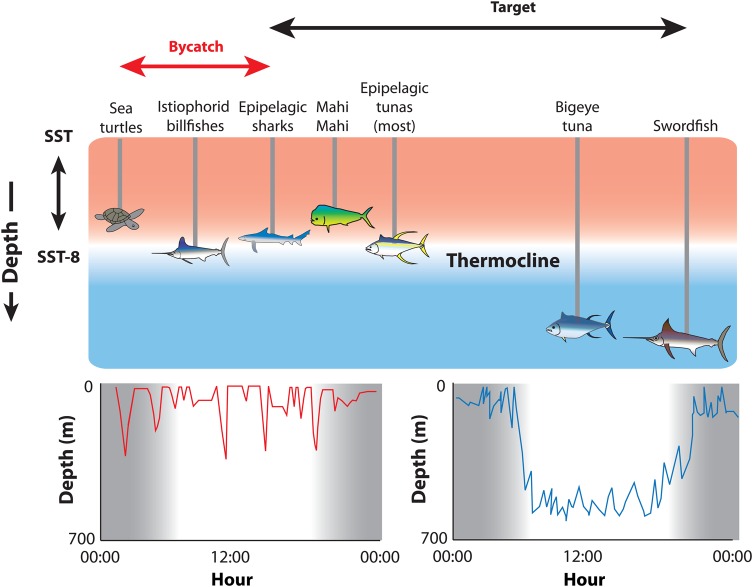
Despite their endothermic abilities, temperature conditions with depth still shape the movements, distributions and gear vulnerability of pelagic fishes (Brill and Lutcavage, 2001). There exist two overarching behavioural guilds in pelagic fish vertical thermal niches: a temperature-limited ‘epipelagic’ group bounded by the sea surface temperature and depth of the thermocline (generally 0–200 m), and a thermocline-penetrating ‘mesopelagic’ group capable of extensive vertical movement patterns that follow the diel vertical migrations of organisms of the deep scattering layer, which they exploit as prey (Fig. 2). A litany of electronic tagging studies has revealed that most species of tunas, billfishes, and pelagic sharks, as well as mahi mahi (Coryphaena hippurus), largely demonstrate the epipelagic pattern, limiting the majority of their vertical movements to the upper 8°C of the water column between sea surface temperature and the thermocline (reviewed by Brill, 1996; Brill and Lutcavage, 2001; Bernal et al., 2009; Braun et al., 2015). In contrast, bigeye tuna, swordfish and bigeye thresher sharks (Alopias superciliosus) demonstrate the mesopelagic pattern, inhabiting much deeper and cooler waters below the thermocline for most of the day and ascending at night (Bernal et al., 2009). Why is a broad taxonomic range of epipelagic-guild endothermic fishes temperature limited, and how do mesopelagic-guild fishes avoid these constraints in spite of phylogeny? There is a simple answer to the first half of the question: the heart lies outside of the influence of countercurrent heat exchangers in all species and thus immediately reflects changes in ambient temperature (Galli et al., 2011). The keys to understanding the depth patterns of these two thermal guilds lie in the effects of temperature on cardiac function.
Figure 2:
Relationship between ambient temperature and vertical movement patterns of commercially targeted and bycatch species because of the effect of temperature on cardiac function. The influence of temperature on calcium cycling in cardiac tissue limits the movements of a broad taxonomic array of pelagic fishes to the shallow, warm waters above the thermocline (Bernal et al., 2009; Galli et al., 2009). The characteristic epipelagic vertical movements (red line, lower lefthand panel) of many billfishes, tunas, and sharks are mostly bounded by the range between sea surface temperature (SST) and 8°C below STT (i.e. SST − 8; Brill et al., 1998; Bernal et al., 2009). Species that exceed this vertical and thermal range to demonstrate a more characteristic mesopelagic pattern (blue line, lower righthand panel) have cardiovascular adaptations to preserve cardiac function at low temperatures (Galli et al., 2009). Illustrated vertical movement patterns are generalized 24 h periods from the biotelemetry literature (Bernal et al., 2009).
Temperature control of cardiac function predicts species-specific vertical movement patterns in a wide taxonomic range of pelagic fishes. Mechanistic understanding of cardiac thermal niches in pelagic fishes is based largely on experiments conducted on tunas, with analogies extended to billfishes and some pelagic sharks (Brill et al., 1998; Weng et al., 2005; Bernal et al., 2009). In the epipelagic guild, reductions in cardiac calcium cycling impede excitation–contraction coupling in cardiac myocytes when instantaneous temperature changes exceed ∼8°C (Galli et al., 2009; Shiels et al., 2011). Epipelagic tunas also have extremely limited abilities to compensate for temperature-induced bradycardia by increasing stroke volume (i.e. the volume of blood pumped per heart beat); therefore, cardiac output decreases with heart rate when fish move into cooler waters (Brill et al., 1998). Accordingly, most forays to depth by the epipelagic guild must be brief and generally above the thermocline. Cardiac muscle must thereafter be warmed, resulting in a vertical movement pattern reminiscent of an air-breathing vertebrate, in which vertical excursions are followed by surface ‘recovery’ periods (Fig. 2; Horodysky et al., 2007). Body size and consequent thermal inertia may influence the variation in vertical movements and ambient temperatures seen among individuals and between species (Goldman et al., 2004; Horodysky et al., 2007). Deeper-dwelling bigeye tuna, swordfish, and bigeye thresher sharks compensate for life histories expressed in cooler, low-oxygen subthermocline waters via increased lipid stores as insulation and specific adaptations in cardiorespiratory physiology (e.g. Brill et al., 1998; Lowe et al., 2000; Blank et al., 2004; Bernal et al., 2009; Galli et al., 2009). These fishes forage extensively below the mixed layer by maintaining cardiac function via greater capacity for calcium cycling in the sarcoplasmic reticulum at reduced temperatures (Landeira-Fernandez et al., 2004, 2012; Bernal et al., 2009; Galli et al., 2009). In summary, a long history of electronic tagging studies demonstrates that daily vertical movements and thermal ranges of large pelagic fishes are species specific and thermal guild specific, and physiological experiments demonstrate the specific mechanistic underpinnings of these behaviours. The collective interdisciplinary synthesis (exemplified by Brill et al., 1999, 2005; Weng et al., 2005; Galli et al., 2009) encapsulates the physical and physiological bounds of fundamental thermal niches, habitats, and vertical movement behaviours in these fishes.
Beyond cardiac performance, there is also a sensory advantage to the endothermy of pelagic fishes that allows for vertical as well as latitudinal niche expansion. Cranial endothermy has evolved by convergence in lamnid and alopiid sharks, billfishes, tunas, butterfly mackerel (Gasterochisma melampus), and opah (Lampris guttatus), making it arguably the most widespread form of regional endothermy in fishes (Runcie et al., 2009). The energetic requirements and advantages underlying both the evolution and the maintenance of endothermy are notable. Neural and ocular endothermy expands the thermal niche in the following two ways: (i) it buffers the central nervous system from rapid changes in ambient temperature, allowing the maintenance of neural function; and (ii) it improves temporal resolution and the detection of rapid motion 10-fold, enhancing the ability to track fast-moving prey relative to unwarmed eyes (Fig. 3; Fritsches et al., 2005). For example, in swordfish that may traverse a temperature gradient of 20°C or more in minutes, the highly specialized extraocular muscle heater and retial system can warm the eyes and brain to 10–15°C above ambient (Carey, 1982). Similar mechanisms operate in billfishes that share the neural ocular heater mechanism (Carey, 1982; Block, 1986) and, to some degree, in the whole-body endothermic tunas and pelagic sharks (Block and Carey, 1985). In the case of swordfish and istiophorid billfishes, thermal niche expansion was enabled simply by the evolution of a neural heater to compensate for the extreme temperature sensitivity of the retina (Fritsches et al., 2005); in tunas and endothermic sharks, thermal niche expansion required whole-body endothermy. Although warmer retinal temperature increases thermal noise, which decreases absolute sensitivity in dim light (Aho et al., 1988), neural warming nonetheless provides the large, fast, and sensitive eyes of billfishes with a crucial advantage for detecting, pursuing, and capturing their fast-moving prey in the cold, dim waters in which they hunt (Fritsches et al., 2005). Feeding motivation may thus be high in dim, cold, oxygen-poor depths even though some of these fishes (i.e. the epipelagic guild) may not spend the majority of time there; an inference that is counter to applications of HBS assumptions that weigh feeding motivation proportionally to time spent at depth.
Figure 3:
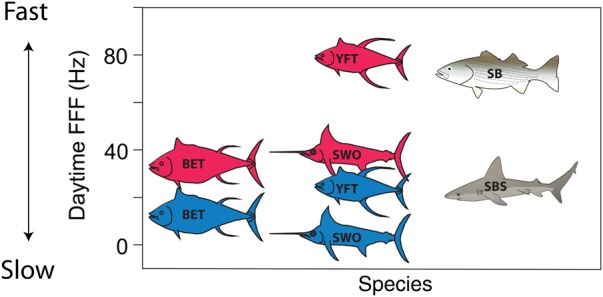
Comparison of temperature effects on temporal resolution (flicker fusion frequency, FFF) in pelagic fishes. Data for swordfishes (SWO), bigeye tuna (BET), and yellowfin tuna (YFT) are from Fritsches et al. (2005). Blue fish symbols represent data in ambient conditions at depth, whereas red fish symbols represent temporal resolution in conditions of cranial endothermy. Results for pelagic fishes are compared with coastal pelagic species including striped bass (SB; Horodysky et al., 2010) and sandbar sharks (SBS; Kalinoski et al., 2014).
