Abstract
Objective
Intestinal metaplasia and spasmolytic polypeptide-expressing metaplasia (SPEM) are considered neoplastic precursors of gastric adenocarcinoma and are both marked by gene expression alterations in comparison to normal stomach. Since miRNAs are important regulators of gene expression, we sought to investigate the role of miRNAs on the development of stomach metaplasias.
Design
We performed miRNA profiling using a qRT-PCR approach on laser capture microdissected human intestinal metaplasia and SPEM. Data integration of the miRNA profile with a previous mRNA profile from the same samples was performed to detect potential miRNA-mRNA regulatory circuits. Transfection of gastric cancer cell lines with selected miRNA mimics and inhibitors was used to evaluate their effects on the expression of putative targets and additional metaplasia markers.
Results
We identified several genes as potential targets of miRNAs altered during metaplasia progression. We showed evidence that HNF4γ (upregulated in intestinal metaplasia) is targeted by miR-30 and that miR-194 targets a known co-regulator of HNF4 activity, NR2F2 (downregulated in intestinal metaplasia). Intestinal metaplasia markers such as VIL1, TFF2 and TFF3 were down-regulated after overexpression of miR-30a in a HNF4γ-dependent manner. In addition, overexpression of HNF4γ was sufficient to induce the expression of VIL1 and this effect was potentiated by down-regulation of NR2F2.
Conclusion
The interplay of the two transcription factors HNF4γ and NR2F2 and their coordinate regulation by miR-30 and miR-194, respectively, represent a miRNA to transcription factor network responsible for the expression of intestinal transcripts in stomach cell lineages during the development of intestinal metaplasia.
Keywords: intestinal metaplasia, SPEM, miRNA, gastric cancer, HNF4γ, NR2F2
Introduction
Gastric cancer development is preceded by a series of alterations affecting the gastric mucosa cell lineages. Those changes are usually triggered by Helicobacter pylori infection, which leads to a chronic inflammatory response and subsequent oxyntic atrophy (loss of acid-secreting parietal cells). In the stomach corpus, parietal cell loss results in two types of metaplasia: intestinal metaplasia (IM), characterized by the presence of cells with intestinal morphologies, and spasmolytic polypeptide-expressing metaplasia (SPEM), which shows morphological characteristics of the deep antral glands and expresses trefoil factor 2 (spasmolytic polypeptide) (for review see1–3). Both types of metaplasia are highly associated with intestinal-type gastric cancer4, 5 and are considered neoplastic precursors, although the mechanisms driving the progression from metaplasia to neoplasia remain unclear. SPEM originates from the transdifferentiation of mature chief cells in animal models.6 Other studies in animal models indicate that after parietal cell loss, SPEM is the first metaplastic lesion to evolve, whereas intestinal metaplasia will develop from SPEM.7, 8 In humans, there is also evidence suggesting that SPEM progresses to intestinal metaplasia.9–11 The type, extent and location of intestinal metaplasia are also important for risk stratification: extensive metaplasia, usually correlated with the incomplete type of intestinal metaplasia, is associated with increased risk for gastric cancer.12, 13 In previous work, our group compared the mRNA profiles of the normal chief cells with those from both SPEM and IM lineages and identified several important expression markers for human metaplasias. Some of those markers, including MUC13 and CDH17, also demonstrated prognostic value for patients with stage I gastric cancer.14 Both metaplasia and cancer development are marked by global gene expression alterations. One important class of gene expression regulators is represented by microRNAs (miRNAs), which are small noncoding RNAs involved in the post-transcriptional regulation of gene expression. They target the 3′untranslated region of mRNA transcripts and down-regulate gene expression by either inducing mRNA degradation or translational repression.15 Aberrant expression of miRNAs has been reported during cancer development and progression, where they regulate proliferation, differentiation, apoptosis and metastatic competence.16 However, the role of miRNAs during metaplasia development in the stomach is poorly characterized.
We sought here to investigate the influence of miRNAs on the establishment of the metaplasia expression profiles. We performed miRNA profiling on laser capture microdissected human SPEM and intestinal metaplasia lesions from human samples overlapping those previously used for the mRNA profiling. We identified a strong association of mRNA transcript levels and changes in miRNAs. In addition, we were able to discern potential miRNA-mRNA regulatory circuits and we validated the influence of miR-30 down-regulation and miR-194 up-regulation on the expression of specific metaplasia markers through regulation of the transcription factors HNF4γ and NR2F2. These results indicate that miRNAs can regulate cascades of gene expression that influence the development of pre-neoplastic metaplastic lineages.
Methods
miRNA tissue profiling
Areas corresponding to SPEM and intestinal metaplasia lesions (five cases each) were microdissected from 10 μm sections of frozen biopsies from gastric resections of intestinal type tumors in the gastric body. Based on hematoxylin and eosin staining of an adjacent section, the areas corresponding to the metaplasias were microdissected by laser capture using a Veritas Microdissection System (Molecular Devices, Sunnyvale, CA). SPEM lesions were also confirmed by TFF2 staining (Supplemental Figure 1). As a control group, chief cells were microdissected from the normal fundic mucosa of non-gastric cancer patient biopsies with no evidence of atrophic gastritis, intestinal metaplasia, SPEM or adenocarcinoma. All samples were obtained from the Department of Surgery at Seoul National University Hospital (SNUH) from July 2007 to July 2008. The study protocol was approved by the institutional review board (IRB) at SNUH, with written informed consent provided by all patients. Use of the deidentified material was also approved by the Vanderbilt IRB.
Total RNA was extracted from microdissected samples using a miRVana miRNA Isolation Kit (Ambion), which preserves the small RNA fraction. MicroRNA expression was determined by Real Time PCR using TaqMan MicroRNA Arrays (Applied Biosystems). Expression data were analyzed by the comparative Ct (2−ΔΔCt) method using the software DataAssist v2.0 (Applied Biosystems). Group fold changes and statistical analysis (Student’s t-test for pairwise comparisons with control group) were also performed using DataAssist.
miRNA-mRNA data integration analysis and promoter analysis
For the integration analysis we used the software MAGIA2. TargetScan and Diana micro-T miRNA were used for target prediction in combination with a direct integration of our miRNA and a previous mRNA expression14 database using a non-parametric measurement (Spearman) of the correlation. The tables containing the top interactions for each group of differentially expressed miRNAs (r > 0.55) were used for the construction of a bipartite network using the platform Cytoscape.17
Analysis of the enrichment of transcription factor binding sites among the genes previously found up-regulated in stomach metaplasia14 was performed using the Webgestalt package (http://bioinfo.vanderbilt.edu/webgestalt/). For a more extensive analysis searching specifically for HNF4 binding elements, 1 kb regions upstream the transcription start site (defined by RefSeq transcripts) of all genes up-regulated in metaplasia were retrieved from Genome Browser (http://genome.ucsc.edu/) and analyzed using the software MATCH (http://www.gene-regulation.com/cgi-bin/pub/programs/match/bin/match.cgi).
qRT-PCR for miRNA expression validation and for miRNA target transcripts
Total RNA was extracted from fresh cell cultures or stomach tissue (frozen or RNAlater preserved) using TRIzol (Invitrogen, Carlsbad, CA). Normal stomach samples were obtained from Vanderbilt University Hospital from organ donor patients. Metaplasia samples from gastric resections were obtained from the Cooperative Human Tissue Network, Western Division at Vanderbilt University Medical Center (Western CHTN) or the Department of Surgery at Seoul National University Hospital (SNUH). cDNA was prepared using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and qPCR was performed using the primers listed on Supplemental Table 1 and Express SYBR GreenER qPCR Super Mix (Invitrogen, Carlsbad, CA), in an ABI StepOne Real Time PCR System (Applied Biosystems, Foster City, CA). Each sample was measured in triplicate. miRNA expression was analyzed using using TaqMan miRNA assays in an ABI StepOne Real Time PCR System.
