Abstract
Objective
The influence of study design variables and publication year on response to medication and placebo was investigated in clinical trials for social anxiety disorder (SAD), generalized anxiety disorder (GAD), and panic disorder (PD).
Method
Hierarchical linear modeling determined whether publication year, treatment assignment (medication vs. placebo), study type (placebo-controlled or active comparator), study duration, and the number of study visits affected the mean change associated with medication and placebo.
Results
In the 66 trials examined, the change associated with both medication and placebo increased over time (t = 4.23, df = 39, P < .001), but average drug–placebo differences decreased over time (t = −2.04, df = 46, P = .047). More severe baseline illness was associated with greater drug–placebo differences for serotonin norepinephrine reuptake inhibitors (SNRIs, t = 3.46, df = 106, P = .001) and selective serotonin reuptake inhibitors (SSRI, t = 10.37, df = 106, P < .001). Improvement with medication was significantly greater in active-comparator studies compared to placebo-controlled trials (t = 3.41, df = 39, P = .002). A greater number of study visits was associated with greater symptom improvement in PD trials relative to SAD (t = 2.83, df = 39, P = .008) and GAD (t = 2.16, df = 39, P= .037).
Conclusions
Placebo response is substantial in SAD, GAD, and PD trials, and its rise over time has been associated with diminished drug–placebo differences. Study design features that influence treatment response in anxiety disorder trials include patient expectancy, frequency of follow-up visits, and baseline illness severity.
Keywords: antidepressants, anxiety/anxiety disorder, clinical trials, pharmacotherapy, treatment
Introduction
Placebo response in randomized controlled trials (RCTs) of psychopharmacologic agents has been increasing over time across diverse psychiatric disorders.[1, 2] High placebo response rates contribute to diminishing average drug–placebo differences and increasing numbers of failed trials, both of which increase the costs of drug development, delay clinical availability of new medications, and precipitate reductions in pharmaceutical company research for psychiatric disorders.[3] However, from a therapeutic perspective, harnessing and enhancing the components leading to placebo response may facilitate improvements in the clinical treatment of patients.[4]
In order to develop means of minimizing placebo response detrimental to novel drug discovery and maximize it in clinical practice, it is imperative to elucidate the mechanisms leading to placebo response. These mechanisms may differ across different psychiatric disorders. To date, conceptual models of placebo response have been developed,[5] and early neuroscientific investigations into the neural mechanisms of placebo response have been conducted in major depressive disorder (MDD).[6] In contrast, little systematic research has been conducted on the magnitude and mechanisms of placebo response in anxiety disorders, which contributes to continuing high placebo response rates, costly failed trials, and ultimately to a paucity of new anxiolytic agents approved by the Food and Drug Administration (FDA) over the past 20 years.[7–9]
The available literature on placebo response in anxiety disorders suggests that disorders such as social anxiety disorder (SAD), generalized anxiety disorder (GAD), and panic disorder (PD) are associated with high placebo response rates, perhaps comparable to those observed in MDD, while obsessive–compulsive disorder (OCD) spectrum illnesses may have lower rates of placebo response.[10] Few if any correlates of placebo response in anxiety disorders have been reported, whether in terms of clinical/demographic characteristics of subjects or study design variables,[8, 11–13] which hampers the efforts of investigators to improve signal detection and clinicians to optimize patient care. In addition, the available studies are limited by nonsystematic and partial reviews of the literature, small sample sizes, and meta-analytic methodology that do not permit the dissection of disparate nonpharmacologic treatment factors.
The goal of the present study was to address these shortcomings in the literature by analyzing treatment response in RCTs for the anxiety disorders with the highest reported rates of placebo response (SAD, GAD, and PD). By means of hierarchical linear modeling (HLM) methods successfully utilized in several previous publications,[2, 14, 15] we estimated the magnitude of placebo responses in SAD, GAD, and PD, and determined their trajectories over time. We sought to illuminate the causes of placebo response in these trials by evaluating the contributions of patient expectancy of improvement and therapeutic contact with health-care providers. We were interested not only in how these factors predicted placebo response, but also in how they combined with medication effects to produce medication response and how they influenced drug–placebo differences.
In line with the results of prior analyses, we hypothesized that the standardized mean change (SMC) observed in placebo-treated patients for the selected anxiety disorders would significantly increase from 1985 to the present, resulting in significantly decreasing drug–placebo differences over time. Similar to findings in trials for MDD, we anticipated that greater SMC would occur during medication treatment in active comparator versus placebo-controlled trials due to the increased expectation of improvement induced by receiving a known active treatment. Finally, we anticipated that a greater number of protocol visits would be associated with increased placebo response relative to medication response, leading to decreased average drug–placebo differences.
Methods
Search Strategy and Selection Criteria
Medline, PsycINFO, and PubMed were searched to identify RCTs contrasting antidepressant medication to placebo or active comparator in adults with SAD, GAD, and PD. The index terms “anxiety disorder—drug therapy,” “anxiety disorder—drug effects,” and “antianxiety agents,” in addition to the class and individual generic names of all antidepressant medications approved for use in the United States were combined using the “or” operator. Limiting these results to humans, English language articles, publication year 1985 or later, and age group ≥ 18 yielded 1,792 journal articles. Three authors (BRR, EP, and VSB) conducted a preliminary review to rule out those which were obviously not clinical trials, resulting in 236 titles. These were then sequentially examined from titles to abstracts and finally paper texts to determine whether they met inclusion or exclusion criteria (see Fig. 1).
Figure 1. Literature review and selection of studies.
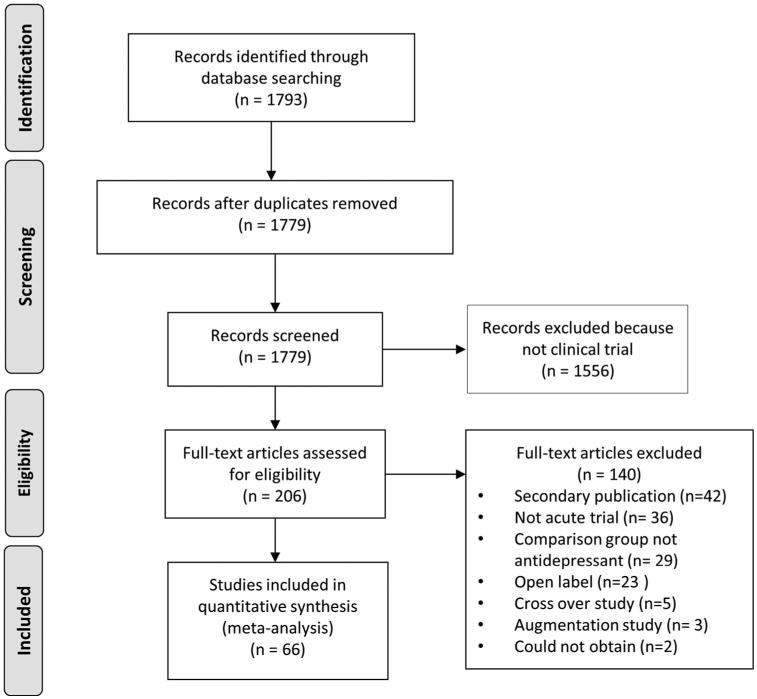
Inclusion criteria stipulated that articles report an RCT of an antidepressant medication to treat SAD, GAD, or PD in adult outpatients. We chose to restrict this analysis to antidepressant medications and exclude other psychopharmacologic treatments for anxiety (e.g., benzodiazepines, antipsychotics, anticonvulsants, nutraceuticals) in order to select for a relatively homogeneous sample of studies differing mainly on our independent variables of interest and to minimize the influence of unblinding effects in the data. We were not primarily interested in calculating effect sizes for different psychopharmacologic treatments, but rather we wished to evaluate the effect of study design characteristics on medication and placebo response. Antidepressant medications are the most frequently studied class of psychopharmacologic agents in anxiety disorders, and restricting this analysis to antidepressants permits comparison with complementary meta-analyses we have performed in MDD trials.
Further criteria required trials to last between 6 and 24 weeks (inclusive), have a comparison group of placebo or another antidepressant medication, be written in English, be published 1985 or later, and have symptom change measured using a standardized outcome measure. Trials also were excluded for enrolling treatment-resistant patients, those requiring as inclusion criteria specific symptoms beyond those used for diagnosis or severity threshold, a specific medical illness, or an Axis I disorder other than those specified above.
Data Extraction
Study information such as the year of publication, sample size, and presence of a lead-in period in addition to the clinical and demographic characteristics of participants, details of the treatment conditions, duration of active treatment in each study, and the number of study visits were entered into a database. Medications were classified as selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), or other (a group that included monoamine oxidase inhibitors [MAOIs] due to the small number of trials as well as atypical antidepressants such as nefazodone and mirtazapine). We started counting the number of visits proscribed in each study with the initiation of treatment (i.e., we began with the week 1 visit and did not count evaluation or screening appointments).
Outcome data were extracted in the form of pre–post change scores as well as response rates. Our primary hypotheses focused on mean symptom change from baseline, but we planned a priori to repeat the analyses using response rate data in order to investigate the robustness of the study results. Because different scales were used to measure pre–post change (particularly across the different anxiety disorders studied), it was necessary to standardize the change scores published for each treatment condition in the studies comprising our sample. Our primary method was to calculate SMC by dividing the pre–post mean difference by the number of total points possible on the scale used. For example, the standardized change for a treatment cell in which subjects improved by 12 points on the Hamilton Anxiety Rating Scale (HARS, each item rated 0–4, maximum score 56) was calculated to be .214. For studies reporting standard deviations of the pre- and posttreatment severity scores, we also calculated SMC by dividing the mean change for a given treatment cell by its pretreatment standard deviation, and then we compared the results obtained with the first method.
