Abstract
We evaluated the anti-oxidant role of peroxiredoxin 6 (Prdx6) in primary lung alveolar epithelial type II cells (AEC II) that were isolated from wild-type, Prdx6−/−, or Prdx6 transgenic overexpressing (Tg) mice and exposed to H2O2 at 50µM - 500µM for 1-24 hours. Expression of Prdx6 in Tg AEC II was 7-fold greater than wild type. Prdx6 null AEC II exposed to H2O2 showed concentration-dependent cytotoxicity indicated by decreased “live/dead” cell ratio, increased propidium iodide staining, increased annexin V binding, increased DNA fragmentation by TUNEL assay, and increased lipid peroxidation by diphenylpyrenylphosphine (DPPP) fluorescence. Compared to Prdx6 null cells, oxidant mediated damage was significantly less in wild type AEC II and was least in Prdx6 Tg cells. Thus, Prdx6 functions as an anti-oxidant enzyme in mouse AEC II. Prdx6 has been shown previously to reduce phospholipid hydroperoxides and we postulate that this activity is a major mechanism for the effectiveness of Prdx6 as an anti-oxidant enzyme.
Keywords: cell viability, apoptosis, lipid peroxidation, diphenylpyrenylphosphine, TUNEL, Annexin V
Introduction
Oxidative stress is widely recognized as a major pathophysiologic mechanism in various injuries that lead to either acute or chronic organ failure. This stress is characterized by the increased production of reactive oxygen species (ROS) such as superoxide radical, H2O2, and hydroxyl radical, which can result in oxidation of macromolecules including nucleic acids, lipids, and proteins [Chow et al., 2003; Fisher et al., 1984; Lang et al., 2002]. The lung is particularly susceptible to oxidative damage caused either by direct contact with oxidants in ambient air or by blood borne oxidants delivered through its extensive capillary perfusion [Fisher et al., 1984; Lang et al., 2002] . A broad spectrum of disease states has been attributed to oxidative stress in the lung including sepsis-induced acute lung injury (ALI) and chronic obstructive pulmonary disease (COPD) associated with smoking of cigarettes [Lang et al., 2002; Rahman and Adcock, 2006].
To protect against the harmful consequences of oxidative stress, cells express a number of antioxidant enzymes including superoxide dismutases (SOD), glutathione (GSH) peroxidases (GPx), catalase, and peroxiredoxins, as well as non-enzymatic antioxidants such as GSH [Forman and Fisher, 1981]. The peroxiredoxins (Prdx), a recent addition to the list of antioxidant enzymes, use cysteine as the catalytic center rather than the selenocysteine that characterizes the GPx enzymes [Rhee et al., 2005; Wood et al., 2003]. These enzymes have been classified as a 1-cys or 2-cys according to the number of conserved cysteine residues that function in catalysis [Rhee et al., 2005; Wood et al., 2003]. The 2-cys Prdxs generally use thioredoxin as a co-factor to reduce H2O2 and organic hydroperoxides [Rhee et al., 2005; Wood et al., 2003]. Prdx6 is a 1-cys enzyme that utilizes GSH as the physiological reductant to reduce H2O2 and organic hydroperoxides. Prdx6 is the only one of the six mammalian peroxiredoxins reported so far with the ability to reduce phospholipid hydroperoxides (PLOOH) at a significant rate [Fisher et al., 1999; Manevich et al., 2004], although this oxidation product also can be reduced by phospholipid hydroperoxide GPx (also called GPx4) [Ursini et al., 1995].
The role of Prdx 6 and other anti-oxidant enzymes in protecting the lung against oxidant injury has been evaluated in cell and animal models by manipulation of their expression levels. These studies include gain and loss of function for SOD 1 and 2 [Asikainen et al., 2002; Danel et al., 1998; Folz et al., 1999; Ho, 2002; Ho et al., 1998], GPx1 [Ho, 2002; Ho et al., 1998], and catalase [Danel et al., 1998]. These studies have given ambiguous results for their role in anti-oxidant defense. Similar studies have been carried out more recently with Prdx6 [Manevich et al., 2002; Pak et al., 2002; Wang et al., 2003; Wang et al., 2004a; Wang et al., 2006a; Wang et al., 2004b; Wang et al., 2006b]. Overexpression of Prdx6 protein in human lung derived NCI-H441 cells attenuated membrane phospholipid peroxidation and apoptosis when cells were subjected to .OH stress by Cu2+/ascorbate treatment [Manevich et al., 2002], while antisense-mediated decrease in expression of Prdx6 in L2 cells, a rat lung epithelial cell line, resulted in increased oxidant sensitivity and cell death [Pak et al., 2002]. Overexpression of Prdx6 in mouse lungs mediated by adenoviral transfection or by gene transfer techniques decreased oxidative stress and increased survival in hyperoxia [Wang et al., 2004b; Wang et al., 2006b], while loss of Prdx6 by gene inactivation resulted in increased lung injury and mouse mortality associated with hyperoxia or paraquat exposure [Wang et al., 2003; Wang et al., 2004a; Wang et al., 2006a]. These results indicate that Prdx6 plays an important role in defense of the lung against oxidative stress.