Immunostaining
Human stomach sections were deparaffinized and submitted to antigen retrieval by hot steaming in the Target Retrieval solution (Dako North America, Inc., Carpinteria, CA). Cells grown on coverslips were fixed in 4% paraformaldehyde and then permeabilized by incubation in 0.3% Triton X-100 for 15 minutes at room temperature. Blocking for tissue section was performed during 1 h at RT in the serum-free protein blocking solution (Dako North America, Inc., Carpinteria, CA). Blocking for cells was performed during 1 h at room temperature in 10% donkey normal serum. The primary antibody incubation was performed in a humid chamber overnight at 4°C. Primary antibodies used were: 1) Mouse anti-HNF4γ (R&D Systems), at the concentration 1:1000 for tissue sections and 1:500 for cells; 2) Mouse anti-NR2F2 (Abcam), at the concentration of 1:500 and 3) Rabbit anti-MUC2 (Santa Cruz), at the concentration of 1:200. Appropriate secondary antibodies conjugated with Alexa 488, Cy3, or Cy5 were used (1h incubation at room temperature).
Plasmid construction
A mCherry-HNF4γ construct was generated by cloning the coding region of HNF4γ, PCR amplified from H. felis-infected mouse stomach tissue cDNA, into a mCherry-C2 vector (Clontech). For generation of luciferase reporter constructs, the PCR amplified full-length 3′UTR regions of both HNF4γ and NR2F2 or 64-mer oligonucleotides corresponding to regions containing the putative binding sites for miR-30 or mir-194 (in wild type and mutated versions) were cloned downstream of the Firefly luciferase gene of the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). The vector also contains a Renilla luciferase gene, whose activity was used for the normalization of the Firefly luciferase signal.
Cell culture and transfections
Human gastric cancer cell lines (KATO III, MKN7 and MKN28) and HEK-293T cells were maintained in DMEM media supplemented with 10% FBS and antibiotics. Cells were transfected with 40 nM of miR-30a or miR-194 miRIDIAN miRNA mimics (Dharmacon, Pittsburgh, PA), 100 nM of miRCURY LNA™ microRNA miR-30 Family Inhibitor (Exiqon), or 10 nM of siRNA against HNF4γ or NR2F2 (Silence Select siRNA, Ambion, Life Technologies, CA) using the reverse transfection protocol of the Lipofectamine RNAiMax reagent, according manufacture’s instructions (Life Technologies, CA). For plasmid transfection, MKN28 cells (1 × 106 cells) were transfected with 2 μg of mCherry-HNF4γ construct or mCherry empty vector as control using the Polyjet hard-to-transfect protocol (SignaGen Laboratories, Rockville, Maryland) and plated in six well plates. After 24h cells were split 1:3 and plated in selection media containing G418 at 0.5 mg/ml.
Luciferase reporter assay
For the luciferase assays, HEK-293T cells plated on 96-well plates (5,000 cells/well) 24h prior transfection were co-transfected with one of the luciferase reporter constructs (100 ng) and the appropriated miR mimics (100 nM) or a miR negative control using DharmaFECT Duo Transfection Reagent (GE Healtcare-Dharmacon Inc., Lafayette, CO), according to the manufacture’s instructions. Luciferase activity was determined using the Dual Luciferase Assay System (Promega). The assays were repeated twice and performed in triplicates each time.
Results
miRNA profile of metaplasias in the stomach
In a previous study, our group investigated the mRNA transcript profile of human SPEM and intestinal metaplasia in comparison with chief cells from normal stomach mucosa.14 Here we sought to expand the molecular characterization of stomach metaplasias and to build a regulatory network of the alterations during metaplasia progression, focusing especially on miRNAs targeting transcription factors, as this could imply an even broader effect for those miRNAs on the establishment of the metaplasia expression profile. For this, we performed a miRNA profile on laser-captured microdissected human stomach cell lineages (Figure 1A), using an overlapping group of samples as used for the transcriptional profile (four out of five for normal mucosa and three out of five for each of the SPEM and intestinal metaplasia groups). As in our previous investigations of mRNA transcript expression,14 we compared the miRNA expression patterns in metaplasias to expression in chief cells, because all of our recent results indicate that metaplasias are derived from transdifferentiation of chief cells.6 We detected a total of 81 differentially expressed miRNAs (fold change ≥ 2.0) in metaplasias in comparison to normal chief cells. In SPEM, 28 miRNAs were up-regulated, whereas no significant down-regulation was detected. The highest number of differentially expressed miRNAs was observed in intestinal metaplasia, which showed 43 up-regulated (14 of which were also up-regulated in SPEM), and 24 down-regulated miRNAs (Figure 1B–C and Supplemental Tables 2–5). Using the software MAGIA2, we integrated our miRNA expression data with the previous mRNA data obtained from the similar patient samples14 and the top interactions (negative correlation >0.55) were used to construct interaction networks using Cytoscape 3.0 (Figure 2).
Figure 1. miRNA profiling of stomach metaplastic lineages.
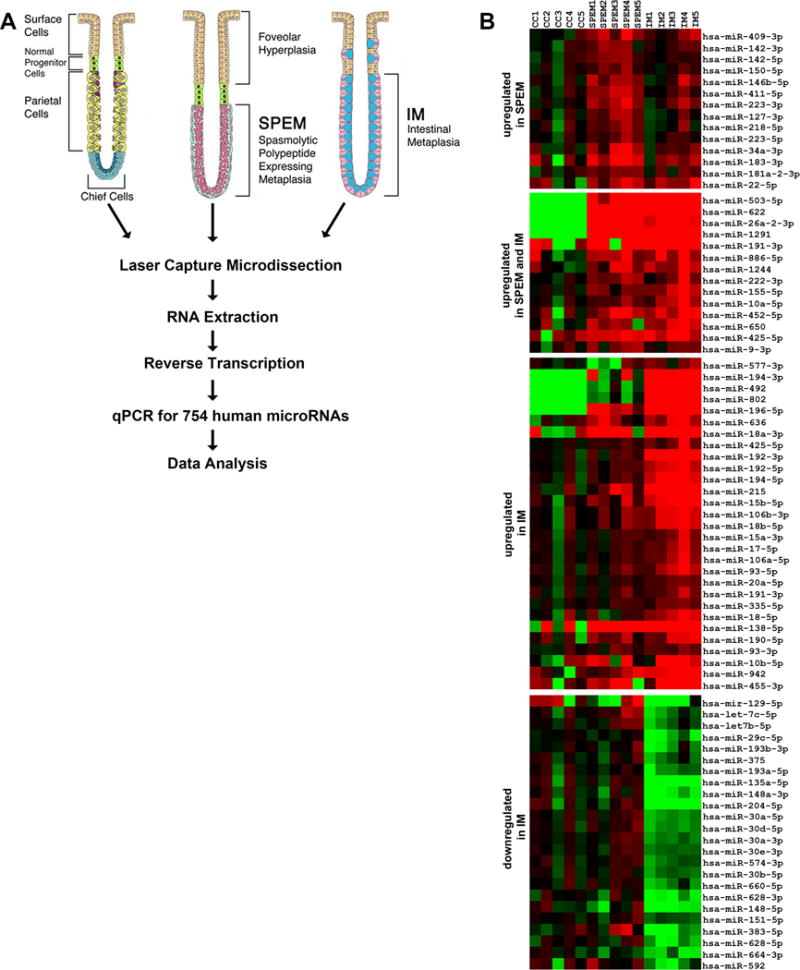
(A) Method schematics. SPEM and intestinal metaplasia lesions (five cases each) were microdissected from 10 μm sections of frozen biopsies from gastric resections of intestinal type tumors in the fundus. As a control comparison group, chief cells were microdissected from normal stomach mucosa. Total RNA was extracted using the mirVana kit (Applied Biosystems) and then, 60 ng of RNA from each sample were converted to cDNA and pre-amplified using Megapool RT and pre-amplification primers (Applied Biosystems). MicroRNA expression was determined by Real Time PCR using TaqMan MicroRNA Arrays (Applied Biosystems). (B) Venn diagram summarizing the overlap of miRNA changes, both up-regulated and down-regulated, in SPEM and intestinal metaplasia (IM). (C) Heat map of the expression profiles of microRNAs differentially expressed in the two types of metaplasias in the human stomach (intestinal metaplasia and SPEM) in comparison with normal chief cells.