Dissolved oxygen also has a substantial influence on the behaviours and distributions of high-oxygen-demand pelagic fishes, increasing the catchability of epipelagic guild fishes by surface fishing gear by constraining available predator and prey habitat to a narrow strip of shallow normoxic surface waters underlain by hypoxic regions (Prince and Goodyear, 2006; Prince et al., 2010). Regions of relatively low dissolved oxygen occur across much of the equatorial Atlantic and eastern tropical Pacific and are affected by depth, temperature, productivity, salinity and upwelling (Stramma et al., 2008). Their volume, extent, and severity are expected to increase with climate change (Stramma et al., 2012). Oxygen is a limiting factor that constrains maximal metabolic rates and metabolic scope of some tunas, and by analogy, billfishes and sharks (Fry, 1947; Bushnell et al., 1990). Although vertical movements of epipelagic guild tunas are limited by dissolved oxygen < 3.5 ml−1, deeper-dwelling bigeye tuna are tolerant of low ambient oxygen levels, routinely inhabiting waters with dissolved oxygen ∼1 ml−1 (Lowe et al., 2000). This difference lies in the significantly higher blood O2 affinity of bigeye tuna relative to yellowfin tuna, skipjack tuna, and kawakawa (Euthynnus affinis) and in potential differences in gill structure that allow mesopelagic guild fishes to extract more oxygen from their oxygen-poor habitat (Bushnell and Brill, 1991, 1992; Lowe et al., 2000; Wegner et al., 2010). The oxygen requirements of istiophorids are poorly known, but experiments with stressed juvenile sailfish (Idrisi et al., 2002) suggest that billfishes have high oxygen requirements typical of tropical epipelagic tunas and are likely to experience hypoxia-based limitation of vertical movements (Brill, 1996; Prince and Goodyear, 2006). Collectively, consideration of temperature, but omission of oxygen, may compromise the habitat standardizations of CPUE trends used by some assessment methods (Bigelow et al., 2002).
Bycatch reduction: keeping gear away from non-target species and vice versa
Bycatch reduction involves two contrasting techniques: (i) keeping the fishing gear away from the bycatch species (i.e. time–area closures, gear deployment strategies); and (ii) keeping bycatch species away from the fishing gear (i.e. making the gear less attractive). The latter bycatch-reduction technologies (i.e. repulsive devices, alternative baits) represent an applied interdisciplinary forum for collaboration. Species-specific sensory insights can be used to enhance the performance or attractiveness of gear for target species (i.e. improving target catchability), a concept that warrants further development. However, although this approach may change target:non-target catch ratios, it is unlikely to change the magnitude of discards or bycatch. Thus, it is an ineffective conservation strategy for commercial pelagic longline, gill net, and purse seine fisheries that interact with protected billfishes and sea turtles. Overly conservative management measures that function by keeping gear away from the bycatch species (e.g. time–area closures) can be economically undesirable. A more economically desirable alternative involves improving selectivity by keeping the non-target species away from fishing gear.
An integrated approach to making gear less attractive to non-target catch requires exploitable differences in the sensory biology and/or behaviours of target and non-target species that allow gear modifications to deter the latter but not the former, lest target catches decline (Southwood et al., 2008; Brill et al., 2009; Wang et al., 2010). Given the broad taxonomic diversity of bycatch (spanning primitive and advanced fishes, reptiles, and birds), it is unlikely that a single solution will reduce all bycatch while simultaneously increasing (or at least not significantly decreasing) the target catch. Rather, a series of strategies is likely to be required for different species, regions, and oceanographic conditions (Hall, 1996). Understanding the sensory abilities of fisheries resources (e.g. Brill et al., 2005; Horodysky et al., 2008a,b) and bycatch species (e.g. Fritsches et al., 2000; Hart and Collin, 2015) is the critical first step to developing a library of potential technologies (Erickson and Berkeley, 2008; Southwood et al., 2008; Stroud et al., 2013; O'Connell et al., 2014a,b; Martin and Crawford, 2015). There are surprisingly few sensory data for many pelagic species (particularly fishes; Brill et al., 2005), perhaps owing to the difficulties of their capture and captive maintenance (Box 1).
Once baseline sensory data are obtained and exported to studies of field-based performance of deterrent and attractant stimuli, the efficacy of bycatch technologies can be iteratively tested with field gear modification trials that generate new hypotheses that can then be addressed in the laboratory (e.g. Mooney et al., 2007; Brill et al., 2009; Hutchinson et al., 2012). Effective deterrents, attractants and bait alternatives must have the following attributes: (i) easy and safe to use; (ii) affordable and exportable on a commercial scale; (iii) functional over a wide range of environmental conditions; and (iv) effective for their intended use with minimal reduction in the catch (Brill et al., 2009).
To date, the interdisciplinary development and testing of sensory-based bycatch-reduction strategies in pelagic fisheries has spanned the auditory, chemoreceptive (olfactory and gustatory), visual, and electroreceptive senses (reviewed by Southwood et al., 2008; Stroud et al., 2013). Fishes, marine mammals and sea turtles are all sensitive to low-frequency acoustic signals, which may have an initial deterrent effect on target and non-target catch but may lead to habituation (Moein Bartol and Musick, 2003; Southwood et al., 2008; Hart and Collin, 2015). Although a litany of chemicals, including natural defensive compounds, alkaloids, and pungent and bitter substances have been assessed, an effective chemical deterrent for turtle, seabird, and billfish bycatch awaits identification (Southwood et al., 2008). Sea turtle bycatch in gill nets may be reduced via visual means, including Plexiglass shark shapes, although with corresponding reductions in target catch, or by the illumination of the net with LED lights or chemical light sticks, which do not affect target catch (Wang et al., 2010). Colouring squid baits blue can reduce seabird bycatch (Cocking et al., 2008) but not that of all sea turtles (Swimmer et al., 2005). Despite dramatic differences in visual spectral sensitivity between mesoplagic-guild and epipelagic-guild fishes, bait colour does not seem to reduce bycatch of fishes or sharks (Brill et al., 2005; Southwood et al., 2008). Electropositive metals, magnets and semiochemical repellents all show some potential in reducing shark bycatch, but results appear to be temperature and species specific and may be overridden by social cues when fishes are at high density (Stoner and Kaimmer, 2008; Brill et al., 2009; Robbins et al., 2011; Hutchinson et al., 2012; Godina et al., 2013; O'Connell et al., 2014b). In the energy-depauperate pelagic environment, visual information may override chemosensory and/or electroreceptive input during predatory choices (Southwood et al., 2008; Wang et al., 2010; Hutchinson et al., 2012). Collectively, sensory bycatch studies show some successes in their stated objectives and have evaluated and eliminated other candidate technologies, providing direction for future avenues of research. Sensory-based bycatch research therefore remains a growth area for collaboration between the disciplines of physiology and fisheries science in the interest of conservation biology (Molina and Cooke, 2012; Jordan et al., 2013; Hart and Collin, 2015).
Enhancing survival following release
Sharks, tunas and billfishes occur in extensive commercial and recreational fisheries throughout the world as both target and non-target species. As bycatch cannot be avoided completely, minimizing the impact of fishing gear on the non-target catch is a critical research area. Over the past 30 years, changes in management regulations and increased conservation awareness have resulted in increased live release of pelagic fishes caught by commercial and recreational gears. In North America, for example, increasing proportions of recreationally caught istiophorid billfishes (Goodyear and Prince, 2003), bluefin tuna Thunnus thynnus (Stokesbury et al., 2011; Marcek and Graves, 2014) and sharks (Sepulveda et al., 2015) are released annually, as are all Atlantic billfishes caught by US commercial fishers (Graves and Horodysky, 2015). Internationally, the member nations of the International Commission for the Conservation of Atlantic Tunas (ICCAT) adopted a measure in 2000 requiring mandatory live release of blue and white marlin (Makaira nigricans and Kajikia albida, respectively) caught in the pelagic longline and purse seine fisheries (Graves and Horodysky, 2015).
The estimation of post-release mortality is a natural collaboration of physiologists and fisheries ecologists. Research into the survival of released fish has identified key predictors of mortality and assessed gear modifications and handling practices that reduce it (Cooke and Schramm, 2007). Standard methods for assessing post-release mortality in fishes, often including confinement (Muoneke and Childress, 1994), are simply not applicable to pelagic fishes given their size, their generally poor success in captivity, and their diversity of complex physiological demands. Efforts to investigate the physical and physiological effects of capture on large pelagic species must therefore include novel techniques used across diverse phylogenetic groups (Skomal, 2007).
The evaluation of the post-release survival of pelagic fishes has been made possible by advances in electronic tagging technologies (e.g. Donaldson et al., 2008; Graves et al., 2012; Hutchinson et al., 2012; Poisson et al., 2014; Graves and Horodysky, 2015). Initial studies of the post-release survival of pelagic fishes sought to demonstrate the efficacy of pop-up satellite archival tag technology, then to generate preliminary estimates of post-release survival for assessment purposes and pelagic fisheries management (e.g. Graves et al., 2002; Kerstetter et al., 2003; Horodysky and Graves, 2005). Investigations of terminal gear configurations (e.g. Prince et al., 2002; Domeier et al., 2003; Horodysky and Graves, 2005; Graves and Horodysky, 2008; Graves and Horodysky, 2010; Afonso et al., 2011) and handling procedures (e.g. Bromhead et al., 2012; Schlenker, 2014; Schlenker et al., in press) eventually came to include collaborative studies among fishers, physiologists, and fisheries biologists directed at predicting, quantifying, and applying the emerging synthesis in the interest of conservation (Moyes et al., 2006; Musyl et al., 2011a, 2011b; Gallagher et al., 2014).