Prdx 6 is enriched in lung compared to other organs and is expressed at the highest levels in alveolar epithelial type II cells, bronchiolar Clara cells, and alveolar macrophages [Kim et al., 1998; Mo et al., 2003]. Alveolar type I and type II epithelial cells (AEC I and II) are major constituents among the 40 or more different cell types that comprise the lung. AEC I cover ~95% of the lung alveolar surface area and form the barrier that is important for gas exchange. AEC II account for ~15% of total lung cells and cover ~5% of the alveolar surface area. These latter cells play a critical role in maintaining lung function by production of surfactant, by their ability to proliferate to restore damaged epithelium, and by their contribution to host defense [Dobbs, 1990] . Because AEC II comprise a relatively small percentage of total lung cells, it is difficult to determine their response to oxidant stress through study of the whole lung. The response to oxidant stress of rat AEC II in primary culture has been studied previously by measuring dye exclusion, generation of thiobarbituric acid reactive substances (TBARS), MTT reduction, cytochrome c release, and propidium iodine staining [Hagen et al., 1986; Piotrowski et al., 2004; Yin et al., 2005]. In the present study, we investigate the role of Prdx6 in anti-oxidant defense using primary isolates of AEC II from lungs of wild type mice and those overexpressing and deficient in Prdx6.
Materials and methods
Animals
The use of animals for these studies was approved by the University of Pennsylvania Animal Care and Use Committee (IACUC). Three experimental groups of mice were studied: a C57BL/6 wild-type (WT) group (n=95), a homozygous Prdx6 −/− group (n=80) and a Prdx6 transgenic (Tg) group (n=82). The generation and genotyping of Prdx6 −/− and Tg Prdx6 mice has been described previously [Mo et al., 2003; Phelan et al., 2003; Wang et al., 2006b]; these mice were bred in the animal facility of the University of Pennsylvania and maintained under HEPA filtered air. The transgene for TgPrdx6 mice was a genomic clone containing the entire Prdx6 gene including 6644 bp of upstream sequence [Phelan et al., 2003]. WT mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice of either sex at 8-11 weeks of age and weighing 24-28g were used to isolate AEC II.
Isolation and culture of AEC II
AEC II were isolated by enzymatic digestion of mouse lungs as described previously [Bortnick et al., 2003]. Briefly, lungs were cleared of blood by perfusion through the pulmonary artery, instilled with 2 ml of dispase (50 U/ml), and incubated at room temperature for 45 min. Lung tissue was separated from large bronchi with forceps and transferred to a Petri dish containing 10 ml DMEM with 0.01% DNase I for 10 min incubation at 37°C; subsequently, the cells were filtered sequentially through 100-, 35-, and 17-µm nylon mesh. The cell pellet was resuspended in 10 ml DMEM, plated on mouse IgG (0.75 mg/ml)-coated Petri dishes, and incubated at 37°C for 1 h to remove adherent macrophages. The non-adherent cells were centrifuged, resuspended in DMEM with 10% FBS, and seeded on tissue culture plastic dishes (Techno Plastic, Trasadingen, Switzerland) for a 1 h incubation at 37°C to remove adherent fibroblasts. The remaining cells were resuspended in Ham's F-12 culture medium supplemented with 15 mM HEPES, 0.8 mM CaCl2, 0.25% BSA, 5 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml sodium selenite, and 4% mouse serum and seeded on 35 mm type I collagen-coated cell culture dishes (BD Biosciences, Franklin Lakes, NJ). After 36 h, cells were washed and medium was replaced with serum-free medium in order to maintain the AEC II phenotype. Dishes at 48 h of culture contained 105-106 AEC II and were used for experiments.
Identification of AEC II
AEC II were identified by staining with modified Nile red (nile blue A oxazone, Sigma, St. Louis, MO), a lipophilic fluorescent dye that can label lipid-rich areas and is used to identify lamellar bodies, the organelle that is characteristic of these cells [Bates et al., 2002; Bortnick et al., 2003; Dobbs, 1990]. Nile red, dissolved in dimethylsulfoxide (DMSO), was added at 1:40 to unfixed cells in PBS and swirled in the dish for 10 min at room temperature to label the cells; the dishes were washed with medium and examined using the Rhodamine filter of the fluorescence microscope (Nikon Eclipse TE 2000-U, Nikon, El Segundo, CA).
Cell viability and apoptosis
Cell viability was detected by the live/dead cell viability and cytotoxicity kit (Molecular Probes, Eugene, OR). Live cells were determined by green fluorescence (ex/em ~495nm/~515nm) obtained by enzymatic conversion of non-fluorescent calcein AM to calcein. Dead cells were indicated by red fluorescence (ex/em ~495nm/~635nm) of ethidium homodimer (EthD-1) which can enter cells through damaged membranes and binds to nucleic acids. AEC II were incubated with 0.5 µM calcein AM, plus 2 µM EthD-1 for 20 mins at 37°C and examined by fluorescence microscopy [Muro et al., 2003].
