Abstract
Heart failure (HF) is an important public health problem in need of strategies to improve outcomes and decrease healthcare resource utilization and costs. The prevalence has risen as the population ages and HF continues to be associated with a high mortality and frequent need for hospitalization. The total cost of care for patients with HF is $30.7 billion, and estimated to more than double to $69.8 billion by 2030. Given this reality, there has been recent investigation into ways of identifying and preventing HF in patients at risk (stage A HF) and those with cardiac structural and functional abnormalities but no clinical HF symptoms (Stage B). For patients who have developed symptoms of HF (Stage C), there has been important research into the most effective ways to decongest patients admitted with acute decompensated HF and prevent future hospital readmissions. We continue to search for successful strategies to treat patients with HF and preserved ejection fraction, which has risen in prevalence. We are in the midst of a rapid evolution in our ability to care for patients with end stage HF (Stage D) due to the introduction and improvement in mechanical circulatory support. Left ventricular assist devices used as destination therapy offer an important therapeutic option to patients who don’t qualify for heart transplantation due to advanced age or excess comorbidity. This review will provide a thorough update on contemporary strategies in the diagnosis and management of HF by stage (A to D) that have emerged in the last several years.
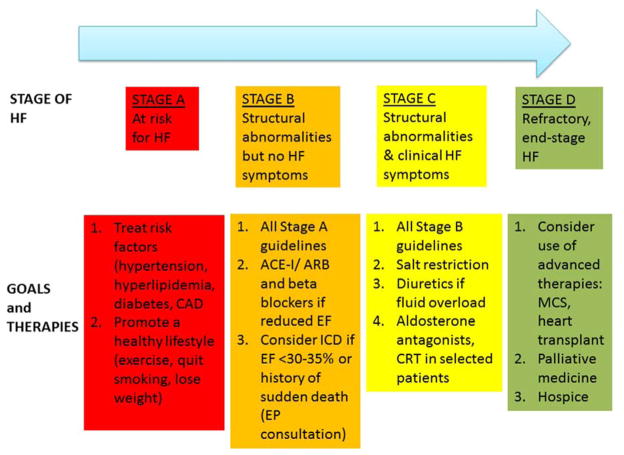
An estimated 5.8 million adults in the United States are currently living with heart failure (HF), and its prevalence is projected to increase to 25% by 2030.1 HF is primarily a disease of the elderly with prevalence increasing from 0.9% in patients aged 55–64 years to 17.4% in those 85 and older.2 The increasing prevalence of HF is attributed to aging of the population and improved survival from HF and other cardiovascular diseases. Given the rise in prevalence and epidemic of hospitalizations in patients with HF, total costs are projected to increase from $30.7 billion in 2012 to $69.8 billion in 2030.1 While most of the focus on HF is aimed at treatment of affected patients, in 2001, the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) revised the HF classification to also include patients who are at high risk for the disease (Stage A, Figure 1), but have not yet developed structural cardiac abnormalities or clinical evidence of HF. In 2010, Ramani et al3 reviewed the contemporary diagnosis and management of HF for this journal, including a review of guideline-based management for patients with HF. Since then, there has been an expansion of indications for drug and device therapy, significant progress made with mechanical circulatory support (MCS) and new clinical trials aimed towards enhancing the care of the HF patient. This complementary review will provide a thorough update on contemporary strategies in the diagnosis and management of HF by stage (A to D) that have emerged in the last several years, with a focus on new guidelines and research results which may affect clinical practice.
Figure 1. Stages in the Development of Heart Failure.
Adapted from J Am Coll Cardiol7 with permission.
ACE-I= angiotensin converting enzyme inhibitor, ARB= angiotensin receptor blocker, CAD= coronary artery disease, CRT= cardiac resynchronization therapy, EP= electrophysiology, ICD= implantable cardioverter defibrillator, MCS= mechanical circulatory support
STAGE A HF: PATIENTS AT RISK
Stage A HF includes patients who have not yet developed HF or cardiac structural abnormalities but are at risk due to coronary artery disease, diabetes, hypertension or other conditions. As many of these predisposing conditions are highly prevalent, patients with stage A HF are very common. In one community study, it was estimated that 56% of the population ≥45 years old had stage A or B HF.4
Patients at Risk for the Development of HF Can Be Predicted with Modest Accuracy
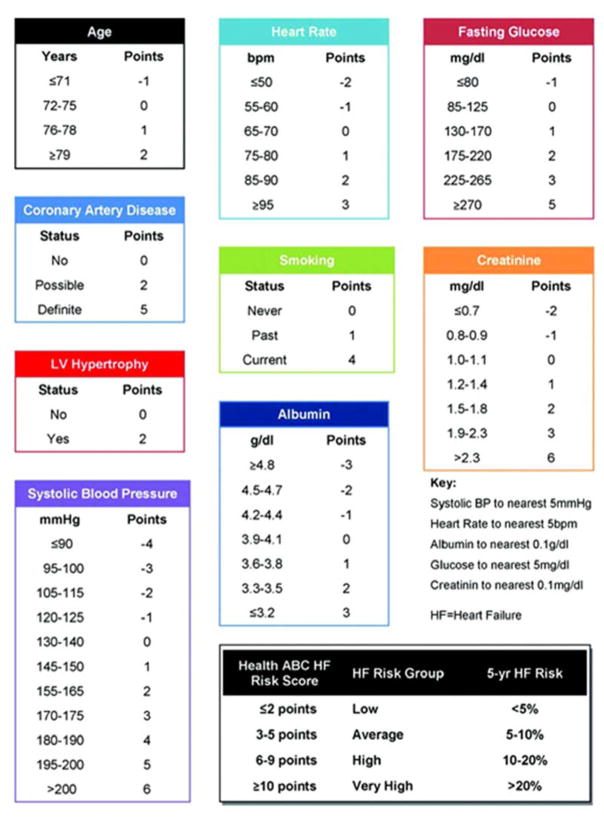
While only Stage C & D patients would meet criteria for HF, this focus on identifying patients at risk for HF (stage A) has prompted the development of several incident HF risk scores. The Health Aging and Body Composition (ABC) study included 3075 community dwelling elderly patients (aged 70–79 years) who were followed for 7 years for clinical events, including the onset of HF, which developed in 258 participants.5 A simple point score based on the following independent predictors of HF was developed (Figure 2). While the risk score is easy to calculate, the ability to discriminate is only acceptable (c statistic 0.72). Similarly, a model to predict incident HF validated in the Atherosclerosis Risk in Communities cohort6 included many of the same variables (age, coronary artery disease, blood pressure, smoking, heart rate), as well as race, sex, diabetes, and body mass index. They reported similar predictive ability to the Health ABC score, and found that both models performed better with the addition of NT-pro BNP. Either risk model would be acceptable to use in clinical practice to help identify patients who may be at higher risk for the development of HF.
