Abstract
With a global prevalence of 9%, diabetes is the direct cause of millions of deaths each year and is quickly becoming a health crisis. Major long-term complications of diabetes arise from persistent oxidative stress and dysfunction in multiple metabolic pathways. The most serious complications involve vascular damage and include cardiovascular disease as well as microvascular disorders such as nephropathy, neuropathy, and retinopathy. Current clinical analyses like glycated hemoglobin and plasma glucose measurements hold some value as prognostic indicators of the severity of complications, but investigations into the underlying pathophysiology are still lacking. Advancements in biotechnology hold the key to uncovering new pathways and establishing therapeutic targets. Metabolomics, the study of small endogenous molecules, is a powerful toolset for studying pathophysiological processes and has been used to elucidate metabolic signatures of diabetes in various biological systems. Current challenges in the field involve correlating these biomarkers to specific complications to provide a better prediction of future risk and disease progression. This review will highlight the progress that has been made in the field of metabolomics including technological advancements, the identification of potential biomarkers, and metabolic pathways relevant to macro- and microvascular diabetic complications.
Introduction
Diabetes, a metabolic disease defined by chronic hyperglycemia, is subcategorized as Type 1 (T1DM), characterized by youth onset and insulin dependence, and Type 2 (T2DM), characterized generally by adult onset, dyslipidemia, and insulin resistance.1 In the short term, extreme hyperglycemia may lead to severe complications such as ketoacidosis. More commonly, long-term hyperglycemia damages the vasculature and nervous system. The major cell types affected by hyperglycemia are endothelial cells and sensory neurons, which are unresponsive to insulin, as well as insulin-responsive cells like podocytes, macrophages, and Schwann cells. The long-term complications of diabetes affect renal function (nephropathy), sensory neurons (neuropathy), vision (retinopathy) and macrovascular events such as heart attack and stroke. Chronic elevation of glucose inherently leads to downstream metabolic dysfunction. The establishment of sensitive biomarkers for early complication onset, discovery of novel therapeutic targets, and characterization of aberrant metabolic pathways involved in diabetic complications remains an urgent undertaking as diabetes is projected to be the 7th leading cause of death worldwide by 2030.2
Metabolomics is the analysis of small endogenous molecules from cells, tissue or biofluids. The analytical approaches for metabolomics are generally subdivided into either untargeted (global profiling) or targeted (the analysis of a defined set of compounds). Both global and targeted metabolite analyses use universal detectors such as mass spectrometry (MS) or nuclear magnetic resonance (NMR). Global profiling aims to capture as many metabolite features as possible. These extremely large data sets are used to compare normal and disease states, followed by identification of metabolite features of interest. A strong advantage of untargeted metabolomics is the ability to uncover novel and potentially relevant metabolites which can be the basis of therapeutic approaches or prognostic indicators. However, this approach often suffers from diminished quantitation as internal standards are not available for the vast majority of metabolites detected. Large replicates are needed to overcome this limitation. Another caveat of global profiling is difficult identification of novel features. In the case of MS, even when the molecular formula is determined, database searches often yield multiple hits or no hits at all. Targeted metabolomics aims at analyzing a distinct set of compounds, often those within the same metabolic pathway or which have structural similarities. The advantage of targeted metabolomics is that commercially available standards can be used as calibrants or isotope labeling can be utilized to establish quantitation. While limited in scope, targeted metabolomics offers a deep understanding of a portion of the metabolome.
This review will focus on the application of metabolomics technologies in the analysis of diabetic complications (DCs). It will cover a general basis of the biochemical understanding of DCs before 2005, the analytical techniques most commonly used, and the implementation of these methods to gain a deeper understanding of metabolic dysfunction that leads to the pathology of DCs. The majority of this review will be confined to publications between 2005 and 2015, when metabolomics was beyond its infancy. All studies addressed here are summarized in the table. For a detailed discussion of the role of metabolomics in general diabetes, not confined to DCs, the reader is advised to seek out the reviews by Sas et al.3 and Bain et al.4
Table 1.