Figure 2. miRNA-mRNA regulatory networks in stomach metaplasias.
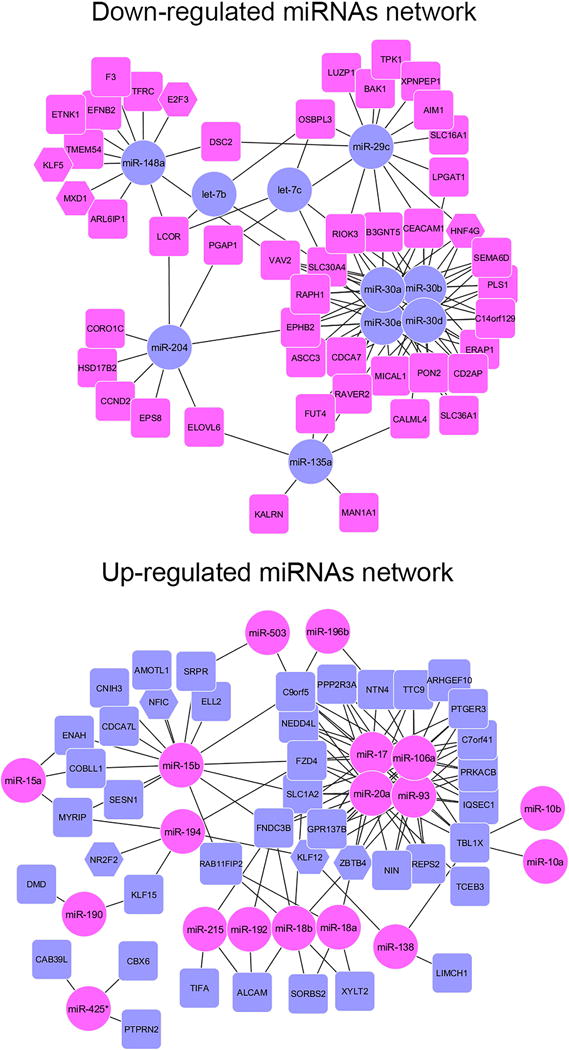
The software MAGIA2 was used to compare our miRNA expression data with a previous mRNA data obtained from the same patient samples14 TargetScan and Diana micro-T miRNA were used for target prediction in combination with a direct integration of our miRNA and a previous mRNA expression data using a non-parametric measurement (Spearman) of the correlation indexes. The top 250 interactions (negative correlation >0.650) were used to construct an interaction network using Cytoscape 3.0. In A-C, Magenta circles represent miRNAs upregulated in the metaplasias, whereas blue rectangles or hexagons (for transcription factors) represent protein-coding mRNAs down-regulated in metaplasia. Comparisons are shown for A. miRNAs up-regulated significantly in SPEM only, B. miRNAs significantly up-regulated in SPEM and intestinal metaplasia (IM) and C. miRNAs up-regulated in intestinal metaplasia. D. depicts miRNAs down-regulated in intestinal metaplasia with Blue circles representing miRNAs down-regulated in the IM, whereas magenta rectangles or hexagons (for transcription factors) represent protein-coding mRNAs upregulated in metaplasia.14
The integration analysis revealed potential miRNA-mRNA regulatory networks operating during metaplasia progression. Since our previous studies of mRNA transcript profiling detected a smaller number of changes in SPEM than in intestinal metaplasia, the analysis of correlations with miRNA changes was less extensive. Four miRNAs up-regulated only in SPEM correlated inversely with six down-regulated mRNAs, including the transcription factor NFIC as a target of miR-150 (Figure 2A). These represent potential regulatory networks involved in the initiation of SPEM. Three of the miRNAs (miR-223, miR-150 and miR-218) have already been connected to gastric cancer, although in opposite ways (miR-22318 and miR-15019 as up-regulated in gastric cancer and miR-21820 as down-regulated). Three of the miRNAs up-regulated in both SPEM and IM correlated with eight mRNAs found progressively down-regulated in these lesions, including the transcription factor FOXN3 (Figure 2B). All three miRNAs have previously been linked to gastric cancer, with reports showing miR-155 up-regulation21 and miR-503 down-regulation22 in gastric cancer and conflicting results for miR-10a. Interestingly, one of the predicted targets for miR-503 in our analysis, IGF1R, has previously been documented as a direct target which is down-regulated in gastric cancer cells22. For intestinal metaplasia, a much higher number of interactions between miRNAs and their putative targets were identified (Figure 2 C–D), including nodes with several members of a miRNA family putatively targeting a high number of transcripts. We therefore focused here a more detailed analysis on miRNA regulation of intestinal metaplasia.
Four members of the miR-30 family were found up-regulated in intestinal metaplasia and were connected to 18 transcripts down-regulated in this lesion, including the transcriptional factor HNF4γ. A bioinformatics analysis using the Webgestalt package and subsequently the software for transcription binding site search, MATCH, showed a significant enrichment for predicted transcriptional targets of HNF4 (HNF4α or HNF4γ) among the gene transcripts previously found up-regulated in intestinal metaplasia14 (Supplemental Table 6). In addition, a known co-regulator of HNF4 activity, NR2F2, was among the transcription factors down-regulated in SPEM and intestinal metaplasia14 and was identified by our integration analysis as a putative target of miR-194, which was up-regulated in intestinal metaplasia (8-fold), but also showed a trend for increased expression in SPEM (1.9-fold). Thus we sought to investigate further the putative counter-regulation of HNF4γ and NR2F2 by their predicted regulatory miRNAs.
Reciprocal expression of the transcriptions factors HNF4γ and NR2F2 and their putative regulatory miRNAs (mir-30 and mir-194) during metaplasia development
We first investigated the mRNA and protein expression levels for the transcription factors HNF4γ and NR2F2 and their putative regulatory miRs in an additional set of biopsied tissue and also in gastric cancer cell lines. We confirmed that miR-30a and miR-30d were down-regulated (Figure 3A and Supplemental Figure 2), whereas HNF4γ mRNA (Figure 3A) and protein (Figure 4A) were up-regulated in stomach samples containing intestinal metaplasia lesions. In addition, MKN28 cells, which express high levels of miR-30a and miR-30d (> 6-fold higher in comparison to KATO III cells), expressed very low levels of HNF4γ mRNA and protein in comparison to KATO III cell lines (Figures 3B and 5A,B). NR2F2 and miR-194 were expressed in the opposite way, with NR2F2 mRNA and protein being down-regulated in intestinal metaplasia areas in comparison to normal stomachs (Figures 3C and 4B) and in KATO III cells in comparison with MKN7 and MKN28 (Figure 5C–D). In contrast, miR-194 was up-regulated in intestinal metaplasia (Figure 3D) and in MKN7 and MKN28 cells (Figure 3D). In spite of different expression levels between normal stomach and intestinal metaplasia for both mRNA and protein, both transcription factors showed detectable levels of mRNA in all tissues. However, by immunofluorescence, HNF4γ was only detected in intestinal metaplasia areas (Figure 4A) and NR2F2 is mostly detected in normal (Figure 4B) and SPEM areas (data not shown).
Figure 3. Expression of two transcription factors, HNF4γ and NR2F2, and their putative regulatory miRNAs (miR-30a and miR-194) in stomach tissue samples and in gastric cancer cell lines.