The change in terminal gear in commercial and recreational fisheries from straight-shank J hooks to circle hooks decreased rates of post-release mortality in many pelagic fishes and other vertebrate bycatch species (Read, 2007; Campana et al., 2009; Povano et al., 2009; Graves et al., 2012; Swimmer et al., 2014). In recreational fisheries for white marlin, circle hooks have significantly reduced deep-hooking, hook-induced trauma, and mortality, resulting in management measures requiring their use with natural baits in US fishing tournaments (Horodysky and Graves, 2005; Graves and Horodysky, 2008; Graves and Horodysky, 2015). Although not universally the case, similar effects of circle hook performance on survival are generally seen with tunas, istiophorid billfishes, elasmobranchs, and sea turtles in commercial pelagic longline gear (Kerstetter et al. 2007; Campana et al., 2009; Povano et al., 2009; Afonso et al., 2011; Pacheco et al., 2011; Graves et al., 2012; Swimmer et al., 2014) and in recreationally caught tuna (Stokesbury et al., 2011). Results from recreational fisheries targeting striped and blue marlin are consistent but more subtle, presumably because of differences in fishing techniques and behavioural differences in how these fishes attack baits (Domeier et al., 2003; Graves and Horodysky, 2010). The collective synthesis is that by virtue of differences in shape, circle hooks are more likely to hook fish and other vertebrates in the jaw than straight-shank J hooks and are thus less likely to cause immediate or delayed internal trauma and physiological wasting (Borucinska et al., 2002; Cooke and Suski, 2004; Swimmer et al., 2014; Graves and Horodysky, 2015). Hook-induced injuries and consequences of capture may range from sublethal (but with fitness repercussions) to lethal, and mortality may be immediate or occur hours to weeks after the capture event (Wilson et al., 2014). Sublethal effects of hooking and capture have thus far received considerably less attention because of the logistic challenges associated with doing so for this group of animals.
To assess mortalities associated with capture stress synoptically, researchers must look beyond mortality that immediately follows the capture event to examine the cumulative impacts of physical trauma and physiological stress (Skomal, 2007). Capture stresses involve: (i) the physical trauma of hooking, fighting, and handling; and (ii) the physiological stresses of catch, exhaustive exercise, handling, and recovery (Cooke and Suski, 2005; Skomal, 2007; Wilson et al., 2014). Released fishes may experience fitness consequences ranging from perturbations in blood acid–base balance and ion levels (e.g. Skomal and Bernal, 2010; Marshall et al., 2012; Kneebone et al., 2013) to physical injury (Pranovi et al., 2001) to mortality (Kaiser and Spencer, 1995). These fitness consequences result from the following factors: (i) interactions with the fishing gear itself (which may result in catch or escapement); (ii) capture by the fishing gear; (iii) landing onto a vessel; (iv) retention on deck during catch-sorting operations; or (v) entanglement in materials used to construct fish aggregation devices (Filmalter et al., 2013; Ingolfsson et al., 2007; Giomi et al., 2008). Physiological techniques continue to provide insights into the effects of interactions with fishing gear, capture and subsequent release (e.g. Moyes et al., 2006; Skomal, 2007; Skomal and Bernal 2010; Cooke et al., 2012a; Marshall et al., 2012; Hutchinson et al., 2015), improving the welfare of fishes and other vertebrates released from commercial gillnets, purse seines, trawls, and longlines (Farrell et al., 2001; Marçalo et al., 2006; Mandelman and Farrington, 2007; Marshall et al., 2012) as well as recreational fishing gear (Cooke and Schramm, 2007). Indeed, such information is increasingly being used in various certification programmes (e.g. Marine Stewardship Council) to evaluate the sustainability of a given fishery.
Physiological studies of pelagic fishes following capture have largely focused on perturbations to homeostasis reflected in blood or muscle chemistry (Wells and Davie, 1985; Mandelman and Skomal, 2009; Skomal and Bernal, 2010; Marshall et al., 2012; Schlenker, 2014). Skomal and Chase (2002) and Skomal (2006) quantified changes in blood acid–base status, metabolites, electrolytes, and proteins in several species of sharks, tunas, and marlin after capture and release on recreational fishing gear, finding significant interspecific differences in the magnitude and source of these disturbances, with greatest disruption in the tunas. Skipjack tuna also display pronounced acidosis after induced exhaustive exercise, but have extremely rapid lactate turnover (∼1 h), rivalling mammals (Perry et al., 1985; Wells and Davie, 1985; Weber et al., 1986, Wells et al., 1986; Arthur et al. 1992). This outcome stands in stark contrast to results observed in sharks that suggest lengthy perturbations for up to 24 h (reviewed by Skomal, 2007). Moyes et al. (2006) delineated five variables linked to strenuous muscular activity and resulting physiological stress (acidosis) and tissue damage (myopathy) that distinguished surviving from moribund longline-caught sharks: Mg2+, lactate, Hsp70 mRNA, K+ and Ca2+.
Novel applications combine field-based tagging and laboratory approaches in the study of fish post-release ecophysiology, and iteratively verify assays with field-based measures and telemetric technologies. In a study of 11 shark species, Marshall et al. (2012) documented species-, family- and ecology-specific responses in plasma electrolyte levels (Na+, Cl−, Mg2+, Ca2+ and K+), metabolites (glucose and lactate), blood haematocrit, and heat shock proteins following longline capture; Marshall (2015) verified survival with satellite tags in a subset of species. A similar biochemical and satellite tagging approach was used for white marlin released from recreational fishing gear by Schlenker (2014), who found elevated K+ concentrations to be a predictor of mortality. Taken together, the results of Marshall et al. (2012) and Schlenker (2014) suggest that hyperkalaemia deserves further investigation as a mechanistic predictor of mortality.
Many studies investigating the effects of angling on the post-release survival of pelagic fishes overlook the fundamental relationships between drag, metabolism, and endurance, assuming that physiological perturbations are proportional to the duration of exertion. Significant positive relationships between stress and the duration of the capture event have been demonstrated in a diverse group of fishes (Skomal, 2006; Suski et al., 2007; Cooke et al., 2008; Heberer et al., 2010; Gallagher et al., 2014). The consideration of fight time as a mechanistic variable has understandable appeal because it can lead to practical management policy outcomes that provide best-practice angling guidelines. Unfortunately, simple correlation of fight time and stress overlooks the fact that hooked fishes can exert maximal fighting effort for only fractions of a minute because of the steep inverse relationship between endurance and swimming performance (Horodysky et al., 2015). Fishes can therefore fight maximally for a very short time, fight minimally for a long time or fight maximally in bursts followed by rest periods for an intermediate time. Although fight time may serve as an adequate stress proxy for the first two fight strategies, the burst–rest strategy confounds the utility of fight time as a predictor. This is likely to be the case in many pelagic fishes that switch between blistering runs, acrobatic aerial displays, and sounding and circling behaviours in deeper, cooler waters during capture on recreational fishing gear. Correlating blood perturbations with cumulative ‘fight time’ without considering the changing nature of the intensity of activity risks potentially biased or confounded outcomes (Horodysky et al., 2015). Triaxial accelerometers present novel options to quantify changes in activity of angled fish (Kneebone et al., 2013), but to our knowledge this technology has not yet been used with pelagic fishes.
Collectively, while assessing post-release mortality in pelagic fishes is difficult and requires multiple laboratory, telemetric, field-based approaches that quantify the extent of physical damage and the level of physiological disruption, such data are critically needed for the conservation of pelagic species. Integrations of the disciplines of molecular biology, telemetry, behaviour, and population modelling have the potential to provide more robust inferences (Davie and Kopf, 2006; Moyes et al. 2006; Musyl et al., 2011a; Cooke et al., 2013) to inform fisheries management and educate fishers about angling and handling best practices (Cooke and Schramm, 2007). Specifically, physiological techniques can be used to develop handling procedures that reduce sublethal stresses and/or avoid lethal outcomes (Cooke et al., 2002; Suski et al., 2007; Mandelman and Skomal, 2009), and may be combined with quantitative techniques to assess the bioenergetics and other fitness consequences of capture (Meka and Margraf, 2007; Musyl et al., 2011a). We view this as a major growth area for the interdisciplinary collaboration of fisheries science and physiology.
Conclusions and future directions
The conservation physiology of pelagic fishes provides both formidable challenges and exciting interdisciplinary opportunities. Pelagic fishes support some of the most important fisheries in the world, and while target species are fished near capacity, many non-target species are overfished. There is thus an exigent need to understand better the mechanistic relationships of pelagic fishes to their environment and fishing gear, the associated feedback mechanisms from the cellular to the population level, and the effects of anthropogenic stressors (i.e. fishers' behaviour) across this continuum. Pelagic habitats and their fishes are remote frontiers for mechanistic research, because access to them is both logistically difficult and expensive. Many of these species cannot tolerate captivity. Despite these challenges, new technologies and interdisciplinary collaborations are inspiring new research directions, disciplinary Rubicons are being crossed, and insights are being obtained from the cellular to the population level. Perhaps the greatest collaborative successes in pelagic fish conservation physiology have resulted from studies of fish–environment relationships, bycatch reduction, and assessment of the factors that affect rates of post-release survival. The iterative testing by one discipline of hypotheses generated by the other is beginning to enclose the fundamental−applied science continuum, leading to the development of robust mechanistic insights that lead to informed management outcomes.