Summary of all metabolomics studies of DCs discussed in this review.
| Complication | Subject type | Sample type | Technique | Metabolite categories | Comments | Ref. |
|---|---|---|---|---|---|---|
| Retinopathy | Human subjects with proliferative DR | Human vitreous fluid | RPLC- and HILIC-MS | Untargeted | Increases in arginine, proline, and acylcarnitine metabolism and decreases in purine metabolism were reported | 107 |
| Retinopathy | Human subjects with proliferative DR | Human vitreous fluid | 1NMR | Untargeted | Galactitol and ascorbic acid were decreased in diabetes; NMR pattern recognition identified patients with DR with 81% specificity | 104 |
| CVD | Malmo diet and cancer cardiovascular cohort | Human plasma | HILIC LC-QQQ-MS | Branched-chain and aromatic amino acids | Branched-chain AAs indicated five-fold greater risk for inducible myocardial ischemia and atherosclerosis | 63 |
| CVD | Insulin resistance atherosclerosis study | Human plasma | NMR | Lipoproteins | Increasing LDL/HDL ratio indicate proatherogenic state and predict subsequent progression to diabetes | 50 |
| CHD, high blood pressure, nonalcoholic fatty liver disease | 292 T2DM subjects in Shanghai, China | Human serum | UPLC-QTOF-MS | Steroid, bile acid, TCA, and fatty acid metabolism | Dodecanoic and decanoic acid were strongly associated with high blood pressure, nonalcoholic fatty liver disease, and CHD. Leucine was elevated in high blood pressure and nonalcoholic fatty liver disease | 59 |
| CHD | T2DM patients with CHD | Human serum | Quantitative NMR | Amino acids, fatty acids, lipoproteins | Rosiglitazone treatment lowered circulating lactate and elevated glutamine concentrations | 52 |
| Heart failure | Heart failure patients (17% with DM) | Human serum | GC-TOF-MS | Nucleosides, sugar alcohols, urea cycle, TCA metabolites | Pseudouridine and α-ketoglutarate were significantly elevated in cases | 76 |
| CVD | T2DM patients | Human serum | 1H NMR | Lipoproteins | Size-modulated lipid distribution was altered in diabetes; pharmacological treatment had no effect on lipid configuration | 51 |
| Neuropathy | Male type 2 diabetic (db/db) mice | Sural and sciatic nerves and dorsal root ganglia of mice | Targeted LC-MS/MS | Glycolytic and TCA cycle intermediates | A proximal-to-distal increase in oxidative stress was reported, with a decrease in aconitase activity | 90 |
| Neuropathy | STZ rats | Rat plasma and spinal cord tissue | LC-MS/MS | 26 bioactive lipids | Inhibitors of soluble epoxide hydrolase elevated plasma and spinal epoxygenated fatty acid levels and resulted in antiallodynic effects | 93 |
| Neuropathy | STZ rats | Rat sciatic nerve, dorsal root ganglia, and trigeminal ganglia | GC- and LC-MS | Untargeted | Increase in polyol and glucose intermediates in all 3 tissues; dysregulation of oxidative phosphorylation and lipid metabolism only in sciatic nerve | 91 |
| Diabetic kidney disease | Patients with diabetes and chronic kidney disease | Human urine and serum | GC-MS | Organic acids and amino acids | 12 of 13 differentially expressed metabolites are linked to mitochondrial metabolism | 87 |
| Diabetic kidney disease | 52 T1DM patients from the FinnDiane study | Human urine | GC-MS and LC-MS (Fourier transform-LIT) | Global profilling | Acylcarnitines, acylglycines and Trp metabolites were capable of discriminating between progressive and normoalbuminuria with 75% accuracy | 80 |
| End-stage renal disease | Joslin kidney study cohort | Human plasma | LC-MS (UPLC/ESI/LIT) | Untargeted | Essential amino acids were depleted, while amino acid-derived acylcarnitines were increased. 16 uremic solutes were elevated years before onset of end-stage renal disease | 75 |
| Diabetic nephropathy | STZ mice | Mouse urine and plasma | RPLC-UV, HILIC-ESI-MS/MS | Untargeted | Rosiglitazone treatment restored urinary levels of glyoxylic acid, methylformamide, formylmethionine, indoxyl sulfate, and more | 78 |
| Diabetic nephropathy | db/db mice | Mouse urine and serum | GC-TOF-MS | TCA cycle metabolites | Citrate and malate levels in urine relative to serum may be indicator of kidney function | 72 |
| Diabetic nephropathy | NADPH-oxidase transgenic mice | Mouse urine | GC-MS | Organic acids | TCA cycle metabolites were increased in diabetes; fumarate levels were restored following NADPH-oxidase inhibition | 85 |
| Diabetic nephropathy | STZ rats | Serum, urine, and renal extracts from diabetic rats | 1H NMR | Untargeted | Elevations in allantoin, uric acid, and xanthine oxidase activity indicates disturbed purine metabolism and oxidative stress | 88 |
| Diabetic nephropathy | Natural history of diabetic nephropathy study | Human plasma and renal biopsies | HPLC-QQQ-MS and electron microscopy | AGEs and oxidation products | MGHI, CEL, and CML levels were higher in fast progressors | 84 |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; STZ, streptozotocin; TOF, time of flight; ELISA, enzyme-linked immunoassay; LIT, linear ion trap.
