Abstract
Purpose
The introduction of effective antiretroviral therapy (ART) has transformed HIV infection from a deadly to a chronic infection. Despite its successes in reducing mortality, ART fails to cure HIV allowing HIV persists in vivo. HIV persistence under ART is thought to be mediated by a combination of latent infection of long-lived cells, homeostatic proliferation of latently infected cells, anatomic sanctuaries and low-level virus replication. To understand the contribution of specific cell types and anatomic sites to virus persistence in vivo animal models are necessary.
Recent findings
The advancements in ART and our understanding of animal models have facilitated the development of models of HIV persistence in nonhuman primates and mice. SIV or SHIV infection of rhesus and pigtail macaques followed by effective ART represents the most faithful animal model of HIV persistence. HIV infection of humanized mice also provides a useful model for answering specific questions regarding virus persistence in a uniquely mutable system.
Summary
In this review we describe the most recent findings using animals models of HIV persistence. We will first describe the important aspects of HIV infection that SIV/SHIV infection of NHP are able to recapitulate, then we will discuss some recent studies that have used these models to understand viral persistence.
Keywords: SIV, latency, HIV, Animal models, eradication strategies
Introduction
Antiretroviral therapy (ART) has transformed HIV into a life-long chronic infection. However, ART has failed to provide a cure for HIV as virus replication rapidly rebounds if ART is interrupted. Additionally, life-long ART does not fully reverse the immunological abnormalities associated with HIV infection, resulting in drug toxicities, other side effects, and creates a massive financial burden for health care systems worldwide. The major barrier to HIV cure is the persistence of latently infected long-lived CD4+ memory T-cells, where latent infection is defined as the presence of stably integrated HIV DNA in absence of active viral production. The study of HIV persistence is limited due to the rarity of latent cells in vivo that harbor replication competent virus, estimated to be ∼1 per million memory CD4+ T-cells, and difficulty in accurately modeling HIV latency and persistence in vitro. In addition, HIV persistence is a very complex virological and immunological phenomenon, with infection of several cell types in a wide array of anatomic tissues that are all regulated differently, including various subsets of CD4+ T-cells, such as central memory T-cells (TCM), follicular helper T-cells (TFH), memory stem T-cells (TSCM), and regulatory T-cells (TREG) in blood and lymphoid tissues, effector memory T-cells (TEM) and T helper-17 cells (TH17) in the gut, microglial cells and astrocytes in the central nervous system and, more controversially, macrophages and dendritic cells (1–5). Therefore a cure to HIV persistence will likely require a combination of several virus elimination strategies, similar to combination ART or poly-chemotherapy for cancer, in order to eliminate the different components of the virus reservoir in different tissues and cells. Nonhuman primate (NHP) models using Indian rhesus macaques(RM, Macaca mulatta) or pigtail macaques (PTM, Macaca nemestrina) experimentally infected with simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus (SHIV) and treated with ART have similar pathology and disease progression compared with ART-treated HIV infection in humans (6–8). The study of HIV/AIDS with NHP models has been instrumental in our current understanding of HIV pathogenesis, previously reviewed by Evans and Silvestri (9). In this review we will summarize the recent progress in the use of animal models to understand HIV persistence and test potential cure strategies.
Models of Nonhuman Primates for HIV infection
Historically, a key limitation of the SIV/RM model to study virus reservoirs has been the lack of optimized ART regimens that fully suppress virus replication. Today, newer ART combinations and formulations can be used in RM and PTM that are extremely effective in reducing plasma viral loads to levels equivalent to what is observed in HIV-1-infected patients on ART, thus providing an excellent in vivo model of HIV persistence (10–15). As summarized in Table 1, there are many benefits to using NHP models in the study of HIV persistence, including the fact that they allow us to extensively sample tissues that are not readily accessible in humans, to study rare cell populations in tissues and blood, such as TSCM, TFH, and TFR (5,16–18), and to deplete or block specifying immune functions, including CD8+ and CD4+ lymphocyte depletion, manipulation of interferons or cytokines, and blockade of co-inhibitory pathways such as PD-1 (6–8,11,15,17,19–32).
Table 1. Benefits of nonhuman primate models in HIV cure studies.
| Benefits of NHP models | Caveats |
|---|---|
| Similar immune systems | NHP have more HLA genes, therefore more variety in Ag recognition |
| Similar pathogenic features including AIDS and virus control on ART | Different viruses. |
| Aggressive tissue sampling and invasive interventions | Need to confirm in human studies. |
| Deeper understanding of mechanisms | Need human in vitro models to replicate mechanisms |
| Normalized route of infection, clonal virus, time with productive infection, ART treatment and time of ART | High cost of animals, long experimental protocols and need for animal resources. |
| Controlled ART adherence | |
| Characterization of cellular and anatomical reservoirs | |
| Test pilot studies that are invasive and ethically impossible in humans. | |
| No barriers to analytical ART interruption. |
