Abstract
Thrombotic microangiopathy (TMA) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, microvascular thrombosis, and various organ dysfunctions. TMA usually occurs in a more advanced stage of HIV disease. TMA as an initial presenting feature is rare. We here report a male patient who presented with oliguric renal failure. Investigations revealed anemia, thrombocytopenia, schistocytes in peripheral smear, and HIV-positive. Renal biopsy revealed TMA. He was treated with hemodialysis and started on highly active antiretroviral therapy.
Keywords: Antiretroviral therapy, HIV, renal failure, thrombotic microangiopathy
Introduction
Thrombotic microangiopathies include thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Lower CD4+ cell counts (<100/µL), higher HIV-1 RNA levels, clinical AIDS, opportunistic infections (cytomegalovirus [CMV] or HHV-8 infection), drugs, and immune reconstitution after highly active antiretroviral therapy (HAART) are the risk factors for thrombotic microangiopathy (TMA) in HIV patients.[1] We describe here a 52-year-old male patient who presented with TMA and later found to have HIV positivity.
Case Report
A 52-year-old-male was admitted to the emergency department with acute breathlessness, edema legs, and oliguria of 3 days duration. He was a nondiabetic with hypertension for 1 year. There was no history of fever or drug usage. On examination, blood pressure was 160/100 mmHg and pulse rate was 88/min. Investigation revealed urine analysis: 1 + proteinuria, no red blood cells; 24 h urine protein: 1.2 g/day; blood hemoglobin: 8 g/dl; platelet count: 80,000 cells/mm3; peripheral smear: Numerous fragmented red blood cells; blood urea: 110 mg/dl; serum creatinine: 7.5 mg/dl; sodium: 130 mEq/l; potassium: 3.5 mEq/L; indirect bilirubin: 1.4 mg/dl; serum lactate dehydrogenase (LDH): 1200 U/L. Prothrombin time, activated partial thromboplastin time and fibrin degradation products were all normal. HIV was found to be positive by Western blot assay. It was negative for hepatitis B and C. CD 4 count was 224 cells/mm3. Serum complements were normal. There was no evidence of any opportunistic infections. He was started on HAART (lamivudine, nevirapine, and zidovudine) and hemodialysis.
After stabilization, renal biopsy was done. Light microscopy showed double contour of the glomerular basement membrane and fibrin thrombi occluding the lumen of arterioles [Figure 1]. Interstitial fibrosis and tubular atrophy were seen in 30% of the core. Immunofluorescence showed no deposits. Diagnosis of chronic TMA was established. He was planned for plasmapheresis, but was not willing for further treatment.
Figure 1.
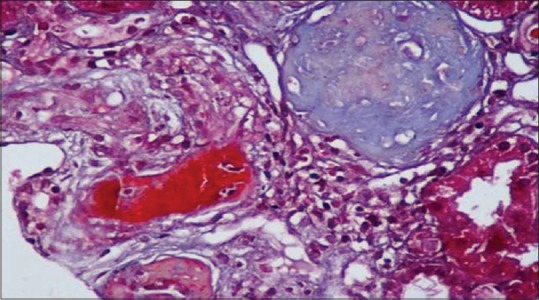
Renal biopsy showing fibrin thrombi in afferent arteriole (trichrome stain)
Discussion
TMA is the most common microvascular injury associated with HIV infection.[2] TMA involving kidney in an AIDS patient was first described by Boccia et al. in 1984.[3] The frequency of TMA was found to be higher in HIV-infected patients than in normal population and greater in those with advanced disease. The clinical spectrum varies from asymptomatic thrombocytopenia with mild renal failure to a serious illness with neurological deficit and dialysis requiring renal failure. Most of them have co-existing AIDS-defining illness such as pneumocystis carinii pneumonia, CMV retinitis, and cryptococcal meningitis.[4] The incidence of TMA in HIV patients is decreasing after the introduction of HAART. In a study conducted in Milan,[5] the incidence of TMA was 1.4% in preHAART era whereas no cases were reported in the HAART era.