Biochemistry of diabetic complications prior to 2005
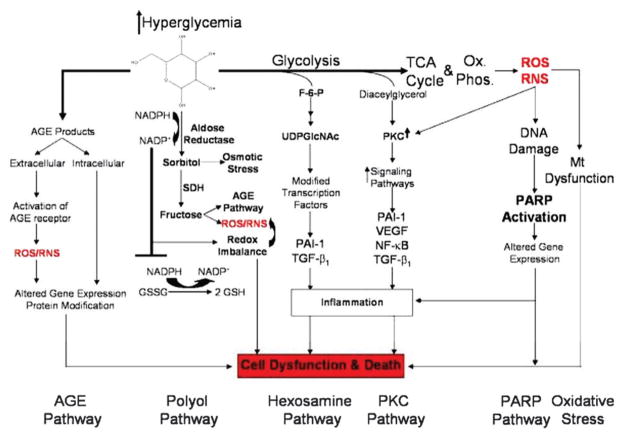
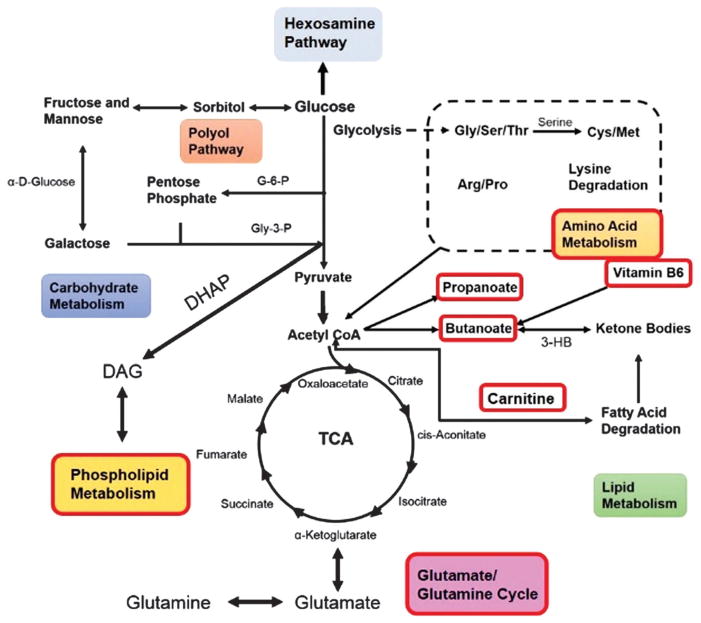
Prior to 2005, the primary theory in the field of diabetic pathophysiology was focused on five distinct pathways which are activated and exacerbated by hyperglycemia-induced oxidative and nitrosative stress: the polyol, poly(ADP-ribose) polymerase (PARP) advanced glycation end product (AGE), hexosamine and protein kinase C (PKC) pathways (Fig. 1). These salient pathways are relevant to both vascular and neuronal complications. Despite the prominence of these pathways, therapeutics which target them have proven largely ineffective in clinical studies and a deeper understanding of their biological effects is required.
Fig. 1.
Schematic of hyperglycemic effects on biochemical pathways in diabetic neuropathy. Excessive glucose metabolism generates excess NADH and leads to overload of the electron transport chain causing oxidative stress, damage to mitochondria, and activation of PARP. PARP activation by ROS acts in conjunction with the hexosamine and PKC pathway to induce inflammation and neuronal dysfunction. A combination of oxidative stress and hyperglycemia activates the detrimental pathways of AGE, polyol, hexosamine and PKC pathways which lead to redox imbalance and further oxidative stress. These pathways also induce inflammation and neuronal dysfunction. Abbreviations: NF-κB: nuclear factor kappa B; PARP: poly(ADP-ribose) polymerase; PKC: protein kinase C; AGE: advanced glycation endproducts; RNS: reactive nitrogen species; ROS: reactive oxygen species, GSH: glutathione; GSSG: oxidized glutathione; UDPGlcNAc: UDP-N-acetylglucosamine; VEGF: vascular endothelial growth factor. From Edwards, et al., Diabetic Neuropathy: Mechanisms to Management, Pharmacol. Ther., 2008, 120(1), 1–34. Used with permission.
