Abstract
Background
Since the 2001 “black box” warning on droperidol, its use in the prehospital setting has decreased substantially in favor of haloperidol. There are no studies comparing the prehospital use of either drug. The goal of this study was to compare QTc prolongation, adverse events, and effectiveness of droperidol and haloperidol among a cohort of agitated patients in the prehospital setting.
Methods
In this institutional review board-approved before and after study, we collected data on 532 patients receiving haloperidol (n = 314) or droperidol (n = 218) between 2007 and 2010. We reviewed emergency department (ED) electrocardiograms when available (haloperidol, n = 78, 25%; droperidol, n = 178, 76%) for QTc length (in milliseconds), medical records for clinically relevant adverse events (defined a priori as systolic blood pressure (SBP) <90 mmHg, seizure, administration of anti-dysrhythmic medications, cardioversion or defibrillation, bag–valve–mask ventilation, intubation, cardiopulmonary arrest, and prehospital or in-hospital death). We also compared effectiveness of the medications, using administration of additional sedating medications within 30 minutes of ED arrival as a proxy for effectiveness.
Results
The mean haloperidol dose was 7.9 mg (median 10 mg, range 4–20 mg). The mean droperidol dose was 2.9 mg (median 2.5 mg, range 1.25–10 mg.) Haloperidol was given IM in 289 cases (92%), and droperidol was given IM in 132 cases (61%); in all other cases, the medication was given IV. There was no statistically significant difference in median QTc after medication administration (haloperidol 447 ms, 95% CI: 440–454 ms; droperidol 454 ms, 95% CI: 450–457). There were no statistically significant differences in adverse events in the droperidol group as compared to the haloperidol group. One patient in the droperidol group with a history of congenital heart disease suffered a cardiopulmonary arrest and was resuscitated with neurologically intact survival. There was no significant difference in the use of additional sedating medications within 30 minutes of ED arrival after receiving droperidol (2.9%, 95% CI: −2.5–8.4%).
Conclusions
In this cohort of agitated patients treated with haloperidol or droperidol in the prehospital setting, there was no significant difference found in QTc prolongation, adverse events, or need for repeat sedation between haloperidol and droperidol.
Keywords: droperidol, haloperidol, emergency medical services
Introduction
Since the 2001 black box warning on droperidol,1 its use in the prehospital setting has decreased2,3 substantially in favor of haloperidol. The 2001 warning for droperidol reports “deaths associated with QT prolongation and torsades de pointes in patients treated with doses of INAPSINE (droperidol) above, within, and even below the approved range.”1 It also recommends a pretreatment electrocardiogram (ECG) be obtained to establish baseline QTc length.1 These recommendations have limited the use of droperidol in the prehospital setting.3
However, there is controversy regarding the black box warning. Horowitz et al. pointed out the limited generalizability of the reported deaths to emergency settings and doses, and questions the appropriateness of the black box warning.4 Kao et al. stated that “the evidence is not convincing for a causal relationship between therapeutic droperidol administration and life-threatening cardiac events.”5 Shale et al. report that “in clinical use there is no pattern of sudden deaths.”6
Droperidol has been studied in the prehospital setting without a control group7 and with a placebo control.8 Droperidol and haloperidol have been compared in the ED setting, with the authors finding more rapid sedation with droperidol.9 However, there are no other trials comparing droperidol with haloperidol in the prehospital setting.
Therefore, the goal of this study was to compare prehospital haloperidol and droperidol with respect to ECG QTc length, adverse events, and need for additional ED sedation.
Methods
Study Design
This was a before–after quasi-experiment of all patients receiving prehospital haloperidol or droperidol from January 1, 2007 through January 30, 2010. The Colorado Multiple Institutional Review Board approved the study.
Study Setting and Population
Denver County has a geographic area of approximately 150 square miles, an estimated county population of 600,000, and an estimated metropolitan population of 2.3 million according to 2010 census data.10 The Denver Health Paramedic Division (DHPD), an urban hospital-based EMS agency, is the primary provider of 9-1-1 ambulance transport for the City and County of Denver. The DHPD responds to approximately 80,000 calls per year, with 55,000 patient transports distributed among 12 receiving hospitals. During the study period, 50% of all transports went to the study hospitals, with 40% of all patients transported to Denver Health Medical Center, and 10% transported to the University of Colorado Hospital. Denver Health is a level I trauma center and by policy the preferred destination for critically injured patients. University of Colorado Hospital is a level II trauma center.
