Combining physiological and morphological measures in the laboratory with registrations of detailed measures of field activity, we tested the hypothesis that individual activity patterns correlate with individual metabolism and morphology as proposed by several conceptual models. We found no evidence indicating an effect of metabolism, whereas morphology correlated with several activity measures.
Keywords: Aerobic metabolic scope, fineness ratio, morphology, OCLTT hypothesis, performance and allocation models, standard metabolic rate
Abstract
Ongoing climate change is affecting animal physiology in many parts of the world. Using metabolism, the oxygen- and capacity-limitation of thermal tolerance (OCLTT) hypothesis provides a tool to predict the responses of ectothermic animals to variation in temperature, oxygen availability and pH in the aquatic environment. The hypothesis remains controversial, however, and has been questioned in several studies. A positive relationship between aerobic metabolic scope and animal activity would be consistent with the OCLTT but has rarely been tested. Moreover, the performance model and the allocation model predict positive and negative relationships, respectively, between standard metabolic rate and activity. Finally, animal activity could be affected by individual morphology because of covariation with cost of transport. Therefore, we hypothesized that individual variation in activity is correlated with variation in metabolism and morphology. To test this prediction, we captured 23 wild European perch (Perca fluviatilis) in a lake, tagged them with telemetry transmitters, measured standard and maximal metabolic rates, aerobic metabolic scope and fineness ratio and returned the fish to the lake to quantify individual in situ activity levels. Metabolic rates were measured using intermittent flow respirometry, whereas the activity assay involved high-resolution telemetry providing positions every 30 s over 12 days. We found no correlation between individual metabolic traits and activity, whereas individual fineness ratio correlated with activity. Independent of body length, and consistent with physics theory, slender fish maintained faster mean and maximal swimming speeds, but this variation did not result in a larger area (in square metres) explored per 24 h. Testing assumptions and predictions of recent conceptual models, our study indicates that individual metabolism is not a strong determinant of animal activity, in contrast to individual morphology, which is correlated with in situ activity patterns.
Introduction
Aerobic metabolism in animals is dependent on several abiotic factors, including ambient water temperature and CO2 and O2 levels. For example, metabolism associated with maintenance increases as a function of temperature in ectothermic animals (Brett, 1964; Tirsgaard et al., 2015). Consequently, metabolic performance, typically measured as oxygen consumption rate, has been identified as a key component in predicting the reaction of aquatic ectothermic animals to climate change and ultimately their conservation through the oxygen- and capacity-limited thermal tolerance (OCLTT) hypothesis (Pörtner, 2010). Specifically, the OCLTT model proposes that the functional capacity of systems supplying and using oxygen sustains the aerobic performance capacity of the organism (Pörtner, 2010) and becomes limiting at high temperature extremes (Bozinovic and Pörtner, 2015). The OCLTT represents a major tool to predict the consequences of variation in temperature, oxygen availability and pH in the aquatic environment within the emerging field of conservation physiology. A central tenet of the OCLTT hypothesis is that aerobic metabolic scope (AMS) is a principal physiological trait governing many other performance traits (e.g. growth, digestion, reproduction, immune function, muscular activity and thermal tolerance). Aerobic metabolic scope is defined as the excess oxygen available above oxygen demand for maintenance and fuels the performance capacity of the animal (Pörtner and Lannig, 2009), which is therefore limited by the maximal aerobic metabolic rate. Although several recent studies question the broad applicability of the OCLTT hypothesis (e.g. Clark et al. 2013; Ern et al. 2014; Norin et al. 2014; Wang et al. 2014), it is conceivable that the link between AMS and performance, as suggested by the OCLTT hypothesis, should be revealed by positive correlations between individual AMS and activity levels. This assumes that individual activity levels exhibit periodic or frequent elevations close to the performance ceiling or that maximal activity levels consistently correspond to a certain fraction of the performance ceiling. Correlations between individual AMS and activity levels have, however, rarely been tested in the wild.
A link between consistent individuality in metabolism and activity has also been suggested by recent studies unrelated to the OCLTT hypothesis (Careau et al., 2008; Biro and Stamps, 2010; Burton et al., 2011; Mathot and Dingemanse, 2015). A mechanism proposed to facilitate this coupling is the concept of an individually sized ‘metabolic machinery’ that on the one hand enables energy output, but on the other hand requires maintenance (the ‘performance model’ sensu Careau et al. 2008). Following this, individuals with relatively large machinery capable of producing more energy to fuel activities (e.g. movement, generation of somatic or gonadal tissue) are faced with a need for a higher energy uptake. For example, as individuals are expected to display behaviour that increase food intake rate, ‘high-energy’ individuals should be more active, be bolder and explore larger areas to sustain their metabolic machinery, assuming they rely on an active food search strategy. In contrast, ‘low-energy’ individuals will have lower amounts of available energy for activity but also have lower maintenance needs, i.e. a lower need to be active. Thus, the performance model predicts a positive relationship between standard metabolic rate (SMR) and activity. Alternatively, a coupling between metabolic traits and activity could exist according to the ‘allocation model’ (sensu Careau et al. 2008), in which an individual animal has a limited amount of energy to allocate between SMR and activity. Individuals with a need for more energy to maintain SMR can allocate less energy to activity than individuals with lower SMR. In contrast to the performance model, therefore, the allocation model predicts a negative relationship between SMR and activity. Regardless of the complex mechanisms linking metabolism, thermal tolerance and animal activity (Horodysky et al., 2015), the examination of related correlations is a useful approach to examine conceptual models and their assumptions and predictions.
Interspecific differences in morphology of fish are known to reflect differences in swimming capabilities and general behaviour. For instance, the posterior positioning of the dorsal and anal fins in northern pike (Esox lucius) reflects an adaptation to sprint-based foraging (Craig, 1996), whereas thunniform body shapes are optimal for cruising (Webb, 1984). Likewise, intraspecific individual variation in body shape could affect the cost of transport and translate into behavioural variation. For fish moving through water, overall body shape is a major determinant of resistive drag and thereby cost of transport. Combining physics theory and hydrodynamic modelling, fish body form may be simplified to a prolate spheroid and described by the fineness ratio (FR), defined as length divided by maximal diameter (i.e. body depth in most fish species). From such modelling, FRs ranging between 4.5 and 8 have been shown to be most efficient (Blake, 1983; Chung, 2009; Langerhans and Reznick, 2010). Given that an optimum exists, it is conceivable that individual morphological differences will influence cost of transport and, subsequently, behaviour. Using swimming respirometry, Ohlberger et al. (2006) found a relationship between swimming costs and FR in common carp (Cyprinus carpio) and roach (Rutilus rutilus). Boily and Magnan (2002) found higher swimming costs for stout than slender individuals of brook char (Salvelinus fontinalis) and yellow perch (Perca flavescens); however, the authors did not report FR for the fish. Using telemetry in the field, Hanson et al. (2007) found that body shape influenced mean swimming speeds of largemouth bass (Micropterus salmoides).
Most studies linking metabolic and morphological traits to activity levels and behaviour have been performed in laboratory settings. However, several studies related to metabolism and behaviour suggest that care should be taken when extrapolating laboratory findings to natural settings (Blake, 1991; Klefoth et al., 2012; Vanin et al., 2012; Fisher et al., 2015). For instance, fish behaviour in laboratory trials might not be a good predictor for fish behaviour in the wild, as indicated by Klefoth et al. (2012). Thus, although correlations between individual metabolism and behaviour are found in laboratory experiments, it is largely unknown whether such correlations exist in nature. Recent technological advents have enabled field studies on detailed in situ fish behaviour (Lucas and Baras, 2000; Cooke et al., 2005; Svendsen et al., 2011; Baktoft et al., 2015), thereby facilitating the inclusion of volitional behaviour of free-swimming animals in this research area.
In the present study, we tested the overarching hypotheses that individual variation in metabolic and morphological traits influence and, consequently, are correlated with in situ behavioural variation. Specifically, we examined predictions derived from the OCLTT hypothesis, the allocation model and the performance model that fish activity levels would correlate with metabolic physiology measured as AMS and SMR. Additionally, we examined the prediction that morphology measured as FR would be correlated with fish activity levels. To this end, we captured European perch (Perca fluviatilis) to quantify their physiology and morphology in the laboratory and returned the fish to their natal lake, where we monitored their activity patterns using high-resolution positional telemetry.