Figure 2. Predicting Risk of Heart Failure: the Health ABC Risk Score.
ABC= Aging and Body Composition, HF= heart failure, LV= left ventricular
Reprinted from Circ Heart Fail with permission.
Consider Genetic Testing in Patients Suspected of Having a Familial Cardiomyopathy
A high proportion (20–35%) of patients with a dilated cardiomyopathy (DCM) may have a familial cardiomyopathy (defined as 2 or more closely related family members with DCM).7 A thorough family history should be obtained with a new diagnosis of DCM. If a familial cardiomyopathy is suggested based on history, genetic testing and referral to a genetic counselor should be considered. However, pathogenic mutations are identified in only 30–35% of familial case genetic causes8, so a negative genetic screen does not eliminate the possibility of an inherited DCM. Unaffected first degree relatives of patients with familial DCM should undergo screening with echocardiography at least every 3–5 years.7 Hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia can also be inherited, and genetic screening, counseling and testing in these conditions are thoroughly covered in a recent review.9
Treatment of Stage A HF Patients Should be Aimed at Controlling Modifiable Risk Factors
Treatment of patients identified to be at high risk for the development of HF should be aimed at reducing their risk by treatment of modifiable risk factors, including aggressive treatment of hypertension, diabetes, hyperlipidemia, and obesity. In particular, long term hypertension control may reduce the risk of incident HF by more than 50%.10,11 The choice of anti-hypertensive therapy should be made according to published guidelines,7,12 though a meta-analysis suggested that diuretics, angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) are the most effective classes of drugs at reducing HF risk.11
STAGE B HF: STRUCTURAL HEART ABNORMALITIES BUT NO CLINICAL HF SYMPTOMS
Stage B HF includes patients with prior myocardial infarction (MI), left ventricular remodeling including left ventricular hypertrophy and reduced ejection fraction (EF), and asymptomatic valvular heart disease that have never had active HF symptoms. The number of patients in stage B is estimated to be 3 to 4 times the number of patients in stage C and D combined.4,13 The prevalence of asymptomatic reduced EF is estimated at 3–6%,14 and increases with age. Asymptomatic diastolic dysfunction is more common, with an estimated prevalence as high as 27%.15 Patients with stage B HF are at high risk for the development of symptomatic (stage C) HF, but strategies exist to reduce that risk. In general, all of the therapies for stage A patients, including aggressive treatment of risk factors, should be used in stage B patients. Additional therapies recommended for patients with stage B HF are shown in Figure 1.
Chemotherapy-Associated Cardiotoxicity Occurs and Should be Treated
The anthracyclines (doxorubicin, daunorubicin, epirubicin, idarubicin) and the anthraquinone mitoxantrone are the most frequently implicated chemotherapeutic agents associated with the development of cardiotoxicity, with an incidence as high as 26%.16 A meta-analysis suggested that the risk of both clinical cardiotoxicity (OR 5.43, 95% CI 2.34–12.62) and subclinical cardiotoxicity (OR 2.88, 95% CI 1.29–6.44) were higher in cancer patients treated with anthracycline vs. non-anthracycline based compounds.17 The risk of cardiotoxicity increases with higher cumulative dose and older age. Use of bolus versus continuous infusions, liposomal vs. non-liposomal doxorubicin, concomitant use of iron-chelating agents, and use of epirubicin or mitoxantrone (lower risk agents for cardiotoxicity) versus doxorubicin may help to mitigate the risk.17 Patients treated with these agents should generally have an assessment of their EF at baseline and repeated periodically based on cumulative dose and risk factors, with discontinuation of chemotherapy if the EF declines by ≥10% to <50%.18 All patients developing a decline in EF should have therapy with ACE-I and beta blockers similar to other stage B patients, though patients with chemotherapy-induced cardiotoxicity are frequently undertreated.19 There are ongoing studies to assess whether patients being treated with anthracyclines should be concomitantly treated with ACE-I to prevent the development of cardiotoxicity.20 Ways of detecting subclinical signs of left ventricular dysfunction, including changes in longitudinal strain on echocardiography and elevation in cardiac troponin I, are being actively investigated.21 Additional chemotherapeutic agents that can cause HF include cyclophosphamide, ifosfamide, trastuzumab and other monoclonal antibody-based tyrosine kinase inhibitors.22
STAGE C HF: STRUCTURAL HEART ABNORMALITIES AND SYMPTOMS OF HF
Once a patient develops clinical signs and symptoms of HF, they become stage C, even if they later become asymptomatic. Important clinical pearls in the general management of patients with stage C HF were included in the prior review by Ramani et al,3 and comprehensive guidelines for the management of patients with HF have been published by the ACCF/AHA.7 This section will focus on highlighting important areas of recent research for stage C patients.