Most studies of HIV cure using NHP models follow a similar protocol, detailed in Figure 1. Either RM or PTM are infected via different routes, usually intravenous or intrarectal, with macaque-adapted viruses such as SIVmac239, SIVmac251 or chimeric simian/human immunodeficiency viruses (SHIV) that are designed to include the HIV RT gene (to improve the susceptibility to certain reverse transcriptase inhibitors) or the HIV-Env gene (for studies of the anti-reservoir effects of HIV-specific broadly neutralizing antibodies). These viruses are typically used at high concentrations to induce robust levels of infection with a single dose challenge. After establishment of infection, ART is administered orally or subcutaneously, with studies reporting ART initiation anywhere from day 3 to day 60 post-infection (or later). The choice of the time of ART initiation should reflect that in HIV-infected humans delayed treatment initiation leads to a greater size of the HIV DNA reservoir (33). ART formulations used in NHP have included two nucleoside reverse transcriptase inhibitors (typically tenofovir [PMPA] and emtricitabine [FTC]), a protease inhibitor (often Darunavir) and an integrase inhibitor (Raltegravir or Dolutegravir) with possible intensification with a CCR5 inhibitor (Maraviroc) or a non-nucleoside reverse transcriptase inhibitor in the case of RT-SHIV infection. While ART formulations differ slightly in various published studies, all ART formulations are highly effective in reducing virus replication by several logs, commonly below detectable levels in plasma, as tested using different viral RNA assays (detection ranges from 3-100 copies/ml). After 6-12 months of virus suppression, which may take variable amounts of time (1-4 months), the cure or latency reversal strategy is tested. It should be noted that while an absolute recapitulation of long-term ART-treated HIV-infected individuals would suggest the use of prolonged ART in SIV/SHIV-infected macaques, logistical and financial considerations may in fact limit the length of treatment in these animals. We propose that 6-12 months of virus suppression is a reasonable approximation of ART-treated patients that allows for more rapid evaluation of potential cure approaches. Finally, to test the efficacy of the used “anti-reservoir” interventionthe size of the virus reservoir can be measured directly using assays such as total and integrated cell-associated SIV-DNA (34, Mavigner at al., unpublished), Tat-Rev-induced limiting dilution assay (TILDA; 35), and quantitative viral outgrowth assays (QVOA;(36,37,10,38)with input cells derived from blood or multiple tissues through biopsies or at necropsy (summarized in Table 2). An alternative study design involves the functional measurement of the virus reservoirs as inferred by the kinetic of virus rebound after ART interruption (11,12,14). Overall, these types of studies involving ART-treated SIV/SHIV-infected macaques are highly informative in the field of HIV cure research and their use has increasing rapidly over the past few years. However, there are biological caveats and limitations to using NHP models for studies of HIV cure. These include the presence of species-specific differences in certain immune function (i.e., MHC genes), as well as high costs associated with animal housing and care (Table 1). As such, while these NHP models provide an excellent tool to study HIV persistence in humans and potential therapeutic strategies that can be used to disrupt persistence, all key findings must ultimately be replicated in the setting of HIV infection of humans.
Figure 1. Schematic of Non-Human Primate Models of HIV/SIV cure.
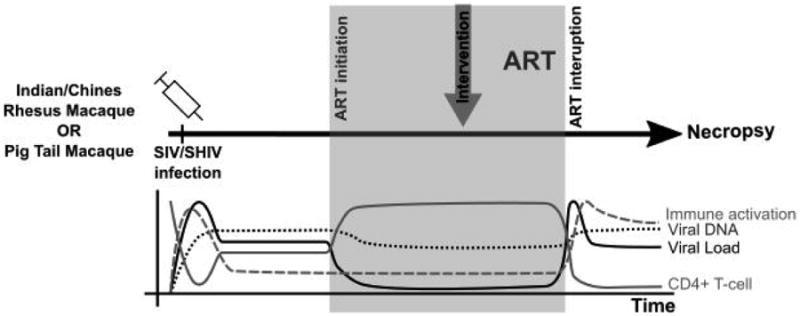
Either A. Indian Rhesus Macaques (RM, Macaca mulatta) or Pigtail Macaques (PTM, Macaca nemestrina) are experimentally infected with simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus (SHIV). After peak viremia, anti-retroviral treatment (ART) is initiated, which usually includes a combination of a two-nucleoside reverse transcriptase inhibitors (NRTI), an integrase inhibitor and either a non-NRTI or a CCR5 inhibitor. Upon virus suppression, an intervention of interest is administered including; latency reversal agents (74), enhancement of immune function (23,38) or elimination of infected cells (11), and ART is continued for some time. To test success of cure strategy and any differences in reservoir size, ART is interrupted and animals are monitored for virus rebound. After viral rebound, animals are sacrificed and tissues are collected for analysis. B. Graph depicts changes in viral load, viral DNA, CD4+ T-cell counts and immune activation during infection, ART treatment and ART interruption.
Table 2. Summary of selected SIV persistence models.
| NHP | Sample number (n) |
Virus | Virus concentration |
Route of Infection | ART formulation | Time on ART (mo) | Virus suppression achieved? |
Limit of detection for SIV RNA assay (copies/ml) |
Intervention tested |
Treatment interruption (post- ART initiation) |
Viral DNA measured? |
Plasma drug concentration measured |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shytaj, 2012, PloS Pathog. (10) | RM | 5 | SIVmac251 | 10ˆ3-10ˆ7 RNA copies/ml | i.r or i.va | FTC, PMPA, RAL for 1.5 mo; DRV/r for +80d; MVC | 5-17 | Y | <40; <3 | N | N | N | Y |
| 2 | SIVmac251 | 10ˆ3-10ˆ7 RNA copies/ml | i.r or i.va | 3wks MRV/r, PMPA, FTC, RAL, DRV | Y | N | N | N | Y | ||||
| 4 | SIVmac251 | 10ˆ3-10ˆ7 RNA copies/ml | i.r or i.va | FTC, PMPA, RAL, DRV/r, MVC | Y | N | N | Total DNA | Y | ||||
| 2 | SIVmac251 | 10ˆ3-10ˆ7 RNA copies/ml | i.r or i.va | FTC, PMPA, RAL, DRV/r | Y | N | N | N | Y | ||||