Since there was also variability in the criteria different studies used to define treatment response, we standardized the response rate data to the extent that was possible. If studies reported multiple response rates based upon different outcome measures, we selected one response rate for extraction according to the following priority list of definitions: HARS ≥ 50% decrease from baseline, clinical global impressions (CGI) improvement score of 1 or 2, and clinical anxiety scale (CAS) ≥ 50% decrease from baseline.
Data Analyses
Differences in study characteristics, patient demographics, and clinical features across the different study types were investigated using two-tailed independent samples t tests for continuous variables and chi-square (χ2) tests for categorical variables (SPSS version 21).
To identify factors significantly associated with the SMC observed in the treatment cells within our sample, we utilized an HLM approach[16–18] similar to that we successfully implemented in several prior manuscripts, where the procedures are described in greater detail.[14, 19, 20] This approach entails first examining the heterogeneity in treatment change across studies by calculating H and I2 statistics for an unconditional model. This model consists of a single level 1 (i.e., within study) equation that assumes the mean change in each treatment cell among the studies in the sample is equal to a constant. At level 2 (i.e., between studies), this constant can be described as varying around a grand mean with error. The H statistic (H = √{χ2/(df – 1)}) can be used to measure this variability in treatment change, approximating 1 when there is only random variation between studies and progressively exceeding 1 as the results of a set of studies lack homogeneity.[21] The I2 statistic(I2 = {H2 – 1}/H2) describes the proportion of total variation in treatment change that is attributable to heterogeneity.[22]
If there was significant variability in mean change across studies (i.e., the 95% confidence interval for H did not include 1), we attempted to explain this variability by means of our hypothesized within- and between-study variables. Within-study (level 1) variables included receiving medication versus placebo, standardized baseline severity score, sample size, and treatment assignment × baseline severity interactions. We then tested year of publication, the number of study sites, diagnosis (SAD, GAD, PD), the presence of single-blind lead-in periods, study type (placebo-controlled vs. comparator), the number of study visits, and study duration as fixed effects in the level 2 equation. Diagnosis × duration, diagnosis × visits, diagnosis × study type, and diagnosis × lead-in period interactions were examined. Finally, we added the cross-level interactions of treatment assignment × visits, treatment assignment × duration, and treatment assignment × publication year. All of the regression models were estimated using HLM 6.08.
Results
Characteristics of Included Studies and Participants
Sixty-six studies met the inclusion and exclusion criteria (Table 1).[23–88]. As shown in Table 2, these included 110 medication conditions (N = 19 SAD, 38 GAD, 53 PD) enrolling 11,435 participants and 59 placebo conditions (N = 14 SAD, 23 GAD, 22 PD) enrolling 6,655 participants. Within each diagnostic group, there were no significant differences between participants receiving medication and placebo in patient age, study duration, the number of study visits, pretreatment symptom severity, or dropout rate. Ninety-one percent (60/66) of the studies in our sample were industry funded, compared to 4.5% (3/66) government-funded. Funding source could not be determined for an additional 3/66.
Table 1. Summary of included studies and participants.
| Study | Treatment | Study duration | N (ITT) | Outcome measure | SMC | Response rate measure | Response rate |
|---|---|---|---|---|---|---|---|
| Allgulander et al.[23] | Paroxetine | 12 | 44 | LSAS | .23 | CGI | 71 |
| Placebo | 48 | .06 | 8 | ||||
| Allgulander et al.[24] | Sertraline | 12 | 184 | HARS | .14 | HARS | 31 |
| Placebo | 189 | .21 | 18 | ||||
| Allgulander et al.[25] | Duloxetine | 10 | 668 | HARS | .2 | HARS | 51 |
| Placebo | 495 | .14 | 33 | ||||
| Allgulander et al.[26] | Venlafaxine ER | 12 | 129 | LSAS | .25 | CGI | 69 |
| Paroxetine | 128 | .13 | 66 | ||||
| Placebo | 132 | .25 | 36 | ||||
| Asakura et al.[27] | Fluvoxamine | 10 | 176 | LSAS | .2 | CGI | 45 |
| Placebo | 89 | .15 | 30 | ||||
| Asnis et al.[28] | Fluvoxamine | 8 | 87 | PDSS | CGI | 64 | |
| Placebo | 92 | 42 | |||||
| Baldwin et al.[29] | Paroxetine | 12 | 139 | LSAS | .2 | CGI | 66 |
| Placebo | 151 | .11 | 32 | ||||
| Baldwin et al.[30] | Placebo | 12 | 138 | HARS | .3 | CGI | 62 |
| Escitalopram 5mg | 134 | .29 | 71 | ||||
| Escitalopram 10 mg | 134 | .28 | 78 | ||||
| Escitalopram 20 mg | 132 | .26 | 72 | ||||
| Paroxetine 20 mg | 136 | .25 | 66 | ||||
| Ball et al.[31] | Paroxetine | 8 | 25 | HARS | HARS | 68 | |
| Sertraline | 28 | 61 | |||||
| Ballenger et al.[32] | Placebo | 10 | 69 | HARS | .13 | CGI | 52 |
| Paroxetine 10 mg | 67 | .13 | 58 | ||||
| Paroxetine 20 mg | 70 | .19 | 75 | ||||
| Paroxetine 40 mg | 72 | .12 | 81 | ||||
| Bandelow et al. [33] | Sertraline | 12 | 112 | HARS | .22 | CGI | 85 |
| Paroxetine | 113 | .22 | 83 | ||||
| Bielski et al.[34] | Escitalopram | 24 | 60 | HARS | .27 | CGI | 78 |
| Paroxetine | 61 | .24 | 62 | ||||
| Bizdan et al.[35] | Placebo | 8 | 151 | HARS | .17 | HARS | 40 |
| Vortioxetine | 150 | .23 | 62 | ||||
| Den Boer and Westenberg[36] | Maprotiline | 6 | 24 | HARS | .23 | HARS | 21 |
| Fluvoxamine | 20 | .13 | 50 | ||||
| Bose et al.[37] | Placebo | 8 | 135 | HARS | HARS | 42 | |
| Escitalopram | 125 | 53 | |||||
| Venlafaxine ER | 125 | 52 | |||||
| Bradwejin et al.[38] | Venlafaxine ER | 10 | 160 | CGI | 55 | ||
| Placebo | 168 | 78 | |||||
| Brawman-Mintzer et al.[39] | Placebo | 10 | 162 | HARS | .2 | HARS | 48 |
| Sertraline | 164 | .23 | 59 | ||||
| Caillard et al.[40] | Clomipramine 75–100 mg | 8 | 62 | HARS | .28 | ||
| Clomipramine 30–60 mg | 61 | .25 | |||||
| Placebo | 57 | .17 | |||||
| Davidson et al.[41] | Placebo | 8 | 153 | HARS | .2 | CGI | 58 |
| Escitalopram | 154 | .13 | 38 | ||||
| Davidson et al.[42] | Fluvoxamine CR | 12 | 131 | HARS | .48 | CGI | 34 |
| Placebo | 126 | .23 | 17 | ||||
| Evans et al.[43] | Zimeldine | 6 | 16 | HARS | .08 | ||
| Imipramine | 19 | .04 | |||||
| Placebo | 9 | .23 | |||||
| Gelenberg et al.[44] | Venlafaxine ER | 28 | 115 | HARS | .16 | HARS | 70 |
| Placebo | 123 | .24 | 40 | ||||
| Kasper et al.[45] | Escitalopram | 12 | 177 | LSAS | .24 | CGI | 54 |
| Placebo | 176 | .19 | 39 | ||||
| Kim et al.[46] | Venlafaxine ER | 8 | 30 | HARS | .36 | HARS | 91 |
| Paroxetine | 30 | .35 | 92 | ||||
| Kobak et al.[47] | Fluoxetine | 14 | 30 | LSAS | .16 | CGI | 40 |
| Placebo | 30 | .16 | 30 | ||||