Biomarkers Can be Useful in Estimating Prognosis in Patients with HF
While biomarkers are most widely used to diagnose HF, they can also help to provide an estimate of prognosis in patients with stage C and D HF. The natriuretic peptides, namely B-type natriuretic peptide (BNP) or its amino-terminal fragment (NT pro BNP), which are released in response to myocardial stretch, and troponins, released in response to myocyte injury, are the most widely reported and used biomarkers for prognosis in HF. Higher natriuretic peptide levels have consistently been shown to predict mortality, but have been less useful in predicting hospital readmissions. One emerging biomarker, ST2, a member of the interleukin-1 receptor family, is predictive of mortality in HF,23 may help in identifying patients with HF who would benefit from beta blocker titration,24 and its use may become more widespread in the coming years. Cystatin C is a marker of acute kidney injury during a HF hospitalization and an increase by >0.3mg/L in the first 48 hours of HF hospitalization is associated with longer length of stay and a four-fold higher in-hospital mortality.25 The clinical value of serial biomarker-guided management of HF remains controversial. While individual trials have often failed to show any reduction in mortality or HF hospitalizations with a natriuretic-peptide guided strategy,26,27 meta-analyses have suggested there may be some mortality benefit to this approach.28,29 The ongoing GUIDE-IT trial may shed some light on this topic, as it investigates the efficacy of a strategy of biomarker-guided therapy compared with usual care in high risk patients with left ventricular systolic dysfunction.
UPDATES ON THE CHRONIC MANAGEMENT of PATIENTS WITH STAGE C HF
HpEF Efficacious Therapies are Still Lacking
Half of patients with HF have preserved EF (HFpEF), which is variably defined across studies, but usually refers to an EF>40–50%. Studies have suggested that the prevalence of HFpEF is increasing over time,30 and is most common in older women. Additional comorbidities including anemia, hypertension, and atrial fibrillation are more common in patients with HFpEF vs. HF with reduced EF (HFrEF).31 Similar to patients with HFrEF, patients with HFpEF are at increased risk for death, with 5 year mortality estimated at around 50% in both groups.32 Compared to patients with HFrEF, patients with HFpEF are more likely to experience a non-cardiovascular cause of death.33
Despite its rising prevalence, there are still no known efficacious pharmacological therapies for HFpEF. Whether renin-angiotensin system antagonists improve outcomes in HFpEF has been highly debated, and a recent analysis of patients enrolled in the Swedish HF registry suggested that there may be mortality reduction associated with renin-angiotensin system inhibition in HFpEF.34 However, randomized controlled trials have consistently failed to demonstrate any improvement in mortality in HFpEF patients treated with ACE-I35 or ARB36,37 compared with placebo. There has been recent interest in using phosphodiesterase-5 inhibitors, a therapy for patients with pulmonary hypertension, to treat patients with HFpEF. However, the RELAX multicenter randomized controlled trial found no change in exercise capacity or clinical status after 24 weeks of sildenafil compared with placebo.38 While aldosterone antagonism with eplerenone did not improve exercise capacity in the recent RAAM-PEF trial, it had favorable effects on diastolic function.39 The TOPCAT trial results, which were presented at the AHA Scientific Sessions in November 2013, found no reduction in the combined risk of cardiovascular mortality, aborted cardiac arrest or HF hospitalization in patients with HFpEF treated with spironolactone compared with placebo, though a reduction in the secondary endpoint of HF hospitalizations was observed. There is currently not sufficient evidence to recommend routine treatment with aldosterone antagonists in patients with HFpEF, though they could be a reasonable choice for patients with another indication for these therapies such as hypertension.
There has been increasing evidence to suggest that activation of the sympathetic nervous system plays a prominent role in the pathophysiology of HFpEF.40 Renal denervation is a transcutaneous catheter-based procedure used to disrupt renal sympathetic nerves. Early studies in hypertensive subjects have demonstrated it to be safe and effective.41,42 There is interest in testing whether renal denervation will be an efficacious therapeutic option in patients with HFpEF, which will be tested in the upcoming DIASTOLE trial.40
Therapy in patients with HFpEF should continue to focus on aggressive management of hypertension, optimizing fluid status with diuretics, and treatment of concomitant comorbidities such as sleep-disordered breathing. While patients with HFpEF often complain of dyspnea with exertion, exercise training is safe and improves exercise capacity.43
Close Monitoring for Hyperkalemia is Necessary for Patients Treated with Aldosterone Antagonists
In randomized controlled trials, eplerenone44 and spironolactone45 markedly reduced death and readmissions in patients with HFrEF. As a result, aldosterone antagonists have received a class I recommendation in the ACCF/AHA guidelines.7 However, they have not been adopted as readily as other guideline-based therapies, in part due to concern over the risk of hyperkalemia, particularly in high-risk patients such as those treated with renin-angiotensin system antagonists or with chronic kidney disease. To address the efficacy and safety of these agents in real-world populations, Hernandez et al published an analysis of 5887 patients enrolled in the Get with the Guidelines HF registry,46 reporting no difference in mortality or all-cause rehospitalization in the 18.2% of patients treated with these medications. While patients treated with aldosterone antagonists did have a lower risk of HF-related rehospitalization, they also had a 2.5-fold increased risk of hospitalization for hyperkalemia within 30 days of initiation. The implications of these data taken in conjunction with those from randomized trials is that aldosterone antagonists can be efficacious therapies, but should be used with caution in patients with a history of hyperkalemia or renal insufficiency (estimated glomerular filtration rate <60mL/min). Potassium and renal function should be monitored at 1 week, 4 weeks, and 3 months after initiation in all patients.
Cardiac Resynchronization Therapy Should be Considered in Patients with Less Severe Symptoms
In previous versions of the ACCF/AHA/HRS guidelines a class I indication for cardiac resynchronization therapy (CRT) was given only for patients with NYHA functional class III symptoms, an EF≤35%, and a QRS duration of ≥120 msec. In the 2012 update, the class I indication was expanded to include patients with NYHA class II symptoms.47 However, the class I recommendation was confined to patients with a left bundle branch block and QRS duration ≥150msec (Table 1). These changes were made based on results of trials including REVERSE,48 MADIT-CRT,49 and RAFT.50
Table 1.