| Mavigner, 2014, PloS Pathog (11) | RM | 3 | RT-SHIV-TC | 10ˆ4 TCID50 | i.r | PMPA, FTC, EFB, RAL | 1-2 | Y | <60 | HSC collection + myeloablative TBI + Stem cell transplant. | Y | Total DNA | N |
| 3 | RT-SHIV-TC | 10ˆ4 TCID50 | i.r | PMPA, FTC, EFB, RAL | 1-2 | Y | <60 | N | N | Total DNA | N | ||
| Whitney, 2014, Nature (12) | RM | 20 | SIVmac251 | 500 TCID50 | i.r | TFV, FTC, DTG | 3, 5, 7, 10 days | Y | <50 | N | Y | Total DNA | N |
| Del Prete, 2015, Aids Res and Hu Retrovirus (13) | RM | 3 | SIVmac239 | 300 IU (TZM-bl reporter cell infection) | i.v | FTC, TDF, DTG | 10 | Y | <30 | +romidepsin | N | N | Y |
| 3 | SIVmac239 | 300 IU (TZM-bl reporter cell infection) | i.v | FTC, TDF, DTG, DRV (DRV dropped after 4 weeks) | N | N | N | Y | |||||
| Micci, 2015, JCI (14) | RM | 8 | SIVmac239 | 300 TCID50 | i.v | PMPA, FTC, RAL, DRV/r | 7 | Y | <60 | weekly IL-21 treatment between day 67-105 and 203-241 | Y | Total DNA | N |
| 7 | SIVmac239 | 300 TCID50 | i.v | PMPA, FTC, RAL, DRV/r | 7 | Y | Control group | Y | Total DNA | N | |||
| Ryan, 2016, PloS Pathog (15) | RM | 16 | SIVmac239 | 300 TCID50 | i.v | PMPA, FTC, RAL, DRV/r | 2 | Y | <60 | N | Y | Total DNA | N |
| Ortiz, 2015, Muc Immun (38) | PTM | 6 | SIVmac239 | 3000 TCID50 | NR | PMPC, FTC, L8 (integrase inhibitor) | 9 | Y | 100 | Daily Probiotic + early and late IL-21 administration | N | Total DNA | N |
| 5 | SIVmac239 | 3000 TCID50 | NR | PMPC, FTC, L8 (integrase inhibitor) | 9 | Y | 100 | Control group | N | Total DNA | N |
Acronyms used: PloS Pathog = Plos Pathogens, JCI = Journal of clinical immunology, Aids res and Hu retrovirus = AIDS research and human retroviruses, RM = Rhesus Macaque, PTM = Pig Tail Macaque, i.r = Intra-rectal, i.va = Intra-vaginal, i.v = Intraveneous, wks = weeks, Mo= months, TCID50 = 50% Tissue culture infection dose, AID50 = 50% Animal infectious doseFTC = emtricitabine, PMPA/TFV = tenofovir, RAL = Raltegravir, DRV/r = ritonavir-boosted darunavir, MVC = maraviroc, EFV = efavirenz, DTG = dolutegravir, TDF = tenofovir disoproxil fumarate, HSC =heamopoetic stem cell, TBI = Total body irradiation, Y= Yes, N=no, NR = not reported.
The humanized mouse model provides a small-scale animal model to study HIV infection, and upon ART treatment mice control viremia and immune function recovers (39–41). Given recent reviews of the humanized mouse models for use in HIV persistence by by Policicchio et al. and Garciahere we will briefly describe two robust mouse models (42,43). The BLT mouse model is the best representative of a humanized mouse with latent HIV infection (44), where irradiated NOD.CB17-PrkdcSCID/J (NSG) are transplanted with human bone marrow, liver and thymus to re-populate a humanized immune system including T-cells, B-cells, monocytes, macrophages and DC (45). Infection progresses similar to humans and latency is established at a similar frequency, allowing potential HIV therapeutics to be tested (Vorinostat, I-BET151 inhibitor, anti-cytotoxic-lymphocyte antigen 4 [CTLA-4]; 46). To only assess T-cell function in HIV persistence, a humanized mouse model has been developed with engraftment of only human T-cells and no myeloid cells, ToM, in a similar method to BLT engraftment(47). Importantly, the To M mice do not develop graft-versus-host disease (GVHD), have potent HIV infection, which is suppressed by ART and rebounds with ART interruption. Additionally, latency is detected at a similar frequency to humans as measured by the QVOA (36). The same group has also developed a model with myeloid cells and no lymphoid cells, MoM (Honeycutt JCI, in press). The ToM and MoM models have allowed researchers to address important questions regarding virus persistence within T-cells or myeloid cells only, allowing differentiation of specific contribution to reservoir or control of HIV persistence. However, due to suboptimal human immune cell engraftment and the small size of mice the latent reservoir remains small and detection of rare immune cell populations, like TSCMand TFHcells, remains difficult. Furthermore, blood volumes obtained are limited leading to reduced ability to measure virus suppression below a threshold of 400 copies/ml(47).
NHP models of SIV persistence
Early establishment of latent infection
In a recent influential study Whitney et al. investigated the early kinetics of reservoir establishment in RM infected with SIVmac251 and early initiation of ART at day 3, 7, 10 and 14 post-infection (12). Interestingly, the authors found that ART initiation at day 3 resulted in detectable SIV RNA and DNA in lymph nodes (LN) and colorectal mucosa, but neither SIV DNA in peripheral blood mononuclear cells (PBMC) nor SIV RNA plasma. In the animals initiated on ART at day 7, 10 and 14 post-infection, i.e., after detection of plasma viremia, and viral RNA and DNA in PBMC, LN and colorectal mucosa, subsequent ART interruption resulted in rapid virus rebound within 7 days. Animals in which treatment was initiated before the presence of detectable SIV RNA in plasma (day 3 ART initiation), a delayed rebound (day 21 post-ART interruption) was also observed. These data are consistent with human studies suggesting that early ART initiation leads to a smaller reservoir size and slower disease progression (33). The findings by Whitney et al. highlight that a small but clearly fully functional SIV reservoir is established very early after experimental inoculation of SIV and before virus detection in plasma and PBMC, with latent virus infection of a critical mass of cells in lymphoid tissues. The implication of these findings for HIV infection in humans is that early ART initiation alone is highly unlikely to represent a potential cure for HIV, but may represent a setting in which additional interventions targeting the established but smaller reservoir are more likely to be effective.
