We estimated maximum metabolic rates (MMR) in four steady-swimming coral reef fishes - two body-caudal and two median-paired fin swimmers - using three different techniques. Differences between MMR estimates were not due to locomotory mode, but rather the method used to induce maximum performance, with swimming respirometry resulting in the highest estimates.
Keywords: Body–caudal fin swimming, carangiform, circular swimming chamber, labriform, median–paired fin swimming, oxygen consumption rate
Abstract
Respirometry is frequently used to estimate metabolic rates and examine organismal responses to environmental change. Although a range of methodologies exists, it remains unclear whether differences in chamber design and exercise (type and duration) produce comparable results within individuals and whether the most appropriate method differs across taxa. We used a repeated-measures design to compare estimates of maximal and standard metabolic rates (MMR and SMR) in four coral reef fish species using the following three methods: (i) prolonged swimming in a traditional swimming respirometer; (ii) short-duration exhaustive chase with air exposure followed by resting respirometry; and (iii) short-duration exhaustive swimming in a circular chamber. We chose species that are steady/prolonged swimmers, using either a body–caudal fin or a median–paired fin swimming mode during routine swimming. Individual MMR estimates differed significantly depending on the method used. Swimming respirometry consistently provided the best (i.e. highest) estimate of MMR in all four species irrespective of swimming mode. Both short-duration protocols (exhaustive chase and swimming in a circular chamber) produced similar MMR estimates, which were up to 38% lower than those obtained during prolonged swimming. Furthermore, underestimates were not consistent across swimming modes or species, indicating that a general correction factor cannot be used. However, SMR estimates (upon recovery from both of the exhausting swimming methods) were consistent across both short-duration methods. Given the increasing use of metabolic data to assess organismal responses to environmental stressors, we recommend carefully considering respirometry protocols before experimentation. Specifically, results should not readily be compared across methods; discrepancies could result in misinterpretation of MMR and aerobic scope.
Introduction
The growing fields of ecological and conservation physiology (Wikelski and Cooke, 2006; Cooke et al., 2013) aim to understand the mechanisms underpinning the behaviour and fitness of organisms in changing environments. As human impacts on global ecosystems continue to increase, greater emphasis will be placed on research that aims to understand whether and how organisms respond and adapt to anthropogenic and environmental stressors (Pörtner and Farrell, 2008; Alcaraz et al., 2013). Choosing appropriate methodologies to study a given species or system is critical to ensure that data are robust and comparisons among studies valid.
The performance of an organism over a range of activities can be tightly linked to oxygen utilization. Respirometry is a tool commonly used to measure oxygen consumption rates () and estimate the metabolic performance of an organism at rest, during exposure to stressors or while performing different locomotory activities. Two important physiological parameters describe the upper and lower bounds of an organism's capacity to metabolize energy. Maximal metabolic rate (MMR) is the maximal amount of energy that can be metabolized aerobically by an organism and can be estimated by measuring an organism's during or immediately after exhaustive exercise (; Norin and Clark, 2016). In contrast, standard metabolic rate (SMR) is the minimal amount of energy required for maintenance and is estimated by measuring in a post-absorptive, resting state (; Nelson and Chabot, 2011; Chabot et al., 2016). The difference between and is the absolute or total scope for aerobic activity (aerobic scope; AS), which is, in essence, the capacity for aerobic metabolism, in excess of basic maintenance costs, for activities essential to support biological fitness, such as swimming, feeding and reproduction (Brett, 1964). Respirometry can therefore provide essential information about the metabolic performance of an organism and is thus rapidly becoming more widely used (Clark et al., 2013; Norin and Clark, 2016).
A particularly interesting model system is fish, where experimental techniques in respiratory physiology have been widely used for nearly a century and increasingly over recent decades (Webb, 1975; Steffensen et al., 1984, 1989; Svendsen et al., 2016). Many of these techniques are used extensively today in ecological and conservation physiology studies (Nilsson et al., 2007, 2009; Donelson et al., 2011; Couturier et al., 2013; McLeod et al., 2013; Rummer et al., 2013, 2014 Trappett et al., 2013; Binning et al., 2014).
The most established method to estimate MMR in fish uses a treadmill-like swimming respirometry chamber (hereinafter, swimming respirometry), where individuals swim against near-laminar water flow at incrementally increased speeds until fatigue is reached (Fig. 1A; Brett, 1964). Using this protocol, SMR can be estimated indirectly by extrapolating the non-linear –swimming speed relationship to zero swimming speed (Steffensen et al., 1984; Steffensen, 2005; Roche et al., 2013).
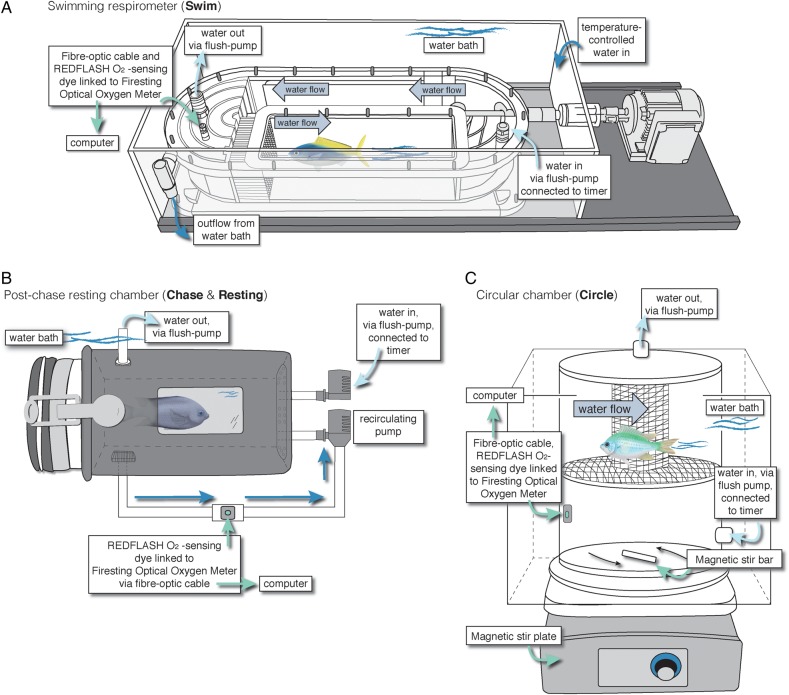
Figure 1:
Schematic diagrams of the three respirometers used in this study: a swimming respirometer (A), a resting respirometer (post exhaustive chase; B) and a circular chamber respirometer (C).
A second common method uses an exhaustive chase protocol (commonly referred to as the ‘stick method’), during which fish are first chased to exhaustion (some protocols also incorporate air exposure) and then placed into a resting respirometer that closely matches the fish's size, such that active swimming is restricted (Fig. 1B; Ferguson and Tufts, 1992; Donaldson et al., 2010; Clark et al., 2012, 2013; Roche et al., 2013). The calculated immediately after this exhaustive exercise (which includes repayment of the oxygen debt resulting from anaerobic activity) is used as an indirect estimate of MMR. After recovery from the chase, SMR can be estimated directly once the fish has fully recovered from exercise (6–24 h depending on species; Milligan and Wood, 1986; Milligan, 1996; Milligan et al., 2000; Chabot et al., 2016).
A third and more recent method uses a circular chamber and a magnetic stir-bar to create a vortex-like water flow, against which a fish must swim facing either clockwise or anticlockwise (Munday et al., 2009; Gardiner et al., 2010; Couturier et al., 2013; Rummer et al., 2013; Trappett et al., 2013; Pope et al., 2014; hereinafter, circular chamber; Fig. 1C, online supplementary material, Fig. S1, but referred to as a ‘swim respirometer’ by Nilsson et al., 2007). The circular chamber combines aspects of both swimming and resting respirometers, but instead the fish swims in tight circles. As in a traditional swimming respirometer, this method allows direct estimates of MMR during exertion. In contrast to traditional swimming respirometry, direct estimates of SMR can also be obtained, as in resting chambers, when the revolutions of the stir-bar are reduced to a level that gently mixes the water but does not induce swimming. All three methods have been used interchangeably to estimate metabolic performance in fishes. However, studies suggest that the choice of method could significantly affect the accuracy (Reidy et al., 1995; Roche et al., 2013; Norin and Clark, 2016; Svendsen et al., 2016) and, perhaps, repeatability of MMR and SMR estimates.