Indications for Cardiac Resynchronization Therapy
| Classification of Recommendation | ||||
|---|---|---|---|---|
| NYHA Functional Class | Class I Benefit ≫> Risk | Class IIa Benefit ≫ Risk | Class IIb Benefit ≥ Risk | Class III No Benefit |
| I | EF≤30% QRS≥150msec LBBB Ischemic |
QRS<150msec Not LBBB |
||
| II | EF≤35% QRS≥150msec LBBB Sinus rhythm |
EF≤35% QRS 120–149msec LBBB Sinus rhythm |
EF≤35% QRS ≥150 msec Non LBBB Sinus rhythm |
QRS<150 msec & Not LBBB |
| III | EF≤35% QRS≥150msec LBBB Sinus rhythm |
EF≤35% Sinus rhythm LBBB + QRS 120–149msec OR Non LBBB + QRS ≥150 msec |
EF≤35% QRS 120–149msec Non LBBB Sinus rhythm |
|
| IV/Stage D | If limited survival to <1 year | |||
| Atrial fibrillation | EF<35% and requires pacing or expected to pace frequently | |||
EF= ejection fraction, LBBB= left bundle branch block, NYHA= New York Heart Association
All patients should be on goal-directed medical therapy prior to consideration of CRT. Data from J Am Coll Cardiol47.
Intravenous Iron Therapy Improves Exercise Capacity in Iron Deficient Patients with HF
Anemia is associated with increased morbidity and mortality among patients with HF,51,52 and is more common in women and patients with HFpEF. Given the prevalence of anemia in HF and associated adverse effects, there has been interest in using iron and erythropoietin therapy. As iron is not well absorbed orally, its safety and efficacy when administered intravenously has been investigated in 3 randomized trials. While no consistent improvements in hard clinical endpoints such as death and hospitalization were seen, intravenous iron therapy was safe and improved New York Heart Association (NYHA) functional class and exercise capacity53–55 in iron-deficient HF patients with and without anemia. Thus, use of intravenous iron therapy should be considered in patients with HF and iron deficiency. Erythropoietin is produced by the kidneys, is often elevated in patients with HF, and is associated with adverse outcomes. In patients with advanced chronic kidney disease, erythropoietin is frequently used to treat anemia, and thus, was of interest for use in patients with HF and anemia. Despite promising results in smaller studies, the recently published RED-HF trial, which randomized 1136 patients with hemoglobin 9–13mg/dL to darbepoetin alfa or placebo, found no difference in clinical outcomes in patients treated with darbepoetin.56 At this time, there is not sufficient data to support the routine use of erythropoietin agents in patients with HF and anemia.
Pharmacogenetics May Help Individualize Treatment of Patients with HF
Pharmacogenetics is the study of the role that inherited factors play in an individual’s response to a drug. Advances in DNA sequencing and genotyping have made it possible to rapidly and accurately identify variation in DNA sequence and structure. As a result, we can now correlate genomic variation with drug response, which helps us to predict individual variation in responses to specific medications, to optimize medication selection and dose, and to avoid adverse medication effects. In HF, one early example of the potential importance of pharmacogenetics is with use of beta blockers. While beta blockers are known to reduce morbidity and mortality in HF, there is heterogeneity in this response. It was hypothesized that this heterogeneity may be explained in part by genetic variation in the β1 adrenergic receptor (ADRβ1). The HF ACTION DNA study found that patients with a specific polymorphism of the ADRβ1 receptor (ARG389) required larger doses of beta blockers to achieve similar effects compared with other patients.57 There are important challenges to address regarding the implementation of pharmacogenetics in clinical practice, including availability of genotyping, insurance coverage of testing, and physician and patient acceptance.58
UPDATES ON THE MANAGEMENT of PATIENTS WITH ACUTE DECOMPENSATED HF (ADHF)
Emergency Department Observation Units May Decrease the Need for Hospital Admission
Each year, nearly 800,000 patients are admitted to the hospital with HF from the Emergency Department (ED). Only a small proportion of patients present in cardiogenic shock or require invasive diagnostic evaluations or intravenous inotrope infusions. However, only 10–20% of the patients who present to the ED with HF are discharged to home,59 with the remainder admitted to the hospital. As some of the patients admitted may only require decongestion and monitoring for a short period of time, there has been interest in understanding whether ED observation protocols can be developed to treat patients with HF who require further evaluation before deciding about disposition. A two-level algorithm to identify low and intermediate-risk candidates for observation has been proposed.60 Patients exhibiting high risk features such as hemodynamic instability, worsening renal function, and elevated troponin would be admitted to an inpatient unit. Those who quickly return to their baseline after initiation of diuretic therapy and have no high risk features could be discharged to home. The third group of low- and intermediate-risk patients would be candidates for a 24 hour observation unit where continued response to therapy could be visualized. Up to 50% of patients who are currently admitted with HF may qualify for observation units and up to 75% of those may be able to be discharged home after observation without requiring hospitalization. However, a randomized-controlled trial would be needed before deciding whether this option can reduce costs and resource use without affecting outcomes.
There are Multiple Equivalent Strategies to Decongest Patients Admitted with HF
Approximately 90% of patients admitted with HF are treated with loop diuretics, but there has been controversy as to whether bolus dosing or continuous infusion resulted in better decongestion. A number of small trials had been published but they were underpowered and reported disparate results.61–63 In 2011, the NIH-sponsored DOSE trial that found no difference in patient-reported symptoms, change in renal function, or net fluid loss in patients treated with bolus vs. continuous infusion of intravenous furosemide.64 A reasonable total daily intravenous furosemide starting dose upon admission would be 2.5 times the patient’s total daily outpatient oral diuretic dose in furosemide equivalents. Lower doses could be employed, but may require longer duration of intravenous diuresis or a dose increase if a lack of response is observed.