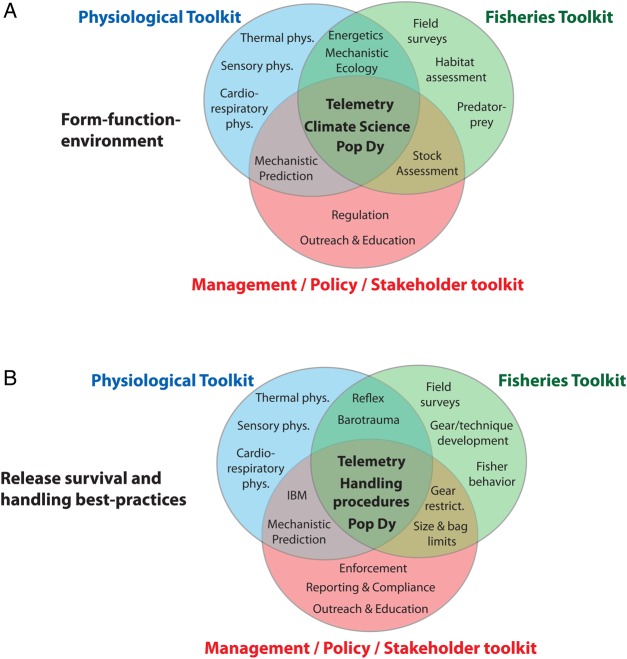
The future of pelagic fish conservation physiology lies in interdisciplinary interactions of the perspectives and toolkits possessed by the disparate disciplines of physiology and fisheries science and their integration with the needs of fisheries managers and stakeholders (Fig. 4). Overcoming logistical challenges to obtain physiological data will help to parameterize stock assessment models to improve mechanistic prediction of populations, responses to environmental change, and the effects of management plans and harvest strategies, ultimately providing better tools to guide management decisions and interventions (Cooke et al., 2014a). In the interests of conservation and stewardship, physiology must be further integrated into pelagic fisheries management and conservation (Cooke and Suski, 2008; Cooke and O'Connor, 2010).
Figure 4:
Venn diagram of the potential toolkits provided to studies of form–function–environment relationships (A) and release survival best practices (B) by both the disciplinary-specific contributions and interdisciplinary interactions of the fields of fish physiology (blue) and fisheries science (green) when combined with the management, policy, and stakeholder arena (red).
We believe, as have others (Cooke and O'Connor, 2010), that fish physiologists, fisheries scientists, and resource managers should further their collaborations to identify, plan, and evaluate future research directions and their products, and this is especially so in the frontier field of pelagic fish conservation physiology. As evidenced in the examples provided above, physiology offers a suite of tools to establish cause-and-effect relationships, provide baseline background data, and suggest and monitor the efficacy of management strategies (Wikelski and Cooke, 2006). Given that protecting all habitats and species is unrealistic, physiological tools can identify critical habitats of functional and temporal importance and the individual-level benefits associated with management recommendations (e.g. improved fitness; Cooke and Suski, 2008; Cooke et al., 2013). Furthermore, the relationship between physiology and the environment can inform management action via the incorporation of such data in population models, individual-based models, species distribution models, and mass- or energy-balance models (Metcalfe et al., 2012). Ultimately, the effective integration of physiological approaches in the synoptic management and sustained use of pelagic fish stocks requires interdisciplinary collaborations between scientists in several subdisciplines, fisheries managers and regional fisheries management agencies, and fisheries stakeholders.
Funding
A.Z.H. acknowledges support from the NOAA Living Marine Resources Cooperative Science Center and NSF Educational Partnership in Climate Change and Sustainability. S.J.C. is supported by NSERC, Ocean Tracking Network Canada, and the Canada Research Chairs Program.
Acknowledgements
This is contribution 3517 from the Virginia Institute of Marine Science. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the US Department of Commerce—National Oceanic and Atmospheric Administration (NOAA) or any of its subagencies. We thank the EU COST Action on the Conservation Physiology of Marine Fishes (especially David McKenzie) for facilitating presentation of this work in front of colleagues in Montpellier, France in the spring of 2015.
References
- Afonso AS, Hazin FHV, Carvalho F, Pacheco JC, Hazin H, Kerstetter DW, Murie D, Burgess GH (2011) Fishing gear modifications to reduce elasmobranch mortality in pelagic and bottom longline fisheries off Northeast Brazil. Fish Res 108: 336–343. [Google Scholar]
- Aho AC, Donner K, Hyden C, Larsen L, Reuter T (1988) Low retinal noise in animals with low body temperatures allows high visual sensitivity. Nature 333: 348–350. [DOI] [PubMed] [Google Scholar]
- Alvarez-Berastegui D, Ciannelli L, Aparicio-Gonzalez A, Reglero P, Hidalgo M, López-Jurado JL, Tintoré J, Alemany F (2014) Spatial scale, means and gradients of hydrographic variables define pelagic seascapes of bluefin and bullet tuna spawning distribution. PLoS ONE 9: e109338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur PG, West TG, Brill RW, Shulte PM, Hochachka PW (1992) Recovery metabolism in skipjack tuna (Katsuwonus pelamis) white muscle; rapid and parallel changes of lactate and phosphocreatine after exercise. Can J Zool 70: 1230–1239. [Google Scholar]
- Barkley RA, Neill WH, Gooding RM (1978) Skipjack tuna, Katsuwonus pelamis, habitat based on temperature and oxygen requirements. Fish Bull 76: 653–662. [Google Scholar]
- Bell J, Reid C, Batty M, Lehodey P, Rodwell L, Hobday A, Johnson JE, Demmke A (2013) Effects of climate change on oceanic fisheries in the tropical Pacific: implications for economic development and food security. Clim Change 119: 199–212. [Google Scholar]
- Bernal D, Sepulveda C, Musyl M, Brill R (2009) The eco-physiology of swimming and movement patterns of tunas, billfishes, and large pelagic sharks. In Domenici P, Kapoor BG, eds, Fish Locomotion – An Etho-Ecological Perspective. Science Publishers, Enfield, NH, USA, pp 436–483. [Google Scholar]
- Bertrand A, Josse E, Bach P, Dagorn L (2003) Acoustics for ecosystem research: lessons and perspectives from a scientific programme focusing on tuna-environment relationships. Aquat Living Resour 16: 197–203. [Google Scholar]
- Bigelow KA, Hampton J, Miyabe N (2002) Application of a habitat-based model to estimate effective longline fishing effort and relative abundance of Pacific bigeye tuna (Thunnus obesus). Fish Oceanogr 11: 143–155. [Google Scholar]
- Blank JM, Morrissette JM, Landeira-Fernandez AM, Blackwell SB, Williams TD, Block BA (2004) In situ cardiac performance of Pacific bluefin tuna hearts in response to acute temperature change. J Exp Biol 207: 881–890. [DOI] [PubMed] [Google Scholar]
- Block BA. (1986) Structure of the brain and eye heater tissues in marlins, sailfish, and spearfish. J Morphol 190: 169–189. [DOI] [PubMed] [Google Scholar]
- Block BA, Carey FG (1985) Warm brain and eye temperatures in sharks. J Comp Physiol B 156: 229–236. [DOI] [PubMed] [Google Scholar]
- Block BA, Finnerty JR (1994) Endothermy in fishes: a phylogenetic analysis of constraints, predispositions and selection pressures. Environ Biol Fish 40: 283–302. [Google Scholar]
- Block BA, Finnerty JR, Stewart AF, Kidd J (1993) Evolution of endothermy in fish: mapping physiological traits on a molecular phylogeny. Science 260: 210–214. [DOI] [PubMed] [Google Scholar]
- Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, Boustany A, Teo SLH, Seitz A, Walli A et al. (2001) Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 293: 1310–1314. [DOI] [PubMed] [Google Scholar]
- Braccini M. (2015) Is a global quantitative assessment of shark populations warranted? Fisheries 40: 492–501. [Google Scholar]
- Borucinska J, Kohler N, Natanson L, Skomal G (2002) Pathology associated with retained fishing hooks in blue sharks (Prionace glauca) with implications for their conservation. J Fish Dis 25: 515–521. [Google Scholar]
- Braun CD, Kaplan MB, Horodysky AZ, Llopiz JK (2015) Satellite telemetry reveals physical processes driving billfish behavior. Anim Biotelem 3: 2. [Google Scholar]
- Brill RW. (1994) A review of temperature and oxygen tolerance studies of tunas pertinent to fisheries oceanography, movement models and stock assessments. Fish Oceanogr 3: 204–216. [Google Scholar]
- Brill RW. (1996) Selective advantages conferred by the high performance physiology of tunas, billfishes, and dolphin fish. Comp Biochem Physiol 113: 3–15. [Google Scholar]
- Brill RW, Bushnell PG (1991) Metabolic and cardiac scope of high energy demand teleosts, the tunas. Can J Zool 69: 2002–2009. [Google Scholar]
- Brill RW, Bushnell PG (2001) The cardiovascular system of tunas. In Block BA, Stevens ED, eds, Fish Physiology, Vol. 19, Tuna – Physiology, Ecology and Evolution. Academic Press, San Diego, CA, USA, pp 79–120. [Google Scholar]
- Brill RW, Lutcavage ME (2001) Understanding environmental influences on movements and depth distributions of tunas and billfishes can significantly improve population assessments. Am Fish Soc Symp 25: 179–198. [Google Scholar]
- Brill RW, Lowe TE, Cousins KL (1998) How water temperature really limits the vertical movements of tunas and billfishes – it's the heart stupid. In Gamperl K, Farrell A, MacKinlay D, eds, International Conference on the Biology of Fish. American Fisheries Society, Baltimore, MD, USA, pp 57–62. [Google Scholar]
- Brill R, Block B, Boggs C, Bigelow K, Freund E, Marcinek D (1999) Horizontal movements and depth distribution of large, adult yellowfin tuna (Thunnus albacares) near the Hawaiian Islands, recorded using ultrasonic telemetry: implications for the physiological ecology of pelagic fishes. Mar Biol 133: 395–408. [Google Scholar]