Virus persistence in CD4+ TSCM
Despite an initial observation of HIV infection of hematopoietic stem cells in vitro (48), most human studies suggest that hematopoietic stem cells do not contribute to the latent reservoir in vivo (49).In contrast, multi-potent CD4+ T memory stem cells, TSCM, harbor high amounts of viral DNA and contribute to the latent reservoir in CD4+ memory T-cells,, in fact, this contribution increases over time in long-term ART-treated HIV-infected humans (3).In addition, studies from our lab of CD4+ TSCM in SIV-infected RM in the absence of ART revealed that these cells are readily infected with SIV in both blood and lymphoid tissues (16). We found that while total TSCM numbers were maintained, the fraction CD4+ TSCM expressing CCR5 was depleted while the percentage of CD4+ TSCM expressing the proliferation antigen Ki-67 was expanded (16). In follow up work, SIV-infected RMs were treated with ART and we found that suppression of virus replication is associated with an improved homeostasis of the CD4+ TSCM compartment but no major decline of the fraction of these cells containing SIV DNA, even though the frequency of the shorter-lived CD4+ TTM and TEM harboring SIV DNA declined significantly under ART (Cartwright, unpublished). Interestingly, Jaafoura et al. reached similar conclusions regarding the role of CD4+ TSCM in virus persistence under ART by using mathematical modeling of integrated HIV DNA levels in CD4+ T-cells subsets from ART-treated patients (50). Collectively, these studies show that CD4+ TSCM may be important contributors to life-long HIV/SIV persistence under ART, and further highlight the importance of targeting cure strategies towards elimination of latent infection in all long-lived cells.
The role of germinal centers (GC) and follicular T helper cells (TFH) in viral persistence
The role of GC and TFH in HIV persistence has been poorly studied until recently due to the lack of accurate in vivo models. Previous work has shown that human follicular dendritic cells (FDC) in GC can harbor HIV on their surface in an archival fashion, where virus on these FDCs persists for months without decay (51–54). Connick et al. found that GC harbor high levels of SIV RNA and proposed that poor CD8+ T-cell infiltration in the lymph node drives persistence of SIV RNA in GC (55). Petrovas et al. was the first to characterize TFH in RM and during SIV infection, showing that activated CD4+ T-cells constantly differentiate into TFH and upon SIV infection TFH adopt a pro-inflammatory phenotype and function but are not depleted, rather they accumulate in the GC (56). In a recent influential study, Fukazawa et al. (2015) showed that low-level viremia in elite controllers originates from TFH as a consequence of the limited access of SIV-specific CD8+ T-cells to the GC (5). We and others have also defined a population of follicular regulatory T-cells (TFR) in SIV-infected and uninfected RM, showing that a reduction in the TFR/TFH ratio after SIV infection was associated with TFH expansion (17,29,57). While both TFH and TFR are known to be important in the development of a strong and balanced humoral response against HIV/SIV antigens during vaccination (and potentially therapeutic vaccination as part of a curative approach), these studies show that TFH and TFR infection are also important for HIV persistence in that they function as viral targets and represent a source of low-level viremia under ART (58). Collectively, these observations suggest that virus-producing TFH in the B-cell area of LN form a continual source of virions that can bind to and interact with FDC, and that, in the context of limited CD8+ T-cell mediated clearance and possibly low bio-distribution of drugs in LN, may crucially contribute to virus persistence under ART.
The role of CD8+ T-cells in controlling viremia under ART
A number of experimental observations demonstrate the role of CD8+ T-cells in controlling HIV and SIV replication in vivo during the natural history of infection. This evidence includes the key observation that in vivo depletion of CD8+ lymphocytes in SIV-infected RM invariably results in increased levels of virus replication (59–61). However, the role of CD8+ T-cells in suppressing virus replication under ART treated was not addressed in these earlier studies. Recently, we performed experimental CD8+ lymphocyte depletion in thirteen ART-treated SIV-infected RM, and found that this procedure resulted in increased virus production in both plasma and lymphoid tissues in 13 out of 13 of the animals, with levels of viremia up to ∼5000 copies/ml from plasma while ART was maintained (Cartwright, unpublished). In most animals we also observed that upon CD8+ T-cell repopulation (but not NK cell repopulation), viremia returned to an undetectable level. Taken together, these data indicate that CD8+ lymphocytes are required to maintain virus suppression in ART-treated SIV-infected RM. Our data reveal a previously unrecognized antiviral function of CD8+ lymphocytes that could be boosted by interventions such as therapeutic vaccination and immune-based interventions to release inhibitory stimuli from CD8+ T-cells (e.g., checkpoint blockade inhibitors; Cartwright, unpublished).
SIV cure strategies
Total body irradiation, bone marrow transplant and hematopoietic stem cell engraftment in NHP
To date only one person, the Berlin patient, appears to have been cured of HIV. This patient was treated for acute myeloid lymphoma (AML) with two rounds of total body irradiation (TBI) followed by bone marrow transplant (BMT) with a HIV resistant, homozygous CCR5δ32 donor (62,63). Follow-up studies have failed to achieve a similar outcome in two HIV-infected individuals that underwent BMT from a CCR5 wild type donor (64,65). To better understand the impact of TBI and autologous hematopoietic stem cell transplant (HSCT) with HSC that were mobilized and collected prior to RT-SHIV infection we designed and conducted a pilot study in ART-treated SHIV-infected RM. We observed that virus rebounded in 2/3 SHIV infected, ART-treated animals that underwent HSCT, engraftment, and, ART interruption, while the third animal was sacrificed at 2 weeks post ART interruption with undetectable viremia, but detectable virus in lymphoid tissues (11). Together, these results and those obtained by other groups in PTMs (66), demonstrate that autologous HSCT can be performed in SHIV-infected RM and PTM, but the protocols used did not result in cure. This model can be further interrogated to ask critical questions regarding the pathogenesis of the apparent HIV cure achieved in the Berlin patient.