Oxidative stress is generated in the mitochondria from the electron transport chain. Under normoglycemic conditions, a small fraction of the electrons used to generate the proton gradient and generate ATP are absorbed by oxygen to form the damaging radical superoxide. Superoxide is detoxified in the cell by glutathione and the enzymes superoxide dismutase, which converts superoxide to hydrogen peroxide, and catalase, which converts hydrogen peroxide to water. Reduced glutathione (GSH) is an endogenous scavenger of reactive oxygen species (ROS) and is converted to glutathione disulfide (GSSG) upon oxidation. GSSG is recycled back to GSH using NADPH as the cofactor. Under hyperglycemic conditions, excess electrons are generated in the electron transport chain owing to increased generation of superoxide and ROS. These ROS saturate the endogenous antioxidant response system and proceed to damage both mitochondrial and cytoplasmic environment. Oxidative stress has also been shown to inhibit the release of nitric oxide-stimulating ATP from erythrocytes, further contributing to vascular dysfunction.5,6
Oxidative stress has a profound influence on metabolism through alterations of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase. Under oxidative stress, damage to DNA occurs and PARP is activated to repair the DNA. Glyceraldehyde-3-phosphate dehydrogenase is translocated to the nucleus, where PARP deactivates it through poly(ADP-ribosyl)ation. The inhibition of glyceraldehyde-3-phosphate dehydrogenase leads to a buildup of upstream glycolytic metabolites.
Under hyperglycemic conditions, instead of being oxidized and released as CO2, glucose is converted to sorbitol via the polyol pathway or forms AGEs. Aldose reductase converts glucose to sorbitol using NADPH as a cofactor. The use of NADPH here depletes the available pool to recycle GSH and therefore increases oxidative stress. The accumulation of sorbitol is thought to damage cells by increasing osmotic stress.7 Aldose reductase inhibitors have had only a tepid effect in preventing complications.8 The combination of glucose with free amino groups of proteins, lipids, and nucleic acids create a heterogenous group of deleterious molecules, known as AGEs. Protein glycosylation in the form of AGEs can lead to protein crosslinking and modifications to the extracellular matrix. Extracellular AGEs can bind to the pro-inflammatory receptor for AGEs.9 Inflammation leading to irreversible vascular damage can persist long after normal glycemic conditions have been restored – a phenomenon known as “metabolic memory.”10
The hexosamine pathway is an offshoot of glycolysis by way of fructose-6-phosphate. The end product of the hexosamine pathway is UDP-N-acetyl glucosamine, which competes for phosphorylation post translation modification sites on transcription factors. These transcription factors regulate inflammatory responses through transcription growth factor-β.
Protein kinase C (PKC) is a serine/threonine kinase which is activated by diacyl glycerol. Diabetic patients have increases in both diacyl glycerol and PKC. PKC has multiple roles which include modulating vascular endothelial growth factors, smooth muscle contraction, haemodynamics and endothelial cell permeability and proliferation.
While vascular and neuronal complications all share common root metabolic dysfunctions, therapeutic approaches to alleviate stress on each of these pathways have generally been successful in laboratory experiments but only moderately effective in human clinical trials. Despite the overwhelming evidence implicating oxidative stress as the underlying lynchpin of DCs, multiple antioxidant therapy regimes have proven ineffective, and in some cases diabetic patients on therapy fared worse than placebo.11 Aldose reductase inhibitors12 have been unsuccessful in treating complications, though recently epalrestat has shown significant benefit to nephropathy and retinopathy.13 Alleviating stress on the hexosamine pathway has been attempted using the nutraceutical benfotiamine, though clinical trials for nephropathy failed.14 Inhibitors of AGEs action have also fallen short in both cardiovascular complications15 and nephropathy.16 Current treatments for DCs include glycemic control, pain management for neuropathy, ACE inhibitors for preventing/treating nephropathy through blood pressure control, and photocoagulation or anti-vascular endothelial growth factor treatments for retinopathy.17 While these treatments provide relief from the pathology, they do not address the underlying metabolic defects inherent to DCs.
Because metabolic dysfunction may persist for several years before manifesting as clinical diabetes,3 an urgent need exists for new biomarkers and risk predictors for early diagnosis as well as a more complete understanding of the ways in which metabolic pathways contribute to diabetic pathophysiology. Metabolomics has emerged as a useful complement to genomics and proteomics for providing insight into a variety of cellular processes and disease states because the metabolome is directly representative of phenotype as well as environmental factors.