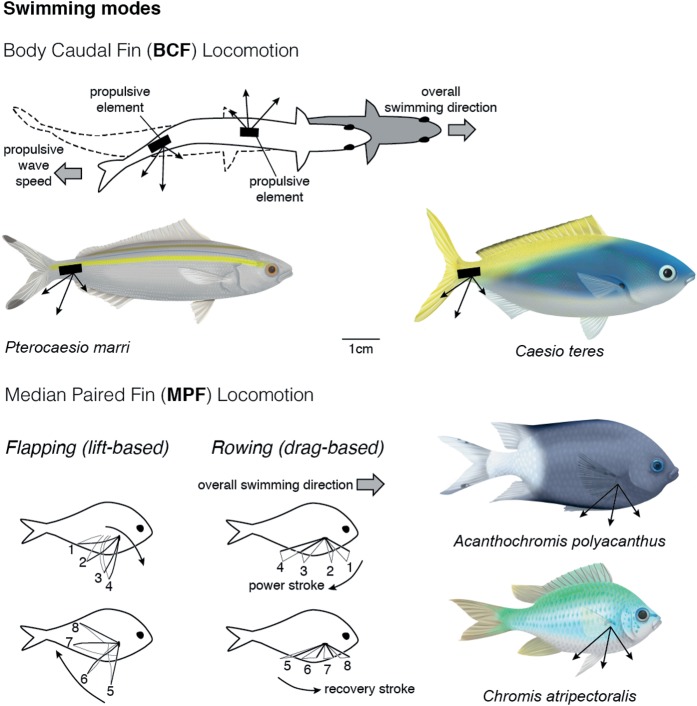
Swimming performance is crucial for nearly every aspect of a fish's ecology, including predator–prey interactions, reproductive behaviour and habitat selection (Breder, 1926; Webb, 1975, 1984; Blake, 2004), and engages a variety of body regions and fin appendages (referred to hereinafter as swimming modes; Breder, 1926). Functional differences in swimming modes may have important implications for determining the best method of obtaining accurate metabolic rate estimates. For instance, body–caudal fin (BCF) swimming (e.g. carangiform) is powered by movements of the caudal (tail) fin and the posterior half of the body (Fig. 2) and is often (but not always) coupled with a long, streamlined body ideal for long-distance and fast-propulsive swimming in open environments (Videler, 1993; Blake, 2004). Alternatively, median–paired fin (MPF; e.g. labriform; pectoral fin) swimming is powered by movements of the median or paired fins, such as the pectoral or dorsal–ventral fins, while maintaining a rigid body (Fig. 2), and is thought to promote manoeuvrability (Korsmeyer et al., 2002). These two swimming modes differ kinematically in how thrust is produced and physiologically in terms of which muscles are used during swimming (Blake, 2004). Although BCF swimming is used by many species during high-speed escapes or chases, species from many families of coral reef fishes (e.g. Acanthuridae, Labridae, Pomacentridae and Scaridae) regularly use an MPF swimming mode during daily activities. Currently, no study has evaluated whether functional differences in routine swimming mode affect which respirometry method should be used to obtain the best metabolic rate estimates.
Figure 2:
Schematic diagram of thrust generation and propulsion produced by body–caudal fin (BCF) and median–paired fin (MPF) swimming. This study focused specifically on BCF and lift-based MPF species.
Previous studies that compare methods or calculations for estimating metabolic rates in fishes are restricted to one or two species and/or a single swimming mode (e.g. Reidy et al., 1995; Schurmann and Steffensen, 1997; Korsmeyer et al., 2002; Roche et al., 2013). This limits our ability to generalize results across taxa with different swimming modes, swimming durations and/or life histories. Additionally, time-sensitive and field-based studies would benefit from more portable and faster protocols for estimating MMR and SMR. The chase and circular chamber methods may therefore provide more rapid options for estimating MMR than traditional swimming respirometry, which is expensive and can require upwards of 12 h per individual. Therefore, the aims of this study were as follows: (i) to compare metabolic rate estimates obtained using three common methods, i.e. a prolonged swim trial using a swimming respirometer, a short-duration exhaustive chase protocol with air exposure followed by resting respirometry, and a short-duration exhaustive swimming trial in a circular chamber followed by resting respirometry; and (ii) to determine whether metabolic rate estimates obtained with each method vary among four fish species, all of which are prolonged swimmers but exhibit different swimming modes (BCF and MPF).
Materials and methods
Experimental animals
We used four species of coral reef fishes, all of which are abundant on the Great Barrier Reef, Australia, to explore differences in MMR and SMR estimates across three respirometry methods and two swimming modes. We chose two predominantly BCF swimming caesionid (fusilier) species [Pterocaesio marri (sample size n = 5; standard length, SL, 85.2 ± 11.4 mm; wet mass 9.89 ± 1.86 g; means ± SD) and Caesio teres (n = 11; 82.2 ± 6.7 mm; 15.07 ± 4.46 g)] that form mixed-species shoals that cruise mid-water along the reef edge (Fig. 2). We also chose two predominantly MPF swimming pomacentrid (damselfish) species [Acanthochromis polyacanthus (n = 11; 74.2 ± 4.8 mm; 16.88 ± 3.38 g) and Chromis atripectoralis (n = 10; 62.5 ± 2.2 mm; 8.10 ± 1.51 g)] that are site attached as adults and territorial (Randall et al., 1997; Fig. 2). The MPF swimmers we chose generate lift-based thrust by flapping their fins, in contrast to other MPF swimmers that produce drag-based thrust by rowing their fins like paddles, a behaviour better suited for low-speed manoeuvring (Vogel, 1994; Walker and Westneat, 2002; Binning and Roche, 2015; Fig. 2). All four species co-occur on the mid-shelf reef crest and feed primarily on plankton in the water column (Randall et al., 1997). Despite differences in their habitat use, all four species are considered steady/prolonged swimmers.
Fishes were collected from reef crest sites around Lizard Island (14°40′08″S; 145°27′34″E) using monofilament barrier and hand nets under Marine Parks Permit #G10/33239.1. Fishes were maintained in flow-through aquaria directly from the reef at the research station laboratory at ambient temperature (∼27°C) and fed to satiation daily (NRD pellets; INVE Aquaculture, Salt Lake City, UT, USA) until being transferred to the James Cook University Marine Aquarium Research Facilities Unit in Townsville, Queensland, Australia, ∼14 days after collection. At James Cook University, fishes were evenly distributed among five aquaria supplied with well-aerated seawater (28.5 ± 0.5°C) and fed to satiation daily (NRD pellets) for a minimum of 19 days before the experiments commenced. Throughout the duration of the project, fishes were maintained under James Cook University Animal Ethics Committee regulations (permit #A1722, approved for this study) according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and the Queensland Animal Care and Protection Act 2001.
Prior to experimental procedures, individual fish were fasted for 36 h in separate aquaria to ensure a post-absorptive state (Niimi and Beamish, 1974). Fishes were tested in the three different respirometry methods in a random order following a repeated-measures design. All experiments were performed between 22 October and 14 November 2012.
Swimming respirometry
Oxygen consumption rates () were measured for solitary individuals swimming in a 4.8 l custom-built Steffensen-type Plexiglas swimming respirometer (Fig. 1A; Steffensen et al., 1984; Johansen and Jones, 2011; Roche et al., 2013), a system that allows oxygen consumption rates and swimming performance to be measured simultaneously while fish are being exercised.