Materials and methods
Fish
Twenty-three European perch [mean body mass 54.2 ± 15.3 g (mean ± SD) and fork length (FL) 16.4 ± 1.4 cm (mean ± SD), range 14.1–19.7 cm] were captured by angling in a lake (Lake Gosmer; 55°55′42N, 10°10′50E; 1 ha; Baktoft et al. 2015) and transferred to the laboratory in mid-September 2010. Fish were kept in flow-through tanks (3 m × 3 m), maintained at 16 ± 1°C and fed daily with roach (Rutilus rutilus). Light regimen was 14 h–10 h (light–dark). Each fish was anaesthetized (benzocaine 300 ppm) and tagged with an acoustic transmitter (Lotek MAP 6_2, burst interval 30 s, 15 mm × 6.2 mm, 1.1 g in air) and a passive integrated transponder tag (12 mm × ’2.12 mm; 95 mg in air; Loligo Systems, Tjele, Denmark) for rapid identification. Both tags were inserted through an incision in the body cavity and closed with a single absorbable suture (Vicryl 5-0FS-2; Ethicon, Piscataway, NJ, USA) following standard procedures (e.g. Boel et al., 2014; Jacobsen et al., 2014). An experienced fish surgeon (Cooke et al., 2003) performed surgical implants in accordance with the guidelines described in permission 2012-DY-2934-00007 from the Danish Experimental Animal Committee. After surgery and recovery (10–20 min), all fish were returned to their holding tank. Fish were then allowed a 10 day period to recover from the surgical procedure (e.g. wound healing) and to acclimate to the laboratory holding facilities before quantification of metabolism and morphology. Fish were isolated and unfed for 24 h before data collection as detailed below. During the holding period in the laboratory (19 days), all fish chased and consumed prey, incisions healed well, and sutures became partly absorbed. Overall fish condition seemed good, although caudal fins were slightly eroded.
Metabolism
Four acrylic respirometer chambers (each 0.54 l) were used concurrently for simultaneous measurements on four fish. Chambers were submerged in aerated water (>95% air saturation) drawn from the recirculating system, i.e. the same water as used for fish holding. Water temperature was kept at 16°C (range 15.9–16.1°C) using a temperature-controlling instrument (TMP-REG; Loligo Systems).
Measurements of oxygen consumption rate (; in milligrams of oxygen per kilogram per hour) were carried out using intermittent flow respirometry (Forstner, 1983; Rosewarne et al., 2015). Each respirometer chamber was fitted with two inlet and two outlet ports and two water pumps (Svendsen et al., 2012; Tirsgaard et al., 2015). Fish did not display rheotaxic responses to chamber flow and remained stationary and calm, apart from intermittent movements. Oxygen partial pressure (in kilopascals) was measured inside the respirometers at 1 Hz using galvanic oxygen sensors (Mini DO Probe; Loligo Systems). Oxygen levels ≥80% air saturation in the respirometers were secured using flush pumps that were activated intermittently and controlled by AutoResp software (Loligo Systems). Between flushings, the declining oxygen partial pressure was recorded to calculate using the following equation:
where K is the linear rate of decline (in kilopascals per hour) in the oxygen content over time (in hours) in the respirometer, V is the volume of the respirometer (in litres) corrected for the volume of fish, β is the solubility of oxygen in the water (in milligrams of oxygen per litre per kilopascal; β = 0.4755) and M is the body mass of the fish (in kilograms). The coefficient of determination (r2) associated with each measurement was always ≥0.90, similar to previous studies (Genz et al., 2013; Svendsen et al., 2013). Corrections of background respiration (i.e. microbial respiration) followed Rosewarne et al. (2015).
Maximal metabolic rate (MMR) was elicited using the chase protocol described previously (Cutts et al., 2002; Norin and Malte, 2011; Svendsen et al., 2014). Individual fish were transferred to a circular trough and chased manually until exhaustion as evidenced by the fish not reacting to being turned upside down and lifted partly out of the water. Chasing lasted for ∼5 min. When exhausted, fish were immediately transferred to the respirometer, and measuring of metabolism commenced within 10 s. The MMR was the highest of three consecutive measurements (Svendsen et al., 2012). It is unlikely that MMR can be sustained beyond a relatively short period (<1 h). In fish, this is probably because of the high ion flux at the gills that is inevitably associated with high or maximal oxygen uptake at the gills.
For each trial, the chase protocol ended around 14.00 h, and fish were left in the chambers for the following 22 h (Fig. 1). During this time, each fish was visually shielded using vertical opaque acrylic screens. Additionally, the entire set-up was shrouded in curtains to exclude visual disturbance of the fish. Pilot experiments showed that European perch generally reached a stable low level within 60 min after the light went off. After each trial, all equipment was disassembled, disinfected and thoroughly rinsed.
Figure 1:
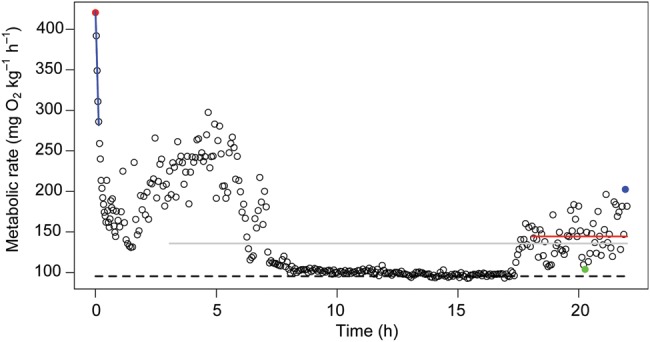
Metabolic Q12 rate (; in milligrams of oxygen per kilogram per hour) over time (in hours) in European perch (Perca fluviatilis) measured using intermittent flow respirometry. A chase protocol was applied to elicit maximal metabolic rate (MMR; red datum), followed by declining values. A linear regression line was fitted to the first five data points recorded immediately after the chase protocol (blue line), and the slope was used to estimate the rate of recovery. Standard metabolic rate (SMR) was estimated as the average of the lowest 10th percentile of all measurements throughout the period of data collection (22 h; dashed line). After 3 h of acclimation, the average was recorded over 19 h (grey line). Routine was recorded as the mean during the last 4 h of respirometer confinement (red line). Likewise, the spontaneous minimal (green datum) and maximal (blue datum) values were measured during the last 4 h of respirometer confinement. Aerobic metabolic scope (AMS) was estimated as the difference between MMR and SMR (i.e. red datum and dashed line), whereas spontaneous aerobic metabolic scope (in milligrams of oxygen per kilogram per hour) was estimated as the difference between the spontaneous minimal and maximal (i.e. between the green and blue data points).
Standard metabolic rate was estimated as the average of the lowest 10th percentile of all measurements within the 22 h of respirometer confinement (Killen et al., 2012b). Aerobic metabolic scope was calculated as the difference between MMR and SMR. In addition to MMR, SMR and AMS, this study quantified the following seven metabolic variables for each individual fish (Fig. 1): (i) rate of recovery was estimated as the slope of the relationship between time (in hours) and the five consecutive measurements of metabolic rate that were recorded immediately after the chase protocol; (ii) average was recorded as the mean during the last 19 h of data collection; (iii) metabolic variability was estimated as the standard deviation of all measurements during the last 19 h of data collection (Careau et al., 2008); (iv) routine was estimated as the average during the last 4 h of respirometer confinement (Killen et al., 2012a); (v) spontaneous minimal and (vi) spontaneous maximal were estimated as the minimal and maximal values, respectively, during the last 4 h of respirometer confinement; and (vii) spontaneous AMS was estimated as the difference between (v and vi).