Apoptosis was evaluated with the ApoAlert Apo 2.7/Annexin V - EGFP kit (Clontech, Mountain View, CA) [Aubry et al., 1999; Martin et al., 1995] Cells that bind annexin V show green staining of the plasma membrane; the red fluorescence of propidium iodide (PI) indicates altered cell membrane permeability. AEC II were incubated with 5 μl Annexin V and 10 μl PI in 200 μl Binding buffer with gentle shaking at room temperature for 10 min in the dark and images were obtained by fluorescence microscopy. For each experiment, the percentage of cells positive for annexin or PI staining was counted for ~1000 cells in 5-6 randomly chosen fields per dish.
Apoptosis also was detected and quantified by fluorescence using the In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) for Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) [Labat-Moleur et al., 1998; Pak et al., 2002] . ATII cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice, and incubated with TUNEL reaction mixture (enzyme and label solutions) in a humidified atmosphere for 60 min at 37°C in the dark. Double-stranded DNA in nuclei was counterstained after TUNEL staining with 4’6’ diaminido -2- phenylindole dihydrochloride (DAPI, 1 ul/ml). Cells were evaluated by fluorescence microscopy (ex/em~495nm/~515 nm); for each experiment, the percentage of TUNEL positive cells was determined by counting ~500 cells per dish from 5-6 randomly chosen fields. For TUNEL positive control staining, the fixed permeabilized ATII cells were incubated with recombinant DNase I (2 U/ml) for 10 min to induce DNA strand breaks. For TUNEL negative control staining, the processed ATII cells were incubated with the labeling solution of the TUNEL reaction mixture minus the enzyme.
Prdx6 protein expression and enzymatic activity
To measure Prdx6 protein content and enzymatic activity, AEC II were treated with lysis buffer (50 mM Tris, 0.1% SDS or 1% NP-40), shaken for 10 mins at 4°C in the presence of Complete Protease Inhibitor Cocktail (Boehringer Mannheim, Indianapolis, IN), and centrifuged at 12,000 x g for 5 min. The supernatant was analyzed for protein concentration by the binding of Coomassie blue (Bio-Rad, Richmond, CA) using bovine gamma globulin as the standard. Samples (20 ug protein per lane) were subjected to 12% SDS-PAGE using a Bio-Rad electrophoresis apparatus (Bio-Rad) and then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). Membranes were incubated for 1 h with Odyssey Blocking Buffer Q diluted 1:1 in PBS, and then probed with polyclonal antibodies to Prdx6 peptide and β-actin followed by fluorescently-labeled (IRDye TM800) affinity purified anti-rabbit IgG secondary antibody (Rockland, Gilbertsville, PA) (1:5000). The membrane was scanned with an OdysseyÔ two-color scanner (LI-COR Biosciences, Lincoln, Nebraska) and protein band density was measured using Odyssey software.
Peroxidase activity was measured in cell sonicates by the decrease in NADPH fluorescence in the presence of aminotriazole (20 mM, to inhibit catalase), reduced glutathione (GSH), an NADPH regenerating system, and 0.66 mM H2O2 or 0.1 mM phospholipid hydroperoxide substrate as previously described; the phospholipid hydroperoxide substrate was prepared by incubation of 1-palmitoyl, 2-linolenoyl sn-phosphorylcholine (PLPC) with lipoxygenase to yield the oxidized product (PLPCOOH) [Fisher et al., 1999].
DPPP detection of cell membrane lipid peroxidation
Diphenyl-1- pyrenylphosphine (DPPP) is a fluorescent probe for detection of cell membrane lipid peroxidation [Matot et al., 2003; Okimotoa et al., 2000]. DPPP is nonfluorescent, but becomes fluorescent when oxidized. DPPP (Dojindo, Gaithersburg, MD) was solubilized in DMSO and stored at −20°C. Storage and all subsequent procedures with DPPP were carried out in the dark. AEC were labeled by incubation at 37° for 10 min with a micellar suspension of DPPP (400 nmol/ml) and washed twice with PBS. DPPP-labeled AEC II from WT, Prdx6−/− or Tg Prdx6 mice then were treated with 500 µM H2O2 for various times as indicated and imaged by fluorescence microscopy (351 nm excitation, 380 nm emission).
DPPP fluorescence of cells also was determined with a PTI spectrofluorometer (Photon Technology Int., Bricktown, NJ) equipped with a single photon counting system. The DPPP-labeled cells were detached from the culture dish with trypsin-EDTA, resuspended in phosphate-buffered saline by gentle swirling in a standard quartz cuvette, and kept on ice until assayed. After recording the initial emission spectrum, the cell suspension was excited at 351 nm (1 nm slit) and fluorescence was measured continuously at 380 nm (3nm slit) over the subsequent 60 sec. The total number of cells in the cuvette was counted using a hemocytometer (Hausser Scientific, Horsham, PA). Fluorescence intensity for DPPP was expressed as the average value during 60 sec and normalized to the total number of cells [Manevich et al., 2002].
Statistical Analysis
Data are expressed as mean ± SEM for 3 or more independent experiments. Statistical significance was assessed with SigmaStat software 9.0/3.1 (Jandel Scientific, San Jose, CA). Group differences were evaluated by one-way ANOVA followed by Bonferroni’s test or by Student’s t test as appropriate. Differences between mean values were considered statistically significant at P<0.05.