- Brill RW, Bigelow K, Musyl M, Fritsches KA, Warrant EJ (2005) Bigeye tuna (Thunnus obesus) behavior and physiology and their relevance to stock assessments and fishery biology. Col Vol Sci Pap ICCAT 57: 142–161. [Google Scholar]
- Brill R, Bushnell P, Smith L, Speaks C, Sundaram R, Stroud W, Wang J (2009) The repulsive and feeding deterrent effects of electropositive metals on captive juvenile sandbar sharks (Carcharhinus plumbeus). Fish Bull 107: 298–307. [Google Scholar]
- Bromhead D, Clarke S, Hoyle S, Muller B, Sharples P, Harley S (2012) Identification of factors influencing shark catch and mortality in the Marshall Islands tuna longline fishery and management implications. J Fish Biol 80: 1870–1894. [DOI] [PubMed] [Google Scholar]
- Bushnell PG, Brill RW (1991) Responses of swimming skipjack (Katsuwonus pelamis) and yellowfin (Thunnus albacares) tunas exposed to acute hypoxia, and a model of their cardio-respiratory function. Physiol Zool 64: 787–811. [Google Scholar]
- Bushnell PG, Brill RW (1992) Oxygen transport and cardiovascular responses in skipjack tuna (Katsuwonus pelamis) and yellowfin tuna (Thunnus albacares) exposed to acute hypoxia. J Comp Physiol B 162: 131–143. [DOI] [PubMed] [Google Scholar]
- Bushnell PG, Brill RW, Bourke RE (1990) Cardiorespiratory responses of skipjack tuna (Katsuwonus pelamis), yellowfin tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus) to acute reductions of ambient oxygen. Can J Zool 68: 1857–1865. [Google Scholar]
- Campana SE, Joyce W, Manning MJ (2009) Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar Ecol Prog Ser 387: 241–253. [Google Scholar]
- Carey FG. (1982) A brain heater in swordfish. Science 216: 1327–1329. [DOI] [PubMed] [Google Scholar]
- Carey FG, Teal JM (1966) Heat conservation in tuna fish muscle. Proc Nat Acad Sci USA 56: 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey FG, Teal JM (1969) Mako and porbeagle: warm bodied sharks. Comp Biochem Physiol 28A: 199–204. [DOI] [PubMed] [Google Scholar]
- Carey FG, Teal JM, Kanwisher JW (1981) The visceral temperatures of mackerel sharks (Lamnidae). Physiol Zool 54: 334–344. [Google Scholar]
- Claireaux G, Lefrancois C (2007) Linking environmental variability and fish performance: integration through the concept of scope for activity. Philos Trans R Soc Lond B Biol Sci 362: 2031–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocking L, Double M, Milburn P, Brando V (2008) Seabird bycatch mitigation and blue-dyed bait: a spectral and experimental assessment. Biol Conserv 141: 1354–1364. [Google Scholar]
- Cooke SJ, O'Connor CM (2010) Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett 2: 159–166. [Google Scholar]
- Cooke SJ, Schramm HL (2007) Catch-and-release science and its application to conservation and management of recreational fisheries. Fish Manage Ecol 14: 73–79. [Google Scholar]
- Cooke SJ, Suski CD (2004) Are circle hooks an effective tool for conserving marine and freshwater recreational catch-and-release fisheries? Aquatic Conserv Mar Freshw Ecosyst 14: 299–326. [Google Scholar]
- Cooke SJ, Suski CD (2005) Do we need species-specific guidelines for catch-and-release recreational angling to conserve diverse fishery resources? Biodiv Conserv 14: 1195–1209. [Google Scholar]
- Cooke SJ, Suski CD (2008) Ecological restoration and physiology: an overdue integration. Biosci 58: 957–968. [Google Scholar]
- Cooke SJ, Schreer JF, Dunmall KM, Philipp DP (2002) Strategies for quantifying sublethal effects of marine catch-and-release angling – insights from novel freshwater applications. Am Fish Soc Symp 30: 121–134. [Google Scholar]
- Cooke SJ, Suski CD, Danylchuk SE, Danylchuk AJ, Dolandson MR, Pullen C, Bulte G, O'Toole A, Murchie KJ, Koppelman JB et al. (2008) Effects of different capture techniques on the physiological condition of bonefish Albula vulpes evaluated using field diagnostic tools. J Fish Biol 73: 1351–1375. [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives of an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Blumstein DT, Buchholz R, Caro T, Fernández-Juricic E, Franklin CJ, Metcalfe J, O'Connor CM, St Clair CC, Sutherland WJ et al. (2014a) Physiology, behaviour and conservation. Physiol Biochem Zool 87: 1–14. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Killen SS, Metcalfe JD, McKenzie DJ, Mouillot D, Jørgensen C, Peck MA (2014b) Conservation physiology across scales: insights from the marine realm. Conserv Physiol 2: doi:10.1093/conphys/cou24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie PS, Kopf RK (2006) Physiology, behavior, and welfare of fish during recreational fishing and after release. N Z Vet J 54: 161–172. [DOI] [PubMed] [Google Scholar]
- Del Raye G, Weng KC (2015) An aerobic scope-based habitat suitability index for predicting the effects of multi-dimensional climate change stressors on marine teleosts. Deep Sea Res II 113: 280–290. [Google Scholar]
- Dewar H, Graham J (1994) Studies of tropical tuna swimming performance in a large water tunnel. J Exp Biol 192: 13–31. [DOI] [PubMed] [Google Scholar]
- Dickson K. (1995) Unique adaptations of the metabolic biochemistry of tunas and billfishes for life in the pelagic environment. Environ Biol Fish 42: 65–97. [Google Scholar]
- Domeier ML, Dewar H, Nasby-Lucas N (2003) Mortality rate of striped marlin (Tetrapturus audax) caught with recreational tackle. Mar Freshw Res 54: 435–445. [Google Scholar]
- Donaldson MR, Arlinghaus R, Hanson KC, Cooke SJ (2008) Enhancing catch-and-release science and biotelemetry. Fish Fish 9: 79–105. [Google Scholar]
- Dulvy NK, Baum JK, Clarke S, Compagno LVJ, Cortés E, Domingo A, Fordham S, Fowler S, Francis MP, Gibson C et al. (2008) You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks. Aquat Conserv 18: 459–482. [Google Scholar]
- Erickson DL, Berkeley SA (2008) Methods to reduce bycatch mortality in longline fisheries. In Camhi MD, Pikitch EK, Babcock EA, eds, Sharks of the Open Ocean: Biology, Fisheries and Conservation. Blackwell Publishing Ltd, Oxford, UK, pp 462–471. [Google Scholar]
- Essington TE, Beaudreau AH, Wiedenmann J (2006) Fishing through marine food webs. Proc Nat Acad Sci USA 103: 3171–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K, Langley A, Clear NP, Williams P, Patterson T, Sibert J, Hampton J, Gunn JS (2008) Behaviour and habitat preferences of bigeye tuna (Thunnus obesus) and their influence on longline fishery catches in the western Coral Sea. Can J Fish Aquat Sci 65: 2427–2443. [Google Scholar]
- Everson JP, Hobday AJ, Hartog JR, Spillman CM, Rough KM (2015) Seasonal forecasting of tuna habitat in the Great Australian Bight. Fish Res 170: 39–49. [Google Scholar]
- Farrell AP, Gallaugher PE, Fraser J, Pike D, Bowering P, Hadwin AKM, Parkhouse W, Routledge R (2001) Successful recovery of the physiological status of coho salmon on board a commercial gillnet vessel by means of a newly designed revival box. Can J Fish Aquat Sci 58: 1932–1946. [Google Scholar]
- Filmalter JD, Capello M, Deneubourg J-L, Cowley PD (2013) Looking behind the curtain: quantifying massive shark mortality in fish aggregating devices. Front Ecol Environ 11: 291–296. [Google Scholar]
- Fritsches KA, Partridge J, Pettigrew JD, Marshall NJ (2000) Colour vision in billfish. Phil Trans R Soc Lond B Biol Sci 355: 1253–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsches KA, Brill RW, Warrant EJ (2005) Warm eyes provide superior vision in swordfishes. Curr Biol 15: 55–58. [DOI] [PubMed] [Google Scholar]
- Fry FEJ. (1947) Effect of environment on animal activity. Univ Toronto Stud Biol Ser 55: 1–62. [Google Scholar]
- Gallagher AJ, Serafy JE, Cooke SJ, Hammerschlag N (2014) Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar Ecol Prog Ser 496: 207–218. [Google Scholar]
- Galli GLJ, Shiels HA, Brill RW (2009) Temperature sensitivity of cardiac function in pelagic fishes with different vertical mobilities: yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus), mahimahi (Coryphaena hippurus), and swordfish (Xiphias gladius). Physiol Biochem Zool 82: 280–290. [DOI] [PubMed] [Google Scholar]
- Galli GLJ, Lipnick MS, Shiels HA, Block BA (2011) Temperature effects on Ca2+ cycling in scombrid cardiomyocytes: a phylogenetic comparison. J Exp Biol 214: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giomi F, Raicevich S, Giovanardi O, Pranovi F, DiMuro P, Beltramini M (2008) Catch me in winter! seasonal variation in air temperature severely enhances physiological stress and mortality of species subjected to sorting operations and discarded during annual fishing activities. Hydrobiologia 606: 195–202. [Google Scholar]
- Godina AC, Wimmer T, Wang JH, Worm B (2013) No effect from rare-earth metal deterrent on shark bycatch in a commercial pelagic longline trial. Fish Res 143: 131–135. [Google Scholar]
- Goldman K, Anderson S, Latour R, Musick J (2004) Homethermy in adult salmon sharks, Lamna ditropis. Environ Biol Fish 71: 403–411. [Google Scholar]