The working section of the swimming chamber was 7.0 cm × 36.0 cm × 7.0 cm (width × length × depth), but the entire respirometer was immersed in a temperature-controlled bath and maintained at 28.5 ± 0.1°C (mean +/− SD) (Fig. 1A). Flow straighteners were used to create laminar flow within the working section, and flow was calibrated from 0 to 86.6 ± 0.5 cm s−1 (mean ± SD) using a digital vane wheel flow sensor (Höntzsch GmbH, Waiblingen, Germany; model #ZS30GFE-md20T). Solid blocking effects of the fish in the working section were corrected following Bell and Terhune (1970) and did not exceed 5% for any individual.
At the beginning of each trial, the respirometer was filled with temperature-controlled, filtered, ultraviolet-sterilized, well-aerated seawater. Next, a fish was placed in the working section of the chamber and left to habituate for 4–8 h at a swimming speed of 0.5 BL s−1 (body length, taken as the standard length of the fish, per second) until the fish had settled into a continuous swimming rhythm and stabilized. Preliminary trials conducted on all four species in the same chamber demonstrated that all reached gait transitions and critical swimming speeds (Ucrit) well above 4 BL s−1. Specifically, gait transitions occur when MPF swimmers transition from using paired fins to caudal-assisted swimming (Up-c; Johansen and Jones, 2011; Binning et al., 2014) or when BCF swimmers transition from steady swimming to anaerobic burst and coast motions (Uburst; Svendsen et al., 2010) as speeds increase. As is common in other fast, steady-swimming coral reef fish species (Johansen and Jones, 2011; Roche et al., 2013), increases in are only marginal at swimming speeds <40% of Ucrit. Therefore, after the initial habituation period at 0.5 BL s−1, swimming protocols commenced at 4 BL s−1, which was substantially below Upc, Uburst, and Ucrit for these species. All fish were swum during daylight hours.
After the 0.5 BL s−1 habituation period, the swimming trial commenced by increasing the water flow speed to 4 BL s−1 over a period of 4 min. Then, starting at 4 BL s−1, was measured, and the swimming speed was incrementally increased (increments of 0.5 BL s−1) following a standard Ucrit protocol (Jain et al., 1997). Fish swam at each speed for three 8 min cycles (i.e. 24 min at each speed). Each 8 min cycle consisted of a 5 min measurement period and a 3 min flush period to replenish the chamber with filtered, well-aerated seawater. Fish were continuously monitored throughout the swimming trial, and the trial was considered finished when the fish could no longer swim against the flow, being swept downstream onto a retaining grid for >5 s (Ucrit; Roche et al., 2013). At this point, the total swimming time and flow speed were recorded, the experiment was terminated, and the fish was returned to its holding tank. Maximal metabolic rate (MMRSwim) was estimated from the at the maximal swimming speed where fish completed at least one full 8 min cycle.
Critical swimming speed (Ucrit) was calculated following Brett (1964): Ucrit = U + Ui × (t/ti), where U is the penultimate swimming speed before the fish fatigued and stopped swimming (Ucrit); Ui is the swimming speed at which the fish was unable to continue swimming (i.e. swimming speed at increment i); t is the length of time the fish swam at the final swimming speed where fatigue occurred; and ti is the amount of time fish were swum at each speed interval in the trial (24 min).
Exhaustive chase protocol and resting respirometry
Individual fish were placed into a 0.6 m (diameter) round aquarium containing well-aerated and temperature-controlled seawater (0.15 m deep) maintained at the same temperature as the respirometer (28.5 ± 0.1°C). Fish were then chased continuously by hand for 3 min, during which time the experimenter would touch the tail of the fish if it slowed down or stopped swimming. All species swam primarily with their caudal fin when chased, repeatedly bursting away from the stimulus. Fish were deemed exhausted when they became unresponsive to chasing, which always occurred by the end of the 3 min chase period. Fish were then scooped into a rubber mesh net and maintained out of the water for 1 min (Clark et al., 2013; Roche et al., 2013). After air exposure, fish were immediately placed in a 1615 ml closed-loop, recirculating, resting respirometry chamber submerged in a temperature-controlled water bath (Fig. 1B; reviewed by Clark et al., 2013). The measurement period commenced within 10 s of placing the fish in the chamber and continued for a period of 5 min. The was calculated from the steepest 1 min slope during this 5 min interval and used to estimate MMR (MMRChase). Standard metabolic rate (SMRRest) was estimated from values obtained after leaving the fish in the chamber for an additional 6–12 h. This time period was deemed sufficient to ensure that stabilized and no longer decreased in each species investigated here (online supplementary material, Fig. S2; also see Roche et al., 2013; Rummer et al., 2013).
Circular chamber respirometry
Individual fish were placed into a 2654 ml (17.2 cm internal height, 14.8 cm internal diameter) o-ring sealed cylinder (Perspex, 0.3 cm thickness) connected via tubing to a submersible pump in a temperature-controlled water bath (28.5 ± 0.1°C; Fig. 1C, online supplementary material, Fig. S1). A magnetic stir plate below the chamber activated a stir bar (1 cm × 6 cm) to create a circular water motion in the chamber. The speed of the rotating stir bar was increased over a period of ∼1 min to the maximal speed at which the fish could only just maintain its position in the chamber (Fig. 1C, online supplementary material, Fig. S1; see also Nilsson et al., 2007). If the fish could no longer hold position in the chamber, the speed was decreased slightly until it was able to maintain position while swimming. The was calculated from the steepest 1 min slope of the change in O2 depletion recorded during the first 5–7 min of measuring (between 5 and 10 min used in previous studies; Nilsson et al., 2009; Gardiner et al., 2010; Couturier et al., 2013; Rummer et al., 2013), which corresponds to MMRCircle. The chamber was then flushed, and the rotational speed of the stir bar was decreased to a minimal speed, at which the fish was able to stop swimming and rest on the false mesh bottom (see Fig. 1C). Similar to the chase protocol, SMR was estimated from values obtained after leaving the fish in the circular chamber for an additional 6–12 h until stabilized and no longer decreased (SMRCircle; online supplementary material, Fig. S2).
General respirometry information and calculations
We used intermittent-flow respirometry (Steffensen, 1989; Svendsen et al., 2016) for all three methods. A digital relay timer (MFRT-1 Multi Function Recycling Timer; Xiamen SUPERPRO Technology Co., Ltd, Xiamen, Fujian, China) was connected to submersible pumps to repeat an 8 min cycle that began with a 5 min measurement period followed by a 3 min flush period. The measurement period was short enough to ensure that oxygen within the chambers remained above 80% air saturation at all times. This is important to ensure that oxygen consumption rates are not influenced by the adrenergic stress response or other metabolic changes associated with hypoxia (Hughes, 1973; Tetens and Lykkeboe, 1985; Boutilier et al., 1988). The flush period was long enough to ensure that oxygen levels returned to 100% air saturation. During the experiments, test fish were shielded from outside stimuli by a dark cloth to avoid unwanted stress. However, there was a small viewing window in each chamber for the researcher to check on the fish and so that the fish could still experience photoperiod (Fig. 1B). Temperature- and barometric pressure-compensated O2 concentration (in milligrams per litre) in the water were continuously measured at 0.5 Hz using oxygen-sensitive REDFLASH dye on contactless spots (2 mm) adhered to the inside of each chamber. Spots were linked to a Firesting Optical Oxygen Meter (Pyro Science e. K., Aachen, Germany) via fibre-optic cables. Each sensor was calibrated using fully aerated seawater (as 100%) prior to each trial and with sodium sulphite (as zero) weekly or as needed.
Text files were imported into LabChart v. 6.1.3 (ADInstruments, Dunedin, New Zealand), and (in milligrams of O2 per kilogram per hour) was calculated as the slope of the linear regression of oxygen concentration decline over time during the measurement period of each cycle using the following equation:
modified from (Bushnell et al., 1994; Schurmann and Steffensen, 1997), where S is the slope (in milligrams of O2 per litre per secondl), Vresp is the volume of the respirometer minus the volume of the fish (in litres), and M is the mass of the fish (in kilograms). We subtracted the proportional background O2 consumption rate (measured as O2 depletion in the empty respirometer before and after each trial, assumed linear) from each measurement. To limit background respiration rates to <5% of a fish's SMR, chambers and pumps were rinsed daily with a 10% bleach solution and fresh water and allowed to dry overnight prior to commencing trials on the next day. The SMR (in milligrams of O2 per kilogram per hour) was estimated from the average of the lowest 10% of values (Clark et al., 2013; Rummer et al., 2013, 2014).