The medications were indicated for patients “behaving in a manner that poses a threat to their own well-being or others” and for combative, head-injured patients (Figure E1, available online). The protocol allowed for intramuscular administration when an IV was not readily obtainable. The protocol called for the use of either medicine as a single agent (that is, without other sedatives). All patients sedated for agitation also were physically restrained in the supine or semi-Fowler’s position using seat belts and Velcro or gauze wrist restraints.
On January 15, 2009, droperidol replaced haloperidol in the DHPD protocol (Figure E1, available online). After that date, haloperidol was not available to paramedics. Droperidol was also available for prehospital use as an anti-emetic after that date. However, ondansetron was the preferred anti-emetic and droperidol was only administered for intractable vomiting with transport time greater than 10 minutes. To be complete in capturing any potential adverse outcomes, we decided in advance to include patients receiving droperidol 1.25 mg IV or IM as an anti-emetic.
Study Procedures
We queried the prehospital patient care report (Health-Ware 5.9.4, HealthWare Solutions, Arcata, CA) to identify patients who received haloperidol or droperidol. For patients who were not transported to one of the study hospitals, we used structured follow-up to seek out adverse events. This consisted of daily electronic communication and weekly in-person communication with EMS liaisons at the other hospitals. The Denver Health Paramedic Division officer for quality assurance (JBJ) actively sought reports of adverse events, and instructed and routinely reminded the EMS liaisons at the other hospitals to report adverse events. For patients transported to Denver Health Medical Center and University of Colorado Hospital the prehospital patient care report was matched with the hospital medical record by searching electronically on medical record number, then name, then date of birth, and then social security number. We required two identifying characteristics to be present to match a hospital record to the prehospital record.
Data on call time, prehospital medication and total dose, and transport destination were taken from the prehospital patient care report. Data on QTc interval and adverse events were collected using structured chart review as detailed below.
Data were abstracted from the hospital chart by two trained medical student abstractors (KDM, AMS). Training consisted of one hour of instruction. The abstractors were blinded to the aims of the study. We used a standardized closed-response data collection instrument with precise definitions of each variable. Data were entered into Microsoft Excel 14.1 (Microsoft, Redmond, Washington). Both abstractors reviewed all charts independently and were monitored by senior investigators doing periodic checks on data abstraction throughout the chart review process. We held an interim meeting with the data abstractors to review protocols. We calculated intra-observer agreement on the coding of the abstracted variables (kappa 0.87, 95% CI: 0.79–0.96). Two investigators (ACM, MM) reviewed differences in abstraction and differences were resolved by consensus. A senior investigator (KEM) was available to adjudicate where the reviewers did not reach consensus. However, the two investigators reached consensus in each case.
Outcomes
The QTc interval (in milliseconds) was obtained from the first 12-lead ECG in the hospital record for each patient. In cases where a 12-lead ECG was not obtained or could not be identified from the medical record, QTc interval was recorded from the physician note. If there was no 12-lead ECG or documentation of QTc interval in the physician note, QTc was recorded as missing. Because drug was given for acute agitation, it was not possible to obtain QTc interval prior to administration of the drug.
We defined adverse events a priori as a systolic blood pressure (SBP) <90 mmHg at any point during the ED stay, seizure, administration of anti-dysrhythmic medication, cardioversion or defibrillation, bag–valve–mask ventilation, intubation, cardiopulmonary arrest, and prehospital or in-hospital mortality. Cardiopulmonary arrest and death were followed until hospital discharge; the remainder of the adverse events was defined as occurring during the patient’s stay in the ED.
We used administration of an additional sedating medication within 30 minutes of ED arrival as a proxy for effectiveness. Patients who received droperidol as an anti-emetic were excluded from this analysis.
Data Management and Statistical Analyses
After matching prehospital reports and hospital records, the data were de-identified. We used Stata 11.2 (StataCorp, College Station, Texas) for analyses. Absolute differences in QTc intervals were calculated, with 95% confidence intervals (CIs) calculated using the “cendif” command. We used Fisher’s exact test to calculate differences in adverse events for the use of additional sedating medications within 30 minutes. For the adverse events for which there were no outcomes, we added 1 to each cell in the 2 × 2 table to estimate confidence intervals.