Endothelial damage is the key feature of pathogenesis of TMA.[6] It can be caused directly by viral invasion as evidenced by the expression of HIV-1 P24 antigen/chemokine receptors CXCR4 in endothelial cells or indirectly by the action of cytokines/HIV-associated proteins such as TaT and gp 120 on endothelial cells.[7] Complete deficiency of the vWF-cleaving protease ADAMTS13 has been reported in HIV-associated TMA due to the development of IgG autoantibodies. Role of anti-phospholipid antibodies is also implicated.[8] Other factors include drugs (valacyclovir, fluconazole, and clofazimine), malignancies, and direct vascular injury by infectious agents. Various inflammatory cytokines such as tumor necrosis factor-α and interleukin-1 are found to be raised, and there is evidence of enhanced apoptosis of microvascular endothelial cells in these patients.[9]
Plasma exchange is the mainstay of treatment and renal involvement is generally last to improve. It should be continued until the resolution of neurological symptoms and both a normal serum LDH and platelet count have been maintained for 3 days.[10] Antiretroviral therapy is an important component of the management. Role of corticosteroids, splenectomy, and anti-platelet agents in patients with HIV-TMA is unknown. Use of corticosteroids should be individualized. Splenectomy and monoclonal antibodies should be reserved for those who are refractory to plasma exchange.[11]
Long-term prognosis of HIV-TMA depends on the stage of HIV infection. Mortality of patients with symptomatic HIV infection is three times higher than mortality of patients with asymptomatic HIV infection. Patients with HIV-TMA, who do not have AIDS, have favorable outcome when treated with plasmapheresis.[12] Our patient presented with TMA with severe renal failure as the initial manifestation of HIV. He was treated with hemodialysis, supportive measures, and HAART, and planned for plasmapheresis, but was not willing for further management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Becker S, Fusco G, Fusco J, Balu R, Gangjee S, Brennan C, et al. HIV-associated thrombotic microangiopathy in the era of highly active antiretroviral therapy: An observational study. Clin Infect Dis. 2004;39(Suppl 5):S267–75. doi: 10.1086/422363. [DOI] [PubMed] [Google Scholar]
- 2.Sacristán Lista F, Saavedra Alonso AJ, Oliver Morales J, Vázquez Martul E. Nephrotic syndrome due to thrombotic microangiopathy (TMA) as the first manifestation of human immunodeficiency virus infection: Recovery before antiretroviral therapy without specific treatment against TMA. Clin Nephrol. 2001;55:404–7. [PubMed] [Google Scholar]
- 3.Boccia RV, Gelmann EP, Baker CC, Marti G, Longo DL. A hemolytic-uremic syndrome with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:716–7. doi: 10.7326/0003-4819-101-5-716_2. [DOI] [PubMed] [Google Scholar]
- 4.de Man AM, Smulders YM, Roozendaal KJ, Frissen PH. HIV-related thrombotic thrombocytopenic purpura: Report of 2 cases and a review of the literature. Neth J Med. 1997;51:103–9. doi: 10.1016/s0300-2977(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 5.Gervasoni C, Ridolfo AL, Vaccarezza M, Parravicini C, Vago L, Adorni F, et al. Thrombotic microangiopathy in patients with acquired immunodeficiency syndrome before and during the era of introduction of highly active antiretroviral therapy. Clin Infect Dis. 2002;35:1534–40. doi: 10.1086/344778. [DOI] [PubMed] [Google Scholar]
- 6.Sutor GC, Schmidt RE, Albrecht H. Thrombotic microangiopathies and HIV infection: Report of two typical cases, features of HUS and TTP, and review of the literature. Infection. 1999;27:12–5. doi: 10.1007/BF02565164. [DOI] [PubMed] [Google Scholar]
- 7.del Arco A, Martinez MA, Peña JM, Gamallo C, González JJ, Barbado FJ, et al. Thrombotic thrombocytopenic purpura associated with human immunodeficiency virus infection: Demonstration of p24 antigen in endothelial cells. Clin Infect Dis. 1993;17:360–3. doi: 10.1093/clinids/17.3.360. [DOI] [PubMed] [Google Scholar]
- 8.Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31:256–63. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 9.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 10.Ruggenenti P, Remuzzi G. The pathophysiology and management of thrombotic thrombocytopenic purpura. Eur J Haematol. 1996;56:191–207. doi: 10.1111/j.1600-0609.1996.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 11.Hymes KB, Karpatkin S. Human immunodeficiency virus infection and thrombotic microangiopathy. Semin Hematol. 1997;34:117–25. [PubMed] [Google Scholar]
- 12.Peraldi MN, Maslo C, Akposso K, Mougenot B, Rondeau E, Sraer JD. Acute renal failure in the course of HIV infection: A single-institution retrospective study of ninety-two patients and sixty renal biopsies. Nephrol Dial Transplant. 1999;14:1578–85. doi: 10.1093/ndt/14.6.1578. [DOI] [PubMed] [Google Scholar]