Statistical analyses
We used linear mixed-effects models (LMM; ‘nlme’ package in R) to compare estimates of MMR and SMR obtained with different respirometry methods on the same individuals. This repeated-measures design minimized inter-individual variation in metabolic rate estimates. Species and respirometry method were specified as fixed factors, and fish identity was included as a random factor to control for the non-independence of data points collected using the same individual (Bolker et al., 2009). Post hoc multiple comparisons were done using the R function ‘ghlt’ in the package ‘multcomp’. This model also allowed for individuals to be included even if they did not complete all three methods. We used two general linear models (LM) and Tukey's tests to examine differences in absolute and relative Ucrit among swimming modes and species. Diagnostic plots were used to ensure that the data met the assumptions of the models. Non-significant interactions were removed for model simplification and fit. Analyses were performed in R v3.0.2 (R Core Team, 2013).
Results
Estimates of MMR differed significantly between respirometry methods (LMM, F2,62 = 11.38, P < 0.001; Fig. 3A and Table 1) and fish species (LMM, F3,33 = 4.13, P = 0.014; Fig. 3B and Table 1). However, the effect of method was the same across all species (species × method interaction not significant, F6,56 = 0.74, P = 0.62; online supplementary material, Fig. S3A). The value of MMRSwim was consistently higher than MMRChase (z = 2.95, P < 0.01) and MMRCircle (z = 4.79, P < 0.001) for all species (BCF and MPF swimmers; Fig. 3A). Specifically, MMRSwim was 20% higher than MMRChase based on model predictions computed with the R package effects (Fox et al., 2014); this difference ranged from 6.3 to 35.3% across the four species. Likewise, MMRSwim was 25% higher than MMRCircle; this difference ranged from 15.5 to 38.3% across the four species. On average, MMRChase was numerically higher than MMRCircle, but this difference was not statistically significant (z = 1.84, P = 0.15; Fig. 3A).
Figure 3:
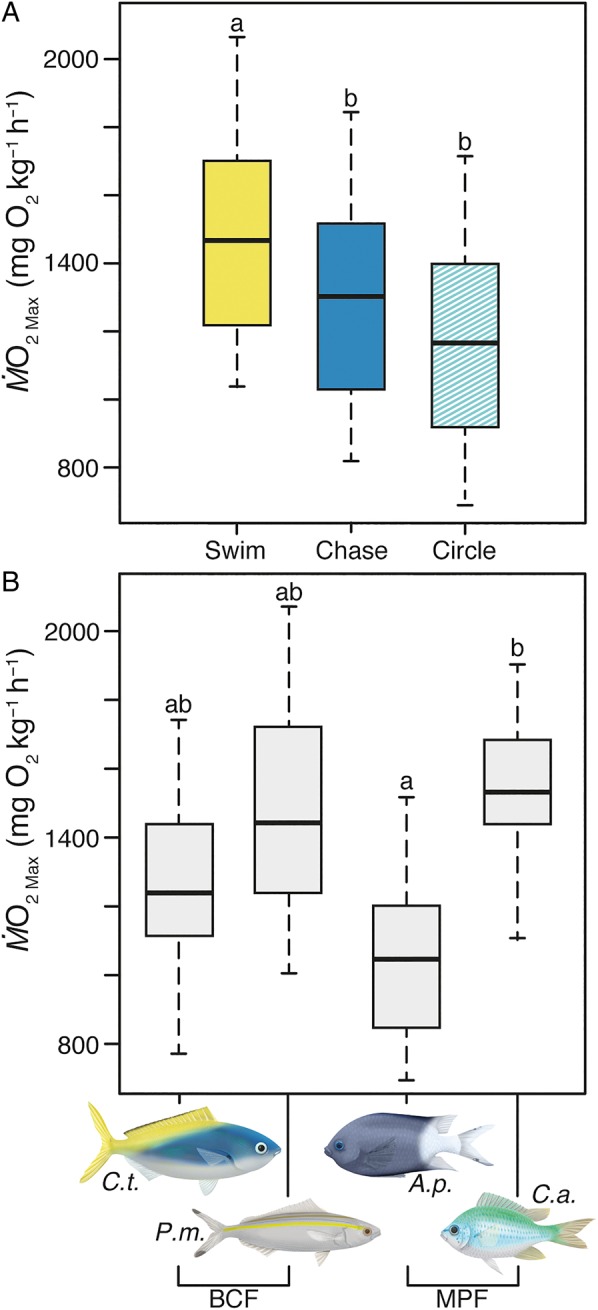
Boxplots showing median and inter-quartile range of (A) maximal metabolic rates (MMR; estimated from the highest value of oxygen consumption rate, ) using three respirometry methods, a critical swimming speed trial in a traditional swimming respirometer (Swim), an exhaustive chase protocol followed by 1 min air exposure (Chase), and an exhaustive swim trial in a circular chamber with a stir bar (Circle), for all species combined that completed at least one method and (B) MMR for all fishes, by species, that completed at least one method. Caesio teres (C.t.) and Pterocaesio marri (P.m.) are body–caudal fin (BCF) swimmers. Acanthochromis polyacanthus (A.p) and Chromis atripectoralis (C.a.) are median–paired fin (MPF) swimmers. Same letters indicate no significant differences (α = 0.05).
Table 1:
Estimates of maximal metabolic rate and standard metabolic rate for all fishes that completed at least one of the three different respirometry methods
| Species | MMR (mg O2 kg−1 h−1) |
SMR (mg O2 kg−1 h−1) |
||||
|---|---|---|---|---|---|---|
| Swim | Chase | Circle | Rest | Circle | ||
| Pterocaesio marri | Mean | 1794.3 | 1376.4 | 1308.1 | 223.3 | 257.9 |
| (n = 5) | SEM | 336.6 | 182.5 | 254.8 | 40.3 | 24.6 |
| Caesio teres | Mean | 1299.4 | 1277.9 | 1175.8 | 167.7 | 176.5 |
| (n = 11) | SEM | 59.4 | 169.8 | 102.5 | 31.3 | 17.5 |
| Acanthochromis polyacanthus | Mean | 1150.7 | 1048.1 | 974.4 | 143.0 | 145.1 |
| (n = 11) | SEM | 112.3 | 164.2 | 80.4 | 26.1 | 7.2 |
| Chromis atripectoralis | Mean | 1768.0 | 1556.7 | 1312.8 | 221.0 | 154.5 |
| (n = 10) | SEM | 112.2 | 134.7 | 104.5 | 35.9 | 10.2 |
Methods included a critical swimming speed trial in a Steffensen-type swimming respirometer (Swim), an exhaustive chase protocol followed by 1 min air exposure (Chase), and an exhaustive swim trial in a circular chamber with a stir bar (Circle). MMR, maximal metabolic rate; and SMR, standard metabolic rate. Values are group means; see the Results section and online supplementary material, Fig. S1 for model predictions that account for repeated measures on the same individuals (i.e. blocking by individual).
Estimates of SMR did not differ between the two respirometry methods assessed, which included the resting and circular chamber protocols (LMM, F1,32 = 0.38, P = 0.54; Fig. 4A; Table 1). However, there were noticeable differences between species (LMM, F3,33 = 9.28, P < 0.001; Fig. 4B), with P. marri having a significantly higher SMR than the other three species. The effect of method was the same across species (species × method interaction not significant, F3,29 = 1.92, P = 0.15; online supplementary material, Fig. S3B; Table 1).