| Lader and Scotto[48] | Hydroxyzine | 4 | 81 | HARS | .16 | HARS | 42 |
| Buspirone | 82 | .19 | 36 | ||||
| Placebo | 81 | .13 | 29 | ||||
| Lader et al.[49] | Placebo | 12 | 166 | LSAS | .24 | CGI | 38 |
| Escitalopram 5mg | 167 | .28 | 57 | ||||
| Escitalopram 10 mg | 167 | .27 | 51 | ||||
| Escitalopram 20 mg | 170 | .27 | 55 | ||||
| Paroxetine 20 mg | 169 | .2 | 58 | ||||
| Lecrubier et al.[50] | Paroxetine | 9 | 123 | HARS | 56 | ||
| Clomipramine | 121 | 51 | |||||
| Placebo | 123 | 35 | |||||
| Leinonen et al.[51] | Placebo | 8 | 96 | MFQ-P | .13 | ||
| Citalopram 10–15 mg | 97 | .15 | |||||
| Citalopram 20–30 mg | 95 | .13 | |||||
| Citalopram 40–60 mg | 89 | .13 | |||||
| Clomipramine 60–90 mg | 98 | .16 | |||||
| Lenox-Smith and Reynolds[52] | Venlafaxine | 24 | 122 | HARS | .21 | HARS | 53 |
| Placebo | 122 | .25 | 48 | ||||
| Lepola et al.[53] | Paroxetine | 12 | 186 | LSAS | .21 | CGI | 57 |
| Placebo | 184 | .13 | 30 | ||||
| Liebowitz et al.[54] | Sertraline | 12 | 205 | HARS | .02 | CGI | 47 |
| Placebo | 196 | .06 | 26 | ||||
| Liebowitz et al.[55] | Placebo | 12 | 144 | LSAS | .27 | CGI | 36 |
| Venlafaxine ER | 133 | .15 | 59 | ||||
| Paroxetine | 136 | .24 | 63 | ||||
| Liebowitz et al.[56] | Venlafaxine ER | 12 | 133 | LSAS | .12 | CGI | 44 |
| Placebo | 138 | .21 | 30 | ||||
| Liebowitz et al.[57] | Placebo | 8 | 28 | CGI | 23 | ||
| Atenolol | 28 | 30 | |||||
| Phenelzine | 29 | 64 | |||||
| Londborg et al.[58] | Placebo | 12 | 45 | PF | 41 | ||
| Sertraline 50 mg | 43 | 57 | |||||
| Sertraline 100 mg | 44 | 57 | |||||
| Sertraline 200 mg | 45 | 57 | |||||
| Lydiard et al.[59] | Desipramine | 12 | 28 | HARS | .25 | PF | 85 |
| Placebo | 28 | .17 | 76 | ||||
| Mavissakalian and Perel[60] | Placebo | 8 | 17 | CGA | .08 | CGA | 14 |
| Imipramine (0.5 mg/kg) | 18 | .23 | 24 | ||||
| Imipramine (1.5 mg/kg) | 20 | .30 | 48 | ||||
| Imipramine (3.0 mg/kg) | 25 | .13 | 43 | ||||
| Michelson et al.[61] | Fluoxetine | 12 | 90 | HARS | .27 | No. panic | 82 |
| Placebo | 90 | .18 | Attacks | 61 | |||
| Michelson et al.[62] | Placebo | 10 | 78 | HARS | .13 | CGI | 46 |
| Fluoxetine 10 mg | 84 | .15 | 64 | ||||
| Fluoxetine 20 mg | 81 | .09 | 55 | ||||
| Modigh et al.[63] | Placebo | 12 | 17 | .14 | |||
| Imipramine | 29 | .09 | |||||
| Clomipramine | 22 | HARS | .04 | ||||
| Nair et al.[64] | Fluvoxamine | 8 | 43 | CAS | .18 | CGI | 37 |
| Imipramine | 42 | .30 | 64 | ||||
| Placebo | 47 | .15 | 47 | ||||
| Nimatoudis et al.[65] | Venlafaxine ER | 8 | 24 | HARS | .19 | HARS | 92 |
| Placebo | 22 | .34 | 27 | ||||
| Oehrberg et al.[66] | Paroxetine | 12 | 60 | HARS | 85 | ||
| Pohl et al.[67] | Buspirone | 8 | 16 | HARS | 25 | ||
| Imipramine | 14 | 7 | |||||
| Placebo | 14 | 14 | |||||
| Pollack et al.[68] | Placebo | 12 | 156 | HARS | .22 | CGI | 56 |
| Venlafaxine ER 75 mg | 158 | .17 | 77 | ||||
| Venlafaxine ER 150 mg | 159 | .22 | 79 | ||||
| Paroxetine | 161 | .21 | 81 | ||||
| Pollack et al.[69] | Placebo | 12 | 157 | PDSS | .43 | CGI | 60 |
| Venlafaxine ER 75 mg | 156 | .31 | 81 | ||||
| Venlafaxine ER 225 mg | 160 | .46 | 85 | ||||
| Paroxetine 40 mg | 151 | .41 | 83 | ||||
| Pollack et al.[70] | Sertraline | 10 | 88 | HARS | .15 | CGI | 57 |
| Placebo | 88 | .17 | 47 | ||||
| Pollack et al.[71] | Placebo | 8 | 163 | HARS | .22 | CGI | 47 |
| Paroxetine | 161 | .18 | 62 | ||||
| Ribeiro et al.[72] | Mirtazapine | 8 | 14 | HARS | .30 | ||
| Fluoxetine | 13 | .27 | |||||
| Rickels et al.[73] | Placebo | 12 | 135 | LSAS | .15 | CGI | 33 |
| Venlafaxine ER | 126 | .23 | 50 | ||||
| Rickels et al.[74] | Placebo | 8 | 96 | HARS | .17 | ||
| Venlafaxine ER 75 mg | 86 | .22 | |||||
| Venlafaxine ER 150 mg | 81 | .21 | |||||
| Venlafaxine ER 225 mg | 86 | .20 | |||||
| Rickels et al.[75] | Placebo | 8 | 180 | HARS | .22 | CGI | 46 |
| Paroxetine 20 mg | 188 | .22 | 62 | ||||
| Paroxetine 40 mg | 197 | .17 | 68 | ||||
| Rothschild et al.[76] | Vortioxetine 5 mg | 8 | 148 | HARS | .24 | HARS | 53 |
| Placebo | 151 | .22 | 50 | ||||
| Rynn et al.[77] | Duloxetine | 10 | 168 | HARS | .15 | HARS | 40 |
| Placebo | 159 | .11 | 32 | ||||
| Sheehan et al.[78] | Buspirone | 8 | 17 | HARS | .11 | ||
| Imipramine | 17 | .13 | |||||
| Placebo | 17 | .08 | |||||
| Stahl et al.[79] | Placebo | 10 | 114 | HARS | .09 | HARS | 38 |
| Escitalopram | 125 | .11 | 50 | ||||
| Citalopramine | 112 | .09 | 39 | ||||
| Stein et al.[80] | Fluvoxamine | 12 | 42 | LSAS | .15 | CGI | 43 |
| Placebo | 44 | .05 | 23 | ||||
| Stein et al.[81] | Paroxetine | 12 | 91 | LSAS | .21 | CGI | 55 |
| Placebo | 92 | .10 | 24 | ||||
| Van Ameringen et al.[82] | Sertraline | 20 | 134 | MFQ-S | .06 | CGI | 53 |
| Placebo | 69 | .19 | 29 | ||||
| Van Ameringen et al.[83] | Nefazodone | 14 | 51 | LSAS | .16 | CGI | 31 |
| Placebo | 51 | .1 | 24 | ||||
| Van Vilet et al.[84] | Fluvoxamine | 12 | 15 | HARS | .15 | LSAS | 46 |
| Placebo | 13 | .04 | 7 | ||||
| Wade et al.[85] | Placebo | 8 | 96 | HARS | .18 | CAS | 34 |
| Citalopram 10–15 mg | 97 | .19 | 44 | ||||
| Citalopram 20–30 mg | 95 | .21 | 59 | ||||
| Citalopram 40–60 mg | 89 | .21 | 51 | ||||
| Clomipramine 60–90 mg | 98 | .13 | 51 | ||||
| Wen-yuan et al.[86] | Duloxetine | 15 | 108 | HARS | .26 | HARS | 69 |
| Placebo | 102 | .21 | 53 | ||||
| Westenberg et al.[86] | Fluvoxamine CR | 12 | 146 | LSAS | .25 | CGI | 48 |
| Placebo | 151 | .19 | 44 |
SMC, pre–post treatment difference in means divided by the number of total points possible on the scale used; HARS, Hamilton Anxiety Rating Scale; LSAS, Liebowitz Social Anxiety Scale; CGI, clinician's global scale of improvement; CAS, clinical anxiety scale; PDSS, panic disorder severity scale; MFQ-S, marks fear questionnaire, social phobia subscale; MFQ-P, marks fear questionnaire, phobia scale; CGA, clinician's global assessment.
Table 2. Clinical characteristics of included patients and methodological features of studies.
| SAD | GAD | PD | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Characteristic | Medication | Placebo | Medication | Placebo | Medication | Placebo |
| No. of treatment groups | 19 | 14 | 38 | 23 | 53 | 22 |
| No. of patients | 2,054 | 1,347 | 5,461 | 3,659 | 3,920 | 1,638 |
| Mean age | 37.1 ± 1.8 | 37.4 ± 1.4 | 40.3 ± 2.8 | 40.3 ± 2.7 | 36.4 ± 2.1 | 36.2 ± 2.0 |
| Mean pretreatment severity | 89.0 ± 6.7a | 87.9 ± 6.7a | 27.4 ± 11.0b | 28.6 ± 13.8b | 20.6 ± 3.3b | 20.6 ± 3.2b |
| Mean study duration | 12.2 ± 2.4 | 12.6 ± 2.5 | 11.1 ± 5.2 | 11.0 ± 5.3 | 9.4 ± 2.0 | 9.7 ± 1.9 |
| Mean number visits | 6.6 ± 1.8 | 6.7 ± 1.6 | 6.4 ± 1.7 | 6.3 ± 1.7 | 6.6 ± 1.8 | 6.8 ± 1.7 |
| Mean dropout rate | 25.6 ± 15.4 | 22.5 ± 16.1 | 27.4 ± 11.1 | 28.6 ± 13.8 | 18.4 ± 13.2 | 21.0 ± 14.8 |
| Mean response rate | 52.5 ± 10.8c | 27.7 ± 10.7c | 59.5 ± 15.6c | 38.9 ± 12.1c | 59.1 ± 20.1c | 46.5 ± 17.6c |
Severity measured by Liebowitz Social Anxiety Scale.
Severity measured by Hamilton Anxiety Rating Scale.
Medication versus placebo comparison within disorder P < .05.