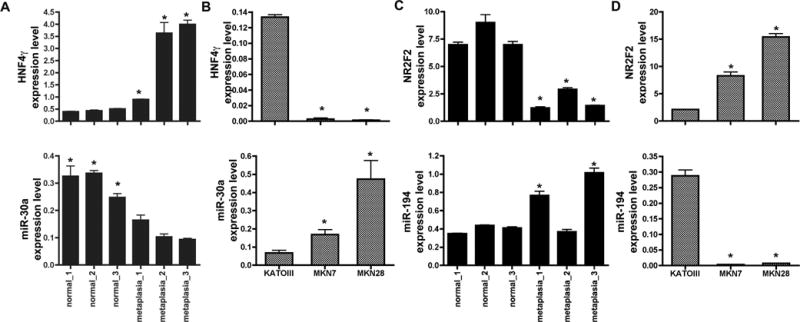
For HNF4γ (A,B, upper panels) and NR2F2 (C,D, upper panels), equal amounts of each cDNA were analyzed by real-time PCR using specific primers. TBP expression was used as an endogenous control for normalization. For miR-30a (A,B, lower panels) and miR-194 (C,D, lower panels), cDNA reactions and qPCR were performed using TaqMan microRNA assays and U6 snRNA levels were measured as an endogenous control. Each sample was measured in three independent cDNA preparations and in triplicate during PCR. All values are shown as means ± SD. Pairwise comparisons were performed by Student’s t-test (* indicates P < 0.05). For comparisons with the tissue samples (A and C), each metaplasia sample was compared to the mean value of all three normal samples.
Figure 4. HNF4γ and NR2F2 protein expression in gastric tissue.
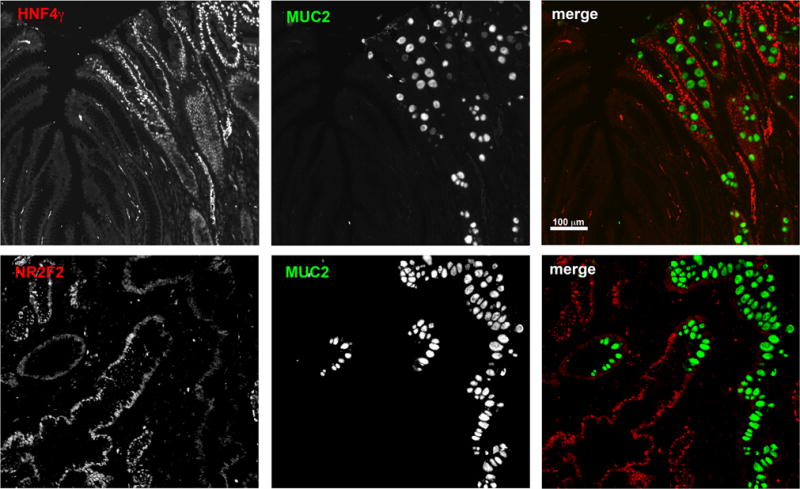
(A) Co-staining of HNF4γ (red) and MUC2 (green) in a section of human stomach displaying both normal and intestinal metaplasia areas. (B) Co-staining of NR2F2 (red) and MUC2 (green) in a section of human stomach displaying both normal and intestinal metaplasia areas. Bar = 50 μm.
Figure 5. HNF4γ and NR2F2 protein expression in gastric cancer cell lines.
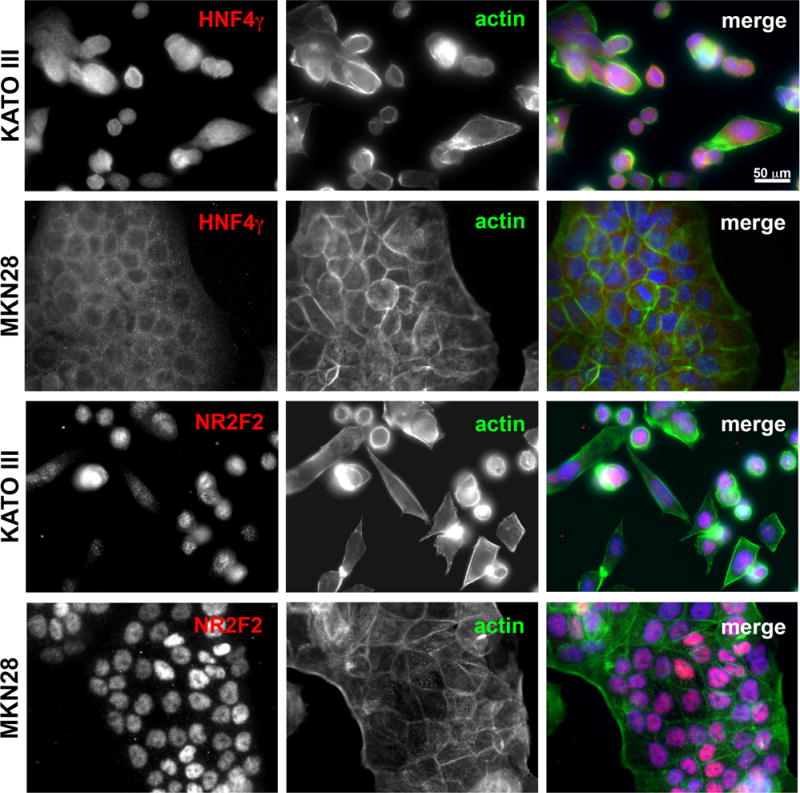
Immunofluorescence using HNF4γ or NR2F2 specific antibodies (red) in the gastric cell lines KATO III and MKN28. A co-labeling with Alexa488-conjugated phaloidin and DAPI was performed for better visualization of cell and nuclear morphology. Images were taken under 40X objective in a Zeiss Axiophot microscope.
miR-30 down-regulates HNF4γ, whereas miR-194 down-regulates NR2F2
We next sought to evaluate the effects of direct manipulation of miR-30a and miR-194 levels on their putative targets. After transfection with miR-30a mimics, the levels of both HNF4γ mRNA and protein decreased in KATO III cells (Figure 6A). On the other hand, when MKN28 cells were transfected with a miR-30 family inhibitor, we observed an increase in HNF4γ mRNA and protein (Figure 6B), although additional regulatory mechanisms for HNF4γ protein may be operating differentially between those two cell lines. In the public sequence databases, there is evidence for two spliced variants of HNF4γ, which would produce proteins with the predicted molecular masses of 44 kDa and 48 kDa. In KATO III cells, we detected one band lower and one slightly higher than 50kDa, compatible with the predicted protein sizes. However, we also detected a higher molecular weight band, which also was affected by the expression of miR-30a and may represent an unknown spliced variant or a post-translational modification of the HNF4γ protein. In MKN28 cells, up-regulation of only one band higher than 50 kDa was observed after inhibition of miR-30 family expression. In contrast, when we expressed a miR-194 mimics in MKN28 cells, NR2F2 mRNA and protein levels were down-regulated in comparison with cells expressing a miR negative control (Figure 6C).
Figure 6. miR-30 targets HNF4γ, whereas miR-194 targets NR2F2.