Figure 4:
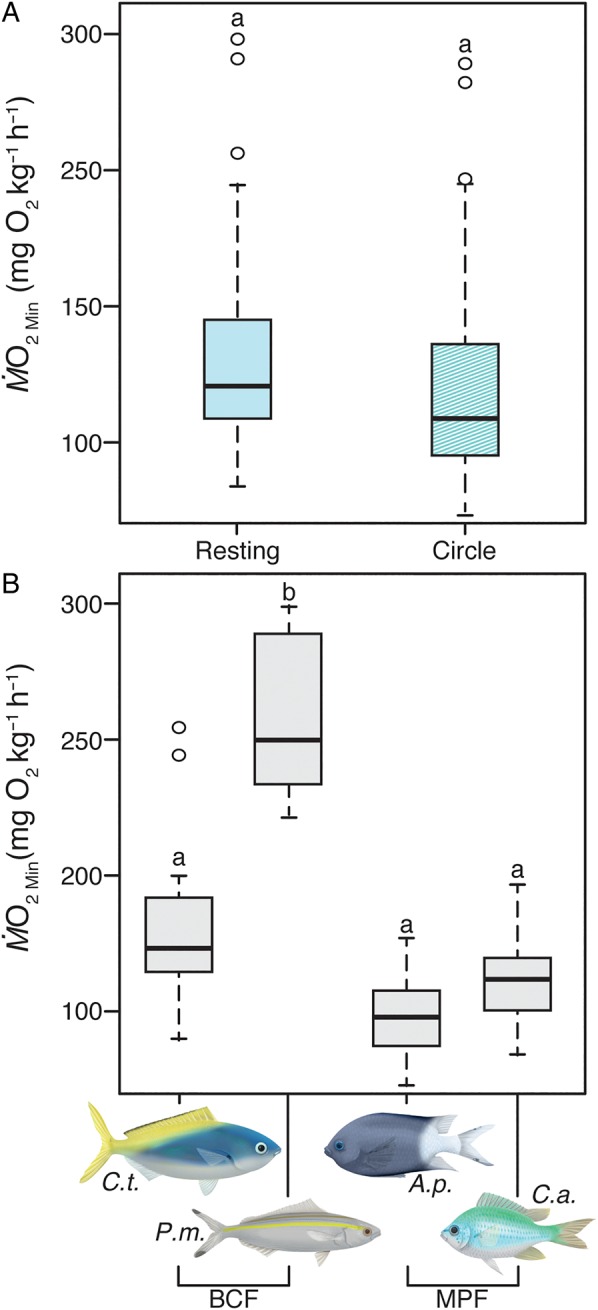
Boxplots showing median and inter-quartile range of (A) standard metabolic rates (SMR; estimated from lowest value of oxygen consumption rate, ; see Materials and methods for further details) using two respirometry methods, a resting respirometer (Resting) and a circular chamber with a stir bar (Circle), for all species combined that completed at least one method and (B) SMR for all fishes that completed at least one method. Caesio teres (C.t.) and Pterocaesio marri (P.m.) are body–caudal fin (BCF) swimmers. Acanthochromis polyacanthus (A.p.) and Chromis atripectoralis (C.a.) are median–paired fin (MPF) swimmers. Same letters indicate no significant differences (α = 0.05).
Body–caudal fin swimming fishes exhibited a significantly higher absolute Ucrit (in centimetres per second) than MPF swimming fishes (LM, F1,31 = 16.1, P < 0.001; Table 2), and absolute Ucrit differed slightly among species within swimming mode (LM, F2,31 = 1.1, P = 0.03; Table 2). Values of absolute and relative Ucrit for all species are presented in Table 2.
Table 2:
Absolute and relative critical swimming speed by species and swimming mode for all fishes that completed the swimming trials
| Mode | Species |
Ucrit (absolute; cm s−1) |
Ucrit (relative; body lengths s−1) |
||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| BCF | Pterocaesio marri | 82.05a | 5.10 | 10.10ab | 1.04 |
| BCF | Caesio teres | 83.96a | 3.20 | 10.13a | 0.30 |
| MPF | Acanthochromis polyacanthus | 63.12b | 1.76 | 8.43a | 0.19 |
| MPF | Chromis atripectoralis | 75.57a | 3.41 | 12.10b | 0.50 |
Swimming modes included body–caudal fin (BCF) and median–paired fin (MPF) swimming. Ucrit, critical swimming speed. Sample sizes are as in Table 1. Common letters indicate no significant differences (α = 0.05).
Discussion
This study demonstrates the importance of choosing the appropriate method when estimating metabolic rates in fishes. Swimming respirometry involves incrementally increasing swimming speeds over several hours until the animal reaches a maximal swimming speed that is unsustainable. This technique has been traditionally referred to as the most accurate means of estimating MMR for steady/prolonged swimmers (Steffensen et al., 1984; Farrell and Steffensen, 1987; Plaut, 2001). The incremental increase in speed allows for the routine gait of a species to be used at low speeds, with transitions to caudal-assisted (Up-c for MPF swimmers; Johansen and Jones, 2011; Binning et al., 2014; Johansen et al., 2015) and/or anaerobic burst-and-coast swimming (Uburst; Svendsen et al., 2010) as speeds increase, during which time substrate limitations will result in fibre and therefore muscle exhaustion. Additionally, MMR is estimated directly while the fish is maximally exercised. Indeed, we found that swimming respirometry consistently gave the highest estimates of MMR in both BCF and MPF swimmers. The two short-duration protocols, where fish are challenged for 3 min with 1 min air exposure (chase protocol) or 5–7 min (circular chamber protocol) both underestimated MMR by as much as 38% for a single species compared with swimming respirometry (online supplementary material, Table S1). This may be because MPF swimmers would be forced to transition immediately to their final swimming mode (caudal burst swimming), which involves different kinematics from their routine, labriform swimming mode (Webb, 1975, 1984; Blake, 2004), in order to undergo the challenge. In contrast, for BCF swimmers, the kinematics of movement between routine and burst swimming are very similar. Therefore, in theory, all methods for estimating MMR should have resulted in similar values for BCF swimmers and different values for MPF swimmers, but this prediction did not hold.
For these prolonged swimmers, swimming mode did not influence the best choice of respirometry method used to estimate MMR. However, other aspects may be important when considering respirometry methods. For example, the duration of the challenge and protocol may play a key role (e.g. incremental increases in speed over several hours in the swimming protocol vs. a few minutes in the chase and circular chamber protocols). Even within a long-duration protocol, such as with swimming respirometry, the time between the incremental increases in water velocity can affect how fish respond in terms of oxygen consumption rates (Kiceniuk and Jones, 1977; Farrell, 2007). The size of the animal and flow encountered in their natural habitat should also be taken into consideration (Nelson, et al., 2002). It should be noted that the effect on unsteady swimming species that perform poorly in a swim tunnel (e.g. burst swimmers and ambush predators; Killen et al., 2007) has not been thoroughly tested across species (see examples for cod in Soofiani and Priede, 1985; Reidy et al., 1995).
Locomotory modes and duration of physiological performance in fishes
Swimming is characterized by the structures required (e.g. fins and muscles) and duration. Body–caudal fin swimmers rely on caudal body musculature and a caudal fin to generate thrust, which can support steady movements over prolonged periods of time in some species (Blake, 1983) and unsteady movements over short periods of time in others (e.g. ambush predators; Fig. 2). In contrast, MPF swimmers employ a lift-based (wing) or rowing (oar) paired fin movement, while the body remains rigid to reduce drag and save energy (Fig. 2; Webb, 1984; Blake, 2004). Median–paired fin swimmers are generally optimized for maintaining position, hovering and manoeuvring in complex environments, especially for those with more rounded fins, and were not traditionally viewed as high-performance endurance swimmers (but, see Fulton et al., 2013; Binning and Roche, 2015). Sustained swimming (via BCF or MPF) is generally considered to last >200 min; in contrast, prolonged swimming (20 s to 200 min) and burst-and-coast swimming (<20 s) both end in fatigue (reviewed by Blake, 2004; but also see Sfakiotakis et al., 1999; Korsmeyer et al., 2002). Sustained or endurance swimming, which can be measured using traditional swimming protocols, is predominantly aerobic, using red muscle and not limited by fuel supply because lipids, proteins and carbohydrates can all be oxidized aerobically (Peake and Farrell, 2004; Fangue et al., 2008). Then, as fish approach maximal swimming speeds, they recruit fast-twitch, glycolytic, white muscle, which generates more thrust (Rome et al., 1996; Korsmeyer et al., 2002; Peake and Farrell, 2004; Svendsen et al., 2010). Consequently, the short-duration protocols (chase or circular chamber) may be exploiting only this final burst-and-coast gait, explaining why MMR can be significantly underestimated.