Results
During the 37-month study period, the Denver Health Paramedic Division responded to 190,292 calls (116,674 [61%] while haloperidol was available and 100,645 [39%] when droperidol was available). In this time, 154,764 patients were transported (100,645 [65%] while haloperidol was available, 54,119 [35%] while droperidol was available). During the study period, 488 patients received haloperidol (0.5% of patients transported during that time) and 361 received droperidol (0.7% of patients transported during that time). Of the 849 patients who received these medications, 166 patients who received haloperidol and 145 who received droperidol were transported to non-study hospitals and were excluded from further analyses (n = 311). No deaths were reported in the excluded group. Of the remaining 538 patients, there were 6 (1%) for whom we were unable to match prehospital records to hospital records by two patient identifiers. All of these patients had received haloperidol. A review of morbidity and mortality case logs for the study hospitals did not identify any adverse effects in patients meeting any of the characteristics of the 6 patients who could not be identified. The final study population consisted of 314 patients who received prehospital haloperidol and 218 patients who received prehospital droperidol (Figure 1). Of these 218 patients who received prehospital droperidol, 11 patients received droperidol 1.25 mg IV or IM as an anti-emetic. The mean haloperidol dose was 7.9 mg (median 10 mg, range 4–20 mg). The mean droperidol dose was 2.9 mg (median 2.5 mg, range 1.25–10 mg).
Figure 1.
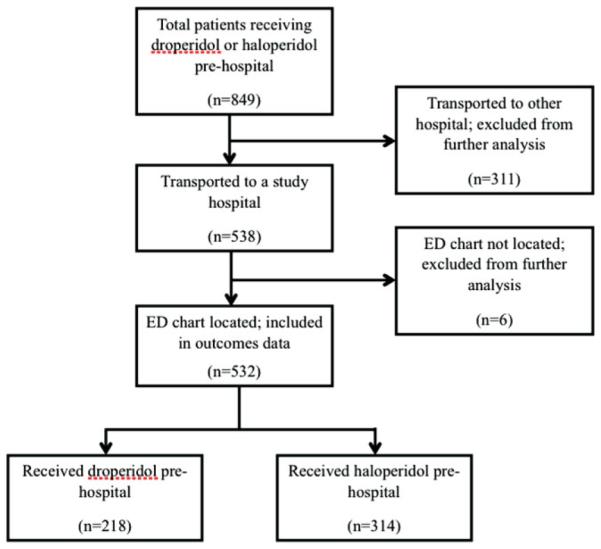
Flowchart illustrating patient flow.
The study groups were similar demographically. For the haloperidol group, median age was 31 (IQR 23–40) years and 69% of patients were male. In the droperidol group, median age was 31 (IQR 24–42) years and 75% of them were male.
QTc Interval Length
The median QTc was 448 (IQR 426–467) ms for patients who received prehospital haloperidol and 453 (IQR 437–469) ms for patients who received prehospital droperidol (Figure 2). Because ECG was obtained at physician discretion during the historical haloperidol period, an initial ED ECG was only recorded on 78 patients in the haloperidol group (25%). Initial ED ECGs were recorded on 166 patients in the droperidol group (76%). The longest QTc interval noted in both groups was 542 ms; neither of these 2 patients experienced an adverse event (Table 1).
Figure 2.
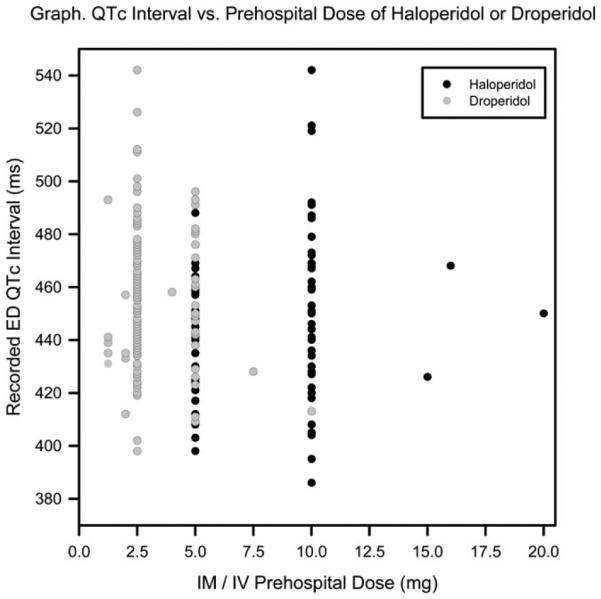
QTc by prehospital medication dose.