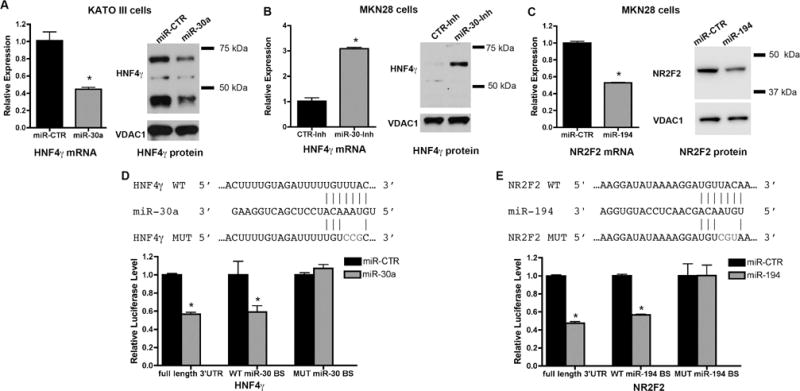
Cells were transfected with a miR-30a mimic (A), a miR-30 family inhibitor (B) or a miR-194 mimic (C), and at 96 hours after transfection, cells were processed for RNA extraction and qRT-PCR for analysis of HNF4γ and NR2F2 message levels or for Western blot for detection of HNF4γ and NR2F2 protein. HNF4γ protein bands in the miR-30 treated samples showed an average reduction of 40% in comparison with the miR-CTR treated samples (with p > 0.001, by Student’s t-test). NR2F2 protein band in the miR-194 treated samples showed an average reduction of 30% in comparison with the miR-CTR treated samples (with p = 0.003, by Student’s t-test). (D,E) Luciferase reporter assays showing the effect of miR-30a (D) or miR-194 (E) on the full-length 3′UTR and on the wild type (WT) miR binding site (BS), but not on the mutant (MUT) sequences within the 3′UTR regions of HNF4g (D) and NR2F2 (E). qRT-PCR analysis of indicated intestinal metaplasia markers after transfection of KATO III cells with either a miR-30 mimic or an siRNA against HNF4γ. All values are shown as means ± SD. Pairwise comparisons (miR-mimics vs. miR-CTR) were performed by Student’s t-test (* indicates P < 0.05).
In addition, we used a luciferase reporter assay to assess the direct effects of the miRs on their putative transcription factor targets. Expression of luciferase fused with either the full HNF4γ 3′UTR or a smaller region of HNF4γ 3′UTR containing a wild type binding site for miR-30 was repressed by a miR-30a mimic, whereas no inhibition was observed for a construct containing a small region of HNF4γ 3′UTR with a mutated miR-30 binding site (Figure 6D). Similar results were observed for NR2F2 and miR-194 (Figure 6E), supporting the notion that miR-30 directly targets HNF4γ, whereas miR-194 targets NR2F2.
Putative transcriptional targets of HNF4γ are also affected by overexpression of miR-30
KATO III cells, which express higher levels of HNF4γ and lower levels of miR-30 members in comparison to MKN7 and MKN28 cells, are the only cell line among the three to maintain the expression of several SPEM and intestinal metaplasia markers (Table 1).23 Some of those markers, including TFF2, TFF3 and VIL1, have predicted HNF4 binding elements in their promoters (Supplemental Table 6), and are not predicted as direct targets of miR-30. We therefore investigated whether miR-30-mediated down-regulation of HNF4γ could affect the expression of those metaplasia markers. We transfected KATO III cells either with a miR-30a mimic or a siRNA against HNF4γ (Figure 7A) and evaluated the mRNA expression of the metaplasia markers 96 hours after transfection (Figure 7B). Expression of all three transcripts (TFF2, TFF3 and VIL1) decreased in KATO III cells transfected either with miR-30 mimics or an siRNA against HNF4γ (Figure 7B), indicating that the effect miR-30a overexpression on those metaplasia markers is likely through the down-regulation of HNF4γ. Expression of the metaplasia marker WFDC2/HE4, which is not a predicted target of miR-30a or HNF4γ, was not significantly affected in either of the transfected cells (Supplemental Figure 3A). Expression of B3GNT5, a putative direct target of miR-30 (Figure 2), but not of HNF4γ, was only decreased in KATO III cells transfected with a miR-30 mimics, but not in cells transfected with the siRNA against HNF4γ (Supplemental Figure 3B). Since CDX1 and CDX2 are critical factors for establishment of the intestinal phenotype, we also evaluated the effects of miR-30 or HNF4γ manipulations on their expression. CDX1 has a very low expression in KATO III cells (Ct > 35) and no change was observed after overexpression of miR-30 or knocking down of HNF4γ expression. However, significant decreases in the expression levels of CDX2 message were detected after overexpression of miR-30 (44% decrease) or knocking down of HNF4γ, (22% decrease) (Figure 7E). The miR-30 effect on CDX2 can be explained by the presence of a non-conserved recognition binding site (not detected in the integration analysis, which considered only highly conserved binding sites). The effect of HNF4γ is probably indirect, as no HNF4 element was predicted in the CDX2 promoter.
Table 1.
Expression of metaplasia markers in gastric cancer cell lines
| Marker | KATO III | AGS | MKN7 | MKN28 |
|---|---|---|---|---|
| TFF2 | + | − | − | − |
| TFF3 | + | − | − | − |
| WFDC2/HE4 | + | +/− | − | − |
| MUC6 | − | − | − | − |
| CLU | + | + | + | + |
| VIL1 | + | + | NT | − |
| DMBT1 | − | + | − | − |
| MUC2 | +/− | − | − | − |
| MUC13 | + | NT | NT | − |
| CDX2 | + | + | + | + |
| CFTR | + | + | − | − |
Expression assessed by PCR and supported by study on molecular profiling of 37 gastric cancer cell lines23.
Figure 7. MiR-30a affects other metaplasia markers in an HNF4γ manner.
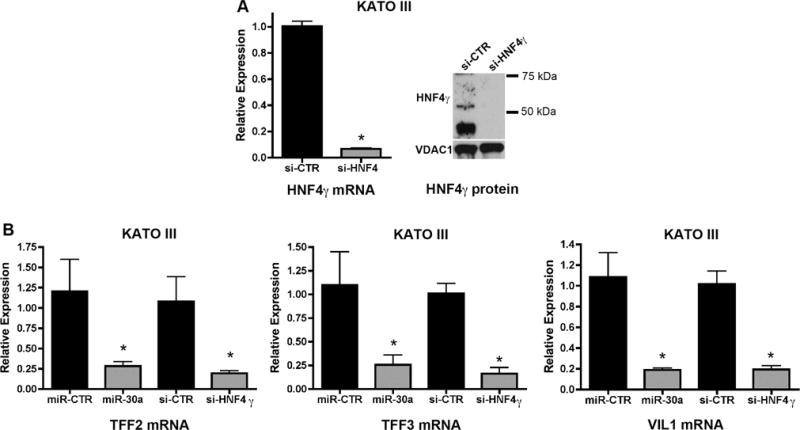
Cells were transfected with a miR-30a mimic or a siRNA against HNF4γ and at 96 hours after transfection, cells were processed for RNA extraction and qRT-PCR for analysis of the expression of metaplasia markers putatively transactivated by HNF4γ. (A) Effectiveness of HNF4γ knockdown at mRNA and protein levels using siRNA. (B-E) qRT-PCR analysis of metaplasia markers TFF2 (B), TFF3 (C), Villin (VIL1, D) and CDX2 (E) after transfection of KATO III cells with either a miR-30 mimic or an siRNA against HNF4γ. All values are shown as means ± SD. Pairwise comparisons were performed by Student’s t-test (* indicates P < 0.05).
To confirm the effect of HNF4γ on its putative transcriptional targets, we then transfected MKN28 cells with an HNF4γ-expression construct (mCherry-HNF4γ) and stable cells lines expressing mCherry-HNF4γ or mCherry alone were established through antibiotic selection. Overexpression of HNF4γ in MKN28 cells was confirmed by qRT-PCR and western blot (Figure 8A). The correct nuclear localization of the mCherry-tagged HNF4γ protein was confirmed by immunocytochemistry (Figure 8B). MKN28 cells overexpressing HNF4γ regained the expression of VIL1 mRNA, but not of TFF2 or TFF3 messages (Figure 8D), indicating that while HNF4γ is necessary and sufficient to induce VIL1 expression, it is necessary, but not sufficient to induce TFF2 and TFF3 expression.
Figure 8. HNF4γ overexpression restores VIL1 expression in MKN28 cells and the effect is potentiated by the down-regulation of NR2F2.