Therapy For Patients Presenting with Cardiorenal Syndrome Remains Challenging
Strategies previously advocated have included use of ultrafiltration, low-dose dopamine, and nesiritide. However, recent results have suggested that none of these therapies is more efficacious than intravenous diuretics. The CARESS-HF trial found that ultrafiltration resulted in similar weight loss compared with diuretics plus inotropes, but was associated with worsening renal function and increased adverse events.65 The efficacy of adding low-dose dopamine to diuretics was tested in the DAD-HF and ROSE-AHF trials. In DAD-HF, patients randomized to a low-dose furosemide infusion and 5μg/kg/min dopamine had improved renal function compared to patients treated with very high-dose furosemide infusion (20mg/hr).66 However, ROSE-AHF found no difference in urine volume or renal function with addition of dopamine to intravenous diuretics in patients with ADHF and renal dysfunction.67 Finally, nesiritide, a recombinant B-type natriuretic peptide (BNP), was approved for use in patients with ADHF in the United States in 2001, as earlier studies demonstrated an improvement in dyspnea and reduction pulmonary capillary wedge pressure after administration.68,69 However, subsequent meta-analyses raised concerns that nesiritide may be associated with worsening renal function and higher mortality.70 Both the ASCEND and ROSE-AHF randomized trials found no increase in the risk of death or worsening renal function in patients treated with nesiritide.67,71 Both studies found a greater risk of hypotension with nesiritide, and only ASCEND reported a small, non-significant improvement in dyspnea with nesiritide compared with placebo. There is no strong evidence to support the routine use of ultrafiltration, dopamine, or nesiritide in the management of ADHF and cardiorenal syndrome. A more prudent approach may be to treat patients with intravenous loop diuretics, and to only consider additional therapies in refractory patients.
There is no Easy Solution to the HF Readmissions Problem
One in four patients discharged from the hospital following an admission for HF are readmitted within 30 days (median cost of $9923 per readmission72), and HF is the most frequent reason for readmission among Medicare beneficiaries.73 Given the economic and public health implications of readmissions, several healthcare related institutes and payers have focused on this metric as an indicator of the quality of the care that is provided. On October 1, 2012, the Centers for Medicare and Medicaid Services (CMS) began to financially penalize hospitals with higher than expected 30-day readmission rates for pneumonia, acute myocardial infarction, and HF. As such, hospitals began scrambling to implement strategies to reduce readmissions and avoid the pay-for-performance penalties. While a wide variety of strategies have been implemented, they can be been categorized into 3 groups: (1) quality improvement efforts and performance monitoring (e.g., presence of a quality improvement team, partnering with community-based agencies to reduce readmission), (2) medication management (e.g. medication reconciliation and patient teaching), and (3) discharge and follow-up procedures (e.g., early outpatient follow-up, care transitions programs).74 The vast majority of hospitals have implemented multiple practices in these domains.74 Perhaps the most enlightening analysis of best practices is by Bradley et al, who recently described 6 strategies which were associated with a significant reduction in readmissions in a national hospital survey (Table 2).75 While the magnitude of readmission reduction for each of these strategies was small, their combined effect may constitute a meaningful difference, and would be an appropriate place to focus readmission reduction efforts.
Table 2.
Six Effective Strategies to Reduce Readmissions in HF
| Strategy to Reduce Readmission | Estimated Absolute Reduction in Risk Standardized 30-Day Readmission Rates |
|---|---|
|
| |
| 1. Partnering with community physicians or physician groups | 0.33% |
| 2. Partnering with local hospitals to reduce readmissions | 0.34% |
| 3. Having nurses responsible for medication reconciliation | 0.18% |
| 4. Arranging follow-up appointments before discharge | 0.18% |
| 5. Having a process in place to send all discharge paper or electronic summaries directly to the patient’s primary physician | 0.21% |
| 6. Assigning staff to follow up on test results that return after the patient is discharged. | 0.26% |
Data from Circ Cardiovasc Qual Outcomes75.
STAGE D HF: REFRACTORY END-STAGE HF
Stage D encompasses patients with refractory HF despite usual medical therapy, and often includes patients with recurrent hospitalizations. These patients experience daily life-limiting symptoms, and are unlikely to resume stable disease with continuation of stage C HF therapies. It is estimated that approximately 5% of the patients with HF are stage D.76 While an individual with stage D HF’s risk of death may vary according to their specific clinical characteristics, the estimated 1- and 5-year mortality in all stage D patients is 28%76 and 80%,4 respectively. In recent years, therapeutic options for patients with stage D HF have increased. However, not all options are medically appropriate for all patients, and some therapies may not be in alignment with an individual’s goals and preferences. Therefore, there has been a recognized need to promote shared decision making and improved patient-provider communication around potential options in stage D patients.77 Referral to an advanced HF provider should be considered at any time when questions arise in the management of patients with HF, but particularly when having difficulty managing a patient’s HF symptoms, when a patient is unable to tolerate HF-related medications such as beta blockers, with complicated or recurrent HF hospitalizations, or when a provider believes that MCS and cardiac transplant should be considered (Table 3).
Table 3.
When to Consider a Patient for Cardiac Transplantation or LVAD as Destination Therapy
| Indications | Contraindications |
|---|---|
| Heart Transplant | |
|
|
| LVAD as Destination Therapyc | |
|
|
LVAD= left ventricular assist device
Indicates a relative contraindication
LVAD as bridge to transplant (BTT) may be used in any patient listed for heart transplantation felt to benefit. The LVAD is removed at the time of heart transplantation. A total artificial heart may be considered in a patient awaiting heart transplant who needs MCS but has very poor RV function
Mechanical Circulatory Support Is an Efficacious Therapy for Selected Patients with Stage D HF
We are in the midst of a rapid evolution in our ability to care for patients with advanced HF due to the introduction and improvement in MCS. Until recently, cardiac replacement therapy was limited to orthotopic heart transplant. While an efficacious therapy, organ supply is limited having remained around 2200 heart transplants per year in the United States, with most organs allocated to younger patients with limited comorbidities. In the past two decades, MCS devices have gotten smaller with a lower rate of complications. The LVADs used most frequently now provide continuous flow from the left ventricle through the pump and into the aorta. They are quite durable and have enabled patients who are not candidates for cardiac transplantation to be implanted with an LVAD to remain in situ until death (“destination therapy”, DT). In addition, they have also allowed patients who are awaiting heart transplantation to reap the benefits of improvement in HF symptoms and quality of life until suitable organs become available (“bridge to transplant”, BTT).