For all estimates of metabolism, body mass and metabolic rates were log10-transformed prior to analyses to normalize and linearize the data (Auer et al., 2015). Residuals generated from each of these analyses differentiated those individuals having higher than expected metabolism for their body size (i.e. positive residuals) from those having metabolic rates lower than expected (i.e. negative residuals). Given that body mass can influence both metabolism and activity patterns, these estimates of mass-independent metabolic rates were used in subsequent analyses (Robertsen et al., 2014). No body mass correction was applied for metabolic variables (i) and (iii) (i.e. rate of recovery and metabolic variability).
The SMR, MMR and AMS were analysed separately in subsequent analyses, and the dimensions of the data set containing the remaining seven metabolic metrics were reduced using a principal component analysis (PCA). Prior to the PCA, data were centred and scaled to have unit variance. The first three axes from the PCA (MET1, MET2 and MET3) were used as explanatory variables in subsequent analyses.
Morphology
In addition to measuring FL, FR was calculated following Blake (1983) as FR = FL/maximal body depth. Fineness ratio is a dimensionless measure of overall body shape, in which low and high values indicate stout and slender individuals, respectively.
Activity measures
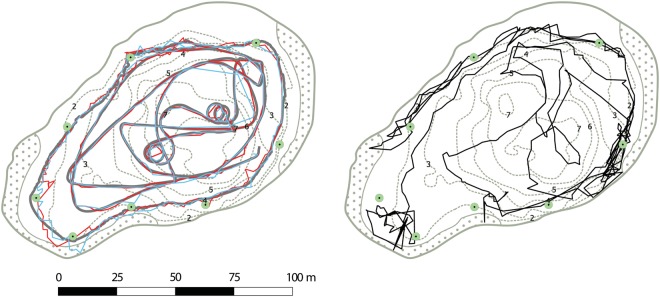
The European perch were returned to their natal lake upon completion of the laboratory protocol (all fish were returned on the same day). An acoustic positional telemetry system was used to record volitional in situ behaviour in Lake Gosmer (Fig. 2). In short, the telemetry system enabled near-continuous monitoring of tagged fish with high temporal and spatial resolution by yielding time-stamped geographical coordinates (mean precision 0.2 m; Baktoft et al., 2015). From these data, we calculated the following: (i) daily individual movement as total moved distance in 24 h (Aday; in metres per 24 h); (ii) instantaneous individual swimming speed (Uinst; in metres per second); (iii) daily maximal individual swimming speed (Umax; in metres per second); and (iv) daily covered area (ARday; in square metres per 24 h). For all analyses the complete data set was used, but note the selection criteria for swimming speed calculations given below. The Aday was calculated as the daily sum of Euclidian distances between consecutive positions. Swimming speeds (i.e. Uinst and Umax) were calculated as the two-dimensional Euclidian distance between two consecutive points divided by change in time. Only instances with maximal obtainable temporal resolution between two registrations, i.e. the transmitter burst interval (=30 s), were used for estimates of swimming speed. Furthermore, only instances where the individual European perch were active were used for the measures of Uinst and Umax. In order to identify these data objectively, we employed a hidden Markov model with location and level of activity as hidden states (Pedersen et al., 2011), providing a measure of activity expressed as the probability that a given fish was active at a given time (pactive). Only observations with pactive ≥ 0.75 were included in analyses of Uinst. The Umax was defined as the individual daily maximum of Uinst. Finally, the ARday was estimated as the area of a 0.5 m buffer applied to daily tracks obtained by connecting consecutive positions with straight lines (Svendsen et al., 2011). Overlapping daily buffers within an individual were merged before area calculation. The ARday represents a measure of exploratory tendency, which may differ from Aday and estimates of swimming speed (Cote et al., 2010; Svendsen et al., 2011). The first 7 days in the lake were excluded to allow the European perch to re-acclimatize to their natural environment. All calculated activity measures were based on data obtained from the following 12 days, during which the lake was left undisturbed. Dissolved oxygen and water temperature were monitored using four optical probes (FDO 700 IQ; WTW, Weilheim, Germany) positioned 1.0, 2.5, 4.0 and 5.5 m below the surface.
Figure 2:
Overview of study lake, telemetry system and example data from test tracks (left) and a 24 h track from a single fish used in the study (right). Green circles indicate positions of the eight hydrophones; dotted areas show the extent of emergent macrophytes (Tyhpa latifolia); and dotted lines are depth isopleths, with depth given in metres. Transmitters surgically implanted in the fish emit acoustic signals. When these signals are detected on more than three hydrophones, a position can be calculated using hyperbolic multilateration based on time differences of arrival on each hydrophone (see Baktoft et al. 2015 and references therein for further details). Test tracks (left) were obtained by moving two transmitters (blue and red lines) attached to a vertical rod mounted on a boat. True trajectory of the test track (thick grey line) was obtained by a hand-held differential GPS unit held directly above the transmitters.
Statistical analysis
Random intercept linear mixed effects models were applied to assess whether individual activity in the lake could be explained by metabolic traits and/or morphology. In addition to FL, the following explanatory variables (X) were tested: FR, SMR, MMR, AMS, MET1, MET2 and MET3. As several of these explanatory variables were correlated, separate models were fitted for each of these to avoid collinearity in the models. Additionally, each activity measure (Aday, Uinst, Umax and ARday) was analysed separately using the same initial model:
in which observation i of activity measure k in fish j modelled by explanatory variable p equals the sum of a kp-specific common intercept (αkp), estimated effects of FL, the focal explanatory variable p of fish j (Xjp), a kp-specific random intercept (ajkp) and kp-specific residual variation (ϵijkp). The random intercepts were assumed to be normally distributed with mean zero and kp-specific variances . Additionally, residual variation in each model was assumed to be normally distributed with mean zero and variances varying with activity measure k, explanatory variable p and fish j. To identify the parsimonious model best explaining the variation in each activity measure, initial models were compared with nested models excluding FL and X using Akaike information criterion (AIC) and likelihood ratio tests (Zuur et al., 2009). The determined optimal models for each activity measure were further analysed to obtain parameter estimates and significance levels of FL and explanatory variable X where relevant. Significance tests were based on likelihood ratio tests using maximum likelihood estimation, whereas parameter estimates were obtained using restricted maximum likelihood estimation. The value of Uinst was log(y + 0.1)-transformed to meet model assumption of normality. Model validation based on informal visual inspection of plots of normalized residuals following Zuur et al. (2009) showed no signs of violation of model assumptions. To assess individual consistency of the activity measures, the intraclass correlation coefficients (ICCs) were calculated based on the optimal model for each activity measure (Zuur et al., 2009). The variance structure allowing different variances for each fish necessitated that ICC was calculated for each fish separately, thus: .
All statistical analysis were done in R version 3.0.2 (R Core Team, 2013) using the nlme-package version 3.1-111 (Pinheiro et al., 2013) in addition to base R functions.
Results
In situ activity
Of the 23 fish returned to the lake, three were consumed by northern pike naturally occurring in the lake and tagged as part of other studies (Baktoft et al., 2013, 2012; Jacobsen et al., 2014). Predation was identified when tracks of tagged European perch and northern pike were merged over several days. Additionally, four transmitters malfunctioned (i.e. no signals were received after release in the lake), leaving 16 fish to be included in analyses. During the tracking period (14–25 October, both days included), which coincided with the autumn turnover in the lake, mean water temperature was 9.1°C (range 7.7–11.4) and mean dissolved oxygen content was 5.9 mg l−1 (4.5–6.8).
Generally, recorded behaviour was variable both within and between individual fish (Fig. 3). Nevertheless, overall individual daily activity levels were partly repeatable over time, as indicated by the relatively high median ICCs found for Aday (median ICC = 0.34) and ARday (median ICC = 0.49). This suggests that individual fish displayed consistent daily routines. However, the degree of this was highly variable between fish, as some individuals were very consistent whereas others showed virtually no day-to-day consistency (for example, ARday individual ICC ranged from 0.01 to 0.81). By comparison, the variation in swimming speeds (Umax and Uinst) was considerable both within and between individuals (median ICCs 0.051 and 0.037, respectively), although a few individual fish displayed highly consistent velocities (maximal individual ICC = 0.55).