Purging the reservoir with latency reactivating agents (LRA)
Current HIV cure efforts are focused around the hypothesis that the latent reservoir can be disrupted and eliminated by the reactivation of latent virus. This is conceived to involved the delivery of a “shock” signal, followed by immune-mediated clearance (“kill”) of the cells expressing reactivated virus. Agents designed to reactivate latent virus (latency reversing agents, LRA), including histone de-acetylase inhibitors (HDACi) such as Vorinostat, Panobinastat, and Romidepsin, and a drug used to treat alcohol addiction Disulfiram, have been used safely in HIV-infected individuals in several pilot studies, although with limited impact on virus reactivation and virtually no effect on the size of the virus reservoirs (67–71).Ling et al. and Del Prete et al. tested Vorinostat in suppressed SIV infection of Chinese and Indian RM, respectively, and obtained results similar to those of the above mentioned human clinical trials (72,73). Del Prete et al. have also recently used a pan HDACi, Romidepsin, to reactivate SIV infection in ART-treated RMs (74). When ART was interrupted, plasma virus rebound was similar in Romidepsin treated SIV-infected RM compared to non-Romidepsin treated control animals. While these initial results with LRA strategies in both pre-clinical NHP studies and human clinical trials have not been very impressive, there is considerable optimism in the field as novel classes of LRAs are being developed and tested, including toll-like receptor agonists, and combinations of LRAs with immune enhancing strategies (i.e., therapeutic vaccines, checkpoint blockade inhibitors, monoclonal antibodies) are being explored in studies that rely heavily on the use of ART-treated SIV-infected RM.
Mapping out HIV/SIV infection in the central nervous system under ART
A subset of ART-treated HIV-infected individuals show CNS symptoms that have been grouped under the term of HIV-associated neurocognitive disorder (HAND; (75,76,Reviewed in 77). However, it remains unclear to what extent the pathogenesis of HAND is due to direct virus replication in the CNS despite ART. Using ART-treated SIV-infected RM, it was shown that SIV-DNA can be detected in the brain tissue and cerebrospinal fluid (CSF), thus indicating that the CNS can serve as a site of SIV persistence (6,7,78,79). While it is challenging to measure neurocognitive dysfunction in NHP, investigations of the CNS in NHP are particularly useful given the extreme difficulty in sampling this anatomic compartment in HIV-infected humans. As such, a number of behavioral and biological abnormalities are currently being investigated in macaques as potential markers of brain dysfunction to be used for studies of the CNS reservoirs in SIV-infected ART-treated RM. Recently, Beck et al. showed a difference in infection efficiency of the virus infecting the CNS compared to the periphery (80). These authors characterized the viral fitness of both wild type SIV and the specific CTL escape mutant (K165) to show that the perivascular CTL response exerts selective pressure on wild type SIV in the CNS but not in the periphery, suggesting that the virus causing CNS infection may be different from its peripheral counterpart. Furthermore, in a brief report Marcondes et al. specifically depleted CD8+ T-cells in the CNS of SIV-infected RMs (non ART-treated) via intra-thecal injection of CD8+ depleting antibody and found that viral loads in the CNS were significantly higher than those observed in control groups, thus highlighting the importance of the CD8+ T-cell response protecting CNS from viral infection (79). Further studies of the CNS-based virus reservoir in ART-treated SIV-infected RM or PTM will likely identify site-specific mechanisms of virus persistence and allow the pre-clinical testing of novel therapeutic approaches that specifically target the CNS reservoir.
Targeting HIV/SIV persistence in the gut
The gut is a unique anatomical reservoir of HIV latency as well as a site of residual immune activation in ART-treated HIV-infected individuals (1,2,81,82). Th17 and Th22 CD4+ T-cells mediate protection against bacteria and fungi in gastrointestinal-associated lymphoid tissues (GALT) but are depleted during HIV/SIV infection and fail to fully reconstitute upon ART (6,8,83). Recently, Ryan et al. have described a set of changes in the Th17 and Th22 frequency and function during SIV infection prior to, during and off ART treatment in blood, LN and the colorectum (15). The authors found that ART failed to restore Th17 and Th22 function and frequency to pre-SIV infection levels, and that these markers were predictive of residual immune activation, SIV persistence, and signs of disease progression in the colorectum and blood. Ortiz et al. has shown that IL-21 and probiotic treatment lead to high frequencies of Th17 cells in the gut and improved gut recovery post ART initiation in SIV-infected RMs (38). Similarly, Micci et al. showed that IL-21 administration to ART-treated SIV-infected RM results in enhanced recovery of Th17 and Th22 cells in the gastro-intestinal tract, attenuated signs of inflammation, and reduced levels of plasma SIV RNA (23). Promisingly, these enhancements in the intestinal mucosal immune function remained stable through treatment interruption, suggesting that IL-21 treatment may represent a promising component of HIV cure interventions.
Conclusion
The use of state-of-the-art NHP models of SIV/SHIV infection under ART in combination with new technologies to assess reservoir size and function, along with novel therapeutic “anti-reservoir” approaches represents a formidable tool for basic and translational studies to develop a cure for HIV infection. We envision that these types of in vivo studies will lead to major advances in our understanding of the mechanisms by which SIV/HIV latency is established and maintained, thus ultimately guiding the pre-clinical and clinical development of a feasible strategy for HIV cure.
Key points.
NHP and Mouse models are an important aspect in building our understanding of HIV persistence and the development of a HIV cure.
Extensive sampling of tissue sites in NHP has allowed the characterization of unique cellular reservoirs like follicular T-helper cells, T-memory stem cells.
Strong understanding of SIV pathogenesis has allowed single and combination therapeutics to successfully reverse SIV mediated pathogenesis.
The development of SIV persistence models will allow testing of combination therapies to eliminate multiple sources of HIV persistence can now be tested.
Acknowledgments
None
Financial support and sponsorship: This work was supported by grant to G.S., and by grant 108905-56-RGRL (American Foundation for AIDS Research) and K12 HD072245 to A.C.
Footnotes
Conflicts of interest: None
Contributor Information
Nitasha Kumar, Email: nitasha.kumar@emory.edu.
Ann Chahroudi, Email: ann.m.chahroudi@emory.edu.