As species can be optimized for steady or short-term burst performance, the most relevant assessments of metabolic performance during swimming must consider the swimming behaviour of the species in their natural habitat and the duration over which it occurs. For instance, some species cannot or will not swim for extended periods and may therefore not be amenable to a protocol where water velocity is incrementally increased over several hours to assess swimming performance and estimate MMR (Reidy et al., 1995; Claireaux et al., 2006; Jordan and Steffensen, 2007; Killen et al., 2007). We investigated steady/prolonged swimmers from both BCF and MPF swimming modes and found that a longer duration protocol provides the most accurate estimates of MMR for these species that can sustain high swimming speeds for long periods.
Other methods for estimating MMR may provide more cost- and time-effective alternatives to swimming respirometry. In chase protocols, the fish is forced to use burst, anaerobic swimming until fatigue. However, it is important to consider that after an exhaustive chase challenge, measurements come from excess post-exercise oxygen consumption (EPOC) or repayment of oxygen debt incurred from anaerobic metabolism. There are at least two assumptions underpinning this protocol. The first assumption is that EPOC represents the highest rate of oxygen consumption and is therefore equal to and thus estimates of MMR. The second assumption is that EPOC occurs immediately after the exercise challenge, and recovery takes long enough for a reliable estimate to be obtained. Neither of these assumptions has been rigorously verified across species (see example for barramundi, Lates calcarifer, by Norin and Clark, 2016). If EPOC occurs immediately after a challenge, but recovery is quick, there is a risk of missing the measurements between the time the fish is challenged and when the measurement begins, as with the chase protocol. Theoretically, chase protocols may also delay EPOC by elevating plasma glucose concentrations and delaying glycogen resynthesis and lactate clearance because of stress (Milligan et al., 2000; Peake and Farrell, 2004). In both of these situations, MMR may be underestimated. Nonetheless, exhaustive chase methods may be ideal for unsteady swimmers, such as ambush predators, and future studies should compare methods in such species and others with more burst-type lifestyles.
Recent studies have used another type of exhaustive chase method with a circular chamber protocol (Nilsson et al., 2009; Donelson et al., 2010, 2011; Gardiner et al., 2010; Couturier et al., 2013; Rummer et al., 2013; Pope et al., 2014) as an alternative to swimming respirometry (e.g. Trappett et al., 2013). However, we generally do not recommend the use of a circular chamber, especially if the goals of the study are to compare estimates of AS or MMR with other studies where other methods have been used or to report some measure of swimming speed. This recommendation is based on several lines of reasoning, as follows: (i) velocity across the diameter of the circular respirometer increases significantly towards the edges of the vortex (also mentioned by Nilsson et al., 2007), meaning that the swimming speed of the individual cannot be measured accurately or compared reliably within/among studies or to field conditions; (ii) fish are constantly swimming in either a clockwise or anticlockwise direction, which will result in an imbalanced use of their musculature, may prevent recruitment of all red muscle and therefore achievement of maximal aerobic metabolism, and is likely to cause premature fatigue of right- or left-side muscles, but not complete exhaustion; and (iii) because measurements are made continuously during the exercise period, the flush pump cycle, which periodically provides the fish with clean, well-aerated seawater, cannot be started until after the fish has fatigued, risking chamber O2 concentrations falling below 80% and initiating a hypoxic stress response (Hughes, 1973; Tetens and Lykkeboe, 1985; Boutilier et al., 1988).
Although the circular chamber protocol cannot be compared directly with swimming respirometry and does not allow manual chasing for species that will not swim for prolonged periods (e.g. serranids and gadids), there may be situations where this protocol can be used effectively. Advantages include the following: (i) fish are not moved between chambers, and the investigator is therefore less likely to ‘miss’ the values because O2 is being monitored continuously; and (ii) unlike chase methods, the water velocity can be increased incrementally to allow fish to transition between gaits gradually in order to achieve maximal swimming speeds. As a result, this protocol may work well for some species that cannot be manipulated easily by the researcher or are too delicate for manual chasing protocols (e.g. larval fishes, as in Nilsson et al., 2007). However, researchers should be aware of the difficulties in comparing and interpreting results obtained with a circular chamber protocol and use this technique only if other options have proved unsuccessful or are not feasible.
The ecology of a species may also be important when assessing SMR. Some species that are optimized for fast, sustained swimming (e.g. mackerel and tuna) have difficulty maintaining very low speeds and can exhibit highly variable oxygen consumption rates owing to stress while trying to maintain position (Korsmeyer and Dewar, 2001). Additionally, species using ram ventilation (e.g. tuna and sharks) have to swim continuously to survive; therefore, it may be nearly impossible to estimate SMR accurately using conventional resting methodologies (Clark and Seymour, 2006). Rather, SMR in these species should be estimated indirectly by extrapolation of the non-linear –swimming speed relationship using swimming respirometry (Bushnell et al., 1994; Reidy et al., 2000; Korsmeyer and Dewar, 2001; Korsmeyer et al., 2002; Clark and Seymour, 2006; Roche et al., 2013; Binning et al., 2014). In other fishes, however, SMR can be estimated directly in a non-swimming, relaxed state using a resting respirometer (reviewed by Clark et al., 2013 and Svendsen et al., 2016; but also see Steffensen, 1989; Roche et al., 2013). Indeed, the two resting protocols trialled (post-chase resting chamber and circular chamber) provided consistent estimates of SMR in the present study.
Conclusions and recommendations
In recent decades, there has been a focus on studies linking swimming mode, physiological performance and environmental stress, emphasizing the importance of choosing appropriate methods for addressing a specific research question. The present study highlights that swimming respirometry appears to provide the most accurate estimates of MMR in fish that are steady/prolonged swimmers, regardless of swimming mode. In addition, swimming respirometry provides additional valuable information about swimming performance beyond critical swimming speed and MMR, such as gait transitions, burst speed, optimal swimming speed and cost of transport, all of which are ecologically relevant and could be influenced by changing environmental conditions. If swimming respirometry is not possible for fishes that are good steady/prolonged swimmers, short-duration chase or circular chamber protocols could be used, but with caution, because they may significantly underestimate MMR and therefore AS (see also Roche et al., 2013). Fish that predominantly use burst swimming (e.g. ambush predators) or unsteady MPF swimming (e.g. labriforms that use ‘rowing’ instead of ‘flapping’; see Binning and Roche, 2015) may do better in short-duration challenges, but these predictions remain to be investigated. Nevertheless, the two short-duration alternative methods investigated here did provide direct measurements of and therefore provided equally reliable SMR estimates.