Figure 3:
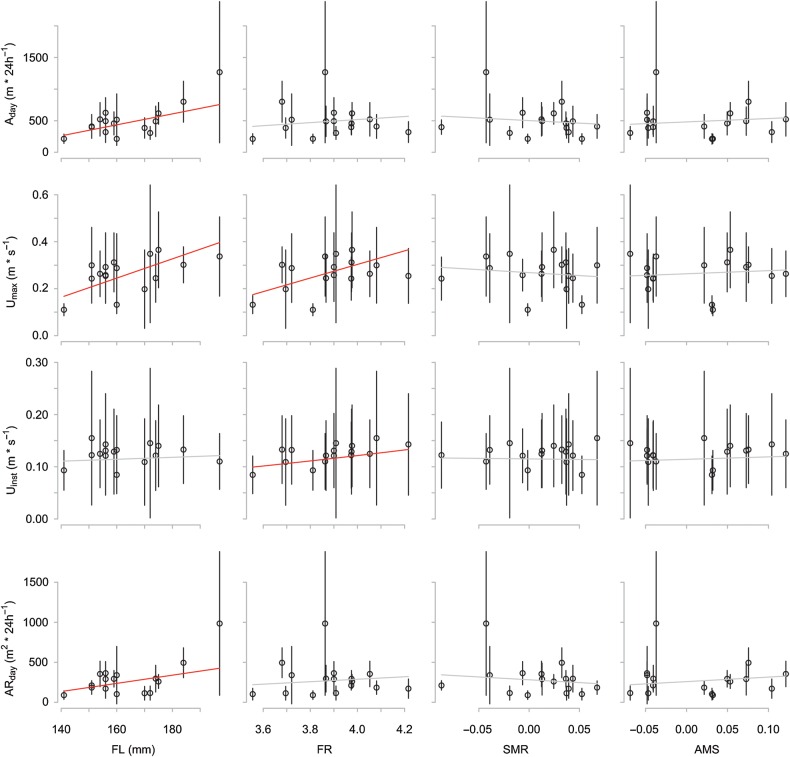
Visualization of raw data (mean values ± SD) and regression lines obtained from the random intercept linear mixed effects models. Significant regression lines (P < 0.05) are shown in red; non-significant in grey. Fork length (FL) and fineness ratio (FR) correlated positively with one or more of the activity measures. None of the metabolic measures [standard metabolic rate (SMR), maximum metabolic rate (MMR), aerobic metabolic scope (AMS), PCA axes representing other metabolic metrics (MET1–3)] was found to explain a significant amount of variation (Table 2; only SMR and AMS are shown in the figure). Metabolic measures were estimated by respirometry and corrected for body mass variation using analysis of residuals when appropriate.
Individual metabolism is not correlated ’with activity
The first three axes from the PCA (MET1, MET2 and MET3) explained 92.7% of the variation in the data constituted by the seven metabolic measures obtained besides SMR, MMR and AMS (Table 1). The statistical models gave no indications suggesting that the metabolic traits influenced in situ activity patterns. Neither SMR, MMR and AMS nor the three PCA axes (MET1, MET2 and MET3) correlated significantly with any of the activity metrics (all P-values > 0.1; Table 2). Consequently, we found no support for either the allocation model or the performance model, because none of the activity metrics correlated with SMR. Likewise, we found no support for the hypothesis derived from the OCLTT model, because AMS did not correlate with the activity metrics (P > 0.1; Table 2). Mean values ± SD of SMR, MMR and AMS were 79.0 ± 9.9, 392.8 ± 61.1 and 313.8 ± 58.9 mg O2 kg−1 h−1, respectively.
Table 1:
Results from the principal component analysis applied on seven metabolic measures
| Metabolic measure | MET1 | MET2 | MET3 |
|---|---|---|---|
| Rate of recovery | −0.24 | 0.19 | 0.84 |
| Average | 0.43 | 0.34 | 0.14 |
| Metabolic variability | 0.38 | −0.02 | 0.44 |
| Routine | 0.45 | 0.23 | 0.05 |
| Spontaneous minimal | 0.35 | 0.52 | −0.27 |
| Spontaneous maximal | 0.41 | −0.42 | 0.06 |
| Spontaneous AMS | 0.35 | −0.59 | 0.07 |
| Cumulative variance explained | 61.6% | 79.8% | 92.7% |
Abbreviations: FL, fork length; FR, fineness ratio; SMR, standard metabolic rate; MMR, maximum metabolic rate; AMS, aerobic metabolic scope; MET1–3, PCA axes representing other metabolic metrics.
The cumulative variance explained by these three axes was 92.7%. Metabolic measures are explained in detail in the text. Abbreviations: AMS, aerobic metabolic scope; and , metabolic rate.
Table 2:
Model comparisons for the four activity metrics modelled as function of FLj and Xp
|
Yk = Aday |
Yk = Umax |
Yk = Uinst |
Yk = ARday |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | FLj | Xp | P-value | ΔAIC | P-value | ΔAIC | P-value | ΔAIC | P-value | ΔAIC |
| M0 | − | − | n.a. | 5.0 | n.a. | 13.1 | n.a. | 1.9 | n.a. | 1.9 |
| M1 | + | − | 0.0083* | 0* | 0.026 | 10.1 | 0.94 | 3.9 | 0.0497* | 0* |
| M1a | + | FRj | 0.24 | 0.6 | 0.0005* | 0* | 0.015* | 0* | 0.37 | 1.2 |
| M1b | + | SMRj | 0.40 | 1.3 | 0.50 | 11.6 | 0.80 | 5.8 | 0.39 | 1.2 |
| M1c | + | MMRj | 0.47 | 1.5 | 0.76 | 12.0 | 0.45 | 5.3 | 0.32 | 1.0 |
| M1d | + | AMSj | 0.35 | 1.1 | 0.65 | 11.9 | 0.38 | 5.1 | 0.21 | 0.4 |
| M1e | + | MET1j | 0.59 | 1.7 | 0.88 | 12.1 | 0.65 | 5.7 | 0.20 | 0.4 |
| M1f | + | MET2j | 0.86 | 2.0 | 0.95 | 12.1 | 0.72 | 5.8 | 0.72 | 1.9 |
| M1g | + | MET3j | 0.30 | 0.9 | 0.74 | 12.0 | 0.33 | 4.9 | 0.21 | 0.4 |
Asterisks indicate optimal models. P-values represent the significance of the respective term tested with each model (i.e. FLj in M1 and Xp in M1a–M1g) obtained using likelihood ratio tests. The ΔAICs were obtained by comparing each model with the optimal model for each respective activity measure.
Individual morphology is correlated ’with activity
Individual morphology correlated significantly with all four activity metrics (Fig. 3 and Tables 2 and 3). Fork length correlated positively with both Aday and ARday. Additionally, the optimal model for Umax found positive significant effects of both FL and FR. Finally, FR but not FL correlated positively with Uinst. These findings indicate that inter-individual variation in swimming activity may be partly predicted by individual morphology. Additionally, body morphology favourable for higher swimming speed (i.e. higher FR) did not result in a larger searched area per 24 h (Fig. 2). Mean ± SD FR was 5.9 ± 0.3.