Results
Identification of AEC II and Prdx6 protein expression
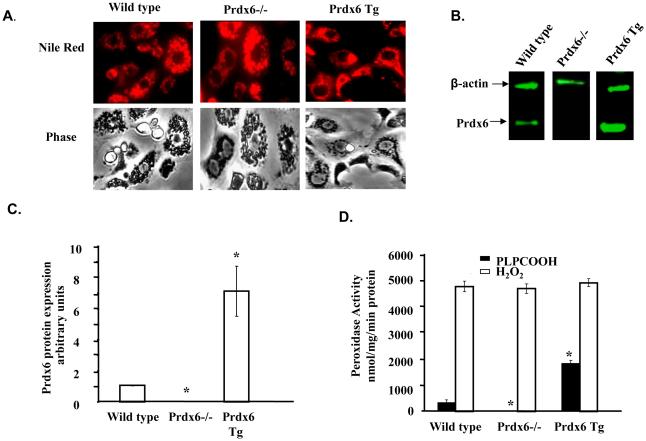
AEC II isolated from WT, Prdx6−/− and Tg Prdx6 mice showed similar morphology and a similar purity by Nile red staining of ~95% (Fig. 1A). We have shown previously that Prdx6 −/− mice as expected do not express Prdx6 message or protein [Mo et al., 2003; Wang et al., 2004a] and this was confirmed for protein expression with AEC II from Prdx6 −/− mice (Fig. 1B). For AEC II from Tg Prdx6 mice, Prdx 6 protein expression by western blot was ~ 7-fold greater as compared to WT mice (Fig 1B, C). Peroxidase activity in sonicated cells when measured with H2O2 substrate showed no significant differences among AEC II from wild type and Prdx6 knock-out or overexpressing mice (Fig. 1D). This indicates that other enzymes (for example, GSH peroxidases, as catalase was inhibited by the presence of aminotriazole) are largely responsible for the degradation of H2O2 in these cells. Peroxidase activity with the phospholipid hydroperoxide substrate (PLPCOOH) was only 10% of the corresponding activity with H2O2 in wild type cells and this activity was abolished by knock-out of Prdx6 (Fig. 1D). This result is consistent with our previous observation that Prdx6 is the only peroxidase in the lung with the capacity to significantly degrade PLPCOOH [Wang et al., 2006a]. Peroxidase activity with PLPCOOH substrate in Tg AECII was increased 5-fold compared to wild type.
Figure 1. Identification of lung alveolar type II alveolar epithelial cells (AEC II).
AEC II were isolated from wild type (WT), Prdx6 null and Prdx6 Tg mice and maintained in culture for 36-48h. A. Fluorescence and phase images after treatment of AEC II with Nile red, a dye that localizes in the lamellar bodies. B. Western Blot following SDS/PAGE (20 mg AEC II protein per lane) using polyclonal antibody to Prdx6 followed by fluorescently-labeled secondary antibody. β-actin was used as a loading control. C. Quantitation of western blots for Prdx6 protein expression under basal conditions showing mean ± SE for n=3. Results were normalized to β-actin. Values were zero for Prdx6 −/−. D. Prdx6 peroxidase activity of sonicated AEC II cells with 660 μM H2O2 or 100 μM 1-palmitoyl-2-linolenoyl phosphatidylcholine hydroperoxide (PLPOOH) as substrate. Aminotriazole (20 mM) was added for assay with H2O2. The value for Prdx6 −/− cells with PLPCOOH substrate was zero. Values are means ± SE for n=3.* p<0.05 compared to wild type for the same substrate.
The change in cellular Prdx6 protein expression after 24 h of treatment with H2O2 at increasing concentrations (50-500 µM) was determined by western blotting (Fig 2A). This oxidant stress induced a dose-dependent increase in Prdx6 expression in both wild type and Prdx6 Tg AECII with an increase in expression levels by 75-100% with 500µM H2O2 treatment (Fig 2B). As Prdx6 −/− AEC did not express Prdx6, they were not evaluated for the effect of H2O2.
Figure 2. Prdx6 expression in wild type and Prdx6 transgenic (Tg) AEC II cells following treatment with H2O2.
A. Cells were treated for 24h with 0-500 μM H2O2 and evaluated by western blot using β-actin as a loading control. B. Quantitation of Western blots for Prdx6 protein expression in AEC II cells. The ratio of Prdx6 to β-actin for each H2O2 concentration was normalized to the respective wild type or Tg control (no H2O2 treatment). Results represent the mean ± SE for 3 experiments. *P<0.05 compared to zero point in the same group; †P<0.05 compared to wild type at the same H2O2 concentration.
Viability and apoptosis of AEC II with H2O2 treatment
Viability of AEC II was studied with calcein and ethidium staining (Fig. 3A). This allowed calculation of the % of “live” (viable) cells, or conversely the % of “dead” cells. The % of “dead” cells with treatment for 24 h varied with the concentration of H2O2 (Fig 3B). The percentage of viable AEC II (100 minus % of “dead” cells) after treatment with 50 µM H2O2 for 24 h was 67% for Prdx6−/− cells, 85% for WT cells, and 95% for Prdx6 Tg overexpressing cells. With 500 μM H2O2, viability decreased to ~50% for Prdx6 −/− cells but was essentially unchanged from control for the Prdx6 overexpressing AEC II (Fig. 3B).