Between diagnostic groups, trials significantly differed in mean study duration (F(2,170) = 7.638, P = .001], sample size [F(2,170) = 14.904, P < .001), dropout rates [F(2,164) = 5.846, P = .004], mean participant age [F(2,160) = 49.068, P < .001], and overall response rates [F(2/146) = 5.566, P = .005]. Compared to trials for SAD and GAD, trials for PD were smaller ([t = 2.402, df = 106, P = .009] and [t = 5.299, df = 138, P < .001], respectively), of shorter duration ([t = 6.534, df = 106, P < .001] and [t = 2.181, df = 138, P = .031], respectively), and enrolled younger participants ([t = 2.162, df = 96, P = .034] and [t = 9.142, df = 131, P < .001], respectively). Additionally, trials for PD had lower dropout rates relative to trials for GAD (t = 3.382, df = 132, P = .001). There were no significant clinical or demographic differences between trials for SAD and GAD.
Treatment Outcomes for Medication and Placebo in SAD, GAD, AND PD
In the unconditional model of treatment change, variability was over 31 times greater than expected by chance alone (H = 31.3, 95% CI = 27.5–35.6), and the proportion of variability in mean change caused by heterogeneity rather than random error was 99.8% (I2 = .998). In the studies examined, overall mean placebo response was 40.6% ± 13.2, compared to a mean active medication response rate of 58.6% ± 13.9. Controlling for all other variables, placebo response was significantly higher in PD trials relative to trials for SAD (OR 2.47, 95% CI = 1.68–3.64, P < .001) and GAD (OR 1.89, 95% CI = 1.27–2.82, P = .003). There was significantly greater change with placebo in PD trials compared to trials for SAD (t = 2.39, df = 39, P = .022) and a trend toward greater change with placebo in PD trials compared to trials for GAD (t = 2.030, df = 39, P = .086).
Controlling for publication year, baseline symptom severity, and diagnosis, each individual antidepressant medication class was associated with significantly greater improvement in anxiety symptoms compared to placebo (TCA: t = 3.10, df = 106, P = .003; SNRI: t = 7.85, df = 106, P < .001; Other AD: t = 3.34, df = 106, P < .001; SSRI: t = 9.67, df = 106, P < .001). The same results in favor of active medication over placebo were obtained by analyzing response rates (TCA: OR = 1.79, 95% CI = 1.46–2.19,P < .001; SNRI: OR2.37, 95% CI = 1.85–3.03, P < .001; Other AD: OR = 1.59, 95% CI = 1.25–2.03, P < .001; SSRI: OR = 2.05, 95% CI = 1.87–2.25, P < .001). For both the SMC and response rate analyses, no antidepressant class was superior to another overall, and no significant medication × diagnosis interactions were observed, indicating that the effect of medication classes on anxiety symptoms did not differ by disorder.
As expected, baseline symptom severity was a significant predictor of SMC (t = 5.25, df = 106, P < .001), likely reflecting the fact that there is more room for change to occur when starting from a higher baseline. More severe baseline illness was associated with greater drug–placebo differences for SNRI and SSRI drug groups (baseline severity × SNRI: t = 3.46, df = 106, P = .001; baseline severity × SSRI: t = 10.37, df = 106, P < .001) but not “Other AD” or TCA (baseline severity × Other AD: t = −1.33, df = 106, P = .18; baseline severity × TCA: t = −0.34, df = 106, P = .74).
Trajectory of Medication and Placebo Treatment Outcomes Over Time
Across disorders, and controlling for other variables, the SMC associated with both medication and placebo increased over time (t = 4.23, df = 39, P < .001), and there was a trend for baseline illness severity to increase over time (t = 1.75, df = 106, P = .082). A significant publication year × treatment assignment interaction (t = −2.04, df = 46, P = .047) indicated that the average drug–placebo difference in the studies examined decreased over time. Further exploration of the model revealed that decreased drug–placebo differences occurred because the change associated with placebo increased at a faster rate than the change associated with active medication. Controlling for other variables, the average subject assigned to placebo experienced 3.4 additional points of improvement on the HARS per decade since 1985, resulting in an average decrease in the drug–placebo difference of 2.3 HARS points per decade.
In the analyses of response rates across the entire sample, there was a significant main effect of publication year on medication and placebo response (OR = 1.03, 95% CI = 1.01–1.06, P = .043), such that participants were increasingly likely to be classified as responders with each 1 year increment after 1985. The rate of rise of placebo response over time outpaced medication response, resulting in the differential odds of treatment response between medication and placebo decreasing over time (treatment assignment × year of publication OR = 0.98, 95% CI = 0.97–0.99, P = .006).
Figure 2 plots the SMC associated with antidepressant medication and placebo against year of publication for each of the individual anxiety disorders. The mean improvement observed in patients receiving medication increased significantly with year of publication for PD (N = 43, r = .45, P = .002) and SAD (N = 17, r = .53, P = .027) but not GAD (N = 34, r = .19, P = .283). Similarly, the mean improvement observed in patients receiving placebo increased significantly with year of publication for PD (N = 16, r = .69, P = .003) and SAD (N = 13, r = .67, P = .012), but not for GAD (N = 22, r = .28, P = .204).
Figure 2.
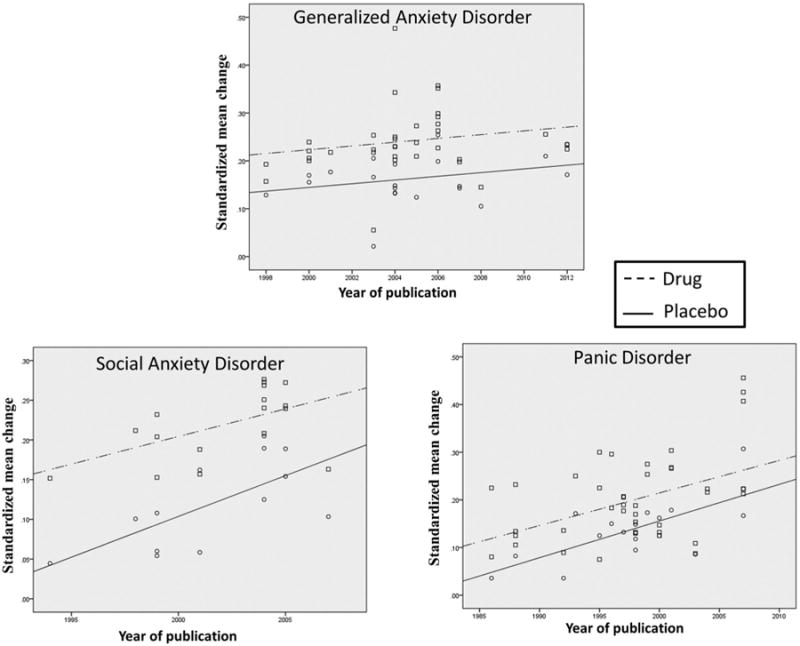
Relationship of standardized mean change (SMC) to year of publication for patients receiving antidepressant medication or placebo. SMC was significantly positively correlated with year of publication for medication cells in Panic Disorder (PD, N = 43, r = 0.45, p = 0.002) and Social Anxiety Disorder (SAD, N = 17, r = 0.53, p = 0.027) but not Generalized Anxiety Disorder (GAD, N = 34, r = 0.19, p = 0.283). The mean change observed in patients receiving placebo also increased significantly with year of publication for PD (N = 16, r = 0.69, p = 0.003) and SAD (N = 13, r = 0.67, p = 0.012) but not for GAD (N = 22, r = 0.28, p = 0.204).
Effect of Study Design Variables on Treatment Outcomes for Medication and Placebo
Coefficients, odds ratios, and accompanying statistical tests for the predictor variables in the final model of SMC are presented in Table 3. Overall, the final mixed model of SMC significantly improved model fit over the unconditional model (χ2 = 65.1, df = 14, P < .001) and explained 72.7% of the original variability in mean change.
Table 3. Multilevel meta-analysis of SMC.