Table 3:
Summaries of optimal models for each activity measure
| Parameter | Estimate | SEM | P-value | |
|---|---|---|---|---|
| Yk = Aday | Optimal model: | M1: Aday = αkp + FLj + ajkp + ϵijkp | ||
| αkp | −954.1 | 519.3 | 0.066 | |
| FLj | 8.7 | 3.2 | 0.0083 | |
| σakp | 132.0 | n.a. | n.a. | |
| σjkp | 185.2 (73.4–1169.4) | n.a. | n.a. | |
| ICCjkp | 0.34 (0.013–0.76) | n.a. | n.a. | |
| Yk = Umax | Optimal model: | M1a: Umax = αkp + FLj + FRj + ajkp + ϵijkp | ||
| αkp | −1.53 | 0.32 | <0.001 | |
| FLj | 0.0041 | 0.00083 | <0.001 | |
| FRj | 0.29 | 0.068 | <0.001 | |
| σakp | 0.029 | n.a. | n.a. | |
| σjkp | 0.12 (0.03–0.28) | n.a. | n.a. | |
| ICCjkp | 0.051 (0.011–0.55) | n.a. | n.a. | |
| Yk = Uinst | Optimal model: | M1a: Uinst = αkp + FLj + FRj + ajkp + ϵijkp | ||
| αkp | −2.60 | 0.47 | <0.001 | |
| FLj | 0.00087 | 0.0011 | 0.44 | |
| FRj | 0.24 | 0.10 | 0.015 | |
| σakp | 0.052 | n.a. | n.a. | |
| σjkp | 0.26 (0.19–0.46) | n.a. | n.a. | |
| ICCjkp | 0.037 (0.013–0.068) | n.a. | n.a. | |
| Yk = ARday | Optimal model: | M1: ARday = αkp + FLj + ajkp + ϵijkp | ||
| αkp | −582.0 | 418.5 | 0.17 | |
| FLj | 5.11 | 2.59 | 0.0497 | |
| σakp | 109.9 | n.a. | n.a. | |
| σjkp | 111.4 (53.5–998.1) | n.a. | n.a. | |
| ICCjkp | 0.49 (0.01–0.81) | n.a. | n.a. | |
Parameter estimates and associated standard errors (where available) are given. Medians are given for and ICCjkp, with the minimum and maximum in parentheses. For all four models, it is assumed that and . P-values are obtained from likelihood ratio tests comparing the optimal model with a nested model excluding the respective parameter.
Discussion
By combining laboratory and field techniques, we tested hypotheses derived from conceptual models (Blake, 1983; Careau et al., 2008; Pörtner, 2010) predicting that individual metabolism and morphology correlate with in situ activity. Surprisingly, we found that individual metabolism and activity are independent, indicating that a strong link between these traits is not universally present. As predicted, we found that individual morphological variation explained variation in activity measures, indicating that morphological variation is a determinant of locomotion patterns.
Metabolism and activity
Defining aerobic performance and being highly dependent on ambient temperature, aerobic metabolism is used as both an indicator and a predictor in relationship to conservation of fish species and their responses to anthropogenic stressors, including rising temperatures mediated by climate change (Horodysky et al., 2015; Schulte, 2015), particularly through the OCLTT hypothesis (Pörtner, 2010). Additionally, metabolism has been recognized in a series of studies as a possible mechanistic link between environmental conditions, life history and behaviour (Brown, 2004; Careau et al., 2008; Biro and Stamps, 2010; Horodysky et al., 2015), although the topic remains debated (Halsey et al., 2015; Mathot and Dingemanse, 2015). The OCLTT hypothesis implies that critical performances, such as growth and locomotion, are causally linked with aerobic scope. It is therefore conceivable that inter-individual variation in AMS should be correlated with individual activity. The present study, however, found no evidence of such correlations. Thus, in alignment with recent studies (Clark et al., 2013; Ern et al., 2014; Norin et al., 2014), this indirect examination of the OCLTT hypothesis suggests that assumptions may not be fulfilled in the wild. While it remains unknown whether temperature-induced variation in AMS corresponding to the phenotypic variation observed in the present study will affect activity patterns, our study suggests that care should be taken if attempting to predict the performance of fish species (e.g. in relationship to climate change) from simple metrics of metabolism.
Standard metabolic rate (and equivalents) is the most-studied aspect of vertebrate metabolism (Careau et al., 2008), and previous laboratory studies have found positive correlations between resting metabolic rate and behavioural parameters such as aggression, dominance and boldness in a number of taxa (reviewed by Biro and Stamps, 2010 and Mathot and Dingemanse, 2015). Although empirical studies on correlations between SMR and activity in fish are scarce, a single study (Farwell and McLaughlin, 2009) was identified by Biro and Stamps (2010) and Mathot and Dingemanse (2015). Using recently emerged brook charr (Salvelinus fontinalis), Farwell and McLaughlin (2009) found no correlation between SMR and activity measured as time spent moving. In contrast, Watz et al. (2015) found correlations between individual resting metabolic rate and swimming activity in brown trout (Salmo trutta) tested in an indoor stream channel. Likewise, Myles-Gonzalez et al. (2015) tested round goby (Neogobius melanostomus) in an artificial flume and found that more active fish also exhibit elevated resting metabolic rate. Interestingly, it has been suggested that environmental stressors such as temperature and hypoxia can reveal, mask and modulate the covariation of physiological and behavioural traits (Killen et al., 2013, 2012a). Thus, although SMR is potentially correlated with some behavioural parameters measured in laboratory settings, the link between SMR and activity in non-stressed conditions can be weak or non-existent, as found in the present study.
Critical and optimal swimming speeds are measures of fish swimming performance obtained using laboratory protocols that involve forced swimming (e.g. Claireaux et al. 2006; Tudorache et al. 2008). Although the mechanisms are not fully understood, these performance measures are often linked to variation in metabolism (Claireaux et al., 2005; Arnott et al., 2006; Binning et al., 2015). For instance, Binning et al. (2015) found MMR to be the best overall predictor of individual swimming performance. These findings could be important for the present study because forced laboratory measures of MMR may correlate positively with MMR measured in spontaneously active fish (Svendsen et al., 2014). A correlation between Umax and MMR (and/or AMS) was therefore expected but not found in the present study. Furthermore, Claireaux et al. (2006) found that European sea bass (Dicentrarchus labrax) reach their maximal aerobic capacity at swimming speeds near the critical swimming speed and that metabolism when swimming at optimal swimming speed represents a consistent percentage of MMR. Thus, under the assumption often used in the literature that free-ranging fish swim at or near optimal swimming speed during routine swimming (Videler, 1993; Claireaux et al., 2006; Tudorache et al., 2011; Svendsen et al., 2015), a correlation between Uinst and MMR and/or AMS was expected but not found in the present study. However, when determining maximal and critical swimming speeds, fish are typically forced to swim until exhaustion. Although these measures give insights into the maximal capacity of the fish, they may not be biologically relevant. For example, it is currently uncertain to what extent fish use their full AMS spontaneously and in natural settings (Lucas et al., 1993; Murchie et al., 2011; Genz et al., 2013). Likewise, the assumed relationship between optimal swimming speed and spontaneous swimming speed of fish in the wild has yet to be confirmed (Claireaux et al., 2006; Tudorache et al., 2011; Svendsen et al., 2015). Finally, the correlation between AMS and swimming performance is not consistent in all fish species (Anttila et al., 2014; Svendsen et al., 2015) and may not be present in European perch.
The complete lack of correlations between metabolism and activity levels was surprising. Besides the possibility that no correlation exists, there are potential sources of error that could enshroud existing correlations. Apart from the inherent uncertainties in both the metabolic measurements and the activity metrics, the combination of laboratory and in situ measurements could introduce context-specific biases. For instance, individual personality might affect the measurement accuracy in metabolic studies through individual differences in reactions to being confined in a respirometry chamber (Careau et al., 2008). Further studies are required to examine stress levels of animals in respirometer chambers and test the hypothesis that disparate behavioural phenotypes react differently to respirometer confinement. Additionally, the translocation of the fish from lake to laboratory and back again could induce stress responses extending the recovery periods and altering both metabolic and behavioural phenotypes. However, the activity levels and spatial distribution of tagged European perch in the present study were comparable (H. Baktoft, unpublished data) to those of conspecifics tagged and immediately returned to Lake Gosmer as part of another study (Jacobsen et al., 2014), suggesting that behaviours were unaffected by the translocation and laboratory tests.
Morphology and activity
Individual FL was positively correlated with Aday, ARday and Umax, but not with Uinst. The positive correlations between FL and Aday, ARday and Umax were expected because larger fish generally use larger areas and are able to swim faster. Following this rationale, a correlation between FL and Uinst was also expected. European perch is a schooling species (Thorpe, 1977), and they often aggregate in foraging groups (Eklöv, 1992), suggesting that the swimming speed of the tagged fish could have been influenced by other school members and not determined solely by individual traits. However, when acknowledging slight differences in body lengths, other studies have found comparable speeds of actively swimming European perch (Zamora and Moreno-Amich, 2002; Linløkken et al., 2010), indicating that Uinst measured in the present study is within a credible range.