- Goodyear CP, Prince ED (2003) US recreational harvest of white marlin. International Commission for the Conservation of Atlantic Tunas. Col Vol Sci Pap 55: 624–632. [Google Scholar]
- Graham JB, Dickson KA (2001) Anatomical and physiological specializations for endothermy. In Block BA, Stevens ED, eds, Fish Physiology, Vol 19, Tuna – Physiology, Ecology and Evolution. Academic Press, San Diego, CA, USA, pp 121–165. [Google Scholar]
- Graves JE, Horodysky AZ (2008) Does hook choice matter? The effects of three circle hook models on post-release survival of white marlin. N Am J Fish Manage 28: 471–480. [Google Scholar]
- Graves JE, Horodysky AZ (2010) Asymmetric conservation benefits of circle hooks in multispecies billfish recreational fisheries: a synthesis of hook performance and analysis of blue marlin post-release survival. Fish Bull 108: 433–441. [Google Scholar]
- Graves JE, Horodysky AZ (2015) The challenges of estimating post-release mortality of istiophorid billfishes caught in the recreational fishery: a review. Fish Res 166: 163–168. [Google Scholar]
- Graves JE, Luckhurst BE, Prince ED (2002) An evaluation of pop-up satellite tags for estimating postrelease survival of blue marlin (Makaira nigricans) from a recreational fishery. Fish Bull 100: 134–142. [Google Scholar]
- Graves JE, Kerstetter DW, Luckhurst BE, Prince ED (2003) Habitat preferences of billfishes in the western North Atlantic: applicability of archival tag data to habitat-based stock assessment methodologies. International Commission for the Conservation of Atlantic Tunas. Coll Vol Sci Pap 55: 594–602. [Google Scholar]
- Graves JE, Horodysky AZ, Kerstetter DW (2012) Incorporating circle hooks into Atlantic pelagic fisheries: case studies from the commercial tuna/swordfish longline and recreational billfish fisheries. Bull Mar Sci 88: 411–422. [Google Scholar]
- Hall MA. (1996) On bycatches. Rev Fish Biol Fish 6: 319–352. [Google Scholar]
- Hara TJ, Zielinski BS (2007) Sensory systems neuroscience. In Farrell AP, Brauner CJ, Hoar WS, Randall DJ, eds, Fish Physiology, Vol 25. Academic Press, San Diego, CA, USA. [Google Scholar]
- Hart NS, Collin SP (2015) Shark senses and shark repellents. Integr Zool 10: 38–62. [DOI] [PubMed] [Google Scholar]
- Heberer C, Albers SA, Bernal D, Kohin S, DiFiore B, Sepulveda CA (2010) Insights into catch-and-release survivorship and stress-induced blood biochemistry of common thresher sharks (Alopias vulpinus) captured in the southern California recreational fishery. Fish 106: 495–500. [Google Scholar]
- Helmuth B. (2009) From cells to coastlines: how can we use physiology to forecast the impacts of climate change? J Exp Biol 212: 753–760. [DOI] [PubMed] [Google Scholar]
- Hinton MG, Nakano H (1996) Standardizing catch and effort statistics using physiological, ecological, or behavioral constraints and environmental data, with an application to blue marlin (Makaira nigricans) catch and effort data from Japanese longline fisheries in the Pacific. Bull IATTC 21: 171–200. [Google Scholar]
- Holland KN, Brill RW, Chang RKC (1990) Horizontal and vertical movements of yellowfin and bigeye tuna associated with fish aggregating devices. Fish Bull 88: 493–507. [Google Scholar]
- Holland KN, Brill RW, Chang RKC, Sibert JR, Fournier DA (1992) Physiological and behavioral thermoregulation in bigeye tuna (Thunnus obesus). Nature 358: 410–412. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Graves JE (2005) Application of pop-up satellite archival tag technology to estimate postrelease survival of white marlin (Tetrapturus albidus) caught on circle and straight-shank (‘J’) hooks in the western North Atlantic recreational fishery. Fish Bull 103: 84–96. [Google Scholar]
- Horodysky AZ, Kerstetter DW, Latour RJ, Graves JE (2007) Habitat utilization and vertical movements of white marlin (Tetrapturus albidus) released from commercial and recreational fishing gears in the western North Atlantic Ocean: inferences from short duration pop-up archival satellite tags. Fish Oceanogr 16: 240–256. [Google Scholar]
- Horodysky AZ, Brill RW, Fine ML, Musick JA, Latour RJ (2008a) Acoustic pressure and acceleration thresholds in six sciaenid fishes. J Exp Biol 211: 1504–1511. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Brill RW, Warrant EJ, Musick JA, Latour RJ (2008b) Comparative visual function in five sciaenid fishes. J Exp Biol 211: 3601–3612. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Brill RW, Warrant EJ, Musick JA, Latour RJ (2010) Comparative visual function in four piscivorous fishes inhabiting Chesapeake Bay. J Exp Biol 213: 1751–1761. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Cooke SJ, Brill RW (2015) Physiology in the service of fisheries science: why thinking mechanistically matters. Rev Fish Biol Fish 25: 425–447. [Google Scholar]
- Humston R, Ault JS, Lutcavage M, Olson DB (2000) Schooling and migration of large pelagic fishes relative to environmental cues. Fish Oceanogr 9: 136–146. [Google Scholar]
- Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fish AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF et al. (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science 348: 1255642. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Wang JH, Swimmer Y, Holland K, Kohin S, Dewar H, Wraith J, Vetter R, Heberer C, Martinez J (2012) The effects of a lanthanide metal alloy on shark catch rates. Fish Res 131–133: 45–51. [Google Scholar]
- Hutchinson MR, Itano DG, Muir JA, Holland KN (2015) Post-release survival of juvenile silky sharks captured in a tropical tuna purse seine fishery. Mar Ecol Prog Ser 521: 143–154. [Google Scholar]
- ICCAT SCRS. 2012. Report of the Standing Committee on Research and Statistics (SCRS), Madrid, Spain, October 1–5, 2012. ICCAT, Madrid, Spain, 300 pp. [Google Scholar]
- Idrisi N, Capo TR, Luthy S, Serafy JE (2002) Behavior, oxygen consumption and survival of stressed juvenile sailfish (Istiophorus platypterus) in captivity. Mar Freshw Behav Physiol 36: 51–57. [Google Scholar]
- Ingolfsson OA, Soldal A, Huse I, Breen MB (2007) Escape mortality of cod, saithe, and haddock in a Barents Sea trawl fishery. ICES J Mar Sci 64: 836–1844. [Google Scholar]
- ISSF (2013) Status of the world fisheries for tuna. ISSF Technical Report 2013-04A. International Seafood Sustainability Foundation, Washington, DC, USA. [Google Scholar]
- Jordan LK, Mandelman JM, McComb DM, Fordham SV, Carlson JK, Werner TB (2013) Linking sensory biology and fisheries bycatch reduction in elasmobranch fishes: a review with new directions for research. Conserv Physiol 1: doi:10.1093/conphys/cot002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen C, Holt RE (2013) Natural mortality: its ecology, how it shapes fish life histories, and why it may be increased by fishing. J Sea Res 75: 8–18. [Google Scholar]
- Josse E, Bach P, Dagorn L (1998) Simultaneous observations of tuna movements and their prey by sonic tracking and acoustic surveys. Hydrobiologia 371/372: 61–69. [Google Scholar]
- Jusup M, Klanjscek T, Matsuda H, Kooijman SALM (2011) A full life cycle bioenergetic model for bluefin tuna. PLoS ONE 6: e21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MJ, Spencer BE (1995) Survival of by-catch from a beam trawl. Mar Ecol Prog Ser 126: 31–38. [Google Scholar]
- Kalinoski M, Hirons A, Horodysky A, Brill R (2014) Spectral sensitivity, light sensitivity, and temporal resolution of the visual systems in three sympatric coastal shark species. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200: 997–1013. [DOI] [PubMed] [Google Scholar]
- Kaijura SM, Cornett AD, Yopak KE (2010) Sensory adaptations to the environment: electroreceptors as a case study. In Carrier JC, Musick JA, Heithaus MR, eds, Sharks and Their Relatives II. Biodiversity, Adaptive Physiology, and Conservation. CRC Press, Boca Raton, FL, USA, pp 393–433. [Google Scholar]
- Kapoor BG, Hara TJ (2001) Sensory Biology of Jawed Fishes: New Insights. Science Publishers, Enfield, NH, USA. [Google Scholar]
- Kearney M, Porter WP (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species' ranges. Ecol Lett 12: 334–350. [DOI] [PubMed] [Google Scholar]
- Kerstetter DW, Luckhurst BE, Prince ED, Graves JE (2003) Use of pop-up satellite archival tags to demonstrate survival of blue marlin (Makaira nigricans) released from pelagic longline gear. Fish Bull 101: 939–948. [Google Scholar]
- Kerstetter DW, Pacheco JC, Hazin FH, Travassos PE, Graves JE (2007) Preliminary results of circle and J-style hook comparisons in the Brazilian pelagic longline fishery. Col Vol Sci Pap ICCAT 60: 2140–2147. [Google Scholar]
- Kneebone J, Chisholm J, Bernal D, Skomal G (2013) The physiological effects of capture stress, recovery, and post-release survivorship of juvenile sand tigers (Carcharias taurus) caught on rod and reel. Fish Res 147: 103–114. [Google Scholar]