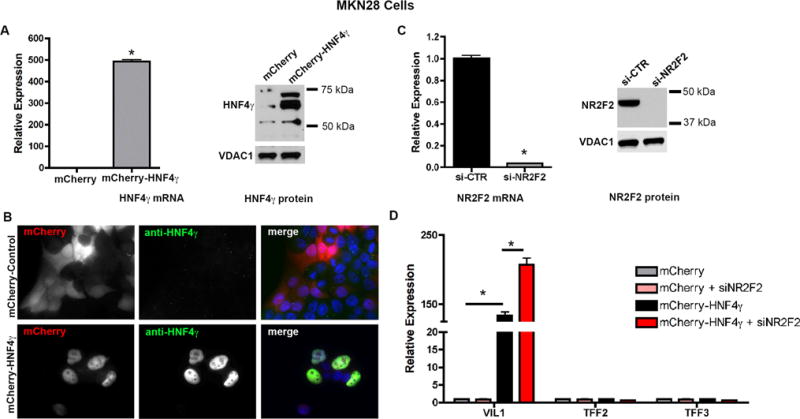
(A) HNF4γ mRNA (by qRT-PCR) and protein levels (by western blot) in MKN28 cells transfected with a control vector (mCherry) or a mCherry-HNF4γ-expression construct. (B) Immunostaining with an HNF4γ-specific antibody in transfected MKN28 cells. Images were taken in a Zeiss Axiophot microscope (20X). (C) Effectiveness of NR2F2 knockdown in MKN28 cells using siRNA. (D) Increased mRNA expression (qRT-PCR) of the intestinal metaplasia marker VIL1, but not TFF2 or TFF3 in MKN28 cells overexpressing HNF4γ or overexpressing HNF4γ and knocked down for NR2F2. All values are shown as means ± SD. Pairwise comparisons, as indicated, were performed by Student’s t-test (* indicates P < 0.05).
NR2F2 is a known co-regulator of several steroid receptor family members, including HNF4. It usually inhibits HNF4 transcriptional activation activity by competing for the same binding sites in the promoters of the target genes,24–26 although sometimes it can work synergistically with HNF4 to activate transcription.27, 28 We investigated if alterations on NR2F2 levels would affect the influence of HNF4γ on the expression of the metaplasia markers VIL1, TFF2 and TFF3. For this, we transfected the MKN28 stable cell line expressing the mCherry-HNF4γ construct with a siRNA against NR2F2. After we confirmed a highly efficient knockdown of NR2F2 by one of the two siRNA we tested (Figure 8C), we observed that a combination of overexpression of HNF4γ and knockdown of NR2F2 in MKN28 resulted in an even higher increase of VIL1 expression than that observed for the cells only expressing the mCherry-HNF4γ fusion protein (Figure 8D). These findings support the concept that for at least one of the metaplasia markers evaluated, VIL1, HNF4γ acts as a transcriptional activator, whereas NR2F2 seems to act as a repressor.
Discussion
Intestinal metaplasia is a well-established precursor for intestinal-type gastric cancer,2, 12 and SPEM has gained increasing attention as a neoplastic precursor.29, 30 Our group has characterized the mRNA and protein expression profiles in metaplasia in both humans and animal models.11, 14, 31, 32 Several studies have reported mRNA and miRNA dysregulation in gastric cancer33–35 and Helicobacter pylori-induced gastritis.36–38 However, no previous studies have evaluated a systematic comparison of the miRNA profiles of SPEM and intestinal metaplasia. Here, we generated a miRNA profile of human SPEM and intestinal metaplasia compared to normal chief cells, the precursor cells of SPEM lesions. This analysis was initiated using a limited set of samples to analyze laser capture microdissected lineages. Thus, we have focused on the identification of alterations of a larger magnitude in these specific isolated cell lineages. Using an integrated miRNA-mRNA data analysis, putative targets for miRNAs differentially expressed in SPEM and intestinal metaplasia were detected, revealing potential miRNA-mRNA regulatory networking involved in metaplasia development. Although the number of connections detected in SPEM was low, they have the potential to represent earlier regulatory events in the cascade to gastric cancer, since most of their up-regulated miRNAs have been linked to gastric cancer. For this study, though, we focused on connections detected in intestinal metaplasia.
The development of intestinal metaplasia in the stomach is believed to recapitulate the intestine developmental process and intestine-specific transcription factors are up-regulated in stomach areas with intestinal metaplasia.39 CDX1 and CDX2 are critical transcription factors for intestinal patterning and, when ectopically expressed in the stomach, they are able to induce the appearance of intestinal metaplasia.40, 41 Although CDX1 and CDX2 are essential for intestine development and maintenance, other transcription factors cooperate to regulate the expression of intestinal genes.42 Therefore, it is likely that additional transcription factors are required for the establishment of the intestinal phenotype during the development of metaplasia in the stomach. We showed evidence here that two types of miRNAs (miR-30 family members and miR-194) differentially expressed during progression from normal stomach to metaplasia control the expression of two of those potential additional transcription factors, HNF4γ and NR2F2.
HNF4γ is the less characterized paralog of HNF4α, a member of the nuclear receptor class of transcription factors with important roles in liver, pancreas and intestine epithelial differentiation. HNF4α regulates the transcriptional profile of differentiated intestinal cells43 and bioinformatics studies showed an over-representation of HNF4 binding sites in genes up-regulated during intestinal epithelium differentiation.44–46 HNF4γ is also highly expressed in intestine and kidney, but in contrast with HNF4α, it is not expressed in mouse liver.47 Although an exact role for HNF4γ in intestinal differentiation has not been identified, it is believed to share the same DNA binding capabilities as HNF4α and one study has suggested that a tightly regulated ratio of HNF4α/HNF4γ is important for the spatial specification of the APOA-IV expression in the intestinal villus48. In addition, HNF4γ is one of the highly up-regulated transcription factors in the duodenum epithelium at the time of the compartmentalization between stomach and intestine during mouse gut development49. Although not essential for the determination of cell fate, HNF4 seems to play a role in controlling the terminal differentiation of the intestinal epithelium, probably downstream of transcription factors required for early intestinal patterning, such as CDX2 and CDX1.
We showed that HNF4γ is upregulated, whereas miR-30 family members are down-regulated in intestinal metaplasia in the stomach. MiR-30 negatively regulated HNF4γ levels in gastric cells and down-regulation of HNF4γ reduces the expression of two intestinal transcripts (VIL1 and TFF3) and a SPEM marker (TFF2). Expression of HNF4γ in the MKN28 gastric cell line was sufficient to induce VIL1 expression. VIL1 is a known transcriptional target of CDX2, although other factors are required for the CDX2-induced activation of VIL1 expression.50 We detected CDX2 expression in both KATO III (HNF4γ expression positive) and MKN28 (HNF4γ expression negative) cells. It is therefore possible that HNF4γ cooperates with CDX2 for the activation VIL1 expression. An interconnection between CDX2 and HNF4γ pathways is reinforced by the fact that CDX2 message expression was altered by either miR-30 overexpression or HNF4γ knock down.
For the expression of TFF3, also a previously described transcriptional target of CDX2, additional factors seem to be required, since the exogenous expression of HNF4γ in MKN28 cells was not sufficient to induce TFF3 expression.
MiR-30 family members are considered tumor suppressor miRNAs, since they are down-regulated in different types of cancer,51–54 including gastric cancer.55–57 A recent study showed that miR-30a is down-regulated in 78% of gastric cancer samples in comparison with normal surrounding tissue and is linked to loss of RUNX3 expression.56. The down-regulation of at least one member, miR-30a, might be related to the down-regulation of RUNX3 also reported in metaplasias in the stomach.58 We suggest that miR-30 down-regulation is an early event in the cascade from metaplasia to gastric cancer and that it contributes to the establishment of an intestinal expression profile through regulation of HNF4γ.