Figure 3. Effect of H2O2 treatment on AEC II viability.
AEC II were treated with 0-500 μM H2O2 for 24 h. A. Fluorescence images with calcein AM to detect “live” cells (green) and ethidium homodimer to detect “dead” cells (red). The calcein stain is diffuse in the cytoplasm while the ethidium stain is concentrated in the nucleus. The scale bar is 100μm. Original magnification x 100. B. Percentage of “dead” cells from experiments shown in A determined from approximately 1,000 total cells in 15 microscopic fields. Values are means ± SE for n=3 separate experiments. * P<0.05 vs. the corresponding zero time value. † P<0.05 vs. wild-type at the same concentration of H2O2.
Staining of wild type AEC II for annexin V under control conditions showed <10% positive and this was not significantly changed with exposure to 50-250 µM H2O2 for 24 h (Fig 4A). However, the percent of annexin positive Prdx6−/−AEC II was 2-fold greater than control at 50 µM H2O2 and increased progressively with increasing H2O2 concentration (Fig. 4A). This result is compatible with the early stages of apoptosis due to oxidant stress. Prdx6 Tg cells were < 5% annexin positive under control conditions and increased only slightly with H2O2 exposure (Fig. 4A). At 500 µM H2O2, PI positive cells were ~ 45% in Prdx6−/−, 23% in WT, and 6% in Prdx6 Tg (Fig 4B). Uptake of PI reflects cell membrane damage indicating late apoptosis or necrotic cell death. These results for PI uptake (Fig. 4B) correlate well with the results for ethidium uptake (Fig. 3) as both reflect increased cell membrane permeability.
Figure 4. The effect of Prdx6 expression on AEC II apoptosis with H2O2 treatment.
AEC II from wild type, Prdx6 −/− and Prdx6 Tg mice were treated for 24 h with 0-500 μM H2O2 and fluorescence staining for cell membrane expression of annexin V, uptake of propidium iodide (PI), and DNA cleavage (TUNEL) were evaluated. Values are means ± SE for n=3-4 experiments. A. Percentage of AEC II positive for annexin V. B. Percentage of AEC II positive for PI uptake. C. Percentage of AEC II positive for TUNEL. * P<0.05 compared to zero point in the same group; #P<0.05 compared to wild type for the same concentration of H2O2; †P<0.05 for Tg vs. Prdx−/− for the same concentration of H2O2.
TUNEL staining was used to determine the effect of H2O2 exposure on DNA cleavage in ATII cells as another index of apoptosis. Under control conditions, the percentage of TUNEL positive cells was ~3% in wild type, 12% in Prdx6 −/− and <1% in Prdx6 Tg AECII (Fig. 4C). At 24 h exposure to H2O2, there was a concentration dependent increase of TUNEL positive cells in the wild type and Prdx6 −/− groups, while the Tg cells showed relatively small changes (Fig. 4C). At 500 μM H2O2, ~40% ATII cells in Prdx6 −/− group were TUNEL positive compared to 20% in the wild type and about 5% in Tg cells.
Lipid peroxidation with H2O2 treatment
DPPP was used as a fluorescent probe to monitor lipid peroxidation in cellular membranes as a more direct index of oxidative stress. AEC II isolated from wild type, Prdx6−/− and Tg Prdx6 mice were treated with 500 μM H2O2 for 1 or 2 h (Fig. 5). DPPP fluorescence was evaluated both by microscopy and by measurement in a fluorometer. Control cellular fluorescence after incubation with DPPP in wild type cells was relatively low but increased progressively with time during the H2O2 exposure (Fig. 5A). AEC II from Prdx6−/− mice showed significantly greater DPPP fluorescence compared to wild type at both time points. The greater change in DPPP fluorescence with the Prdx6 null cells was confirmed by measurements in the spectrofluorometer (Fig. 5B). The fluorescence signal from Tg and wild type cells appeared similar by microscopy (Fig 5A), although DPPP fluorescence by fluorometry indicated less lipid peroxidation in Prdx6 overexpressing cells (Fig 5B).
Figure 5.
The effect of Prdx6 on AEC II cellular membrane lipid peroxidation with H2O2 treatment as indicated by DPPP fluorescence. DPPP fluorescence was measured by microscopy (A) or by a spectrofluorometer (B). A. Fluorescence images of DPPP labeled AEC II from WT, Prdx6−/− and Prdx6 Tg mice after treatment with 500 μM H2O2 for either 1 or 2 h. The scale bar is 50 μm. Original magnification x 200. B. DPPP fluorescence measured by spectrofluorometry in AEC II cells from wild type, Prdx6−/−, and Prdx6 Tg mice. Cells were exposed to 500 μM H2O2 for 1 or 2 h. Values are means ± SE for n=3-4 experiments. * p<0.05 compared to control (zero H2O2) in the same group; † P<0.05 compared to wild type at the same time point.