Table 1.
QTc interval for prehospital haloperidol and droperidol
| Droperidol (n = 166) N (%) |
Haloperidol (n = 78) N (%) |
Difference (95%CI) |
|
|---|---|---|---|
| Median QTc interval in ms (range, IQR) | 453 (398–542, 437–469) | 448 (386–542, 426–467) | 5 ms (−10–6 ms) |
| QTc 450–474 ms | 59 (36) | 23 (29) | 6% (−6–19%) |
| QTc 475–499 ms | 27 (16) | 9 (12) | 5% (−4–14%) |
| QTc >500 ms | 5 (3) | 3 (2) | 1% (−6–4%) |
Adverse Events
There were no seizures, no uses of cardioversion or defibrillation, and no deaths. There was one cardiopulmonary arrest in the droperidol group (0.4% of the droperidol group, absolute difference from haloperidol group 0.5%, 95% CI: −0.04–1.3%). This cardiopulmonary arrest immediately followed a struggle with staff while the patient was combative. The patient had return of spontaneous circulation after 1 minute of CPR (before an initial cardiac arrest rhythm was obtained). The post-arrest QTc was 481 ms; there were no other abnormal features of the ECG. The patient was discharged from the hospital neurologically intact.
Of the other adverse events, there were fewer adverse events in the droperidol group but no statistically significant differences. There were 6 patients with SBP <90 mmHg in the droperidol group and 13 in the haloperidol group (3% versus 4%, absolute difference 1.3%, 95% CI: −1.7–4.5%). All patients who received bag–valve–mask ventilation were also intubated. This comprised 4 in the droperidol group and 12 in the haloperidol group (2% versus 4%, absolute difference 1.9%, 95% CI: −1.0–4.8%). Table 2 summarizes adverse events.
Table 2.
Observed emergency department complications in patients receiving prehospital droperidol or haloperidola
| Droperidol (n = 218) N (%) |
Haloperidol (n = 314) N (%) |
Difference (95%CI) |
|
|---|---|---|---|
| SBP <90 mmHg | 6 (3) | 13 (4) | 1.3% (−1.7–4.5%) |
| Seizure | 0 (0) | 0 (0) | 0 (−0.01–1.2%) |
| Anti-arrhythmic | 1 (0.5) | 5 (2) | 1.1% (−0.1–2.7%) |
| Cardioversion/ defibrillation |
0 (0)8 | 0 (0) | 0 (−0.01–1.2%) |
| BVM | 4 (2) | 12 (4) | 1.9% (−1.0–4.8%) |
| Intubation | 4 (2) | 12 (4) | 1.9% (−1.0–4.8%) |
| Cardiopulmonary arrest |
1 (0.4) | 0 (0) | −0.5% (−1.3–0.04%) |
| Expired in hospital |
0 (0) | 0 (0) | 0 (−0.01–1.2%) |
Occurring at any time during emergency department stay, unless otherwise noted.
Need for Repeat Sedation
In the droperidol group, 22 patients (10%) received an additional sedating medication within 30 minutes of ED arrival, compared to 41 patients (13%) in the haloperidol group (absolute difference 2.9%, 95% CI: −2.5–8.4%). In the droperidol group, 12 patients (6%) received an additional butyrophenone, compared with 22 patients (7%) in the haloperidol group (absolute difference 1.5%, 95% CI: −2.6–5.6%). In the droperidol group, 14 patients (6%) received a benzodiazepine within 30 minutes of ED arrival, compared to 20 patients (6%) in the haloperidol group (absolute difference 0%, 95% CI: −4.2–4.1%) (Table 3).
Table 3.