Fineness ratio explained significant proportions of the variation in both Umax and Uinst. Previous theoretical and empirical studies have shown that higher FR (or equivalent measures) up to a given threshold are generally associated with lower swimming costs (Blake, 1983; Boily and Magnan, 2002; Ohlberger et al., 2006; Chung, 2009). A previous field study linking detailed fish behaviour with morphological characteristics found correlations between a composite morphometric measure comparable to FR and mean speed and mean daily distance in largemouth bass (Hanson et al., 2007). The present study adds empirical field evidence emphasizing the biological relevance of individual morphological differences in relation to swimming speeds. Interestingly, recent studies have concluded that FR is not a strong predictor of swimming performance (Fisher and Hogan, 2007; Hendry et al., 2011; Dalziel and Schulte, 2012; Walker et al., 2013; Binning et al., 2015); nonetheless, we found that FR predicts variation in both Umax and Uinst, perhaps indicating that spontaneous activity is not always predicted by maximal activity measured in the laboratory.
Although the prospect of a purely physical explanation (i.e. the hydrodynamic effects of FR affecting swimming costs) of parts of the individual variability in activity is alluring, this correlation could include biological components as well. For instance, European perch morphology is known to be plastic and correlated with habitat structure, feeding mode and temperature (Olsson and Eklöv, 2005; Rowiński et al., 2015). Generally, deep-bodied (i.e. lower FR) and thus more manoeuvrable European perch are associated with the benthic niche, whereas slender European perch (i.e. higher FR) are associated with the pelagic (Hjelm et al., 2000; Svanbäck and Eklöv, 2004). This differentiated niche association per se could entail behavioural differences influencing activity levels. Thus, the effects of FR on activity levels might not be directly exerted through hydrodynamic effects, but could instead operate indirectly through evolutionary processes shaping the morphology–niche correlation. However, irrespective of the causal mechanism driving the correlations between FR and activity, it is interesting that a simple morphological metric can explain a significant amount of phenotypic behavioural variation in the wild.
Study assumptions
The present study relies on several assumptions, including the following.
First, the tagged European perch behaved naturally, i.e. as untagged conspecifics. This is a fundamental assumption in most studies using telemetry but impossible to validate in the present study.
Second, measured metabolic rates reflected the amount of adenosine triphosphate (ATP) generated aerobically (Salin et al., 2015) and were repeatable and temporally consistent. The latter could not be verified owing to time restrictions set by the transmitter battery life, but previous studies suggest that this is a valid assumption (Nespolo and Franco, 2007; Maciak and Konarzewski, 2010; Norin and Malte, 2011; Svendsen et al., 2014), although repeatability in tagged and translocated fish has not been tested. Moreover, repeatability of the individual metabolic traits can be influenced by environmental factors, such as temperature and hypoxia (Careau et al., 2014; Norin et al., 2015), possibly affecting our findings.
Third, the activity metrics obtained using the telemetry system truthfully reflected fish activity patterns and were relevant in terms of fish balancing their energy budgets in an adaptive fashion (Mathot and Dingemanse, 2015). The validity of data produced by the system has previously been assessed by standardized tests using stationary transmitters and by towing transmitters to mimic swimming fish (Fig. 2). These tests showed very good performance in terms of efficiency, accuracy and precision, and the tow tracks showed very good alignment with true tracks obtained using a differential GPS (Baktoft et al., 2015). However, the estimation accuracy of activity metrics (especially Uinst and Umax) might have been influenced by the transmitter burst intervals (30 s) because any movement beyond straight-line distance between consecutive positions cannot be detected by the system. The selected burst interval was chosen as a compromise between battery life expectancy (i.e. longevity of the study period) and transmitter size.
Fourth, the difference in water temperature between the laboratory facility and the study lake could potentially enshroud links between metabolism and activity. However, water temperature in the laboratory had to be decided a priori and kept fixed for the duration of the laboratory work. Moreover, if shifts in temperature well within the natural range of a species (Thorpe, 1977) dramatically alter linkage between metabolism and activity, the entire concept of causal or mechanistic links between these parameters seems tenuous, particularly in relation to animal conservation and evolutionary patterns.
Fifth and finally, it should be noted that the results in the present study are based on a relatively low sample size, partly owing to predation events and transmitter failure. Therefore, care should be taken when interpreting the results. However, even when correcting for pseudoreplication by using a mixed model approach, several of the findings were highly significant, adding credibility to the results.
Conclusions
The present study suggests that although metabolism is closely related to energy use in individual animals, a direct link to volitional activity is missing. Thus, we found no support for the overarching hypothesis that individual metabolic traits influence individual activity, suggesting that causal links between metabolism and activity derived from the OCLTT hypothesis and the allocation and performance models are not always present in natural settings. In contrast, we found several indications that fish size and morphology are correlated with fish activity, suggesting a stronger link between these factors. Although the conceptual models discussed here represent powerful tools to understand the intricate links between metabolism, environmental variation and animal performance, this study adds to the body of evidence that animal activity patterns are highly complex and variable and are difficult to capture and explain using relatively simple metrics. The complex nature of animal activity patterns remains a challenge for studies providing data for science-based strategies related to management and conservation.
Funding
This work was supported by the Danish Rod and Net Fish License Funds and the Foundation for Science and Technology (FCT) in Portugal [SFRH/BPD/89473/2012] to J.C.S.
Acknowledgements
The authors thank Søren Anton Hansen, owner of Lake Gosmer, for access to the lake and Hans Malte (Department of Bioscience, Aarhus University) for lending parts of the equipment used for the respirometry. The authors thank anonymous reviewers for constructive and helpful comments on an earlier version of the manuscript.