Discussion
It is now recognized that ROS, including superoxide radicals and H2O2, are produced biologically by many different types of cells and can serve as signaling molecules [Thannickal and Fanburg, 2000]. However, ROS when generated at greater than normal concentrations are considered to be agents of cell injury [Chow et al., 2003; Fisher et al., 1984; Forman and Fisher, 1981; Lang et al., 2002] H2O2 exposure has been widely used as a model of cellular oxidant stress as its interactions can generate more potent oxidizing radicals. Enzymes that directly scavenge H2O2 include GPxs, catalase, and the Prdxs [Fisher et al., 1999; Forman and Fisher, 1981; Rhee et al., 1999]. We have extensively studied the anti-oxidant role of Prdx6, one of the six mammalian Prdxs [Manevich et al., 2002; Pak et al., 2002; Wang et al., 2004a; Wang et al., 2006a; Wang et al., 2004b; Wang et al., 2006b]. This enzyme differs from the other mammalian Prdxs in that it uses GSH following glutathionylation mediated by GSH S- transferase pi (πGST) as the physiological co-factor reductant [Manevich et al., 2004; Ralat et al., 2006]. Further, Prdx6 is able to directly reduce phospholipid hydroperoxides (PLOOH) to the less toxic alcohol [Fisher et al., 1999], a pathway that has been estimated as 104 times more efficient than the alternate pathway of PLA2-mediated hydrolysis followed by reduction of the fatty acyl hydroperoxide [Zhao et al., 2003]. Both isolated cell and intact mouse models with Prdx6 overexpression or deletion have demonstrated that this enzyme has a major role in anti-oxidant defense [Manevich et al., 2002; Pak et al., 2002; Wang et al., 2003; Wang et al., 2004a; Wang et al., 2006a; Wang et al., 2004b; Wang et al., 2006b].
AEC II are important for lung homeostasis as they are the cells primarily responsible for lung surfactant production and for repair of the alveolar epithelial surface associated with lung injury. Prdx6 is prominently expressed in AEC II cells [Kim et al., 1998]. However, the antioxidant role of Prdx6 in primary AEC II cells, or for that matter, in any primary cell, has not previously been reported. The present study with H2O2 as the oxidant stress revealed that AEC II from Prdx6 null mice are significantly more sensitive to injury compared to cells from wild type mice while AEC II from Tg Prdx6 overexpressing mice are relatively resistant. The increased resistance to H2O2 shown by Tg AEC II cells indicates that the overexpressed protein is targeted to the relevant compartment(s) for anti-oxidant protection. The induction of Prdx6 in Tg cells with H2O2 exposure (Fig. 2) is compatible with presence of the endogenous promoter in the >6000 base pairs of upstream sequence used to generate the overexpressing construct [Phelan et al., 2003]. Finally, these results suggest availability of sufficient πGST and GSH, necessary co-factor for the Prdx6 catalytic cycle [Manevich et al., 2004; Ralat et al., 2006].
Evidence for toxicity of H2O2 in the present studies was the decreased cell viability indicated by increased uptake of ethidium homodimer (Fig. 3) and propidium iodide (Fig. 4B), decreased uptake of calcein (Fig. 3), increased cellular binding of annexin V (Fig. 4A), increased DNA fragmentation indicated by TUNEL staining (Fig. 4C), and increased DPPP fluorescence indicating lipid peroxidation (Fig. 5). Uptake of ethidium and propidium iodide indicate increased permeability of the plasma membrane suggesting peroxidation of plasma membrane lipid components [Muro et al., 2003]. The increased annexin V binding indicates increased accessibility of phosphatidyl serine to the extracellular milieu [Aubry et al., 1999; Martin et al., 1995]; this change plus the positive TUNEL assay are compatible with cell death by apoptosis [Labat-Moleur et al., 1998]. Recent evidence implicates the endogenous production of cell membrane-derived lipid metabolites including ceramide or the aldehyde 4-hydroxy-2-nonenal (4-HNE) as influencing the signaling pathway for apoptosis and promoting cell death by attacking nucleophilic amino acids such as Cys, His, and Lys [Castillo et al., 2007; Esterbauer et al., 1991; Pettus et al., 2002; Ryter et al., 2007]. For each of the parameters that were evaluated, the response to H2O2 was greater with the Prdx6 null cells and was lessened with Prdx6 overexpression compared to wild type.