Additional chemical sedation received within 30 minutes of arrival to the ED, after prehospital sedation with droperidol or haloperidol
| Droperidol (n = 207) N (%) |
Haloperidol (n = 314) N (%) |
Difference (95% CI) | |
|---|---|---|---|
| Any sedation within 30 min |
21 (10) | 41 (13) | 2.9% (−2.6–8.5%) |
| Butyrophenone within 30 min |
11 (5) | 22 (7) | 1.7% (−2.5–5.9%) |
| Benzodiazepine within 30 min |
14 (6) | 20 (6) | 0.0% (−4.8–3.9%) |
Which butyrophenone was available varied during the droperidol and haloperidol study periods.
Discussion
The present study represents the largest cohort to date comparing haloperidol to droperidol for the sedation of patients with undifferentiated agitation in the prehospital setting. Other studies have compared droperidol with other agents. Droperidol has been reported to be superior to midazolam in several studies with respect to need for rescue medications and need for air-way management.11–13 In the only prior comparisons of haloperidol and droperidol, droperidol has been reported to produce more rapid sedation.9,14
QTc prolongation is the prime concern in the FDA warnings for both haloperidol and droperidol. While exceeding 440 and 460 ms in men and women, respectively, is considered abnormal, the risk for developing torsades de pointes becomes significant when QTc exceeds 500 ms.15 The precise mechanism of butyrophenone-induced QTc interval prolongation is unknown; however, abnormal ventricular repolarization and the development of early after-depolarizations are likely involved.16 This study suggests that haloperidol does not have a significantly different effect on the QTc than does droperidol. However, our group received significantly lower doses of droperidol than haloperidol, and it is possible that a dose-dependent QTc prolongation exists and would have been apparent with a larger dose. Also, we did not have complete ECG data for either group, with a significant number missing in the haloperidol group. It is possible that spectrum bias made patients more prone to adverse events more likely to have ECGs obtained.
Our findings suggest that droperidol does not have a worse side-effect profile than haloperidol. We do, however, recognize that a patient in the droperidol group suffered cardiopulmonary arrest. This patient had history of congenital heart disease and a median sternotomy scar from a surgery at age 3, and did not require defibrillation or anti-dysrhythmic drugs for resuscitation, suggesting that torsades de pointes was a less-likely cause of his cardiopulmonary arrest. Clearly, careful monitoring of the use of sedating agents in this group of high-risk patients is indicated.
Our study did not demonstrate a statistically significant difference between droperidol and haloperidol in effectiveness, measured by use of further medications within 30 minutes of ED arrival. Thomas found more patients receiving equivalent doses of droperidol as compared to haloperidol to be more sedated at 15 and 30 minutes,9 and Resnick found more patients receiving droperidol to be sedated at 30 minutes.14 Our results may differ because of the lower doses of droperidol used as compared to haloperidol. Furthermore, this study only measured in-hospital effectiveness (as defined by the aforementioned criteria of additional medication requirements within 30 minutes of arrival to the ED).
Acute undifferentiated agitation can be difficult to treat. The exact frequency is unknown, and research is limited by the lack of precise definitions. In a database of 698 police use-of-force incidents, 3.4% had several features of excited delirium.17 In a Maryland database of 45 in-custody deaths over 14 years, 5 deaths were attributed to excited delirium (however, in 33 cases the medical examiner could not determine a cause of death).18
There are other pharmacologic options. Benzodiazepines, primarily midazolam, have been used both alone3,11–13and in combination with antipsychotics.12,19,20 Patients treated with midazolam 10 mg IM alone were observed to have higher need for repeat sedation than patients treated with droperidol 10 mg IM or droperidol 5 mg IM and midazolam 5 mg IM.12 Both Isbister and Martel describe increased difficulty related to oversedation or respiratory depression when midazolam was used as a single agent.3,12
Other options for treatment of acute agitation include atypical antipsychotics, diphenhydramine, and ketamine. Atypical antipsychotics often have higher cost than haloperidol or droperidol, and have been observed to have slower time to onset and no significant in adverse reactions as compared to typical antipsychotics.11,19,21 There is little literature to support the use of diphenhydramine for sedation. Although it may decrease dystonic reactions from phenothiazines, it may also worsen anticholinergic delirium and has been associated with paradoxical excitation.22,23 Finally, ketamine may present an option for the most severe cases of excited delirium, with the advantage of rapid sedation and preserved airway reflexes but the disadvantage of hypersalivation and, rarely, laryngospasm.24–30
Limitations
This study has several limitations. As a chart review, it is subject to selection and ascertainment bias. We were only able to obtain hospital records on 63% of the patients who received prehospital haloperidol or droperidol, as we did not have access to medical records at other receiving hospitals. These patients were also less likely to have had a traumatic injury, as patients requiring a trauma center were preferentially transported to the study hospitals. Other bias in destination choice is also possible. We only have QTc data for 29% of patients who received the medications during the study period. The missing data and spectrum bias severely limit the strength of our conclusion regarding QTc prolongation. We also were not able to confirm patient identity in 6 patients in the haloperidol group who were transferred to our study hospitals, and it is possible that we missed ED and in-hospital adverse events in these patients. Of the droperidol group, 11 patients received droperidol as an anti-emetic. These patients were included to maximize capture of adverse events. Our patients were not randomized, and the groups were separated in time; it is possible that secular trends could have contributed to or obscured differences between the groups. Although the protocol was for droperidol or haloperidol as a single agent, we did not control for other potentially sedating medications given in the prehospital setting, and this could have confounded the results. Given the rare outcomes of interest, there is a possibility of type II error. We did not measure the frequency of extrapyramidal side effects, and these may have occurred both during the ED visit and after.