From a technical perspective, both swimming respirometry and the chase protocol can easily be standardized and automated (e.g. using commercially available software) to maintain oxygen concentrations above 80% saturation at all times while calculating oxygen consumption rates at regular intervals (Svendsen et al., 2016). These tools can ease user implementation and could minimize the subjectivity or bias in data collection (i.e. manually selected oxygen consumption rate slopes, duration of slope to use, r2 of slopes, etc.). The circular chamber, however, cannot be automated as easily because the point at which maximal swimming performance and fatigue will occur cannot be anticipated easily. Some methods may also be financially less costly (e.g. chase protocol), and with shorter protocols, more fish can be tested in a limited amount of time. However, if the method chosen is not a good match for the species, then the quality of measurements will be sacrificed for quantity. In particular, this may pose a significant problem if the goal of the study is to estimate AS, because our data suggest that using chase or circular chamber protocols for prolonged swimmers can underestimate MMR (online supplementary material, Table S1) and therefore AS. Additionally, because general correction factors cannot be applied to results post hoc owing to inter-individual, species-specific and/or temperature-driven differences in the degree of underestimation, caution is warranted if MMR and aerobic scope estimates are compared across studies that use different methods. Indeed, this has been a topic that has recently received a lot of attention and discussion, given that these measurements are being extensively used to understand organismal responses to contemporary issues, such as climate change (see Special Issue: Metabolic Rate in Fishes. Definitions, Methods and Significance for Conservation Physiology, volume 88, in Journal of Fish Biology). Ultimately, from our findings, we suggest that researchers consider the following factors: (i) the swimming mode/duration/lifestyle of the species; (ii) the constraints of the methods available; and (iii) potentially cross-validating results between methods to determine the most appropriate method for the species of interest.
Supplementary material
Supplementary material is available at Conservation Physiology online. The data for this study are also publicly archived on the repository figshare. doi: 0.6084/m9.figshare.2060022: https://figshare.com/articles/Methods_matter_Considering_locomotory_mode_and_respirometry_technique_when_estimating_metabolic_rates_of_fishes/2060022?
Funding
Funding for this project was provided by an Australian Research Council Super Science Fellowship and Australian Research Council early career Discovery Fellowship (J.L.R.), the Australian Research Council Centre of Excellence for Coral Reef Studies (J.L.R., D.G.R., S.A.B.) the Australian National University (S.A.B., D.G.R.), the Natural Sciences and Engineering Research Council of Canada (S.A.B., D.G.R.), Total Diving in Montréal (D.G.R., S.A.B.) and the Ian Potter Doctoral Fellowship at Lizard Island (a facility of the Australian Museum; D.G.R., S.A.B.).
Supplementary Material
Acknowledgements
We thank S. Heatwole and the Lizard Island Research Station staff for field support, J. Atherton for laboratory and animal husbandry assistance, P. L. Munday for providing aquarium facilities, C. J. Brauner and T. Norin for helpful comments and E. Walsh for fish illustrations. We also acknowledge R. B. Langerhans, T. Vines and the team at Axios Review for organizing anonymous referees who provided valuable feedback on a previous version.
References
- Alcaraz M, Almeda R, Saiz E, Calbet A, Duarte CM, Agusti S, Santiago R, Alonso A (2013) Effects of temperature on the metabolic stoichiometry of Arctic zooplankton. Biogeosciences 10: 689–697. [Google Scholar]
- Bell WH, Terhune LDB (1970) Water tunnel design for fisheries research. Fisheries Research Board of Canada 195: 1–69. [Google Scholar]
- Binning SA, Roche DG (2015) Water flow and fin shape polymorphism in coral reef fishes. Ecology 96: 828–839. [DOI] [PubMed] [Google Scholar]
- Binning S, Roche D, Fulton C (2014) Localised intraspecific variation in the swimming phenotype of a coral reef fish across different wave exposures. Oecologia 174: 623–630. [DOI] [PubMed] [Google Scholar]
- Blake RW. (1983) Median and paired fin propulsion. In Webb PW, Weihs D, eds, Fish Biomechanics. Praeger, New York, pp 214–247. [Google Scholar]
- Blake RW. (2004) Fish functional design and swimming performance. J Fish Biol 65: 1193–1222. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24: 127–135. [DOI] [PubMed] [Google Scholar]
- Boutilier RG, Dobson G, Hoeger U, Randall DJ (1988) Acute exposure to graded levels of hypoxia in rainbow trout (Salmo gairdneri): metabolic and respiratory adaptations. Respir Physiol 71: 69–82. [DOI] [PubMed] [Google Scholar]
- Breder CM., Jr (1926) The locomotion of fishes. Zoologica 4: 159–296. [Google Scholar]
- Brett JR. (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21: 1183–1226. [Google Scholar]
- Bushnell PG, Steffensen JF, Schurmann H, Jones DR (1994) Exercise metabolism in two species of cod in Arctic waters. Polar Biol 14: 43–48. [Google Scholar]
- Chabot D, Steffensen JF, Farrell AP (2016) The determination of standard metabolic rate in fishes. J Fish Biol 88: 81–121. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Couturier C, Groison AL (2006) Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J Exp Biol 209: 3420–3428. [DOI] [PubMed] [Google Scholar]
- Clark TD, Seymour RS (2006) Cardiorespiratory physiology and swimming energetics of a high-energy-demand teleost, the yellowtail kingfish (Seriola lalandi). J Exp Biol 209: 3940–3951. [DOI] [PubMed] [Google Scholar]
- Clark TD, Donaldson MR, Pieperhoff S, Drenner SM, Lotto A, Cooke SJ, Hinch SG, Patterson DA, Farrell AP (2012) Physiological benefits of being small in a changing world: responses of coho salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event. PLoS ONE 7: e39079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier CS, Stecyk JAW, Rummer JL, Munday PL, Nilsson GE (2013) Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp Biochem Physiol A Mol Integr Physiol 166: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson MR, Clark TD, Hinch SG, Cooke SJ, Patterson DA, Gale MK, Frappell PB, Farrell AP (2010) Physiological responses of free-swimming adult Coho salmon to simulated predator and fisheries encounters. Physiol Biochem Zool 83: 973–983. [DOI] [PubMed] [Google Scholar]
- Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM (2010) Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar Ecol Prog Ser 401: 233–243. [Google Scholar]
- Donelson JM, Munday PL, McCormick MI, Pitcher CR (2011) Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Clim Change 2: 30–32. [Google Scholar]
- Fangue NA, Mandic M, Richards JG, Schulte PM (2008) Swimming performance and energetics as a function of temperature in killifish Fundulus heteroclitus. Physiol Biochem Zool 81: 389–401. [DOI] [PubMed] [Google Scholar]
- Farrell AP. (2007) Cardiorespiratory performance during prolonged swimming tests with salmonids: a perspective on temperature effects and potential analytical pitfalls. Philos Trans R Soc Lond B Biol Sci 362: 2017–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell AP, Steffensen JF (1987) An analysis of the energetic cost of the branchial and cardiac pumps during sustained swimming in trout. Fish Physiol Biochem 4: 73–79. [DOI] [PubMed] [Google Scholar]
- Ferguson RA, Tufts BL (1992) Physiological effects of brief air exposure in exhaustively exercised rainbow trout (Oncorhynchus mykiss): implications for ‘catch and release’ fisheries. Can J Fish Aquat Sci 49: 1157–1162. [Google Scholar]
- Fulton CJ, Johansen JL, Steffensen JF (2013) Energetic extremes in aquatic locomotion by coral reef fishes. PLoS ONE 8: e54033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox F, Weisberg S, Friendly M, Hong J, Andersen R, Firth D, Taylor S (2014) Effects: effect displays for linear, generalized linear, and other models. https://cran.r-project.org/web/packages/effects/effects.pdf.