As with any therapy, there are risks and benefits with MCS. While survival (1-year survival 68% vs. 25%, respectively78,79) and quality of life are both improved in patients with advanced HF treated with MCS compared with medical therapy, complications are common. In patients with LVAD implanted as DT, both device related infection (incidence 8.01 per 100 patient months) and stroke (incidence 0.13 per patient-year ) are still very common.79 The most common reason for readmission after LVAD is gastrointestinal bleeding,80 and a growing body of evidence has implicated acquired Von Willebrand Factor deficiency with a loss of large von Willebrand multimers.81,82 Severe device malfunction requiring pump exchange is rare (incidence 0.06 per patient-year).79 It is estimated that only 30% of MCS patients are free from any adverse event (infection, bleeding, device malfunction, stroke, or death) within the first year of implantation.83 Furthermore, nearly all patients require one or more readmissions early after implantation, with an average of 2 readmissions in the first 6 months.80,84
As our experience in managing patients with LVADs grows, we learn more about optimal strategies for follow-up. Echocardiographic monitoring is an important component of the longitudinal care of LVAD recipients, and there is a growing body of literature advising us on both normal and abnormal echocardiographic values post-LVAD (Table 4).85–87
Table 4.
Echocardiographic Parameters in Patients with Left Ventricular Assist Devices
Changes in Echocardiographic Variables Postoperatively in Patients with Normal LVAD Function
|
While the cost effectiveness has improved with the current continuous flow LVADs, costs remain high ($198,184 per quality adjusted life year88) compared with other cardiac device-related therapies such as CRT.89 The cost effectiveness may continue to improve if the cost of devices declines and as we continue to achieve better patient outcomes. Attaining the best patient outcomes requires both optimal patient selection and appropriate timing of implantation along the HF trajectory. As device technology improves, we may be able to offer MCS to “less sick” patients with advanced HF,90 while still improving their outcomes compared with usual medical therapy. In addition, while LVADs have historically been used as a treatment for patients with dilated and ischemic cardiomyopathy, some centers are implanting LVADs in selected patients with hypertrophic and restrictive cardiomyopathy.91
MCS technology continues to advance and evolve.92 Implantable miniature pumps, such as the Circulite Synergy,93 are being developed that may provide long-term partial support (less liters per minute than the current LVADs), but have the benefit of not requiring cardiopulmonary bypass or a sternotomy for implantation. Thoratec’s HeartMate III has a compact design but can still provide full support (up to 10 liters per minute). Biventricular support is already being provided in some patients with the total artificial heart, a pump that is implanted with removal of both ventricles and the majority of both atria. Syncardia’s total artificial heart has been approved as bridge to transplant therapy since 2001, and more recently as compassionate use in DT patients by the Food and Drug Administration. Furthermore, there have been case reports of successful use of continuous flow pumps in both ventricles.94,95
All Patients with Advanced HF Should Participate in Advanced Care Planning
The highest rate of hospitalizations and cumulative resource utilization in patients with HF occurs at the end of life.96,97 This is despite the fact that the vast majority of patients with chronic conditions say they would want to avoid hospitalization as they near death.98 There are numerous models available to predict death and readmission in HF, with the Seattle HF model99 being the most widely used in clinical practice. While models are imperfect at predicting outcomes for an individual, they are generally more accurate than clinical judgment, which tends to overestimate prognosis. Combining risk prediction models with adaptation based on clinical knowledge of an individual’s situation may be the best approach to providing accurate individualized risk prediction.77 As HF is a clinical syndrome that often follows an unpredictable trajectory, it is important for providers to periodically review HF patients’ preferences for care in the case of both expected and unexpected occurrences. Advanced directives, an important component of advanced care planning and documentation of wishes regarding care, have only been completed in 41% of patients with HF,100 and should ideally be completed and revised as needed after discussion between patients, providers, and families. Palliative care, whose aim is to improve quality of life and support patients’ and families as they deal with chronic and complex illnesses,101 is associated with improved patient and family satisfaction and decreased healthcare utilization and costs102,103 and should be considered as an option in patients with stage D HF. In addition to enhancing transitions to end of life care and hospice when appropriate, palliative medicine can also help with preparedness planning prior to use of advanced therapies such as LVAD104 and transplant.
Conclusion
Since 2010, while there have been no revolutionary new therapies to treat HF, there have been ongoing advances in our ability to diagnose and treat patients with HF. In particular, the ongoing improvement in MCS has offered some patients with end-stage HF new life-prolonging options. As the prevalence of HF has continued to rise, moving forward it will be important to remain committed to searching for effective ways to prevent the development of active HF in patients with stage A and B HF, and to finding efficacious therapies to treat the growing number of patients with HFpEF.
Take Home Points.
HF is becoming more common and associated with rising costs of care
Treatment of patients at risk for developing HF (stage A) should be aimed at controlling modifiable risk factors
Patients with structural heart abnormalities but no clinical symptoms of HF (stage B) are 3–4 times more common than patients with a clinical diagnosis of HF (stage C and D).
Referral to an advanced HF provider should be considered at any time when questions arise in the diagnosis and management of patients with HF, but particularly when having difficulty managing a patient’s HF symptoms, when a patient is unable to tolerate HF-related medications such as beta blockers, with complicated or recurrent HF hospitalizations, or when mechanical circulatory support and cardiac transplant may be an option.