Bibliography
- 1.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis. 2013 Oct 15;208(8):1212–20. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzón MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014 Feb;20(2):139–42. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bednar MM, Sturdevant CB, Tompkins LA, Arrildt KT, Dukhovlinova E, Kincer LP, et al. Compartmentalization, Viral Evolution, and Viral Latency of HIV in the CNS. Curr HIV/AIDS Rep. 2015 Jun;12(2):262–71. doi: 10.1007/s11904-015-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015 Feb;21(2):132–9. doi: 10.1038/nm.3781. This study identified the B-cell folicule as a reservoir of SIV. Authors analysed virus persistence in elite controllers and typical progressors showing that follicular T-cells in germinal centres are infected and can harbour SIV in both groups of patients, however elite controllers eliminate virus from extra follicular regions more effectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinoso JB, Rabi SA, Blankson JN, Gama L, Mankowski JL, Siliciano RF, et al. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol. 2009;83:9247–57. doi: 10.1128/JVI.00840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84:2913–22. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kline C, Ndjomou J, Franks T, Kiser R, Coalter V, Smedley J, et al. Persistence of Viral Reservoirs in Multiple Tissues after Antiretroviral Therapy Suppression in a Macaque RT-SHIV Model. PLoS ONE. 2013 Dec 18;8(12):e84275. doi: 10.1371/journal.pone.0084275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DT, Silvestri G. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS. 2013 Jul;8(4):255–61. doi: 10.1097/COH.0b013e328361cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shytaj IL, Norelli S, Chirullo B, Della Corte A, Collins M, Yalley-Ogunro J, et al. A Highly Intensified ART Regimen Induces Long-Term Viral Suppression and Restriction of the Viral Reservoir in a Simian AIDS Model. [cited 2016 Feb 3];PLoS Pathog [Internet] 2012 Jun;8(6) doi: 10.1371/journal.ppat.1002774. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3380955/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Mavigner M, Watkins B, Lawson B, Lee ST, Chahroudi A, Kean L, et al. Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant. PLoS Pathog. 2014 Sep;10(9):e1004406. doi: 10.1371/journal.ppat.1004406. This study showed that experimental, autologous hematopoietic stem cell transplant (HSCT) in Indian rhesus macaques is safe, however does not reduce the size of SIV reservoir. This study shows that HSCT is feasible in NHP, and future studies can build on this with additional cure strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. [cited 2014 Aug 18];Nature [Internet] 2014 Jul 20; doi: 10.1038/nature13594. advance online publication. Available from: http://www.nature.com/nature/journal/vaop/ncurrent/full/nature13594.html. This study found that SIV reservoir is established within 3 days of infection, before SIV RNA is detected in plasma and PBMC. While early treatment is not possible in humans, this study informs us about the kinetics of latency establishment. [DOI] [PMC free article] [PubMed]
- 13*.Del Prete G, Smedley J, Macallister R, Jones G, Li B, Hattersley J, et al. Comparative evaluation of co-formulated injectable combination antiretroviral therapy regimens in SIV-infected rhesus macaques. AIDS Res Hum Retroviruses. 2015 Jul 6; doi: 10.1089/aid.2015.0130. This study specifically tested 3 and 4 drugs ART regeiments with and without Darunavir showing that 3 drug regimen, including Tenofovir, emtricitabine, and Dolutegravir, was just as effective as the 4 drug regimen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Micci L, Ryan ES, Fromentin R, Bosinger SE, Harper JL, He T, et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J Clin Invest. 2015 Dec 1;125(12):4497–513. doi: 10.1172/JCI81400. This study treated ART supressed rhesus macaques with IL-21 to show that inflammation was reduced in the gut, but more importantly, the SIV reservoir was size was decreased. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Ryan ES, Micci L, Fromentin R, Paganini S, McGary CS, Easley K, et al. Loss of Function of Intestinal IL-17 and IL-22 Producing Cells Contributes to Inflammation and Viral Persistence in SIV-Infected Rhesus Macaques. PLoS Pathog. 2016 Feb;12(2):e1005412. doi: 10.1371/journal.ppat.1005412. This study found that IL-17 and IL-22 drove the loss of CD4+ T-cells in the gut which leads to microbial translocation and chronic inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Cartwright EK, McGary CS, Cervasi B, Micci L, Lawson B, Elliott STC, et al. Divergent CD4+ T memory stem cell dynamics in pathogenic and nonpathogenic simian immunodeficiency virus infections. J Immunol Baltim Md 1950. 2014 May 15;192(10):4666–73. doi: 10.4049/jimmunol.1303193. This study characterized T-memory-stem-cells (TSCM) in rhesus macaques to find that rhesus macaque CCR5+ TSCM were depleted, had higher rates of proliferation and SIV infection compared to sooty managbeys that have non-pathogenic SIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury A, Del Rio PME, Tharp GK, Trible RP, Amara RR, Chahroudi A, et al. Decreased T Follicular Regulatory Cell/T Follicular Helper Cell (TFH) in Simian Immunodeficiency Virus-Infected Rhesus Macaques May Contribute to Accumulation of TFH in Chronic Infection. J Immunol Baltim Md 1950. 2015 Oct 1;195(7):3237–47. doi: 10.4049/jimmunol.1402701. This study characterized follicular regulatory T-cells (TRF), which was found to drive TFH expansion and CD4+ T-cell infiltration into the germinal centre, which drives SIV infection of TFH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013 Feb 1;123(2):594–9. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carruth LM, Zink MC, Tarwater PM, Miller MD, Li M, Queen LA, et al. SIV-specific T lymphocyte responses in PBMC and lymphoid tissues of SIV-infected pigtailed macaques during suppressive combination antiretroviral therapy. J Med Primatol. 2005;34:109–21. doi: 10.1111/j.1600-0684.2005.00103.x. [DOI] [PubMed] [Google Scholar]