| Model of SMC | |||||
|---|---|---|---|---|---|
|
| |||||
| Fixed effects | Coefficient (SE) | t | df | P | |
| Within study (level 1) predictors | |||||
| TCA | .085 (.027) | 3.103 | 106 | .003 | |
| SNRI | .076 (.0096) | 7.850 | 106 | < .001 | |
| SSRI | .074 (.0077) | 9.667 | 106 | < .001 | |
| Other AD | .060 (.018) | 3.343 | 106 | < .001 | |
| Baseline severity | .20 (.038) | 5.246 | 106 | < .001 | |
| Severity × TCA | −.016 (.048) | −.336 | 106 | .74 | |
| Severity × SNRI | .25 (.073) | 3.464 | 106 | .001 | |
| Severity × SSRI | .16 (.0149) | 10.369 | 106 | < .001 | |
| Severity × Other AD | −.37 (.273) | −1.337 | 106 | .18 | |
| Severity × year | .022 (.013) | 1.75 | 106 | .084 | |
| Between study (level 2) predictors | |||||
| Intercept | .15 (.015) | 10.108 | 39 | < .001 | |
| Year | .0060 (.0014) | 4.225 | 39 | < .001 | |
| Design | .050 (.028) | 3.411 | 39 | .002 | |
| Duration | .0020 (.0012) | 1.617 | 39 | .114 | |
| Visits | −.009 (.0049) | −1.752 | 39 | .087 | |
| SP | −.019 (.012) | −1.553 | 39 | .128 | |
| PD | .037 (.019) | 1.876 | 39 | .068 | |
| Visits SP | −.0106 (.0095) | −1.119 | 39 | .270 | |
| Visits × PD | .019 (.007) | 2.826 | 39 | .008 | |
|
| |||||
| Random effect | Coefficient | Variance | df | χ2 | P |
|
| |||||
| Intercept | .037 | .00126 | 39 | 146.15 | < .001 |
This table provides coefficients, odds ratios, and statistical tests for the predictor variables examined in the full model of treatment change. “Year” refers to the year of publication for each study in the sample, centered on the year 1985. “PD” and “SP” are dummy variables coded 1 if the diagnosis is present or 0 otherwise (in this classification of the data, GAD is the reference group). “Design” is a dummy variable coded one for comparator trials and zero otherwise, making the statistics associated with it relative to placebo-controlled trials. “Visits” denotes the number of clinic visits in each study, centered on the overall mean for visits in the sample (mean visits = 6.4 ± 1.7 visits). The statistics associated with “visits” provide the difference in mean change between one additional visit relative to the mean visits. “Duration” is the duration of treatment in each study, centered on the overall mean for duration in the sample (mean duration = 10.0 ± 3.4 weeks). The statistics associated with “duration” provide the difference in mean change between one additional week duration relative to the mean duration. “Baseline severity” is the pretreatment symptom score, standardized by dividing by the maximum number of points possible on the scale used. “TCA” is a dummy variable coded 1 for TCAs and 0 otherwise, and this classification was also used to create dummy variable for “SSRI,” “SNRI,” and “Other AD.” The coefficients associated with “TCA,” “SSRI,” “SNRI,” and “Other AD” represent the difference in mean change between patients receiving these medication classes compared to placebo, controlling for other study variables. Positive coefficients indicate greater pre–post change (i.e., greater improvement relative to baseline), whereas negative numbers indicate less pre–post change.
Medication treatment in comparator study designs was associated with significantly more improvement (t = 3.41, df = 39, P = .002) and increased response rates (OR = 1.79, 95% CI = 1.01–3.19, P = .045) relative to medication treatment in placebo-controlled trials. Longer study durations were associated with increased medication and placebo response (OR = 1.04, 95% CI = 1.01–1.08, P = .034) but not greater SMC for medication and placebo (t = 1.62, df = 39, P = .114). This effect of study duration was not significantly different across diagnostic groups or between medication and placebo.
Controlling for study type and duration, a greater number of study visits was associated with a trend toward decreased SMC (t = −1.752, df = 39, P = .087), but not decreased response rates (OR = 0.94, 95% CI = 0.82–1.07, P = .317) across all disorders. However, the effect of study visits differed by diagnostic group, as more study visits were associated with significantly greater improvement in PD trials relative to trials for SAD (t = 2.27, df = 39, P = .028) and GAD (t = 2.83, df = 39, P = .008). There was no significant effect of study visits on SMC in GAD trials relative to trials for SAD (t = 0.549, df = 40, P = .586). No significant study visits × treatment assignment or visits × treatment assignment × diagnosis interactions were observed, indicating that the number of study visits did not significantly influence signal detection.
Although the number of study sites was significantly correlated with both the mean change (N = 50, r = .361, P = .019) and response rates (N = 53, r = .542, P < .001) associated with placebo, it did not explain significant additional variability in mean change scores when added to the mixed models containing year of publication (t = 0.967, df = 45, P = .339). This likely occurred because year of publication and the number of study sites were themselves significantly correlated (N = 62, r = .468, P < .001), and year of publication had the stronger relationship with mean change. Single-blind lead-in periods did not explain significant variability in SMC (t = 0.070, df = 45, P = .945), nor were there any significant lead-in × treatment assignment interactions, suggesting that the presence or absence of lead-in periods did not influence signal detection in the trials analyzed.
To investigate the robustness of these findings across different methods of standardizing mean treatment change, we repeated the above analyses after recalculating SMC using the standard deviation statistics for each treatment cell. Forty-one (62.1%) of the 66 studies provided pretreatment standard deviations or information on variability that could be used to calculate standard deviations. We found the results obtained by computing SMC by dividing the pre–post mean difference by the pooled pretreatment standard deviation were highly correlated with the results of our preferred method of calculating mean change (r = .70, P < .001). Additionally, the overall pattern of results obtained by using pooled standard deviations to calculate SMC was similar to the above.
Effect of Study Design Variables on Dropout Rates
In order to more comprehensively understand the influence of study design on treatment outcome in these anxiety disorder trials, we also examined its relationship to attrition in a parallel HLM analysis. Results showed dropout rates were not significantly different between each individual antidepressant medication class relative to placebo (TCA: t = −1.481, df = 147, P = .140; SNRI: t = −1.436, df = 147, P = .663; Other AD: t = −0.405, df = 147, P = .685; SSRI: t = 0.390, df = 147, P = .696). Publication year was not a significant predictor of dropout rates overall (t = −0.420, df = 57, P = .676) or for drug–placebo differences in dropout. Neither study design (t = −1.106, df = 50, P = .275), duration (t = 1.112, df = 50, P = .272), nor lead-in periods (t = 0.734, df = 50, P = .402) influenced dropout rates, but a greater number of study visits was found to significantly decrease dropout rates for SNRIs relative to placebo (t = −1.746, df = 137, P = .023).
Discussion
This analysis found that the mean symptom improvement observed in subjects assigned to placebo in RCTs of antidepressant medications for anxiety disorders has been significantly increasing over the past 30 years. Placebo response rose across disorders, but was greatest for PD relative to trials for SAD and GAD. Controlling for other factors, the average improvement associated with placebo for patients with PD nearly doubled in the 30 years between 1985 and 2015 (from a mean of 8.6 points on the HARS to 16.7 points). This striking increase in placebo response was associated with significant decreases in drug–placebo differences over time. Despite this pattern of placebo response, there remained a clear benefit in favor of antidepressant medication over placebo in the treatment of anxiety disorders, and this benefit was particularly pronounced in more severely ill patients.
Rising placebo response in clinical trials for anxiety disorders parallels contemporaneous rises observed in MDD, schizophrenia, and bipolar disorder, suggesting that common factors across psychiatric diagnostic groups may explain this trend.[1, 2, 89] One study design factor correlated with rising placebo response in MDD and schizophrenia has been the number of study sites, which have generally increased over time as RCTs have shifted from smaller, academic, single-site trials toward larger, commercially operated, multicenter trials.[90, 91] Academic sites often entail increased time and expense associated with institutional review board approval, but commercial sites, particularly those operated by contract research organizations (CROs), have arguably more powerful financial incentives to enroll patients, which can result in baseline score inflation by raters followed by a rapid decline in scores once the restrictive entrance criterion has been passed.[92] The number of study sites was found to increase over time in this analysis, but this variable did not remain a significant predictor of mean change once year of publication was taken into account.
Greater patient expectancy of improvement has been linked to rising placebo response in MDD,[14] and results from this analysis provide supporting evidence for this relationship in anxiety disorder trials. Medication response was significantly higher in active comparator studies (in which subjects know they are receiving a medication believed to be effective for their condition) relative to placebo-controlled study designs (in which patients are aware they may receive placebo). Increased expectation of improvement based on this knowledge may lead to improved treatment response in patients with anxiety disorders, just as appears to be the case in MDD. Another possibility is that direct advertising and educational campaigns for the first drug to be approved for SAD (paroxetine) might have increased expectations of improvement among patients entering later SSRI and SNRI trials.
Despite earlier suggestions in the literature to the contrary,[93, 94] the presence of single-blind lead-ins did not significantly affect the average pre–post treatment change observed, and there were no lead-in × treatment assignment interactions to suggest that placebo response was preferentially reduced. Prior analyses have similarly shown that lead-ins are not effective in increasing drug–placebo differences in clinical trials for MDD.[19, 20, 95] One possible explanation is that raters could be biased in favor of higher ratings during the lead-in period in order to maintain study eligibility for the maximum number of subjects. Consequently, lead-ins in which subjects experiencing significant early symptom decreases are removed from the analysis thus may not be beneficial from a study design perspective, since they may function to reduce available power without enhancing signal detection.
Other potential sources of increased placebo response that may be shared across psychiatric disorders include rater bias and recruitment of symptomatic volunteers using advertising. Rater bias occurs when an individual's rating of symptom severity in an antidepressant clinical trial is influenced by underlying beliefs or motivations with respect to the treatments under study.[96] One approach to limiting rater bias is to utilize centralized raters to perform the screening and outcome measures in clinical trials, since centralized raters are less prone to bias by virtue of their off-site location and blinding to study entry criteria, patient phase of treatment, and treatment assignment.[97] Finally, whereas most research participants in the 1960s and 1970s were recruited from in-patient psychiatric units, current participants are symptomatic volunteers responding to advertisements.[98] Studies are needed to compare the baseline characteristics, treatment response, and attrition rates between self-referred depressed patients and those who respond to advertisements, since it is possible the latter group's symptoms are more variable and transient, resulting in increased placebo response rates.
In contrast to these common sources of increased placebo response between clinical trials for anxiety disorders and other psychiatric conditions, important differences also were observed. The amount of supportive care provided to clinical trial participants (operationalized in this analysis by the number of study visits) was associated with greater symptom improvement in PD trials but exhibited a trend toward symptom worsening in trials for SAD and GAD. Multiple prior reports in MDD have found that increasing numbers of study visits increase placebo response.[20, 99] Therapeutic aspects of more frequent clinical management may involve increased empathic support (akin to that provided in supportive psychotherapy), behavioral activation, and exposure to symptom assessments, as well as finer grained titration of medication dosages (for flexible-dose study designs). Although speculative, it may be the case that some of these elements, such as behavioral activation and exposure, are more effective in the treatment of MDD and PD than in SAD and GAD.