Conclusions
In this cohort of agitated patients treated with haloperidol or droperidol in the prehospital setting, there was no significant difference in QTc prolongation, adverse events, or need for repeat sedation between haloperidol and droperidol. There was a trend toward fewer adverse events and less need for repeat sedation in the droperidol group. Further study with larger patient groups is needed to better define the safest and most effective method to sedate agitated patients in the prehospital setting.
Supplementary Material
Acknowledgments
Supported, in part, by K02HS01726 from the Agency for Healthcare Research and Quality and R01AI106057 from the National Institutes of Health to Dr. Haukoos.
Footnotes
SUPPLEMENTARY MATERIAL AVAILABLE ONLINE
Figure E1: Droperidol protocol.
Supplementary material can be viewed and downloaded at http://informahealthcare.com/pec.
This paper was presented, in part, at the National Association of EMS Physicians Annual Meeting, Tucson, Arizona, January 13, 2012.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Inapsine (droperidol) Dear Healthcare Professional Letter Dec 2001. Food and Drug Administration. 2001 Available at: www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm173778.htm. Accessed October 19, 2012.
- 2.Colwell C. Managing the acutely agitated patient. EMS World. 2010 Available at: www.emsworld.com/article/10319462/managing-the-acutely-agitated-patient. Accessed October 22, 2012. [PubMed]
- 3.Martel M, Miner J, Fringer R, et al. Discontinuation of droperidol for the control of acutely agitated out-of-hospital patients. Prehosp Emerg Care. 2005;9(1):44–8. doi: 10.1080/10903120590891723. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz BZ, Bizovi K, Moreno R. Droperidol—behind the black box warning. Acad Emerg Med. 2002;9(6):615–8. doi: 10.1197/aemj.9.6.615. [DOI] [PubMed] [Google Scholar]
- 5.Kao LW, Kirk MA, Evers SJ, Rosenfeld SH. Droperidol, QT prolongation, and sudden death: what is the evidence? Ann Emerg Med. 2003;41(4):546–58. doi: 10.1067/mem.2003.110. [DOI] [PubMed] [Google Scholar]
- 6.Shale JH, Shale CM, Mastin WD. A review of the safety and efficacy of droperidol for the rapid sedation of severely agitated and violent patients. J Clin Psychiatry. 2003;64(5):500–5. doi: 10.4088/jcp.v64n0502. [DOI] [PubMed] [Google Scholar]
- 7.Hick JL, Mahoney BD, Lappe M. Prehospital sedation with intramuscular droperidol: a one-year pilot. Prehosp Emerg Care. 2001;5(4):391–4. doi: 10.1080/10903120190939571. [DOI] [PubMed] [Google Scholar]
- 8.Rosen CL, Ratliff AF, Wolfe RE, Branney SW, Roe EJ, Pons PT. The efficacy of intravenous droperidol in the prehospital setting. J Emerg Med. 1997;15(1):13–7. doi: 10.1016/s0736-4679(96)00259-4. [DOI] [PubMed] [Google Scholar]
- 9.Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407–13. doi: 10.1016/s0196-0644(05)82660-5. [DOI] [PubMed] [Google Scholar]
- 10.2010 Census Gazetteer Files. US Census Bureau. Available at: www.census.gov/geo/www/gazetteer/gazetteer2010.html. Accessed October 19, 2012.