- Gardiner NM, Munday PL, Nilsson GE (2010) Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS ONE 5: e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GM. (1973) Respiratory responses to hypoxia in fish. Amer Zool 13: 475–489. [Google Scholar]
- Jain KE, Hamilton JC, Farrell AP (1997) Use of a ramp velocity test to measure critical swimming speed in rainbow trout (Onchorhynchus mykiss). Comp Biochem Physiol A Mol Integr Physiol 117: 441–444. [Google Scholar]
- Johansen JL, Jones GP (2011) Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Global Change Biol 17: 2971–2979. [Google Scholar]
- Johansen JL, Steffensen JF, Jones PG (2015) Winter temperatures decrease swimming performance and limit distributions of tropical damselfishes. Conserv Physiol 3: doi:10.1093/conphys/cov039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AD, Steffensen JF (2007) Effects of ration size and hypoxia on specific dynamic action in the cod. Physiol Biochem Zool 80: 178–185. [DOI] [PubMed] [Google Scholar]
- Kiceniuk JW, Jones DR (1977) The oxygen transport system in trout (Salmo gairdneri) during sustained exercise. J Exp Biol 69: 247–260. [Google Scholar]
- Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc Biol Sci 274: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer KE, Dewar H (2001) Tuna metabolism and energetics. In Block B, Stevens E, eds, Fish Physiology, Vol 19. Academic Press, New York, pp 35–78. [Google Scholar]
- Korsmeyer KE, Steffensen JF, Herskin J (2002) Energetics of median and paired fin swimming, body and caudal fin swimming, and gait transition in parrotfish (Scarus schlegeli) and triggerfish (Rhinecanthus aculeatus). J Exp Biol 205: 1253–1263. [DOI] [PubMed] [Google Scholar]
- McLeod I, Rummer JL, Clark TD, Jones G, McCormick M, Wenger A, Munday PL (2013) Climate change and the performance of larval coral reef fishes: the interaction between temperature and food availability. Conserv Physiol 1: doi:10.1093/conphys/cot024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan CL. (1996) Metabolic recovery from exhaustive exercise in rainbow trout. Comp Biochem Physiol A Mol Integr Physiol 113: 51–60. [Google Scholar]
- Milligan C, Wood C (1986) Intracellular and extracellular acid–base status and H+ exchange with the environment after exhaustive exercise in the rainbow trout. J Exp Biol 123: 93–121. [DOI] [PubMed] [Google Scholar]
- Milligan C, Hooke G, Johnson C (2000) Sustained swimming at low velocity following a bout of exhaustive exercise enhances metabolic recovery in rainbow trout. J Exp Biol 203: 921–926. [DOI] [PubMed] [Google Scholar]
- Munday PL, Crawley NE, Nilsson GE (2009) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388: 235–242. [Google Scholar]
- Nelson JA, Chabot D (2011) General energy metabolism. In Farrell AP. eds, Encyclopedia of Fish Physiology: from Genome to Environment, Vol 3. Academic Press, San Diego, pp 1566–1572. [Google Scholar]
- Nelson JA, Gotwalt PS, Webber DW, Reidy S (2002) Beyond Ucrit: matching swimming performance tests to the physiological ecology of the animal, including a new fish ‘drag strip’. Comp Biochem Physiol A Mol Integr Physiol 133: 289–302. [DOI] [PubMed] [Google Scholar]
- Niimi AJ, Beamish FWH (1974) Bioenergetics and growth of largemouth bass (Micropterus salmoides) in relation to body weight and temperature. Can J Zool 52: 447–456. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Östlund-Nilsson S, Penfold R, Grutter AS (2007) From record performance to hypoxia tolerance: respiratory transition in damselfish larvae settling on a coral reef. Proc Biol Sci 274: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson GE, Crawley N, Lunde IG, Munday PL (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Global Change Biol 15: 1405–1412. [Google Scholar]
- Norin T, Clark TD (2016) Measurement and relevance of maximum metabolic rate in fishes. J Fish Biol 88: 122–151. [DOI] [PubMed] [Google Scholar]
- Peake SJ, Farrell AP (2004) Locomotory behaviour and post-exercise physiology in relation to swimming speed, gait transition and metabolism in free-swimming smallmouth bass (Micropterus dolomieu). J Exp Biol 207: 1563–1575. [DOI] [PubMed] [Google Scholar]
- Plaut I. (2001) Critical swimming speed: its ecological relevance. Comp Biochem Physiol A Mol Integr Physiol 131: 41–50. [DOI] [PubMed] [Google Scholar]
- Pope EC, Ellis RP, Scolamacchia M, Scolding JWS, Keay A, Chingombe P, Shields RJ, Wilcox R, Speirs DC, Wilson RW et al. (2014) European sea bass, Dicentrarchus labrax, in a changing ocean. Biogeosciences 11: 2519–2530. [Google Scholar]
- Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: http://wwwR-projectorg/. [Google Scholar]
- Randall JE, Allen GR, Steene RC (1997) Fishes of the Great Barrier Reef and Coral Sea. In Randall JE, Allen GR, Steene RC, eds. University of Hawaii Press, Crawford House Publishing, Bathurst, NSW, 557 p. [Google Scholar]
- Reidy SP, Nelson JA, Tang Y, Kerr SR (1995) Post exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J Fish Biol 47: 377–386. [Google Scholar]
- Reidy SP, Kerr SR, Nelson JA (2000) Aerobic and anaerobic swimming performance of individual Atlantic cod. J Exp Biol 203: 347–357. [DOI] [PubMed] [Google Scholar]
- Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216: 2103–2110. [DOI] [PubMed] [Google Scholar]
- Rome LC, Syme DA, Hollingworth S, Lindstedt SL, Baylor SM (1996) The whistle and rattle: the design of sound producing muscles. Proc Natl Acad Sci USA 93: 8095–8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummer JL, Stecyk JAW, Couturier CS, Watson S-A, Nilsson GE, Munday PL (2013) Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv Physiol 1: doi:10.1093/conphys/cot023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, Munday PL (2014) Life on the edge: thermal optima for aerobic scope of Equatorial reef fishes are close to current day temperatures. Global Change Biol 20: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann H, Steffensen JF (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50: 1166–1180. [Google Scholar]
- Sfakiotakis M, Lane DM, Davies JBC (1999) Review of fish swimming modes for aquatic locomotion. IEE J Oceanic Eng 24: 237–252. [Google Scholar]
- Soofiani NM, Priede IG (1985) Aerobic metabolic scope and swimming performance in juvenile cod, Gadus morhua L. J Fish Biol 26: 127–138. [Google Scholar]
- Steffensen JF. (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct them. Fish Physiol Biochem 6: 49–59. [DOI] [PubMed] [Google Scholar]
- Steffensen JF. (2005) Respiratory systems and metabolic rates. In Farrell AP, Steffensen JF, eds, Fish Physiology, Vol 22. Academic Press, New York, pp 203–238. [Google Scholar]
- Steffensen JF, Johansen K, Bushnell PG (1984) An automated swimming respirometer. Comp Biochem Physiol A Mol Integr Physiol 79: 437–440. [Google Scholar]
- Svendsen JC, Tudorache C, Jordan AD, Steffensen JF, Aarestrup K, Domenici P (2010) Partition of aerobic and anaerobic swimming costs related to gait transitions in a labriform swimmer. J Exp Biol 213: 2177–2183. [DOI] [PubMed] [Google Scholar]
- Svendsen MBS, Bushnell PG, Steffensen JF (2016) Design and set up of intermittant-flow respirometry system for aquatic organisms. J Fish Biol 88: 26–50. [DOI] [PubMed] [Google Scholar]
- Tetens V, Lykkeboe G (1985) Acute exposure of rainbow trout to mild and deep hypoxia: O2 affinity and O2 capacitance of arterial blood. Respir Physiol 61: 221–235. [DOI] [PubMed] [Google Scholar]
- Trappett A, Condon CH, White C, Matthews P, Wilson RS (2013) Extravagant ornaments of male threadfin rainbowfish (Iriatherina werneri) are not costly for swimming. Funct Ecol 27: 1034–1041. [Google Scholar]
- Videler JJ. (1993) Fish Swimming. Chapman & Hall, London. [Google Scholar]
- Vogel S. (1994) Life in Moving Fluids: the Physical Biology of Flow. Princeton University Press, Princeton. [Google Scholar]
- Walker JA, Westneat MW (2002) Kinematics, dynamics, and energetics of rowing and flapping propulsion in fishes. Integr Comp Biol 42: 1032–1043. [DOI] [PubMed] [Google Scholar]
- Webb PW. (1975) Hydrodynamics and energetics of fish propulsion. Bull Fish Res Board Can 190: 137–144. [Google Scholar]
- Webb PW. (1984) Form and function in fish swimming. Sci Am 251, 72–82. [Google Scholar]
- Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.