A number of limitations should be considered when interpreting the findings of this study. One of these concerns the use of trial-level summary data, as we were unable to test for associations between individual patient characteristics and the effects of study design features. Publication bias may have affected which studies were included in these analyses, since RCTs failing to demonstrate significant differences between medication and placebo may not have been published. Unpublished studies would tend to limit the power available to detect impact of differences in study design. Study quality was not formally assessed for the studies meeting our selection criteria, so it is possible that between-study quality differences played a role in the results obtained. Also, we determined the number of visits based upon the designed visit schedule for each study rather than upon the actual number of visits that each participant attended or the actual quantity of time each participant spent with study clinicians, which could not be ascertained from the published data. Finally, outcomes were standardized across a heterogeneous set of measures that may have differing sensitivities for separating medication and placebo responses in these disorders, especially when the symptoms in question are shared with those of normative anxiety.
One clinical lesson to be drawn from these results is that enhancing patient expectancy may help improve treatment outcomes. Although specific means of increasing expectancy remain to be studied, helpful approaches may include therapeutic optimism on the part of the clinician as well as proper patient education about the likelihood of response to medication treatment. Moreover, frequent study visits may be helpful for patients with PD, who may benefit from vigorous dose titration, side effect monitoring, and the exposure therapy implicit in discussing their symptoms. Conversely, strategies suggested by these results that may improve signal detection in RCTs include dispensing with single-blind lead-in periods, minimizing patient expectancy by maximizing the probability of receiving placebo, and powering studies appropriately given the high anticipated rates of placebo response. More research is needed at the individual patient level to identify individual characteristics associated with decreased propensity to respond to placebo.
In summary, results from this meta-analysis confirm that placebo response is substantial in SAD, GAD, and PD, and its rise over time has been associated with diminished drug–placebo differences. Study design features that influence treatment response in anxiety disorder trials include patient expectancy, the frequency of follow-up visits, and baseline illness severity. Clinicians as well as researchers may keep these variables in mind as potential means of manipulating placebo response to suit the goals of their treatment setting (i.e., clinical practice vs. drug development).
Acknowledgments
Dr. Rutherford had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Rutherford reports receiving consulting fees from Pfizer. Dr. Schneier reports receiving research funding from Forest Labs (now known as Actavis), serving on a scientific advisory board for Genetech, and receiving payment for writing for UpToDate.
Footnotes
Conflict of interest. Bailey, Pott, Dr. Brown, and Dr. Roose have no disclosures or conflicts of interest to report. This paper has not been previously presented.
References
- 1.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. J Am Med Assoc. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford BR, Pott E, Tandler JM, et al. Placebo response in antipsychotic clinical trials: a meta-analysis. J Am Med Assoc Psychiatry. 2014;71:1409–1421. doi: 10.1001/jamapsychiatry.2014.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72:115–127. doi: 10.1159/000069738. [DOI] [PubMed] [Google Scholar]
- 4.Andrews G. Placebo response in depression: bane of research, boon to therapy. Br J Psychiatry. 2001;178:192–194. doi: 10.1192/bjp.178.3.192. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford BR, Roose SP. A model of placebo effects in antidepressant clinical trials. Am J Psychiatry. 2013;170:723–733. doi: 10.1176/appi.ajp.2012.12040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 7.Schweizer E, Rickels K. Placebo response in generalized anxiety: its effect on the outcome of clinical trials. J Clin Psychiatry. 1997;58(Suppl 11):30–38. [PubMed] [Google Scholar]
- 8.Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B. Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of Escitalopram. J Clin Psychiatry. 2006;67:1741–1746. doi: 10.4088/jcp.v67n1111. [DOI] [PubMed] [Google Scholar]
- 9.Piercy MA, Sramek JJ, Kurtz NM, Cutler NR. Placebo response in anxiety disorders. Ann Pharmacother. 1996;30:1013–1019. doi: 10.1177/106002809603000917. [DOI] [PubMed] [Google Scholar]
- 10.Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug–placebo differences across psychiatric disorders. Psychol Med. 2005;35:743–749. doi: 10.1017/s0033291704003873. [DOI] [PubMed] [Google Scholar]
- 11.Feltner D, Hill C, Lenderking W, Williams V, Morlock R. Development of a patient-reported assessment to identify placebo responders in a generalized anxiety disorder trial. J Psychiatr Res. 2009;43:1224–1230. doi: 10.1016/j.jpsychires.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Uhlenhuth EH, Matuzas W, Warner TD, Thompson PM. Growing placebo response rate: the problem in recent therapeutic trials? Psychopharmacol Bull. 1997;33:31–39. [PubMed] [Google Scholar]
- 13.Cohen D, Consoli A, Bodeau N, et al. Predictors of placebo response in randomized controlled trials of psychotropic drugs for children and adolescents with internalizing disorders. J Child Adolesc Psychopharmacol. 2010;20:39–47. doi: 10.1089/cap.2009.0047. [DOI] [PubMed] [Google Scholar]
- 14.Rutherford BR, Sneed JR, Roose SP. Does study design affect outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom. 2009;78:172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutherford BR, Sneed JR, Tandler J, Peterson BS, Roose SP. Deconstructing pediatric depression trials: an analysis of the effects of expectancy and therapeutic contact. J Am Acad Child Adolesc Psychiatry. 2011;50:782–795. doi: 10.1016/j.jaac.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryk AS, Raudenbush SW. Hierarchical Linear Models. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- 17.Hox J. Multilevel Analysis: Techniques and Applications. Mahwah, NJ: Lawrence Erlbaum Publishers; 2002. [Google Scholar]
- 18.Haddock CK, Rindskopf D, Shadish WR. Using odds ratios as effect sizes for meta-analysis of dichotomous data: a primer on methods and issues. Psychol Methods. 1998;3:339–353. [Google Scholar]
- 19.Rutherford BR, Sneed JR, Tandler J, Peterson BS, Roose SP. Deconstructing pediatric depression trials: an analysis of the effects of expectancy and therapeutic contact. J Am Acad Child Adolesc Psychiatry. 2011;50:782–795. doi: 10.1016/j.jaac.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutherford BR, Tandler J, Brown P, Sneed JR, Roose SP. Clinic visits in late life depression trials: effects on signal detection and therapeutic outcome. Am J Geriatr Psychiatry. 2014;22:1452–1461. doi: 10.1016/j.jagp.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Takkouche B, Cadarso Surez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150:206–215. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 23.Allgulander C. Paroxetine in social anxiety disorder: a randomized placebo-controlled study. Acta Psychiatr Scand. 1999;100:193–198. doi: 10.1111/j.1600-0447.1999.tb10845.x. [DOI] [PubMed] [Google Scholar]
- 24.Allgulander C, Dahl AA, Austin C, et al. Efficacy of sertraline in a 12-week trial for generalized anxiety disorder. Am J Psychiatry. 2004;161:1642–1649. doi: 10.1176/appi.ajp.161.9.1642. [DOI] [PubMed] [Google Scholar]
- 25.Allgulander C, Hartford J, Russell J, et al. Pharmacotherapy of generalized anxiety disorder: results of duloxetine treatment from a pooled analysis of three clinical trials. Curr Med Res Opin. 2007;23:1245–1252. doi: 10.1185/030079907X182202. [DOI] [PubMed] [Google Scholar]
- 26.Allgulander C, Mangano R, Zhang J, et al. Efficacy of venlafaxine ER in patients with social anxiety disorder: a double-blind, placebo-controlled, parallel-group comparison with paroxetine. Hum Psychopharmacol. 2004;19:387–396. doi: 10.1002/hup.602. [DOI] [PubMed] [Google Scholar]
- 27.Asakura S, Tajima O, Koyama T. Flovoxamine treatment of generalized social anxiety disorder in Japan: a randomized double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2007;10:263–274. doi: 10.1017/S1461145706006602. [DOI] [PubMed] [Google Scholar]