- 20.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008 Oct;14(10):1077–87. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, et al. Depletion of CD4+ T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest. 2011 Nov;121(11):4433–45. doi: 10.1172/JCI46023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horiike M, Iwami S, Kodama M, Sato A, Watanabe Y, Yasui M, et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012 Feb 20;423(2):107–18. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Micci L, Cervasi B, Ende ZS, Iriele RI, Reyes-Aviles E, Vinton C, et al. Paucity of IL-21-producing CD4(+) T cells is associated with Th17 cell depletion in SIV infection of rhesus macaques. Blood. 2012 Nov 8;120(19):3925–35. doi: 10.1182/blood-2012-04-420240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micci L, Alvarez X, Iriele RI, Ortiz AM, Ryan ES, McGary CS, et al. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog. 2014 Oct;10(10):e1004467. doi: 10.1371/journal.ppat.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012 Jun;33(6):306–14. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog. 2013;9(7):e1003471. doi: 10.1371/journal.ppat.1003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandler NG, Bosinger SE, Estes JD, Zhu RTR, Tharp GK, Boritz E, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014 Jul 31;511(7511):601–5. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jochems SP, Jacquelin B, Chauveau L, Huot N, Petitjean G, Lepelley A, et al. Plasmacytoid dendritic cell infection and sensing capacity during pathogenic and non-pathogenic SIV infection. J Virol. 2015 Apr 22; doi: 10.1128/JVI.00332-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Miles B, Miller SM, Folkvord JM, Kimball A, Chamanian M, Meditz AL, et al. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat Commun. 2015;6:8608. doi: 10.1038/ncomms9608. This study characterized regulatory follicular T-cells (TFR) in HIV and SIV infection, showing that infection drives increase in TFR population. In turn TFR impair TFH and germinal centre function thereby inhibiting SIV and HIV clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer JL, Ries M, Guha N, Connole M, Colantonio AD, Wiertz EJ, et al. Suppression of a Natural Killer Cell Response by Simian Immunodeficiency Virus Peptides. PLoS Pathog. 2015 Sep;11(9):e1005145. doi: 10.1371/journal.ppat.1005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wonderlich ER, Wu WC, Normolle DP, Barratt-Boyes SM. Macrophages and Myeloid Dendritic Cells Lose T Cell-Stimulating Function in Simian Immunodeficiency Virus Infection Associated with Diminished IL-12 and IFN-α Production. J Immunol Baltim Md 1950. 2015 Oct 1;195(7):3284–92. doi: 10.4049/jimmunol.1500683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009 Mar 12;458(7235):206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PloS One. 2012;7(3):e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Doherty U, Swiggard WJ, Jeyakumar D, McGain D, Malim MH. A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. J Virol. 2002;76:10942–50. doi: 10.1128/JVI.76.21.10942-10950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Procopio FA, Fromentin R, Kulpa DA, Brehm JH, Bebin AG, Strain MC, et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine. 2015 Aug;2(8):872–81. doi: 10.1016/j.ebiom.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laird GM, Eisele EE, Rabi SA, Lai J, Chioma S, Blankson JN, et al. Rapid Quantification of the Latent Reservoir for HIV-1 Using a Viral Outgrowth Assay. [cited 2013 Sep 27];PLoS Pathog [Internet] 2013 May;9(5) doi: 10.1371/journal.ppat.1003398. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3667757/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laird GM, Bullen CK, Rosenbloom DIS, Martin AR, Hill AL, Durand CM, et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest. 2015 Mar 30; doi: 10.1172/JCI80142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Ortiz AM, Klase ZA, DiNapoli SR, Vujkovic-Cvijin I, Carmack K, Perkins MR, et al. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. 2015 Aug 19; doi: 10.1038/mi.2015.75. This study used a combination of probiotics and IL-21, which improved Th17 number and function in the gut, reversed microbial translocation. Additionally, authors found that improvements were independent of loss of viral control and immune activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks DG, Kitchen SG, Kitchen CM, Scripture-Adams DD, Zack JA. Generation of HIV latency during thymopoiesis. Nat Med. 2001 Apr;7(4):459–64. doi: 10.1038/86531. [DOI] [PubMed] [Google Scholar]
- 40.Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. Latent HIV-1 infection of resting CD4+ T cells in the humanized Rag2−/− γc−/− mouse. J Virol. 2012 Jan;86(1):114–20. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, Cortado R, et al. HIV Latency in the Humanized BLT Mouse. J Virol. 2012 Jan;86(1):339–47. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Policicchio BB, Pandrea I, Apetrei C. Animal Models for Hiv Cure Research. Front Immunol. 2016 Jan;28:2–15. doi: 10.3389/fimmu.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia JV. In vivo platforms for analysis of HIV persistence and eradication. J Clin Invest. 2016 Feb 1;126(2):424–31. doi: 10.1172/JCI80562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, et al. Generation of HIV latency in humanized BLT mice. J Virol. 2012 Jan;86(1):630–4. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006 Jul 15;108(2):487–92. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 46.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, et al. Broadly Neutralizing Antibodies and Viral Inducers Decrease Rebound from HIV-1 Latent Reservoirs in Humanized Mice. Cell. 2014 Aug 28;158(5):989–99. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honeycutt JB, Wahl A, Archin N, Choudhary S, Margolis D, Garcia JV. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology. 2013 Oct 24;10(1):121. doi: 10.1186/1742-4690-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jth, Bixby D, Savona MR, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–51. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Josefsson L, Eriksson S, Sinclair E, Ho T, Killian M, Epling L, et al. Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA. J Infect Dis. 2012 Jul 1;206(1):28–34. doi: 10.1093/infdis/jis301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Jaafoura S, de Goër de Herve MG, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo MC, et al. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4+ memory T Cells. Nat Commun. 2014;5:5407. doi: 10.1038/ncomms6407. This study used a mathematical model to show that the HIV reservoir decays slowly around a core of stably infected memory-T-stem-cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, et al. Persistence of infectious HIV on follicular dendritic cells. J Immunol Baltim Md 1950. 2001 Jan 1;166(1):690–6. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 52.Smith-Franklin BA, Keele BF, Tew JG, Gartner S, Szakal AK, Estes JD, et al. Follicular dendritic cells and the persistence of HIV infectivity: the role of antibodies and Fcgamma receptors. J Immunol Baltim Md 1950. 2002 Mar 1;168(5):2408–14. doi: 10.4049/jimmunol.168.5.2408. [DOI] [PubMed] [Google Scholar]