ABBREVIATIONS
- ACCF
American College of Cardiology Foundation
- ACE-I
angiotensin converting enzyme inhibitor
- ADHF
acute decompensated HF
- AHA
American Heart Association
- ARB
angiotensin receptor blocker
- DCM
dilated cardiomyopathy
- HF
heart failure
- LVAD
left ventricular assist device
- MCS
mechanical circulatory support
- HFpEF
heart failure with preserved EF
- HFrEF
heart failure with reduced EF
Footnotes
Financial Support and Disclosures: Dr. Dunlay is supported by an NHLBI Career Development Award (1K23 HL116643). The authors have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25(18):1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Ramani GV, Uber PA, Mehra MR. Chronic heart failure: contemporary diagnosis and management. Mayo Clin Proceed. 2010;85(2):180–195. doi: 10.4065/mcp.2009.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12):1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 5.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1(2):125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5(4):422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57(16):1641–1649. doi: 10.1016/j.jacc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershberger RE, Cowan J, Morales A, Siegfried JD. Progress with genetic cardiomyopathies: screening, counseling, and testing in dilated, hypertrophic, and arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Heart Fail. 2009;2(3):253–261. doi: 10.1161/CIRCHEARTFAILURE.108.817346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. New Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 11.Sciarretta S, Palano F, Tocci G, Baldini R, Volpe M. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med. 2011;171(5):384–394. doi: 10.1001/archinternmed.2010.427. [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 13.Frigerio M, Oliva F, Turazza FM, Bonow RO. Prevention and management of chronic heart failure in management of asymptomatic patients. Am J Cardiol. 2003;91(9A):4F–9F. doi: 10.1016/s0002-9149(02)03335-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138(11):907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kuznetsova T, Herbots L, Lopez B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2(2):105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 16.Belham M, Kruger A, Mepham S, Faganello G, Pritchard C. Monitoring left ventricular function in adults receiving anthracycline-containing chemotherapy. Eur J Heart Fail. 2007;9(4):409–414. doi: 10.1016/j.ejheart.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010:10337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nuclear Cardiol. 2012;19(2):377–388. doi: 10.1007/s12350-012-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? J Am Coll Cardiol. 2010;56(20):1644–1650. doi: 10.1016/j.jacc.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 21.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bovelli D, Plataniotis G, Roila F. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncology. 2010;21(Suppl):5v277–282. doi: 10.1093/annonc/mdq200. [DOI] [PubMed] [Google Scholar]
- 23.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaggin HK, Motiwala S, Bhardwaj A, Parks KA, Januzzi JL., Jr Soluble Concentrations of the Interleukin Receptor Family Member ST2 and Beta Blocker Therapy in Chronic Heart Failure. Circ Heart Fail. 2013;6(6):1206–1213. doi: 10.1161/CIRCHEARTFAILURE.113.000457. [DOI] [PubMed] [Google Scholar]
- 25.Lassus JP, Nieminen MS, Peuhkurinen K, et al. Markers of renal function and acute kidney injury in acute heart failure: definitions and impact on outcomes of the cardiorenal syndrome. Eur Heart J. 2010;31(22):2791–2798. doi: 10.1093/eurheartj/ehq293. [DOI] [PubMed] [Google Scholar]
- 26.Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol. 2010;56(25):2090–2100. doi: 10.1016/j.jacc.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Shah MR, Califf RM, Nohria A, et al. The STARBRITE trial: a randomized, pilot study of B-type natriuretic peptide-guided therapy in patients with advanced heart failure. J Card Fail. 2011;17(8):613–621. doi: 10.1016/j.cardfail.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Felker GM, Hasselblad V, Hernandez AF, O’Connor CM. Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J. 2009;158(3):422–430. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med. 2010;170(6):507–514. doi: 10.1001/archinternmed.2010.35. [DOI] [PubMed] [Google Scholar]
- 30.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 31.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119(24):3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296(18):2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 33.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1(2):91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA. 2012;308(20):2108–2117. doi: 10.1001/jama.2012.14785. [DOI] [PubMed] [Google Scholar]
- 35.Kitzman DW, Hundley WG, Brubaker PH, et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010;3(4):477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. New Engl J Med. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 37.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 38.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF) J Card Fail. 2011;17(8):634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Verloop WL, Beeftink MM, Nap A, et al. Renal denervation in heart failure with normal left ventricular ejection fraction. Rationale and design of the DIASTOLE (DenervatIon of the renAl Sympathetic nerves in hearT failure with nOrmal Lv Ejection fraction) trial. Eur J Heart Fail. 2013 doi: 10.1093/eurjhf/hft119. [DOI] [PubMed] [Google Scholar]
- 41.Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 42.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 43.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3(6):659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. New Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 45.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez AF, Mi X, Hammill BG, et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308(20):2097–2107. doi: 10.1001/jama.2012.14795. [DOI] [PubMed] [Google Scholar]
- 47.Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. [corrected] Circulation. 2012;126(14):1784–1800. doi: 10.1161/CIR.0b013e3182618569. [DOI] [PubMed] [Google Scholar]
- 48.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52(23):1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 49.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. New Engl J Med. 2009;361(14):1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 50.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. New Engl J Med. 2010;363(25):2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 51.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008;121(8):726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52(10):818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 53.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New Engl J Med. 2009;361(25):2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 54.Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51(2):103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 55.Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50(17):1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 56.Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. New Engl J Med. 2013;368(13):1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 57.Fiuzat M, Neely ML, Starr AZ, et al. Association between adrenergic receptor genotypes and beta-blocker dose in heart failure patients: analysis from the HF-ACTION DNA substudy. Eur J Heart Fail. 2013;15(3):258–266. doi: 10.1093/eurjhf/hfs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira NL, Weinshilboum RM. The impact of pharmacogenomics on the management of cardiac disease. Clin Pharmacol Therapeutics. 