We also showed that NR2F2, a known co-regulator of HNF4, is down-regulated during metaplasia progression and is a direct target of miR-194, one of the miRNAs overexpressed in intestinal metaplasia. Previous studies showed that miR-194 is induced during intestinal epithelium differentiation and is transcriptionally regulated by HNF1α, one of the transcription factors implicated in the regulation of the intestinal expression pattern.59 NR2F2 modifies HNF4 transcriptional activity either negatively or positively depending on the cell type and promoter context. It acts as a repressor of HNF4 activity on apolipoprotein gene promoters APOA1, APOA2, APOB, APOC324–26, and hepatic lipase.60 Conversely, NR2F2 works synergistically with HNF4 to activate the transcription of CYP7A128 and HNF1.27 Our data support the notion that in the stomach, NR2F2 interferes negatively with HNF4γ transactivation of metaplasia marker genes. Overexpression of HNF4γ in a cell line normally expressing NR2F2, restored VIL1 expression and the effect of HNF4γ activation of VIL1 was significantly potentiated when NR2F2 levels were down-regulated. Our data support the notion that high levels of NR2F2 are important for the maintenance of the normal stomach expression pattern, whereas increasing the levels of HNF4γ and decreasing the levels of NR2F2 (probably initiated in SPEM) push the cells towards an intestinal-like phenotype. Thus, a tightly regulated balance of expression levels between HNF4γ and NR2F2 contributes to the regulation of the stomach cell lineage identities, and that balance between these two transcription factors is, at least in part, regulated by the miR-30 family and miR-194. It is possible that the influence of miR-194 on NR2F2 may be initiated in SPEM lesions, since a trend for alteration in their expression could already be noticed in SPEM samples (2-fold change for NR2F2 and 1.9 fold change for miR-194). It is interesting to note that two other co-repressors, NFIC and FOXN3, were identified as targets of up-regulated miRNAs correlating with their progressive down-regulation in SPEM and intestinal metaplasia. This suggests that the down-regulation of co-repressors via miRNAs is a contributing event for the establishment of a new expression pattern that will reflect the transdifferentiation of stomach lineages during metaplasia development.
In conclusion, we have demonstrated that synergistic miRNA alterations are present in metaplastic lineages. Two of these alterations, the down-regulation of different members of miR-30 family and the upregulation of miR-194 likely contribute to the expression of intestinal transcripts that are characteristic of intestinal metaplasia through the regulation of HNF4γ and NR2F2. This network of two competing transcription factors and their regulatory miRNAs promotes the “intestinalization” of metaplastic stomach lineages. Therefore, miRNAs may act as coordinated regulators of the transcriptional phenotypes necessary for the evolution and progression metaplastic lineages in the stomach. While the present studies have focused on just two competing regulatory cascades, these investigations have identified a number of further possible interactions between miRNAs and mRNA transcript expression related to the induction of metaplasias that will require further explication in the future.
Supplementary Material
Supplemental Figure 1: TFF2 staining for confirmation of SPEM lesion. Frozen sections of patient biopsies used for laser capture microdissection of SPEM lesions were stained with an anti-TFF2 primary antibody detected with Cy3-conjugated anti-mouse IgM. SPEM lesions were characterized by the expression of TFF2 towards the base of the gastric glands, which normally do not express TFF2.
Supplemental Figure 2: miR-30d expression in gastric tissue and gastric cancer cell lines. cDNA reactions and qPCR were performed using TaqMan microRNA assays and U6 snRNA levels were measured as an endogenous control. Each sample was measured in three independent cDNA preparations and in triplicate during PCR. All values are shown as means ± SD.
Supplemental Figure 3: Expression analysis of metaplasia markers not predicted to be affected by the miR-30/HNF4γ circuit. Cells were transfected with a miR-30a mimic or an HNF4γ siRNA and at 96 hours after transfection, cells were processed for RNA extraction and qRT-PCR for analysis of WFDC2/HE4 (A), a non-predicted target of either miR-30 or HNF4γ, and G3GNT5 (B), a predicted target of miR-30, but not of HNF4γ. All values are shown as means ± SD. Pairwise comparisons, as indicated, were performed by Student’s t-test (* indicates P < 0.05).
Significance of this study.
What is already known on this subject?
Gastric cancer is one of the leading causes of cancer-related death worldwide, making early diagnosis critical to increase patient survival rates.
SPEM and intestinal metaplasia in the stomach are considered preneoplastic lesions and are also marked by an altered expression profile in comparison to the normal stomach, although they are in general considered less heterogeneous than gastric cancer.
miRNAs are important regulators of gene expression and several alterations in miRNA expression have been described in gastric cancer, although miRNA alterations in stomach metaplasias are less characterized.
What are the new findings?
This is the first study showing a comprehensive integration of miRNA and mRNA profiles of two preneoplastic metaplasias, spasmolytic polypeptide-expressing metaplasia (SPEM) and intestinal metaplasia, in the stomach.
We present evidence that the expression levels of two transcription factors (HNF4γ and NR2F2) altered during progression from normal stomach to intestinal metaplasia are regulated by miR-30 and miR-194, respectively.
Our results also indicate for the first time that a regulated balance between the expression levels of HNF4γ and NR2F2 contributes to the development of intestinal metaplasia, through the coordinated upregulation of intestinal markers.
How might it impact on clinical practice in the foreseeable future?
Our study highlights miRNA alterations in stomach metaplasias that are potential early events in the cascade leading to development of gastric cancer. The identification of early miRNA alterations and the characterization of their molecular interactions in preneoplastic lesions have the potential to lead to the development of suitable biomarkers and therapeutic targets for early detection and treatment of gastric cancer.
Acknowledgments
Grant support: These studies were supported by grants to J.R.G. from NIH RO1 DK071590, ARRA Supplemental Funding from RO1 DK071590-S1, from CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences, a Veterans Affairs Merit Review and from a grant from the T.J. Martell Foundation. J.F.S was supported by a fellowship from the Prevent Cancer Foundation. This work was supported by core resources of the Vanderbilt Digestive Disease Center (P30 DK058404), and the Vanderbilt-Ingram Cancer Center through NCI Cancer Center Support Grant P30 CA068485 utilizing the Translational Pathology Shared Resource.
Footnotes
Disclosure: All authors have no conflict of interest.