- 53.Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol. 2008 Jun;82(11):5548–61. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thacker TC, Zhou X, Estes JD, Jiang Y, Keele BF, Elton TS, et al. Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells. J Virol. 2009 Jan;83(1):150–8. doi: 10.1128/JVI.01652-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol Baltim Md 1950. 2014 Dec 1;193(11):5613–25. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012 Sep 4;122(9):3281–94. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blackburn MJ, Zhong-Min M, Caccuri F, McKinnon K, Schifanella L, Guan Y, et al. Regulatory and Helper Follicular T Cells and Antibody Avidity to Simian Immunodeficiency Virus Glycoprotein 120. J Immunol Baltim Md 1950. 2015 Oct 1;195(7):3227–36. doi: 10.4049/jimmunol.1402699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci. 2014 Feb 11;111(6):2307–12. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999 Feb 5;283(5403):857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 60.Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998 Jan;72(1):164–9. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lifson JD, Rossio JL, Piatak M, Parks T, Li L, Kiser R, et al. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001 Nov;75(21):10187–99. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011 Mar 10;117(10):2791–9. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 63.Hütter G, Nowak D, Mossner M, Ganepola S, Müß;ig A, Allers K, et al. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. N Engl J Med. 2009 Feb 12;360(7):692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 64.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014 Sep 2;161(5):319–27. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henrich TJ, Hanhauser E, Harrison LJ, Palmer CD, Romero-Tejeda M, Jost S, et al. CCR5-Δ32 Heterozygosity, HIV-1 Reservoir Size, and Lymphocyte Activation in Individuals Receiving Long-term Suppressive Antiretroviral Therapy. J Infect Dis. 2015 Oct 28; doi: 10.1093/infdis/jiv504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Younan PM, Polacino P, Kowalski JP, Hu SL, Kiem HP. Combinatorial hematopoietic stem cell transplantation and vaccination reduces viral pathogenesis following SHIV89.6P-challenge. Gene Ther. 2015 Dec;22(12):1007–12. doi: 10.1038/gt.2015.83. This study combined HSCT and vaccinia based vaccination with SIV-gag, pol in Pigtail Macaques to show a reduced viremia during both acute and chronic infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliot J, Solomon A, Wightman F, Smith M, Palmer S, Prince M, et al. Effect of multiple doses of vorinostat on HIV transcription in HIV- infected patients receiving combination antiretroviral therapy. Altanta. 2013 [Google Scholar]
- 68.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012 Jul 26;487(7408):482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014 Oct;1(1):e13–21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 70.Wei DG, Chiang V, Fyne E, Balakrishnan M, Barnes T, Graupe M, et al. Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing. PLoS Pathog. 2014 Apr 10;10(4):e1004071. doi: 10.1371/journal.ppat.1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014 Mar;58(6):883–90. doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Prete GQ, Shoemaker R, Oswald K, Lara A, Trubey CM, Fast R, et al. Effect of suberoylanilide hydroxamic acid (SAHA) administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected indian rhesus macaques. Antimicrob Agents Chemother. 2014 Nov;58(11):6790–806. doi: 10.1128/AAC.03746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ling B, Piatak M, Rogers L, Johnson AM, Russell-Lodrigue K, Hazuda DJ, et al. Effects of treatment with suppressive combination antiretroviral drug therapy and the histone deacetylase inhibitor suberoylanilide hydroxamic acid; (SAHA) on SIV-infected Chinese rhesus macaques. PloS One. 2014;9(7):e102795. doi: 10.1371/journal.pone.0102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.Del Prete GQ, Oswald K, Lara A, Shoemaker R, Smedley J, Macallister R, et al. Elevated plasma viral loads in romidepsin treated SIV-infected rhesus macaques on suppressive combination antiretroviral therapy. Antimicrob Agents Chemother. 2015 Dec 28; doi: 10.1128/AAC.02625-15. This study replicated Romidepsin treatment of ART-treated Rhesus Macaques showing an increase in SIV but no decrease in reservoir size. This study shows that LRA treatments are safe and effective in NHP, showing similar results to human clinical trials, therefore other combinations on top of Romidepsin can be tested in NHP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005 Jun;64(6):529–36. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 76.Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2014 Jul;35(7):1755–68. doi: 10.1016/j.neurobiolaging.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zayyad Z, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND) Curr HIV/AIDS Rep. 2015 Mar;12(1):16–24. doi: 10.1007/s11904-014-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clements JE, Li M, Gama L, Bullock B, Carruth LM, Mankowski JL, et al. The central nervous system is a viral reservoir in simian immunodeficiency virus--infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J Neurovirol. 2005;11:180–9. doi: 10.1080/13550280590922748-1. [DOI] [PubMed] [Google Scholar]
- 79**.Marcondes MCG, Morsey B, Emanuel K, Lamberty BG, Flynn CT, Fox HS. CD8+ T cells maintain suppression of simian immunodeficiency virus in the central nervous system. J Infect Dis. 2015 Jan 1;211(1):40–4. doi: 10.1093/infdis/jiu401. This study specifically depleted CD8+ T-cells in the brain of Rhesus Macaques which lead to increased viremia, indicating that even when virus cannot be detected in the CNS, host immune mechanisms maintain virus suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beck SE, Queen SE, Viscidi R, Johnson D, Kent SJ, Adams RJ, et al. Central nervous system-specific consequences of simian immunodeficiency virus Gag escape from major histocompatibility complex class I-mediated control. J Neurovirol. 2016 Jan 4; doi: 10.1007/s13365-015-0420-5. This study found that SIV virus from MHC-I Mane-A1 084:01 Rhesus Macaques (RM) had virus in the CNS was that mutated to become less infectious. When this virus was infected into uninfected RM, mutated virus did not efficiently infect the periphery, but did cause viremia in the CNS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010 Nov 15;202(10):1553–61. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008 Oct 1;112(7):2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]