- 28.Asnis GM, Hameedi FA, Goddard AW, et al. Fluvoxamine in the treatment of panic disorder: a multi-center, double-blind, placebo-controlled study in outpatients. Psychiatry Res. 2001;103:1–14. doi: 10.1016/s0165-1781(01)00265-7. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin D, Bobes J, Stein DJ, Scharwachter I, Faure M. Paroxetine in social phobia/social anxiety disorder. Randomised, double-blind, placebo-controlled study. Paroxetine study group Br J Psychiatry. 1999;175:120–126. doi: 10.1192/bjp.175.2.120. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin DS, Huusom AK, Maehlum E. Escitalopram and paroxetine in the treatment of generalised anxiety disorder: randomised, placebo-controlled, double-blind study. Br J Psychiatry. 2006;189:264–272. doi: 10.1192/bjp.bp.105.012799. [DOI] [PubMed] [Google Scholar]
- 31.Ball SG, Kuhn A, Wall D, Shekhar A, Goddard AW. Selective serotonin reuptake inhibitor treatment for generalized anxiety disorder: a double-blind, prospective comparison between paroxetine and sertraline. J Clin Psychiatry. 2005;66:94–99. doi: 10.4088/jcp.v66n0113. [DOI] [PubMed] [Google Scholar]
- 32.Ballenger JC, Wheadon DE, Steiner M, Bushnell W, Gergel IP. Double-blind, fixed dose, placebo-controlled study of paroxetine in the treatment of panic disorder. Am J Psychiatry. 1998;155:36–42. doi: 10.1176/ajp.155.1.36. [DOI] [PubMed] [Google Scholar]
- 33.Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65:405–413. doi: 10.4088/jcp.v65n0317. [DOI] [PubMed] [Google Scholar]
- 34.Bielski RJ, Bose A, Chang CC. A double-blind comparison of escitalopram and paroxetine in the long-term treatment of generalized anxiety disorder. Ann Clin Psychiatry. 2005;17:65–69. doi: 10.1080/10401230590932326. [DOI] [PubMed] [Google Scholar]
- 35.Bidzan L, Mahableshwarkar AR, Jacobsen P, Yan M, Sheehan DV. Vortioxetine (Lu AA21004) in generalized anxiety disorder: results of an 8-week, multinational, randomized, double-blind, placebo-controlled clinical trial. Eur Neuropsychopharma-col. 2012;22:847–857. doi: 10.1016/j.euroneuro.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Den Boer JA, Westenberg HG. Effect of a serotonin and noradrenaline uptake inhibitor in panic disorder; a double-blind comparative study with fluvoxamine and maprotiline. Int Clin Psychopharmacol. 1988;3:59–74. doi: 10.1097/00004850-198801000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Bose A, Korotzer A, Gommoll C, Li D. Randomized placebo-controlled trial of escitalopram and venlafaxine XR in the treatment of generalized anxiety disorder. Depress Anxiety. 2008;25:854–861. doi: 10.1002/da.20355. [DOI] [PubMed] [Google Scholar]
- 38.Bradwejn J, Ahokas A, Stein DJ, et al. Venlafaxine extended-release capsules in panic disorder: flexible-dose, double-blind, placebo-controlled study. Br J Psychiatry. 2005;187:352–359. doi: 10.1192/bjp.187.4.352. [DOI] [PubMed] [Google Scholar]
- 39.Brawman-Mintzer O, Knapp RG, Rynn M, Carter RE, Rickels K. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67:874–881. doi: 10.4088/jcp.v67n0603. [DOI] [PubMed] [Google Scholar]
- 40.Caillard V, Rouillon F, Viel JF, Markabi S. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. French University Antidepressant Group. Acta Psychiatr Scand. 1999;99:51–58. doi: 10.1111/j.1600-0447.1999.tb05384.x. [DOI] [PubMed] [Google Scholar]
- 41.Davidson JR, Bose A, Korotzer A, Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depress Anxiety. 2004;19:234–240. doi: 10.1002/da.10146. [DOI] [PubMed] [Google Scholar]
- 42.Davidson J, Yaryura-Tobias J, DuPont R, et al. Fluvoxamine-controlled release formulation for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:118–125. doi: 10.1097/01.jcp.0000106222.36344.96. [DOI] [PubMed] [Google Scholar]
- 43.Evans L, Kenardy J, Schneider P, Hoey H. Effect of a selective serotonin uptake inhibitor in agoraphobia with panic attacks. A double-blind comparison of zimeldine, imipramine and placebo. Acta Psychiatr Scand. 1986;73:49–53. doi: 10.1111/j.1600-0447.1986.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 44.Gelenberg AJ, Lydiard RB, Rudolph RL, et al. Efficacy of venlafaxine extended-release capsules in nondepressed outpatients with generalized anxiety disorder: a 6-month randomized controlled trial. J Am Med Assoc. 2000;283:3082–3088. doi: 10.1001/jama.283.23.3082. [DOI] [PubMed] [Google Scholar]
- 45.Gommoll C, Durgam S, Mathews M, et al. A double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone inpatients with generalized anxiety disorder. Depress Anxiety. 2015;32:451–459. doi: 10.1002/da.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasper S, Stein DJ, Loft H, Nil R. Escitalopram in the treatment of social anxiety disorder: randomised, placebo-controlled, flexible-dosage study. Br J Psychiatry. 2005;186:222–226. doi: 10.1192/bjp.186.3.222. [DOI] [PubMed] [Google Scholar]
- 47.Kim TS, Pae CU, Yoon SJ, et al. Comparison of venlafaxine extended release versus paroxetine for treatment of patients with generalized anxiety disorder. Psychiatry Clin Neurosci. 2006;60:347–351. doi: 10.1111/j.1440-1819.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 48.Kobak KA, Gerist JH, Jefferson JW, Katzelnick DJ. Fluoxetine in social phobia: a double-blind, placebo-controlled pilot study. J Clin Psychopharmacol. 2002;22:257–262. doi: 10.1097/00004714-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Lader M, Scotto JC. A multicentre double-blind comparison of hydroxyzine, buspirone and placebo in patients with generalized anxiety disorder. Psychopharmacology (Berl) 1998;139:402–406. doi: 10.1007/s002130050731. [DOI] [PubMed] [Google Scholar]
- 50.Lader M, Stender K, Burger V, Nil R. Efficacy and tolerability of escitalopram in 12- and 24-week treatment of social anxiety disorder: randomised, double-blind, placebo-controlled, fixed-dose study. Depress Anxiety. 2004;19:241–248. doi: 10.1002/da.20014. [DOI] [PubMed] [Google Scholar]
- 51.Lecrubier Y, Bakker A, Dunbar G, Judge R. A comparison of paroxetine, clomipramine and placebo in the treatment of panic disorder. Collaborative Paroxetine Panic Study Investigators. Acta Psychiatr Scand. 1997;95:145–152. doi: 10.1111/j.1600-0447.1997.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 52.Leinonen E, Lepola U, Koponen H, et al. Citalopram controls phobic symptoms in patients with panic disorder: randomized controlled trial. J Psychiatry Neurosci. 2000;25:24–32. [PMC free article] [PubMed] [Google Scholar]
- 53.Lenox-Smith AJ, Reynolds A. A double-blind, randomised, placebo controlled study of venlafaxine XL in patients with generalised anxiety disorder in primary care. Br J Gen Pract. 2003;53:772–777. [PMC free article] [PubMed] [Google Scholar]
- 54.Lepola U, Bergtholdt B, St Lambert J, Davy KL, Ruggiero L. Controlled-release paroxetine in the treatment of patients with social anxiety disorder. J Clin Psychiatry. 2004;65:222–229. doi: 10.4088/jcp.v65n0213. [DOI] [PubMed] [Google Scholar]
- 55.Liebowitz MR, DeMartinis NA, Weihs K, et al. Efficacy of sertraline in severe generalized social anxiety disorder: results of a double-blind, placebo-controlled study. J Clin Psychiatry. 2003;64:785–792. doi: 10.4088/jcp.v64n0708. [DOI] [PubMed] [Google Scholar]
- 56.Liebowitz MR, Gelenberg AJ, Munhack D. Venlafaxine extended release vs. placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry. 2005;62:190–198. doi: 10.1001/archpsyc.62.2.190. [DOI] [PubMed] [Google Scholar]
- 57.Liebowitz MR, Mangano RM, Bradwejn J, Asnis G SAD study group. A randomized controlled trial of venlafaxine extended release in generalized anxiety disorder. J Clin Psychiatry. 2005;66:238–247. doi: 10.4088/jcp.v66n0213. [DOI] [PubMed] [Google Scholar]
- 58.Liebowitz MR, Schneier F, Campeas R, et al. Phenelzine vs. atenolol in social phobia. A placebo-controlled comparison. Arch Gen Psychiatry. 1992;49:290–300. doi: 10.1001/archpsyc.49.4.290. [DOI] [PubMed] [Google Scholar]
- 59.Londborg PD, Wolkow R, Smith WT, et al. Sertraline in the treatment of panic disorder. A multi-site, double-blind, placebo-controlled, fixed-dose investigation. Br J Psychiatry. 1998;173:54–60. doi: 10.1192/bjp.173.1.54. [DOI] [PubMed] [Google Scholar]
- 60.Lydiard RB, Morton WA, Emmanuel NP, et al. Preliminary report: placebo-controlled, double-blind study of the clinical and metabolic effects of desipramine in panic disorder. Psychopharmacol Bull. 1993;29:183–188. [PubMed] [Google Scholar]
- 61.Mavissakalian MR, Perel JM. Imipramine treatment of panic disorder with agoraphobia: dose ranging and plasma level-response relationships. Am J Psychiatry. 1995;152:673–682. doi: 10.1176/ajp.152.5.673. [DOI] [PubMed] [Google Scholar]
- 62.Michelson D, Allgulander C, Dantendorfer K, et al. Efficacy of usual antidepressant dosing regimens of fluoxetine in panic disorder: randomised, placebo-controlled trial. Br J Psychiatry. 2001;179:514–518. doi: 10.1192/bjp.179.6.514. [DOI] [PubMed] [Google Scholar]