References
- 1.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–72. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenring JR, Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 4.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–60. [PubMed] [Google Scholar]
- 5.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46. [PubMed] [Google Scholar]
- 6.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam KT, Lee HJ, Mok H, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–96. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshizawa N, Takenaka Y, Yamaguchi H, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–76. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 9.Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–10. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lennerz JK, Kim SH, Oates EL, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–33. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sousa JF, Ham AJ, Whitwell C, et al. Proteomic profiling of paraffin-embedded samples identifies metaplasia-specific and early-stage gastric cancer biomarkers. Am J Pathol. 2012;181:1560–72. doi: 10.1016/j.ajpath.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–8. doi: 10.1038/ajg.2009.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenring JR, Nam KT. Oxyntic atrophy, metaplasia, and gastric cancer. Prog Mol Biol Transl Sci. 2010;96:117–31. doi: 10.1016/B978-0-12-381280-3.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ, Nam KT, Park HS, et al. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology. 2010;139:213–25. doi: 10.1053/j.gastro.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 17.Cline MS, Smoot M, Cerami E, et al. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2:2366–82. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Zhang Y, Zhang H, et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–33. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Jin H, Yang Z, et al. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem Biophys Res Commun. 2010;392:340–5. doi: 10.1016/j.bbrc.2009.12.182. [DOI] [PubMed] [Google Scholar]
- 20.Tie J, Pan Y, Zhao L, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6:e1000879. doi: 10.1371/journal.pgen.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link A, Schirrmeister W, Langner C, et al. Differential expression of microRNAs in preneoplastic gastric mucosa. Sci Rep. 2015;5:8270. doi: 10.1038/srep08270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Ge G, Ding Y, et al. MiR-503 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J (Engl) 2014;127:2357–62. [PubMed] [Google Scholar]
- 23.Tan IB, Ivanova T, Lim KH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–85. doi: 10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan J, Nakabayashi H, Wong NC. HNF-4 increases activity of the rat Apo A1 gene. Nucleic Acids Res. 1993;21:1205–11. doi: 10.1093/nar/21.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladias JA, Hadzopoulou-Cladaras M, Kardassis D, et al. Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J Biol Chem. 1992;267:15849–60. [PubMed] [Google Scholar]
- 26.Mietus-Snyder M, Sladek FM, Ginsburg GS, et al. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992;12:1708–18. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ktistaki E, Talianidis I. Chicken ovalbumin upstream promoter transcription factors act as auxiliary cofactors for hepatocyte nuclear factor 4 and enhance hepatic gene expression. Mol Cell Biol. 1997;17:2790–7. doi: 10.1128/mcb.17.5.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroup D, Chiang JY. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7alpha-hydroxylase gene (CYP7A1) J Lipid Res. 2000;41:1–11. [PubMed] [Google Scholar]
- 29.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res. 2011;317:2759–64. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12:189–97. doi: 10.1007/s10120-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–22. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weis VG, Sousa JF, Bonnie JL, et al. Heterogeneity in mouse SPEM lineages identifies markers of metaplastic progression. Gut. 2012;62:1270–9. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–46. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasui W, Oue N, Ito R, et al. Search for new biomarkers of gastric cancer through serial analysis of gene expression and its clinical implications. Cancer Sci. 2004;95:385–92. doi: 10.1111/j.1349-7006.2004.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasui W, Oue N, Sentani K, et al. Transcriptome dissection of gastric cancer: identification of novel diagnostic and therapeutic targets from pathology specimens. Pathol Int. 2009;59:121–36. doi: 10.1111/j.1440-1827.2009.02329.x. [DOI] [PubMed] [Google Scholar]
- 36.Matsushima K, Isomoto H, Inoue N, et al. MicroRNA signatures in Helicobacter pylori-infected gastric mucosa. Int J Cancer. 2011;128:361–70. doi: 10.1002/ijc.25348. [DOI] [PubMed] [Google Scholar]
- 37.Petrocca F, Visone R, Onelli MR, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–86. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Zabaleta J. MicroRNA: A Bridge from H. pylori Infection to Gastritis and Gastric Cancer Development. Front Genet. 2012;3:294. doi: 10.3389/fgene.2012.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez-Gonzalez L, Wright NA. Biology of intestinal metaplasia in 2008: more than a simple phenotypic alteration. Dig Liver Dis. 2008;40:510–22. doi: 10.1016/j.dld.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Mutoh H, Sakurai S, Satoh K, et al. Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice. Gut. 2004;53:1416–23. doi: 10.1136/gut.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silberg DG, Sullivan J, Kang E, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–96. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 42.Boudreau F, Rings EH, van Wering HM, et al. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909–17. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 43.Garrison WD, Battle MA, Yang C, et al. Hepatocyte nuclear factor 4alpha is essential for embryonic development of the mouse colon. Gastroenterology. 2006;130:1207–20. doi: 10.1053/j.gastro.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyd M, Bressendorff S, Moller J, et al. Mapping of HNF4alpha target genes in intestinal epithelial cells. BMC Gastroenterol. 2009;9:68. doi: 10.1186/1471-230X-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegmann A, Hansen M, Wang Y, et al. Metabolome, transcriptome, and bioinformatic cis-element analyses point to HNF-4 as a central regulator of gene expression during enterocyte differentiation. Physiol Genomics. 2006;27:141–55. doi: 10.1152/physiolgenomics.00314.2005. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Madison BB, Zacharias W, et al. Deconvoluting the intestine: molecular evidence for a major role of the mesenchyme in the modulation of signaling cross talk. Physiol Genomics. 2007;29:290–301. doi: 10.1152/physiolgenomics.00269.2006. [DOI] [PubMed] [Google Scholar]
- 47.Taraviras S, Mantamadiotis T, Dong-Si T, et al. Primary structure, chromosomal mapping, expression and transcriptional activity of murine hepatocyte nuclear factor 4gamma. Biochim Biophys Acta. 2000;1490:21–32. doi: 10.1016/s0167-4781(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 48.Sauvaget D, Chauffeton V, Citadelle D, et al. Restriction of apolipoprotein A-IV gene expression to the intestine villus depends on a hormone-responsive element and parallels differential expression of the hepatic nuclear factor 4alpha and gamma isoforms. J Biol Chem. 2002;277:34540–8. doi: 10.1074/jbc.M206074200. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Udager AM, Hu C, et al. Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn. 2009;238:3205–17. doi: 10.1002/dvdy.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamichi N, Inada K, Furukawa C, et al. Cdx2 and the Brm-type SWI/SNF complex cooperatively regulate villin expression in gastrointestinal cells. Exp Cell Res. 2009;315:1779–89. doi: 10.1016/j.yexcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Kao CJ, Martiniez A, Shi XB, et al. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene. 2013;33:2495–503. doi: 10.1038/onc.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouzounova M, Vuong T, Ancey PB, et al. MicroRNA miR-30 family regulates non-attachment growth of breast cancer cells. BMC Genomics. 2013;14:139. doi: 10.1186/1471-2164-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porkka KP, Pfeiffer MJ, Waltering KK, et al. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–5. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 54.Van der Auwera I, Limame R, van Dam P, et al. Integrated miRNA and mRNA expression profiling of the inflammatory breast cancer subtype. Br J Cancer. 2010;103:532–41. doi: 10.1038/sj.bjc.6605787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Zhang Y, Zhang Y, et al. Survival prediction of gastric cancer by a seven-microRNA signature. Gut. 2010;59:579–85. doi: 10.1136/gut.2008.175497. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Chen L, Zhang X, et al. RUNX3 regulates vimentin expression via miR-30a during epithelial-mesenchymal transition in gastric cancer cells. J Cell Mol Med. 2014;18:610–23. doi: 10.1111/jcmm.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu ED, Li N, Li BS, et al. miR-30b, down-regulated in gastric cancer, promotes apoptosis and suppresses tumor growth by targeting plasminogen activator inhibitor-1. PLoS One. 2014;9:e106049. doi: 10.1371/journal.pone.0106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito K, Chuang LS, Ito T, et al. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology. 2011;140:1536–46 e8. doi: 10.1053/j.gastro.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 59.Hino K, Tsuchiya K, Fukao T, et al. Inducible expression of microRNA-194 is regulated by HNF-1alpha during intestinal epithelial cell differentiation. RNA. 2008;14:1433–42. doi: 10.1261/rna.810208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rufibach LE, Duncan SA, Battle M, et al. Transcriptional regulation of the human hepatic lipase (LIPC) gene promoter. J Lipid Res. 2006;47:1463–77. doi: 10.1194/jlr.M600082-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: TFF2 staining for confirmation of SPEM lesion. Frozen sections of patient biopsies used for laser capture microdissection of SPEM lesions were stained with an anti-TFF2 primary antibody detected with Cy3-conjugated anti-mouse IgM. SPEM lesions were characterized by the expression of TFF2 towards the base of the gastric glands, which normally do not express TFF2.
Supplemental Figure 2: miR-30d expression in gastric tissue and gastric cancer cell lines. cDNA reactions and qPCR were performed using TaqMan microRNA assays and U6 snRNA levels were measured as an endogenous control. Each sample was measured in three independent cDNA preparations and in triplicate during PCR. All values are shown as means ± SD.
Supplemental Figure 3: Expression analysis of metaplasia markers not predicted to be affected by the miR-30/HNF4γ circuit. Cells were transfected with a miR-30a mimic or an HNF4γ siRNA and at 96 hours after transfection, cells were processed for RNA extraction and qRT-PCR for analysis of WFDC2/HE4 (A), a non-predicted target of either miR-30 or HNF4γ, and G3GNT5 (B), a predicted target of miR-30, but not of HNF4γ. All values are shown as means ± SD. Pairwise comparisons, as indicated, were performed by Student’s t-test (* indicates P < 0.05).