References
- Anttila K, Jørgensen SM, Casselman MT, Timmerhaus G, Farrell AP, Takle H (2014) Association between swimming performance, cardiorespiratory morphometry, and thermal tolerance in Atlantic salmon (Salmo salar L.). Front Mar Sci 1: 76 doi:10.3389/fmars.2014.00076. [Google Scholar]
- Arnott SA, Chiba S, Conover DO (2006) Evolution of intrinsic growth rate: metabolic costs drive trade-offs between growth and swimming performance in Menidia menidia. Evolution 60: 1269–1278. [PubMed] [Google Scholar]
- Auer SK, Salin K, Rudolf AM, Anderson GJ, Metcalfe NB (2015) The optimal combination of standard metabolic rate and aerobic scope for somatic growth depends on food availability. Funct Ecol 29: 479–486. [Google Scholar]
- Baktoft H, Aarestrup K, Berg S, Boel M, Jacobsen L, Jepsen N, Koed A, Svendsen JC, Skov C (2012) Seasonal and diel effects on the activity of northern pike studied by high-resolution positional telemetry. Ecol Freshw Fish 21: 386–394. [Google Scholar]
- Baktoft H, Aarestrup K, Berg S, Boel M, Jacobsen L, Koed A, Pedersen MW, Svendsen JC, Skov C (2013) Effects of angling and manual handling on pike behaviour investigated by high-resolution positional telemetry. Fish Manag Ecol 20: 518–525. [Google Scholar]
- Baktoft H, Zajicek P, Klefoth T, Svendsen JC, Jacobsen L, Pedersen MW, Morla DM, Skov C, Nakayama S, Arlinghaus R (2015) Performance assessment of two whole-lake acoustic positional telemetry systems - is reality mining of free-ranging aquatic animals technologically possible? PLoS ONE 10: e0126534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binning SA, Ros AFH, Nusbaumer D, Roche DG (2015) Physiological plasticity to water flow habitat in the damselfish, Acanthochromis polyacanthus: linking phenotype to performance. PLoS ONE 10: e0121983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol Evol 25: 653–659. [DOI] [PubMed] [Google Scholar]
- Blake RW. (1983) Functional design and burst-and-coast swimming in fishes. Can J Zool 61: 2491–2494. [Google Scholar]
- Blake RW. (1991) On the efficiency of energy transformations in cells and animals. In Blake RW, eds, Efficiancy and Economy in Animal Physiology. Cambridge University Press, Cambridge, pp 13–32. [Google Scholar]
- Boel M, Aarestrup K, Baktoft H, Larsen T, Madsen SS, Malte H, Skov C, Svendsen JC, Koed A (2014) The physiological basis of the migration continuum in brown trout (Salmo trutta). Physiol Biochem Zool 87: 334–345. [DOI] [PubMed] [Google Scholar]
- Boily P, Magnan P (2002) Relationship between individual variation in morphological characters and swimming costs in brook charr (Salvelinus fontinalis) and yellow perch (Perca flavescens). J Exp Biol 205: 1031–1036. [DOI] [PubMed] [Google Scholar]
- Bozinovic F, Pörtner H-O (2015) Physiological ecology meets climate change. Ecol Evol 5: 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett JR. (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21: 1183–1226. [Google Scholar]
- Brown JH. (2004) Toward a metabolic theory of ecology. Ecology 85: 1771–1789. [Google Scholar]
- Burton T, Killen SS, Armstrong JD, Metcalfe NB (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc Biol Sci 278: 3465–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V, Thomas D, Humphries M, Réale D (2008) Energy metabolism and animal personality. Oikos 117: 641–653. [Google Scholar]
- Careau V, Gifford ME, Biro PA (2014) Individual (co)variation in thermal reaction norms of standard and maximal metabolic rates in wild-caught slimy salamanders. Funct Ecol 28: 1175–1186. [Google Scholar]
- Chung M-H. (2009) On burst-and-coast swimming performance in fish-like locomotion. Bioinspir Biomim 4: 036001. [DOI] [PubMed] [Google Scholar]
- Claireaux G, McKenzie DJ, Genge AG, Chatelier A, Aubin J, Farrell AP (2005) Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J Exp Biol 208: 1775–1784. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Couturier C, Groison A-L (2006) Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J Exp Biol 209: 3420–3428. [DOI] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Graeb BDS, Suski CD, Ostrand KG (2003) Effects of suture material on incision healing, growth and survival of juvenile largemouth bass implanted with miniature radio transmitters: case study of a novice and experienced fish surgeon. J Fish Biol 62: 1366–1380. [Google Scholar]
- Cooke SJ, Niezgoda GH, Hanson KC, Suski CD, Phelan FJS, Tinline R, Philipp DP (2005) Use of CDMA acoustic telemetry to document 3-D positions of fish: relevance to the design and monitoring of aquatic protected areas. Mar Technol Soc J 39: 17–27. [Google Scholar]
- Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc Biol Sci 277: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JF. (1996) Pike: Biology and Exploitation. Chapman & Hall, London. [Google Scholar]
- Cutts CJ, Metcalfe NB, Taylor AC (2002) Juvenile Atlantic Salmon (Salmo salar) with relatively high standard metabolic rates have small metabolic scopes. Funct Ecol 16: 73–78. [Google Scholar]
- Dalziel AC, Schulte PM (2012) Correlates of prolonged swimming performance in F2 hybrids of migratory and non-migratory threespine stickleback. J Exp Biol 215: 3587–3596. [DOI] [PubMed] [Google Scholar]
- Eklöv P. (1992) Group foraging versus solitary foraging efficiency in piscivorous predators: the perch, Perca fluviatilis, and pike, Esox lucius, patterns. Anim Behav 44: 313–326. [Google Scholar]
- Ern R, Huong DTT, Phuong NT, Wang T, Bayley M (2014) Oxygen delivery does not limit thermal tolerance in a tropical eurythermal crustacean. J Exp Biol 217: 809–814. [DOI] [PubMed] [Google Scholar]
- Farwell M, McLaughlin RL (2009) Alternative foraging tactics and risk taking in brook charr (Salvelinus fontinalis). Behav Ecol 20: 913–921. [Google Scholar]
- Fisher R, Hogan JD (2007) Morphological predictors of swimming speed: a case study of pre-settlement juvenile coral reef fishes. J Exp Biol 210: 2436–2443. [DOI] [PubMed] [Google Scholar]
- Fisher DN, James A, Rodríguez-Muñoz R, Tregenza T (2015) Behaviour in captivity predicts some aspects of natural behaviour, but not others, in a wild cricket population. Proc Biol Sci 282: 20150708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner H. (1983) An automated multiple-chamber intermittent-flow respirometer. In Gnaiger E, Forstner H, eds, Polarographic Oxygen Sensors. Springer-Verlag, Berlin, pp 111–126. [Google Scholar]
- Genz J, Jyde MB, Svendsen JC, Steffensen JF, Ramløv H (2013) Excess post-hypoxic oxygen consumption is independent from lactate accumulation in two cyprinid fishes. Comp Biochem Physiol A Mol Integr Physiol 165: 54–60. [DOI] [PubMed] [Google Scholar]
- Halsey LG, Matthews PGD, Rezende EL, Chauvaud L, Robson AA (2015) The interactions between temperature and activity levels in driving metabolic rate: theory, with empirical validation from contrasting ectotherms. Oecologia 177: 1117–1129. [DOI] [PubMed] [Google Scholar]
- Hanson KC, Hasler CT, Suski CD, Cooke SJ (2007) Morphological correlates of swimming activity in wild largemouth bass (Micropterus salmoides) in their natural environment. Comp Biochem Physiol A Mol Integr Physiol 148: 913–920. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Hudson K, Walker JA, Räsänen K, Chapman LJ (2011) Genetic divergence in morphology–performance mapping between Misty Lake and inlet stickleback. J Evol Biol 24: 23–35. [DOI] [PubMed] [Google Scholar]
- Hjelm J, Persson L, Christensen B (2000) Growth, morphological variation and ontogenetic niche shifts in perch (Perca fluviatilis) in relation to resource availability. Oecologia 122: 190–199. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Cooke SJ, Brill RW (2015) Physiology in the service of fisheries science: why thinking mechanistically matters. Rev Fish Biol Fish 25: 425–447. [Google Scholar]
- Jacobsen L, Baktoft H, Jepsen N, Aarestrup K, Berg S, Skov C (2014) Effect of boat noise and angling on lake fish behaviour. J Fish Biol 84: 1768–1780. [DOI] [PubMed] [Google Scholar]
- Killen SS, Marras S, Ryan MR, Domenici P, McKenzie DJ (2012a) A relationship between metabolic rate and risk-taking behaviour is revealed during hypoxia in juvenile European sea bass. Funct Ecol 26: 134–143. [Google Scholar]
- Killen SS, Marras S, Steffensen JF, McKenzie DJ (2012b) Aerobic capacity influences the spatial position of individuals within fish schools. Proc Biol Sci 279: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P (2013) Environmental stressors alter relationships between physiology and behaviour. Trends Ecol Evol 28: 651–658. [DOI] [PubMed] [Google Scholar]