- Korsmeyer K, Dewar H (2001) Tuna metabolism and energetics. In Block BA, Stevens ED, eds, Fish Physiology, Vol 19, Tuna – Physiology, Ecology and Evolution. Academic Press, San Diego, CA, USA, pp 35–78. [Google Scholar]
- Ladich F, Collin SP, Moller P, Kapoor BG (2006) Communication in Fishes, Vol 1 and 2. Science Publishers, Enfield, NH, USA. [Google Scholar]
- Landeira-Fernandez AM, Morrissette JM, Blank JM, Block BA (2004) Temperature dependence of the Ca2+-ATPase (SERCA2) in the ventricles of tuna and mackerel. Am J Physiol Regul Integr Comp Physiol 286: R398–R404. [DOI] [PubMed] [Google Scholar]
- Landeira-Fernandez AM, Castilho PC, Block BA (2012) Thermal dependence of cardiac SR Ca2+-ATPase from fish and mammals. J Therm Biol 37: 217–233. [Google Scholar]
- Lehodey P, Senina I, Murtugudde R (2008) A spatial ecosystem and populations dynamics model (SEAPODYM) – modeling of tuna and tuna-like populations. Prog Oceanogr 78: 304–318. [Google Scholar]
- Lehodey P, Senina I, Calmettes B, Hampton J, Nicol S (2013) Modelling the impact of climate change on Pacific skipjack tuna population and fisheries. Clim Change 119: 95–109. [Google Scholar]
- Lehodey P, Senina I, Nicol S, Hampton J (2015) Modelling the impact of climate change on South Pacific albacore tuna. Deep Sea Res II 113: 246–259. [Google Scholar]
- Lesser Antilles Pelagic Ecosystem (LAPE) project - Web site. Pelagic Fisheries in the LAPE area. FI Institutional Websites. In: FAO Fisheries and Aquaculture Department [online]. Rome. Updated 28 November 2008. [Google Scholar]
- Linthicum DS, Carey FG (1972) Regulation of brain and eye temperatures of the bluefin tuna. Comp Biochem Physiol 43A: 425–433. [DOI] [PubMed] [Google Scholar]
- Lowe TE, Brill RW, Cousins KL (2000) Blood oxygen-binding characteristics of bigeye tuna (Thunnus obesus), a high-energy-demand teleost that is tolerant of low ambient oxygen. Mar Biol 136: 1087–1098. [Google Scholar]
- Lynch PD, Graves JE, Latour RJ (2011) Challenges in the assessment and management of highly migratory bycatch species: a case study of Atlantic marlins. In Taylor WW, Schechter MG, Lynch AJ, eds, Sustainable Fisheries: Multilevel Approaches to a Global Problem. American Fisheries Society, Bethesda, MD, USA, pp 197–226. [Google Scholar]
- Lynch PD, Shertzer KW, Latour RJ (2012) Performance of methods used to estimate indices of abundance for highly migratory species. Fish Res 125–126: 27–39. [Google Scholar]
- Madigan DJ, Carlisle AB, Gardner LD, Jayasundara N, Micheli F, Shaefer KM, Fuller DW, Block BA (2015) Assessing niche width of endothermic fish from genes to ecosystem. Proc Nat Acad Sci USA 112: 8350–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelman JW, Farrington MA (2007) The physiological status and mortality associated with otter-trawl capture, transport, and captivity of an exploited elasmobranch, Squalus acanthias. ICES J Mar Sci 64: 122–130. [Google Scholar]
- Mandelman JW, Skomal GB (2009) Differential sensitivity to capture stress assessed by blood acid–base status in five carcharhinid sharks. J Comp Physiol B 179: 267–277. [DOI] [PubMed] [Google Scholar]
- Marçalo A, Mateus L, Duarte Correia JH, Serra P, Fryer R, Stratoudakis Y (2006) Sardine (Sardina pilchardus) stress reactions to purse seine fishing. Mar Biol 149: 1509–1518. [Google Scholar]
- Marcek BJ, Graves JE (2014) An estimate of postrelease mortality of school-size bluefin tuna in the U.S. recreational troll fishery. N Am J Fish Manage 34: 602–608. [Google Scholar]
- Marshall HM. (2015) Investigations in the stress physiology and survival of elasmobranch fishes, with applications to fisheries management. PhD Dissertation University of Massachusetts, Dartmouth, 133 pp. [Google Scholar]
- Marshall H, Field L, Afiadata A, Sepulveda C, Skomal G, Bernal D (2012) Hematological indicators of stress in longline-captured sharks. Comp Biochem Physiol A Mol Integr Physiol 162: 121–129. [DOI] [PubMed] [Google Scholar]
- Martin GR, Crawford R (2015) Reducing bycatch in gillnets: a sensory ecology perspective. Global Ecol Conserv 3: 28–50. [Google Scholar]
- Maury O. (2010) An overview of APECOSM, a spatialized mass balanced ‘Apex Predators ECOSystem Model’ to study physiologically structured tuna population dynamics in their ecosystem. Progr Oceanogr 84: 113–117. [Google Scholar]
- Meka JM, Margraf FJ (2007) Using a bioenergetics model to assess growth reduction from catch-and-release fishing and hooking injury in rainbow trout, Oncorhynchus mykiss. Fish Manage Ecol 14: 131–139. [Google Scholar]
- Metcalfe JD, Le Quesne WJF, Cheung WWL, Righton DA (2012) Conservation physiology for applied management of marine fish: an overview with perspectives on the role and value of telemetry. Philos Trans R Soc B Biol Sci 367: 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein Bartol S, Musick JA (2003) Sensory biology of sea turtles. In Lutz PL, Musick JA, Wyneken J, eds, The Biology of Sea Turtles, Vol 2. CRC Press, Boca Raton, FL, USA, pp 79–102. [Google Scholar]
- Molina JM, Cooke SJ (2012) Trends in shark bycatch research: current status and research needs. Rev Fish Biol Fish 22: 719–737. [Google Scholar]
- Mooney TA, Au WWL, Nachtigall PE, Trippel EA (2007) Acoustic and stiffness properties of gillnets as they relate to small cetacean bycatch. ICES J Mar Sci 64: 1–9. [Google Scholar]
- Moyes CD, Fragoso N, Brill RW, Musyl MK (2006) Predicting postrelease survival in large pelagic fish. Trans Am Fish Soc 135: 1389–1397. [Google Scholar]
- Muhling BA, Lee S-K, Lamkin JT, Liu Y (2011) Predicting the effects of climate change on bluefin tuna (Thunnus thynnus) spawning habitat in the Gulf of Mexico. ICES J Mar Sci 68: 1051–1062. [Google Scholar]
- Muoneke MI, Childress WM (1994) Hooking mortality: a review for recreational fisheries. Rev Fish Sci 2: 123–156. [Google Scholar]
- Musyl MK, Domeier ML, Nasby-Lucas N, Brill RW, McNaughton LM, Swimmer JY, Lutcavage MS, Wilson SG, Galuardi B, Liddle JB (2011a). Performance of pop-up satellite archival tags. Mar Ecol Prog Ser 433: 1–28. [Google Scholar]
- Musyl MK, Brill RW, Curran DS, Fragoso NM, McNaughton LM, Nielsen A, Kikkawa BS, Moyes CD (2011b) Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish Bull 109: 341–368. [Google Scholar]
- Nisbet RM, Jusup M, Klanjscek T, Pecquerie L (2012) Integrating dynamic energy budget (DEB) theory with traditional bioenergetic models. J Exp Biol 215: 892–902. [DOI] [PubMed] [Google Scholar]
- NOAA HMS SAFE Report (2013) Stock Assessment and Fishery Evaluation Report. Status of the U.S. west coast fisheries for highly migratory species through 2013. Pacific Fishery Management Council, Portland, OR, USA, 93 pp. [Google Scholar]
- NOAA HMS SAFE Report (2014) Stock Assessment and Fishery Evaluation Report. Atlantic Highly Migratory Species. NOAA, Silver Spring, MD, USA, 195 pp. [Google Scholar]
- NOAA HMS SAFE Report (2015) Stock assessment and fishery evaluation Report. Status of the U.S. west coast fisheries for highly migratory species through 2013. Portland, OR, 93 PP. [Google Scholar]
- O'Connell CP, Andreotti S, Rutzen M, Meÿer M, He P (2014a) The use of permanent magnets to reduce elasmobranch encounter with a simulated beach net. 2. The great white shark (Carcharodon carcharias). Ocean Coast Manag 97: 20–28. [Google Scholar]
- O'Connell CP, Stroud EM, He P (2014b) The emerging field of electrosensory and semiochemical shark repellents: mechanisms of detection, overview of past studies, and future directions. Ocean Coast Manag 97: 2–11. [Google Scholar]
- Oliver S, Braccini M, Newman SJ, Harvey ES (2015) Global patterns in the bycatch of sharks and rays. Mar Policy 54: 86–97. [Google Scholar]
- Pacheco JC, Kerstetter DW, Hazin FH, Hazin H, Segundo RSSL, Graves JE, Carvalho F, Travassos PE (2011) A comparison of circle hook and J hook performance in a western equatorial Atlantic Ocean pelagic longline fishery. Fish Res 107: 39–45. [Google Scholar]
- Patterson JC, Sepulveda CA, Bernal D (2011) The vascular morphology and in vivo muscle temperatures of thresher sharks (Alopiidae). J Morphol 272: 1353–1364. [DOI] [PubMed] [Google Scholar]
- Perry SF, Daxboeck C, Emmett B, Hochachka PW, Brill RW (1985) Effects of exhausting exercise on acid-base regulation in skipjack tuna (Katsuwonus pelamis) blood. Physiol Zool 58: 421–429. [DOI] [PubMed] [Google Scholar]
- Poisson F, Filmalter JD, Vernet A-L, Laurent Dagorn L (2014) Mortality rate of silky sharks (Carcharhinus falciformis) caught in the tropical tuna purse seine fishery in the Indian Ocean. Can J Fish Aquat Sci 71: 795–798. [Google Scholar]
- Polovina JJ. (2007) Decadal variation in the trans-Pacific migration of northern bluefin tuna (Thunnus thynnus) coherent with climate-induced change in prey abundance. Fish Oceanogr 5: 114–119. [Google Scholar]
- Pörtner HO. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- Pranovi F, Raicevich S, Franceschini G, Torricelli P, Giovanardi O (2001) Discard composition and damage to non-target species in the “rapido” trawl fishery. Mar Biol 139: 863–875. [Google Scholar]