- 11.Martel M, Sterzinger A, Miner J, Clinton J, Biros M. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167–72. doi: 10.1197/j.aem.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Isbister GK, Calver LA, Page CB, Stokes B, Bryant JL, Downes MA. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392–401.e1. doi: 10.1016/j.annemergmed.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Knott JC, Taylor DM, Castle DJ. Randomized clinical trial comparing intravenous midazolam and droperidol for sedation of the acutely agitated patient in the emergency department. Ann Emerg Med. 2006;47(1):61–7. doi: 10.1016/j.annemergmed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45(7):298–9. [PubMed] [Google Scholar]
- 15.Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol. 1993;72(6):23B–5B. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence KR, Nasraway SA. Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature. Pharmacotherapy. 1997;17(3):531–7. [PubMed] [Google Scholar]
- 17.ACEP Excited Delirium White Paper. 2009:1–22. [Google Scholar]
- 18.Southall P, Grant J, Fowler D, Scott S. Police custody deaths in Maryland, USA: an examination of 45 cases. J Forensic Legal Med. 2008;15(4):227–30. doi: 10.1016/j.jflm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Wilson MP, Macdonald K, Vilke GM, Feifel D. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2011 doi: 10.1016/j.jemermed.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Gillies D, Beck A, McCloud A, Rathbone J, Gillies D. Benzodiazepines alone or in combination with antipsychotic drugs for acute psychosis. Cochrane Database Syst Rev. 2005;(4):CD003079. doi: 10.1002/14651858.CD003079.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594. doi: 10.1002/14651858.CD005594.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Cheng KL, Dwyer PN, Amsden GW. Paradoxic excitation with diphenhydramine in an adult. Pharmacotherapy. 1997;17(6):1311–4. [PubMed] [Google Scholar]
- 23.de Leon J, Nikoloff DM. Paradoxical excitation on diphenhydramine may be associated with being a CYP2D6 ultrarapid metabolizer: three case reports. CNS Spectr. 2008;13(2):133–5. doi: 10.1017/s109285290001628x. [DOI] [PubMed] [Google Scholar]
- 24.Burnett AM, Salzman JG, Griffith KR, Kroeger B, Frascone RJ. The emergency department experience with prehospital ketamine: a case series of 13 patients. Prehosp Emerg Care. 2012;16(4):553–559. doi: 10.3109/10903127.2012.695434. [DOI] [PubMed] [Google Scholar]
- 25.Burnett AM, Watters BJ, Barringer KW, Griffith KR, Frascone RJ. Laryngospasm and hypoxia after intramuscular administration of ketamine to a patient in excited delirium. Prehosp Emerg Care. 2012;16(3):412–414. doi: 10.3109/10903127.2011.640766. [DOI] [PubMed] [Google Scholar]
- 26.Thakurta RG, Das R, Bhattacharya AK, et al. Rapid response with ketamine on suicidal cognition in resistant depression. Indian J Psychol Med. 2012;34(2):170–5. doi: 10.4103/0253-7176.101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Cong M, Gynther B, Hunter E, Schuller P. Ketamine sedation for patients with acute agitation and psychiatric illness requiring aeromedical retrieval. Emerg Med J. 2012;29(4):335–7. doi: 10.1136/emj.2010.107946. [DOI] [PubMed] [Google Scholar]
- 28.Ho JD, Smith SW, Nystrom PC, et al. Successful management of excited delirium syndrome with prehospital ketamine: two case examples. Prehosp Emerg Care. 2012;17(2):274–9. doi: 10.3109/10903127.2012.729129. [DOI] [PubMed] [Google Scholar]
- 29.Melamed E, Oron Y, Ben-Avraham R, Blumenfeld A, Lin G. The combative multitrauma patient: a protocol for prehospital management. Eur J Emerg Med. 2007;14(5):265–8. doi: 10.1097/MEJ.0b013e32823a3c9b. [DOI] [PubMed] [Google Scholar]
- 30.Hick JL, Ho JD. Ketamine chemical restraint to facilitate rescue of a combative “jumper”. Prehosp Emerg Care. 2005;9(1):85–9. doi: 10.1080/10903120590891859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