2011;90(4):493–495. doi: 10.1038/clpt.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122(19):1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 60.Collins SP, Pang PS, Fonarow GC, Yancy CW, Bonow RO, Gheorghiade M. Is hospital admission for heart failure really necessary?: the role of the emergency department and observation unit in preventing hospitalization and rehospitalization. J Am Coll Cardiol. 2013;61(2):121–126. doi: 10.1016/j.jacc.2012.08.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen LA, Turer AT, Dewald T, Stough WG, Cotter G, O’Connor CM. Continuous versus bolus dosing of Furosemide for patients hospitalized for heart failure. Am J Cardiol. 2010;105(12):1794–1797. doi: 10.1016/j.amjcard.2010.01.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail. 2010;16(3):188–193. doi: 10.1016/j.cardfail.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Salvador DR, Rey NR, Ramos GC, Punzalan FE. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochr Syst Rev. 2005;(3):CD003178. doi: 10.1002/14651858.CD003178.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. New Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bart BA, Goldsmith SR, Lee KL, et al. Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS-HF, for the Heart Failure Clinical Research Network. J Card Fail. 2012;18(3):176–182. doi: 10.1016/j.cardfail.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. 2010;16(12):922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 67.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. New Engl J Med. 2000;343(4):246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 69.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 70.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111(12):1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 71.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. New Engl J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 72.Eapen ZJ, Reed SD, Curtis LH, Hernandez AF, Peterson ED. Do heart failure disease management programs make financial sense under a bundled payment system? Am Heart J. 2011;161(5):916–922. doi: 10.1016/j.ahj.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 73.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. New Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 74.Bradley EH, Curry L, Horwitz LI, et al. Contemporary evidence about hospital strategies for reducing 30-day readmissions: a national study. J Am Coll Cardiol. 2012;60(7):607–614. doi: 10.1016/j.jacc.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6(4):444–450. doi: 10.1161/CIRCOUTCOMES.111.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM) Am Heart J. 2008;155(2):339–347. doi: 10.1016/j.ahj.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 77.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125(15):1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. New Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 79.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. New Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 80.Hasin T, Marmor Y, Kremers W, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61(2):153–163. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 81.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90(4):1263–1269. doi: 10.1016/j.athoracsur.2010.04.099. discussion 1269. [DOI] [PubMed] [Google Scholar]
- 82.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56(15):1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Forest SJ, Bello R, Friedmann P, et al. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95(4):1276–1281. doi: 10.1016/j.athoracsur.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 85.Fine NM, Topilsky Y, Oh JK, et al. Role of Echocardiography in Patients With Intravascular Hemolysis Due to Suspected Continuous-Flow LVAD Thrombosis. JACC Cardiovasc Imaging. 2013 doi: 10.1016/j.jcmg.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 86.Topilsky Y, Hasin T, Oh JK, et al. Echocardiographic variables after left ventricular assist device implantation associated with adverse outcome. Circ Cardiovasc Imaging. 2011;4(6):648–661. doi: 10.1161/CIRCIMAGING.111.965335. [DOI] [PubMed] [Google Scholar]
- 87.Topilsky Y, Oh JK, Atchison FW, et al. Echocardiographic findings in stable outpatients with properly functioning HeartMate II left ventricular assist devices. J Am Soc Echocardiogr. 2011;24(2):157–169. doi: 10.1016/j.echo.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 88.Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail. 2012;5(1):10–16. doi: 10.1161/CIRCHEARTFAILURE.111.962951. [DOI] [PubMed] [Google Scholar]
- 89.Noyes K, Veazie P, Hall WJ, et al. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol. 2013;24(1):66–74. doi: 10.1111/j.1540-8167.2012.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeevanandam V. Are we ready to implant left ventricular assist devices in “less sick” patients? Sem Cardiothorac Surg. 2012;24(1):8–10. doi: 10.1053/j.semtcvs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Topilsky Y, Pereira NL, Shah DK, et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. 2011;4(3):266–275. doi: 10.1161/CIRCHEARTFAILURE.110.959288. [DOI] [PubMed] [Google Scholar]
- 92.Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: patients and technology in evolution. Circulation. 2012;125(10):1304–1315. doi: 10.1161/CIRCULATIONAHA.111.060830. [DOI] [PubMed] [Google Scholar]
- 93.Meyns B, Klotz S, Simon A, et al. Proof of concept: hemodynamic response to long-term partial ventricular support with the synergy pocket micro-pump. J Am Coll Cardiol. 2009;54(1):79–86. doi: 10.1016/j.jacc.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 94.Saito S, Sakaguchi T, Miyagawa S, et al. Biventricular support using implantable continuous-flow ventricular assist devices. J Heart Lung Transplant. 2011;30(4):475–478. doi: 10.1016/j.healun.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 95.Hetzer R, Krabatsch T, Stepanenko A, Hennig E, Potapov EV. Long-term biventricular support with the heartware implantable continuous flow pump. J Heart Lung Transplant. 2010;29(7):822–824. doi: 10.1016/j.healun.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 97.Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ESF . The Dartmouth Atlas of Health Care. In: KKB, editor. End of Life Care. 2007. [Google Scholar]
- 99.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113(11):1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 100.Dunlay SM, Swetz KM, Mueller PS, Roger VL. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5(3):283–289. doi: 10.1161/CIRCOUTCOMES.112.966036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE. Palliative care in the treatment of advanced heart failure. Circulation. 2009;120(25):2597–2606. doi: 10.1161/CIRCULATIONAHA.109.869123. [DOI] [PubMed] [Google Scholar]
- 102.Whitford K, Shah ND, Moriarty J, Branda M, Thorsteinsdottir B. Impact of a Palliative Care Consult Service. Am J Hospice Palliative Care. 2013 doi: 10.1177/1049909113482746. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 103.Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 104.Swetz KM, Kamal AH, Matlock DD, et al. Preparedness Planning Before Mechanical Circulatory Support: A “How-To” Guide for Palliative Medicine Clinicians. J Pain Symptom Manage. 2013 doi: 10.1016/j.jpainsymman.2013.06.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]