- Prince ED, Goodyear CP (2006) Hypoxia-based habitat compression of tropical pelagic fishes. Fish Oceanogr 15: 451–464. [Google Scholar]
- Prince ED, Ortiz M, Venizelos A (2002) A comparison of circle and J hook performance in recreational catch-and-release fisheries for billfish. In Lucy JA, Studholme A, eds, Catch and Release in Marine Recreational Fisheries, Vol 30 Am. Fish. Soc. Symp, Bethesda, MD, pp 66–79. [Google Scholar]
- Prince EP, Luo J, Goodyear CP, Hoolihan JP, Sondgrass D, Orbesen ES, Serafy JS, Ortiz M, Schirripa MJ (2010) Ocean scale hypoxia-based compression of Atlantic istiophorid billfishes. Fish Oceanogr 19: 448–462. [Google Scholar]
- Punt AE, Su N-J, Sun C-L (2015) Assessing billfish stocks: a review of current methods and some future directions. Fish Res 166: 103–118. [Google Scholar]
- Read AJ. (2007) Do circle hooks reduce the mortality of sea turtles in pelagic longlines? A review of recent experiments. Biol Conserv 135: 155–169. [Google Scholar]
- Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17: 462–468. [Google Scholar]
- Robbins WD, Peddemors VM, Kennelly SJ (2011) Assessment of permanent magnets and electropositive metals to reduce the line-based capture of Galapagos sharks, Carcharhinus galapagensis. Fish Res 109: 100–106. [Google Scholar]
- Runcie RM, Dewar H, Hawn DR, Frank LR, Dickson KA (2009) Evidence for cranial endothermy in the opah (Lampris guttatus). J Exp Biol 212: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer KM, Fuller DW (2010) Vertical movements, behavior, and habitat of bigeye tuna (Thunnus obesus) in the equatorial eastern Pacific Ocean, ascertained from archival tag data. Mar Biol 157: 2625–2642. [Google Scholar]
- Schlenker LS. (2014) Physiological stress and post-release mortality of white marlin (Kajikia albida) caught in the US recreational fishery. MS Thesis College of William & Mary, Williamsburg, Virginia, USA, 87 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker LS, Latour RJ, Brill RW, Graves JE (in press) Physiological stress and post-release mortality of white marlin (Kajikia albida) caught in the US recreational fishery. Conserv Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, Franklin CE (2012) Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil Trans R Soc Lond B Biol Sci 367: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda CA, Heberer C, Aalbers SA, Spear N, Kinney M, Bernal D, Kohin S (2015) Post-release survivorship studies on common thresher sharks (Alopias vulpinus) captured in the southern California recreational fishery. Fish Res 161: 102–108. [Google Scholar]
- Shiels HA, Di Maio A, Thompson S, Block BA (2011) Warm fish with cold hearts: thermal plasticity of excitation-contraction coupling in bluefin tuna. Proc Biol Sci 278: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels HA, Galli GLJ, Block BA (2015) Cardiac function in an endothermic fish: cellular mechanisms for overcoming acute thermal challenges during diving. Proc Biol Sci 282: 20141989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skomal G. (2006) The physiological effects of capture stress on post-release survivorship of sharks, tunas, and marlin. PhD Dissertation Boston University, Boston, MA, USA, 304 pp. [Google Scholar]
- Skomal GB. (2007) Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fish Manage Ecol 14: 81–89. [Google Scholar]
- Skomal G, Bernal D (2010) Physiological responses to stress in sharks. In Carrier JC, Musick JA, Heithaus MR, eds, Sharks and Their Relatives II. Biodiversity, Adaptive Physiology, and Conservation. CRC Press, Boca Raton, FL, USA, pp 460–490. [Google Scholar]
- Skomal G, Chase B (2002) The physiological effects of angling on post-release surviorship in tunas, sharks, and marlin. In Lucy JA, Studholme AL, eds, Catch and Release in Marine Recreational Fisheries. Am Fish Soc Symp 30, pp 135–138. [Google Scholar]
- Sloman KA, Wilson RW, Balshine S (2006) Behavior and physiology. In Farrell AP, Brauner CJ, Hoar WS, Randall DJ, eds, Fish Physiology, Vol 24, Academic Press, San Diego, CA, USA. [Google Scholar]
- Southwood A, Fritsches K, Brill R, Swimmer Y (2008) Sound, chemical, and light detection in sea turtles and pelagic fishes: sensory-based approaches to bycatch reduction in longline fisheries. Endang Species Res 5: 225–238. [Google Scholar]
- Stokesbury MJW, Neilson JD, Susko E, Cooke SJ (2011) Estimating mortality of Atlantic bluefin tuna (Thunnus thynnus) in an experimental recreational catch-and-release fishery. Biol Conserv 144: 2684–2691. [Google Scholar]
- Stoner AW, Kaimmer SM (2008) Reducing elasmobranch bycatch: laboratory investigation of rare earth metal and magnetic deterrents with spiny dogfish and Pacific halibut. Fish Res 92: 162–168. [Google Scholar]
- Stramma L, Johnson GC, Sprintall J, Mohrholz V (2008) Expanding oxygen-minimum zones in the tropical oceans. Science 320: 655–658. [DOI] [PubMed] [Google Scholar]
- Stramma L, Prince ED, Schmidtko S, Luo J, Hoolihan JP, Visbeck M (2012) Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nat Clim Change 2: 33–37. [Google Scholar]
- Stroud EM, O'Connell CP, Rice PH, Snow NH, Barnes BB, Elshaer MR, Hanson JE (2013) Chemical shark repellent: myth or fact? The effect of a shark necromone on shark feeding behavior. Ocean Coast Manag 97: 50–57. [Google Scholar]
- Suski CD, Cooke SJ, Danylchuk AJ, O'Connor CM, Gravel M-A, Redpath T, Hanson KC, Gingerich AJ, Murchie KJ, Danylchuk SE et al. (2007) Physiological disturbance and recovery dynamics of bonefish (Albula vulpes), a tropical marine fish, in response to variable exercise and exposure to air. Comp Biochem Physiol A Mol Integr Physiol 148: 664–673. [DOI] [PubMed] [Google Scholar]
- Swimmer Y, Arauz R, Higgins B, McNaughton L, McCracken M, Ballestro J, Brill R (2005) Food color and marine turtle feeding behavior: can blue bait reduce turtle bycatch in commercial fisheries? Mar Ecol Prog Ser 295: 273–278. [Google Scholar]
- Swimmer Y, Empey CE, McNaughton L, Musyl M, Pargae M (2014) Post-release mortality estimates of loggerhead sea turtles (Caretta caretta) caught in pelagic longline fisheries based on satellite data and hooking location. Aquat Conserv Mar Freshw Ecosyst 24: 498–510. [Google Scholar]
- Uozumi Y. (2003) Historical perspective of global billfish stock assessment. Mar Freshw Res 54: 555–565. [Google Scholar]
- Wang JH, Fisler S, Swimmer Y (2010) Developing visual deterrents to reduce sea turtle bycatch in gillnet fisheries. Mar Ecol Progr Ser 408: 241–250. [Google Scholar]
- Ward P, Myers RA (2005) Inferring the depth distribution of catchability for pelagic fishes and correcting for variations in the depth of longline fishing gear. Can J Fish Aquat Sci 62: 1130–1142. [Google Scholar]
- Weber JM, Brill RW, Hochachka PW (1986) Mammalian metabolic flux rates in a teleost: lactate and glucose turnover in tuna. Am J Physiol Regul Integr Comp Physiol 250: R452–R458. [DOI] [PubMed] [Google Scholar]
- Wegner NC, Sepulveda CA, Bull KB, Graham JB (2010) Gill morphometrics in relation to gas transfer and ram ventilation in high-energy demand teleosts: scombrids and billfishes. J Morphol 271: 36–49. [DOI] [PubMed] [Google Scholar]
- Wegner NC, Snodgrass OE, Dewar H, Hyde JR (2015) Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus. Science 348: 786–789. [DOI] [PubMed] [Google Scholar]
- Weissburg MJ, Browman HI (2005) Sensory biology: linking the internal and external ecologies of marine organisms. Mar Ecol Progr Ser 287: 263–265. [Google Scholar]
- Wells RMG, Davie PS (1985) Oxygen binding by the blood and hematological effects of capture stress in two big gamefish: mako shark and striped marlin. Comp Biochem Physiol A Comp Physiol 81: 643–646. [DOI] [PubMed] [Google Scholar]
- Wells RMG, McIntyre RH, Morgan AK, Davie PS (1986) Physiological stress responses in big gamefish after capture: observations on plasma chemistry and blood factors. Comp Biochem Physiol A Comp Physiol 84: 565–571. [DOI] [PubMed] [Google Scholar]
- Weng KC, Castilho PC, Morrissette JM, Landeira-Fernandez AM, Holts DB, Schallert RJ, Goldman KJ, Block BA (2005) Satellite tagging and cardiac physiology reveal niche expansion in salmon sharks. Science 310: 104–106. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Raby GD, Burnett NJ, Hinch SG, Cooke SJ (2014) Looking beyond the mortality of bycatch: sublethal effects of incidental capture on marine animals. Biol Conserv 171: 61–72. [Google Scholar]
- Worm B, Hilborn R, Baum JK, Branch TA, Collie JS, Costello C, Fogarty MJ, Fulton EA, Hutchings JA, Jennings S et al. (2009) Rebuilding global fisheries. Science 325: 578–585. [DOI] [PubMed] [Google Scholar]
- Worm B, Davis B, Kettemer L, Ward-Paige C-A, Chapman D, Heithaus MR, Kessel ST, Gruber SH (2013) Global catches, exploitation rates, and rebuilding options for sharks. Mar Policy 40: 194–204. [Google Scholar]