The mechanism for cellular toxicity of H2O2 is not clear. H2O2 itself is a mild oxidant although in the presence of O2−· and/or transition metals it can generate the very toxic ·OH [Forman and Fisher, 1981]. The latter is a powerful oxidant that can readily oxidize cellular proteins and lipids. H2O2 is also a signaling molecule and its effect on signaling cascades could contribute to its toxicity [Thannickal and Fanburg, 2000]. Thus, removal of excess H2O2 would be important for the prevention of cellular damage. However, the present results (Fig. 1) indicate that Prdx6 plays a minor role in H2O2 removal in the AEC II and suggest that the ability to reduce H2O2 is unlikely to be the mechanism for Prdx6-mediated protection against cell injury. We have suggested that reduction of phospholipid hydroperoxides may be the major role for Prdx6 in anti-oxidant protection [Manevich et al., 2002; Pak et al., 2002]. Evidence for lipid peroxidation due to H2O2 in the present study is the demonstration of increased DPPP fluorescence [Matot et al., 2003; Okimotoa et al., 2000]. Although DPPP reacts with oxidized lipids in solution, its fluorescence yield is considerably enhanced by reaction with oxidized membrane lipids [Okimotoa et al., 2000]. Also, oxidation of membrane phospholipids is a likely mechanism for the increased cell permeability and increased incidence of apoptosis observed with H2O2 treatment in the present study. We have previously obtained similar evidence for lipid peroxidation associated with oxidant stress and protection by Prdx6 in studies of cell lines [Manevich et al., 2002; Pak et al., 2002] and intact lungs [Wang et al., 2004a; Wang et al., 2006a; Wang et al., 2006b]. These results suggest that the ability of Prdx6 to reverse cell membrane lipid peroxidation may be the predominant mechanism for protecting cells from irreversible cell injury related to H2O2 treatment and other forms of oxidative stress.
Previous studies of oxidant stress have utilized models for varied expression of the classical antioxidant enzymes such as SODs, catalase, and GPxs [Asikainen et al., 2002; Danel et al., 1998; Folz et al., 1999; Ho, 2002; Ho et al., 1997; Ho et al., 1998]. Unlike the results for altered expression of Prdx6, studies of these other enzymes generally have yielded ambiguous results for their role in defense of the lung against oxidative stress. Thus, Prdx6 appears to be an especially important anti-oxidant enzyme and may play a critical role in the lung. While SOD can remove O2-. (thereby generating H2O2) and catalase and GPxs can reduce H2O2 to H2O, these enzymes forestall but are unable to reverse ROS-mediated damage. As described above, we have postulated that the basis for this special protective effect of Prdx6 is its ability to reduce PLOOH. Phospholipids are an important component of cell membranes and their oxidation to PLOOH can result in altered transport and other functions of the plasma membrane and/or disruption of cellular organelles. Like Prdx6, GPx4 has the ability to reduce PLOOH [Ursini et al., 1995]. However, we have found that peroxidase activity with PLOOH as substrate is reduced by 96% in Prdx6 null lung homogenate [Wang et al., 2004a] and was undetectable in Prdx6 null AEC II cells (Fig. 1). These results indicate that Prdx6 is mainly responsible for PLOOH reduction activity in lung and that GPx4 has at best minor role. Thus, we conclude that Prdx6 plays a unique role in protection of AEC II and the lung against oxidant stress.
Acknowledgements
We thank Ms. Susan Turbitt for typing the manuscript, Drs. Shelley Phelan, Beverly Paigen, and Xiasong Wang for providing breeding pairs of transgenic mice, Dr. Sandra Bates and Jian-Qin Tao for assistance with type II cell isolation, Dr. Yefim Manevich for peroxidase assays, and Kevin Yu for advice regarding microscopy. Grant support was provided by HL-65543 from the National Institutes of Health. Presented in part at the 2007 Experimental Biology Annual meeting in San Francisco, CA
References
- Asikainen TM, Huang TT, Taskinen E, Levonen AL, Carlson E, Lapatto R, Epstein CJ, Raivio KO. Increased sensitivity of homozygous Sod2 mutant mice to oxygen toxicity. Free Radic Biol Med. 2002;32:175–86. doi: 10.1016/s0891-5849(01)00776-6. [DOI] [PubMed] [Google Scholar]
- Aubry JP, Blaecke A, Lecoanet-Henchoz S, Jeannin P, Herbault N, Caron G, Moine V, Bonnefoy JY. Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry. 1999;37:197–204. doi: 10.1002/(sici)1097-0320(19991101)37:3<197::aid-cyto6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bates SR, Gonzales LW, Tao JQ, Rueckert P, Ballard PL, Fisher AB. Recovery of rat type II cell surfactant components during primary cell culture. Am J Physiol Lung Cell Mol Physiol. 2002;282:L267–76. doi: 10.1152/ajplung.00227.2001. [DOI] [PubMed] [Google Scholar]
- Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L869–78. doi: 10.1152/ajplung.00077.2003. [DOI] [PubMed] [Google Scholar]
- Castillo SS, Levy M, Wang C, Thaikoottathil JV, Khan E, Goldkorn T. Nitric oxide-enhanced caspase-3 and acidic sphingomyelinase interaction: a novel mechanism by which airway epithelial cells escape ceramide-induced apoptosis. Exp Cell Res. 2007;313:816–23. doi: 10.1016/j.yexcr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–31. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- Danel C, Erzurum SC, Prayssac P, Eissa NT, Crystal RG, Herve P, Baudet B, Mazmanian M, Lemarchand P. Gene therapy for oxidant injury-related diseases: adenovirus-mediated transfer of superoxide dismutase and catalase cDNAs protects against hyperoxia but not against ischemia-reperfusion lung injury. Hum Gene Ther. 1998;9:1487–96. doi: 10.1089/hum.1998.9.10-1487. [DOI] [PubMed] [Google Scholar]
- Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–47. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem. 1999;274:21326–34. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Forman HJ, Glass M. Mechanisms of pulmonary oxygen toxicity. Lung. 1984;162:255–9. doi: 10.1007/BF02715655. [DOI] [PubMed] [Google Scholar]
- Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–66. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H, Fisher AB. Antioxidant Defense. In: Gilbert DL, editor. Oxygen and Living Processes. Springer-Verlag; New York: 1981. pp. 235–249. [Google Scholar]
- Hagen TM, Brown LA, Jones DP. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986;35:4537–42. doi: 10.1016/0006-2952(86)90776-8. [DOI] [PubMed] [Google Scholar]
- Ho YS. Transgenic and knockout models for studying the role of lung antioxidant enzymes in defense against hyperoxia. Am J Respir Crit Care Med. 2002;166:S51–6. doi: 10.1164/rccm.2206017. [DOI] [PubMed] [Google Scholar]
- Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–51. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- Ho YS, Vincent R, Dey MS, Slot JW, Crapo JD. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am J Respir Cell Mol Biol. 1998;18:538–47. doi: 10.1165/ajrcmb.18.4.2959. [DOI] [PubMed] [Google Scholar]
- Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB. Cloning and expression of rat lung acidic Ca(2+)-independent PLA2 and its organ distribution. Am J Physiol. 1998;274:L750–61. doi: 10.1152/ajplung.1998.274.5.L750. [DOI] [PubMed] [Google Scholar]
- Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Brambilla E, Negoescu A. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J Histochem Cytochem. 1998;46:327–34. doi: 10.1177/002215549804600306. [DOI] [PubMed] [Google Scholar]
- Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A. 2004;101:3780–5. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci U S A. 2002;99:11599–604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matot I, Manevich Y, Al-Mehdi AB, Song C, Fisher AB. Fluorescence imaging of lipid peroxidation in isolated rat lungs during nonhypoxic lung ischemia. Free Radic Biol Med. 2003;34:785–90. doi: 10.1016/s0891-5849(02)01435-1. [DOI] [PubMed] [Google Scholar]
- Mo Y, Feinstein SI, Manevich Y, Zhang Q, Lu L, Ho YS, Fisher AB. 1-Cys peroxiredoxin knock-out mice express mRNA but not protein for a highly related intronless gene. FEBS Lett. 2003;555:192–8. doi: 10.1016/s0014-5793(03)01199-2. [DOI] [PubMed] [Google Scholar]
- Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285:C1339–47. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- Okimotoa Y, Watanabea A, Nikia E, Yamashitab T, Noguchia N. A novel fluorescent probe diphenyl-1-pyrenylphosphine to follow lipid peroxidation in cell membranes. FEBS Lett. 2000;474:137–40. doi: 10.1016/s0014-5793(00)01587-8. [DOI] [PubMed] [Google Scholar]
- Pak JH, Manevich Y, Kim HS, Feinstein SI, Fisher AB. An antisense oligonucleotide to 1-cys peroxiredoxin causes lipid peroxidation and apoptosis in lung epithelial cells. J Biol Chem. 2002;277:49927–34. doi: 10.1074/jbc.M204222200. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochimica et Biophysica Acta. 2002;1585:114–25. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Phelan SA, Wang X, Wallbrandt P, Forsman-Semb K, Paigen B. Overexpression of Prdx6 reduces H2O2 but does not prevent diet-induced atherosclerosis in the aortic root. Free Radic Biol Med. 2003;35:1110–20. doi: 10.1016/s0891-5849(03)00462-3. [DOI] [PubMed] [Google Scholar]
- Piotrowski WJ, Marczak J, Kurmanowska Z, Gorski P. Concentration of TBA-reactive substances in type II pneumocytes exposed to oxidative stress. Archivum Immunologiae et Therapiae Experimentalis. 2004;52:435–40. [PubMed] [Google Scholar]
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–42. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- Ralat LA, Manevich Y, Fisher AB, Colman RF. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45:360–72. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–52. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Netto LE, Seo MS, Stadtman ER. A family of novel peroxidases, peroxiredoxins. Biofactors. 1999;10:207–9. doi: 10.1002/biof.5520100218. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Brigelius-Flohe R, Aumann KD, Roveri A, Schomburg D, Flohe L. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–90. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Radic Biol Med. 2004a;37:1736–43. doi: 10.1016/j.freeradbiomed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid Redox Signal. 2006a;8:229–37. doi: 10.1089/ars.2006.8.229. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manevich Y, Feinstein SI, Fisher AB. Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2004b;286:L1188–93. doi: 10.1152/ajplung.00288.2003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Phelan SA, Manevich Y, Feinstein SI, Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol. 2006b;34:481–6. doi: 10.1165/rcmb.2005-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Yin L, Stearns R, Gonzalez-Flecha B. Lysosomal and mitochondrial pathways in H2O2-induced apoptosis of alveolar type II cells. Journal of Cellular Biochemistry. 2005;94:433–45. doi: 10.1002/jcb.20277. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang HP, Zhang HJ, Weydert CJ, Domann FE, Oberley LW, Buettner GR. L-PhGPx expression can be suppressed by antisense oligodeoxynucleotides. Arch Biochem Biophys. 2003;417:212–8. doi: 10.1016/s0003-9861(03)00342-4. [DOI] [PubMed] [Google Scholar]