- Klefoth T, Skov C, Krause J, Arlinghaus R (2012) The role of ecological context and predation risk-stimuli in revealing the true picture about the genetic basis of boldness evolution in fish. Behav Ecol Sociobiol 66: 547–559. [Google Scholar]
- Langerhans RB, Reznick DN (2010) Ecology and evolution of swimming performance in fishes: predicting evolution with biomechanics. In Domenici P, Kapoor BG, eds, Fish Locomotion: an Etho-Ecological Perspective. Science Publishers, Enfield, pp 200–248. [Google Scholar]
- Linløkken AN, Bergman E, Greenberg L (2010) Effect of temperature and roach Rutilus rutilus group size on swimming speed and prey capture rate of perch Perca fluviatilis and R. rutilus. J Fish Biol 76: 900–912. [Google Scholar]
- Lucas MC, Baras E (2000) Methods for studying spatial behaviour of freshwater fishes in the natural environment. Fish Fish 1: 283–316. [Google Scholar]
- Lucas MC, Johnstone ADF, Priede IG (1993) Use of physiological telemetry as a method of estimating metabolism of fish in the natural environment. Trans Am Fish Soc 122: 822–833. [Google Scholar]
- Maciak S, Konarzewski M (2010) Repeatability of standard metabolic rate (SMR) in a small fish, the spined loach (Cobitis taenia). Comp Biochem Physiol A Mol Integr Physiol 157: 136–141. [DOI] [PubMed] [Google Scholar]
- Mathot KJ, Dingemanse NJ (2015) Energetics and behavior: unrequited needs and new directions. Trends Ecol Evol 30: 199–206. [DOI] [PubMed] [Google Scholar]
- Murchie KJ, Cooke SJ, Danylchuk AJ, Suski CD (2011) Estimates of field activity and metabolic rates of bonefish (Albula vulpes) in coastal marine habitats using acoustic tri-axial accelerometer transmitters and intermittent-flow respirometry. J Exp Mar Biol Ecol 396: 147–155. [Google Scholar]
- Myles-Gonzalez E, Burness G, Yavno S, Rooke A, Fox MG (2015) To boldly go where no goby has gone before: boldness, dispersal tendency, and metabolism at the invasion front. Behav Ecol 26: 1083–1090. [Google Scholar]
- Nespolo RF, Franco M (2007) Whole-animal metabolic rate is a repeatable trait: a meta-analysis. J Exp Biol 210: 2000–2005. [DOI] [PubMed] [Google Scholar]
- Norin T, Malte H (2011) Repeatability of standard metabolic rate, active metabolic rate and aerobic scope in young brown trout during a period of moderate food availability. J Exp Biol 214: 1668–1675. [DOI] [PubMed] [Google Scholar]
- Norin T, Malte H, Clark TD (2014) Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J Exp Biol 217: 244–251. [DOI] [PubMed] [Google Scholar]
- Norin T, Malte H, Clark TD (2015) Differential plasticity of metabolic rate phenotypes in a tropical fish facing environmental change. Funct Ecol. 29: 931–940. [Google Scholar]
- Ohlberger J, Staaks G, Hölker F (2006) Swimming efficiency and the influence of morphology on swimming costs in fishes. J Comp Physiol B Biochem Syst Environ Physiol 176: 17–25. [DOI] [PubMed] [Google Scholar]
- Olsson J, Eklöv P (2005) Habitat structure, feeding mode and morphological reversibility: factors influencing phenotypic plasticity in perch. Evol Ecol Res 7: 1109–1123. [Google Scholar]
- Pedersen MW, Patterson TA, Thygesen UH, Madsen H (2011) Estimating animal behavior and residency from movement data. Oikos 120: 1281–1290. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, The R Development Core Team (2013) nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-111. http://CRAN.R-project.org/package=nlme. [Google Scholar]
- Pörtner HO. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Lannig G (2009) Oxygen and capacity limited thermal tolerance. In Richards JG, Farrell AP, Brauner CJ, eds, Fish Physiology. Academic Press, Burlington, pp 143–191. [Google Scholar]
- R Core Team (2013) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Robertsen G, Armstrong JD, Nislow KH, Herfindal I, McKelvey S, Einum S (2014) Spatial variation in the relationship between performance and metabolic rate in wild juvenile Atlantic salmon. J Anim Ecol 83: 791–799. [DOI] [PubMed] [Google Scholar]
- Rosewarne PJ, Wilson JM, Svendsen JC (2015) Measuring maximum and standard metabolic rates using intermittent flow respirometry: a student laboratory investigation of aerobic metabolic scope and environmental hypoxia in aquatic breathers. J Fish Biol. doi:10.1111/jfb.12795. [DOI] [PubMed] [Google Scholar]
- Rowiński PK, Mateos-Gonzalez F, Sandblom E, Jutfelt F, Ekström A, Sundström LF (2015) Warming alters the body shape of European perch Perca fluviatilis. J Fish Biol 87: 1234–1247. [DOI] [PubMed] [Google Scholar]
- Salin K, Auer SK, Rey B, Selman C, Metcalfe NB (2015) Variation in the link between oxygen consumption and ATP production , and its relevance for animal performance. Proc Biol Sci 282: 20151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PM. (2015) The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218: 1856–1866. [DOI] [PubMed] [Google Scholar]
- Svanbäck R, Eklöv P (2004) Morphology in perch affects habitat specific feeding efficiency. Funct Ecol 18: 503–510. [Google Scholar]
- Svendsen JC, Aarestrup K, Malte H, Thygesen UH, Baktoft H, Koed A, Deacon MG, Cubitt KF, McKinley RS (2011) Linking individual behaviour and migration success in Salmo salar smolts approaching a water withdrawal site: implications for management. Aquat Living Resour 24: 201–209. [Google Scholar]
- Svendsen JC, Steffensen JF, Aarestrup K, Frisk M, Etzerodt A, Jyde M (2012) Excess posthypoxic oxygen consumption in rainbow trout (Oncorhynchus mykiss): recovery in normoxia and hypoxia. Can J Zool 90: 1–11. [Google Scholar]
- Svendsen JC, Banet AI, Christensen RH, Steffensen JF, Aarestrup K (2013) Effects of intraspecific variation in reproductive traits, pectoral fin use and burst swimming on metabolic rates and swimming performance in the Trinidadian guppy (Poecilia reticulata). J Exp Biol 216: 3564–3574. [DOI] [PubMed] [Google Scholar]
- Svendsen JC, Genz J, Anderson WG, Stol JA, Watkinson DA, Enders EC (2014) Evidence of circadian rhythm, oxygen regulation capacity, metabolic repeatability and positive correlations between forced and spontaneous maximal metabolic rates in lake sturgeon Acipenser fulvescens. PLoS ONE 9: e94693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen JC, Tirsgaard B, Cordero GA, Steffensen JF (2015) Intraspecific variation in aerobic and anaerobic locomotion: gilthead sea bream (Sparus aurata) and Trinidadian guppy (Poecilia reticulata) do not exhibit a trade-off between maximum sustained swimming speed and minimum cost of transport. Front Physiol 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe J. (1977) Synopsis of biological data on the perch, Perca fluviatilis, and Perca flavescens. FAO Fisheries Synopsis 113 Rome, Italy, 138 pp. [Google Scholar]
- Tirsgaard B, Svendsen JC, Steffensen JFL (2015) Effect of temperature on specific dynamic action in the Atlantic cod Gadus morhua. Fish Physiol Biochem 41: 41–50. [DOI] [PubMed] [Google Scholar]
- Tudorache C, Viaene P, Blust R, Vereecken H, De Boeck G (2008) A comparison of swimming capacity and energy use in seven European freshwater fish species. Ecol Freshw Fish 17: 284–291. [Google Scholar]
- Tudorache C, O’Keefe RA, Benfey TJ (2011) Optimal swimming speeds reflect preferred swimming speeds of brook charr (Salvelinus fontinalis Mitchill, 1874). Fish Physiol Biochem 37: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP (2012) Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484: 371–375. [DOI] [PubMed] [Google Scholar]
- Videler JJ. (1993) Fish Swimming. Chapman and Hall, London. [Google Scholar]
- Walker JA, Alfaro ME, Noble MM, Fulton CJ (2013) Body fineness ratio as a predictor of maximum prolonged-swimming speed in coral reef fishes. PLoS ONE 8: e75422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Lefevre S, Iversen NK, Findorf I, Buchanan R, McKenzie DJ (2014) Anaemia only causes a small reduction in the upper critical temperature of sea bass: is oxygen delivery the limiting factor for tolerance of acute warming in fishes? J Exp Biol 217: 4275–4278. [DOI] [PubMed] [Google Scholar]
- Watz J, Bergman E, Calles O, Enefalk Å, Gustafsson S, Hagelin A, Nilsson PA, Norrgård JR, Nyqvist D, Österling EM et al. (2015) Ice cover alters the behavior and stress level of brown trout Salmo trutta. Behav Ecol 26: 820–827. [Google Scholar]
- Webb PW. (1984) Body form, locomotion and foraging in aquatic vertebrates. Am Zool 24: 107–120. [Google Scholar]
- Zamora L, Moreno-Amich R (2002) Quantifying the activity and movement of perch in a temperate lake by integrating acoustic telemetry and a geographic information system. Hydrobiologia 483: 209–218. [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed Effects Models and Extensions in Ecology with R. Springer, New York. [Google Scholar]