- 63.Michelson D, Lydiard RB, Pollack MH, et al. Outcome assessment and clinical improvement in panic disorder: evidence from a randomized controlled trial of fluoxetine and placebo. The Fluoxetine Panic Disorder Study Group. Am J Psychiatry. 1998;155:1570–1577. doi: 10.1176/ajp.155.11.1570. [DOI] [PubMed] [Google Scholar]
- 64.Modigh K, Westburg P, Eriksson E. Superiority of clomipramine over imipramine in the treatment of panic disorder: a placebo-controlled trial. J Clin Psychopharmacol. 1992;12:251–261. [PubMed] [Google Scholar]
- 65.Nair NP, Bakish D, Saxena B, et al. Comparison of fluvoxamine, imipramine, and placebo in the treatment of outpatients with panic disorder. Anxiety. 1996;2:192–198. doi: 10.1002/(SICI)1522-7154(1996)2:4<192::AID-ANXI6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 66.Nimatoudis I, Zissis NP, Kogeorgos J, et al. Remission rates with venlafaxine extended release in Greek outpatients with generalized anxiety disorder. A double-blind, randomized, placebo controlled study. Int Clin Psychopharmacol. 2004;19:331–336. doi: 10.1097/00004850-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Oehrberg S, Christiansen PE, Behnke K, et al. Paroxetine in the treatment of panic disorder. A randomised, double-blind, placebo-controlled study. Br J Psychiatry. 1995;167:374–379. doi: 10.1192/bjp.167.3.374. [DOI] [PubMed] [Google Scholar]
- 68.Pohl R, Balon R, Yeragani VK, Gershon S. Serotonergic anxiolytics in the treatment of panic disorder: a controlled study with buspirone. Psychopathology. 1989;22(Suppl 1):60–67. doi: 10.1159/000284627. [DOI] [PubMed] [Google Scholar]
- 69.Pollack MH, Lepola U, Koponen H, et al. A double-blind study of the efficacy of velafaxine extended-release, paroxetine, and placebo in the treatment of panic disorder. Depress Anxiety. 2007;24:1–14. doi: 10.1002/da.20218. [DOI] [PubMed] [Google Scholar]
- 70.Pollack M, Mangano R, Entusuah R, et al. A randomized controlled trial of velafaxine ER and paroxetine in the treatment of outpatients with panic disorder. Psychopharmacology (Berl) 2007;194:233–242. doi: 10.1007/s00213-007-0821-0. [DOI] [PubMed] [Google Scholar]
- 71.Pollack MH, Otto MW, Worthington JJ, Manfro GG, Wokow R. Sertraline in the treatment of panic disorder: a flexible-dose multicenter trial. Arch Gen Psychiatry. 1998;55:1010–1016. doi: 10.1001/archpsyc.55.11.1010. [DOI] [PubMed] [Google Scholar]
- 72.Pollack MH, Zaninelli R, Goddard A, et al. Paroxetine in the treatment of generalized anxiety disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:350–357. doi: 10.4088/jcp.v62n0508. [DOI] [PubMed] [Google Scholar]
- 73.Ribeiro L, Busnello JV, Kauer-Sant'Anna M, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Braz J Med Biol Res. 2001;34:1303–1307. doi: 10.1590/s0100-879x2001001000010. [DOI] [PubMed] [Google Scholar]
- 74.Rickels K, Mangano R, Khan A. A double-blind, placebo-controlled study of a flexible dose of venlafaxine ER in adult outpatients with generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:488–496. doi: 10.1097/01.jcp.0000138764.31106.60. [DOI] [PubMed] [Google Scholar]
- 75.Rickels K, Pollack MH, Sheehan DV, Haskins JT. Efficacy of extended-release venlafaxine in nondepressed outpatients with generalized anxiety disorder. Am J Psychiatry. 2000;157:968–974. doi: 10.1176/appi.ajp.157.6.968. [DOI] [PubMed] [Google Scholar]
- 76.Rickels K, Zaninelli R, McCafferty J, Bellew K, Iyengar M, Sheehan D. Paroxetine treatment of generalized anxiety disorder: a double-blind placebo-controlled study. Am J Psychiatry. 2003;160:749–756. doi: 10.1176/appi.ajp.160.4.749. [DOI] [PubMed] [Google Scholar]
- 77.Rothschild AJ, Mahableshwarkar AR, Jacobsen P, Yan M, Sheehan DV. Vortioxetine (Lu AA21004) 5 mg in generalized anxiety disorder: results of an 8-week randomized, double-blind, placebo-controlled clinical trial in the United States. Eur Neuropsychopharmacol. 2012;22:858–866. doi: 10.1016/j.euroneuro.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Rynn M, Russell J, Erikson J, et al. Efficacy and safety of duloxetine in the treatment of generalized anxiety disorder: a flexible-dose, progressive-titration, placebo-controlled trial. Depress Anxiety. 2008;25:182–189. doi: 10.1002/da.20271. [DOI] [PubMed] [Google Scholar]
- 79.Sheehan DV, Raj AB, Harnett-Sheehan K, Soto S, Knapp E. The relative efficacy of high-dose buspirone and alprazolam in the treatment of panic disorder: a double-blind placebo-controlled study. Acta Psychiatr Scand. 1993;88:1–11. doi: 10.1111/j.1600-0447.1993.tb03405.x. [DOI] [PubMed] [Google Scholar]
- 80.Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64:1322–1327. doi: 10.4088/jcp.v64n1107. [DOI] [PubMed] [Google Scholar]
- 81.Stein MB, Fyer AJ, Davidson JR, Pollack MH, Wiita B. Fluvoxamine treatment of social phobia (social anxiety disorder): a double-blind, placebo-controlled study. Am J Psychiatry. 1999;156:756–760. doi: 10.1176/ajp.156.5.756. [DOI] [PubMed] [Google Scholar]
- 82.Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. J Am Med Assoc. 1998;280:708–713. doi: 10.1001/jama.280.8.708. [DOI] [PubMed] [Google Scholar]
- 83.Van Ameringen MA, Lane RM, Walker JR, et al. Sertraline treatment of generalized social phobia: a 20-week, double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:275–281. doi: 10.1176/appi.ajp.158.2.275. [DOI] [PubMed] [Google Scholar]
- 84.Van Ameringen M, Mancini C, Oakman J, et al. Nefazodone in the treatment of generalized social phobia: a randomized, placebo-controlled trial. J Clin Psychiatry. 2007;68:288–295. doi: 10.4088/jcp.v68n0215. [DOI] [PubMed] [Google Scholar]
- 85.Van Vliet IM, Westenberg HG, Slaap BR, den Boer JA, Ho Pian KL. Anxiogenic effects of pentagastrin in patients with social phobia and healthy controls. Biol Psychiatry. 1997;42:76–78. doi: 10.1016/S0006-3223(97)00185-6. [DOI] [PubMed] [Google Scholar]
- 86.Wade AG, Lepola U, Koponen HJ, Pederson V, Pederson T. The effect of citalopram in panic disorder. Br J Psychiatry. 1997;170:549–553. doi: 10.1192/bjp.170.6.549. [DOI] [PubMed] [Google Scholar]
- 87.Westenberg HG, Stein DJ, Yang H, Li D, Barbato LM. A double-blind placebo-controlled study of controlled release fluvoxamine for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24:49–55. doi: 10.1097/01.jcp.0000104906.75206.8b. [DOI] [PubMed] [Google Scholar]
- 88.Wu W, Wang G, Ball S, Desaiah D, Ang Q. Duloxetine versus placebo in the treatment of patients with generalized anxiety disorder in China. Chin Med J. 2011;124:3260–3268. [PubMed] [Google Scholar]
- 89.Sysko R, Walsh BT. A systematic review of placebo response in studies of bipolar mania. J Clin Psychiatry. 2007;68:1213–1217. doi: 10.4088/jcp.v68n0807. [DOI] [PubMed] [Google Scholar]
- 90.Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166:42–49. doi: 10.1176/appi.ajp.2008.08020247. [DOI] [PubMed] [Google Scholar]
- 91.Agid O, Siu CO, Potkin SG, et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry. 2013;170:1335–1344. doi: 10.1176/appi.ajp.2013.12030315. [DOI] [PubMed] [Google Scholar]
- 92.Leucht S, Heres S, Davis JM. Increasing placebo response in antipsychotic drug trials: let's stop the vicious circle. Am J Psychiatry. 2013;170:1232–1234. doi: 10.1176/appi.ajp.2013.13081129. [DOI] [PubMed] [Google Scholar]
- 93.Dager SR, Khan A, Cowley D, et al. Characteristics of placebo response during long-term treatment of panic disorder. Psychopharmacol Bull. 1990;26:273–278. [PubMed] [Google Scholar]
- 94.Albus M, Lecrubier Y, Maier W, et al. Drug treatment of panic disorder: early response to treatment as a predictor of final outcome. Acta Psychiatr Scand. 1990;82:359–365. doi: 10.1111/j.1600-0447.1990.tb01401.x. [DOI] [PubMed] [Google Scholar]
- 95.Trivedi M, Rush J. Does a placebo run-in or a placebo treatment cell affect the efficacy of antidepressant medications? Neuropsychopharmacology. 1995;11:33–43. doi: 10.1038/npp.1994.63. [DOI] [PubMed] [Google Scholar]
- 96.Marcus SM, Gorman JM, Tu X. Rater bias in a blinded randomized placebo-controlled psychiatry trial. Stat Med. 2006;25:2762–2770. doi: 10.1002/sim.2405. [DOI] [PubMed] [Google Scholar]
- 97.Kobak KA, Kane JM, Thase ME, Nierenberg AA. Why do clinical trials fail? The problem of measurement error in clinical trials: time to test new paradigms? J Clin Psychopharmacol. 2007;27:1–5. doi: 10.1097/JCP.0b013e31802eb4b7. [DOI] [PubMed] [Google Scholar]
- 98.Brody B, Leon AC, Kocsis JH. Antidepressant clinical trials and subject recruitment: just who are symptomatic volunteers? Am J Psychiatry. 2011;168:1245–1247. doi: 10.1176/appi.ajp.2011.11060864. [DOI] [PubMed] [Google Scholar]
- 99.Posternak MA, Zimmerman M. Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials. Br J Psychiatry. 2007;190:287–292. doi: 10.1192/bjp.bp.106.028555. [DOI] [PubMed] [Google Scholar]