Analytical platforms for metabolomics
The sample preparation steps required for metabolomics analyses largely depend on the type of sample matrix (cells, tissues, or biofluid) and the chemical functionality of the analytes of interest. Urine is generally treated by solid phase extraction before analysis. Mammalian tissue samples are prepared for extraction by lyophilization and pulverization. For cell cultures, a scraping step is required to detach adherent cells. Sonication/lysis of cells, plasma, and homogenized tissue is performed in an organic solvent such as acetonitrile or methanol.18,19 Proteins and large lipids are precipitated via centrifugation, and the remaining supernatant is evaporated to dryness and reconstituted in an appropriate buffer for analysis. Variations in the composition of lysing solvent have been developed for the preferential extraction of particular classes of metabolites. For example, polar lipids are often isolated using a methanol/chloroform mixture.20 In all cases, metabolite extraction should take place as quickly as possible and should proceed at low temperatures in order to effectively quench metabolism.3 This is accomplished by chilling samples and solvents with dry ice; techniques which utilize liquid nitrogen to rapidly freeze cell cultures have also been described.19
Because metabolite structures range from highly polar to hydrophobic and their molecular weights can vary by hundreds of Daltons, finding a single analytical method by which to study the wide variety of analytes involved in a particular metabolic pathway presents a challenge.21 Furthermore, metabolite concentrations can span from picomolar to millimolar levels, so detection systems capable of quantifying analytes present in extremely low abundance are necessary.22 NMR is a common analytical method used in metabolomics and is ideal for performing rapid analyses as it requires minimal sample preparation and does not require a prior chromatographic separation. However, this method suffers from relatively poor sensitivity (millimolar to high micromolar).23 Such a disadvantage means that less abundant species may not be detected, leading to an incomplete picture of the metabolic system of interest.
Gas chromatography coupled to mass spectrometry (GC-MS) is a well-established technique in metabolic research; it is used in many clinical laboratories to diagnose metabolic diseases due to high chromatographic resolution and low detection limits.24 However, many metabolites are not sufficiently volatile to be introduced directly into the GC and extensive derivatization and sample preparation are often required. This limits the analysis times achievable with this technique to ~30 minute range.23
Many of the drawbacks associated with NMR and GC-MS can be overcome by using liquid chromatography to separate the metabolites prior to analysis with mass spectrometry (LC-MS). Although the resolution achievable in LC is typically lower than that of GC, LC-MS allows a wide range of metabolites including polar species to be analyzed without the need for prior derivatization, which is a significant advantage that is capable of providing a more complete picture of the both the lower and higher molecular weight metabolites. MS is operated in either positive mode for analysis of basic species or in negative mode for measuring acidic metabolites; the polarity can be rapidly changed as the separation proceeds. The chromatographic separation minimizes the number of analytes that enter the ESI at any given time, preventing them from outcompeting each other for ionization so that a high degree of sensitivity (pg mL−1) is maintained.23 Both reverse phase (RP) LC, which separates based on hydrophobicity, and hydrophilic interaction LC (HILIC), which separates based on polarity, are common in metabolomics. When coupled with high resolution mass analyzers, the molecular formula of metabolites can be determined and subsequent fragmentation can lead to identification. The downside to direct infusion LC-MS is competing ionization which diminishes quantitative ability and often requires the use of isotope labeling or tagging to overcome these challenges.
Metabolomics and diabetes biomarkers
First proposed as a tool for monitoring blood glucose control in 1976, the most widely used biomarker of long-term glycemic control in diabetes is glycosylated hemoglobin (HbA1c).25 During exposure to plasma glucose, the hemoglobin component of red blood cells undergoes irreversible glycosylation to form a non-enzymatic AGE. Because the glycated hemoglobin persists in the red blood cell for the duration of its life cycle, HbA1c levels are directly proportional to the average blood glucose concentration during the previous 8 weeks.26 HbA1c levels of ≥6.5% and 5.7–6.4% are used to diagnose diabetes and prediabetes, respectively.27 Because HbA1c measures prolonged glucose exposure, it is considered more accurate than short-term blood glucose measurements in predicting DC onset. Furthermore, HbA1c has been shown to be a reliable marker of insulin resistance in prediabetic individuals.28
While HbA1c provides some prognostic information regarding diabetes progression and risk of developing vascular complications, HbA1c itself is not considered a causal agent of vascular damage and suffers from certain limitations including ethnic and age variability.29 The emergence of new metabolomics technologies has seen a push to identify new biomarkers that not only hold diagnostic value, but also contribute to our understanding of diabetes pathology. For example, GC-MS and LC-MS/MS studies have identified significantly elevated glyoxylate, another AGE, in human serum up to 3 years prior to diabetes diagnosis.30 Other popular research avenues in the field of diabetes metabolomics include a focus on identifying metabolic “signatures” consisting of a group of molecules which have improved predictive capability over traditional measures of diabetes risk. Still other approaches aim to move away from circulating biomarkers in favor of studying metabolites in cells and tissues most affected by diabetic complications.
Biomarkers of insulin resistance
Insulin resistance is defined by an increase in the amount of insulin released by pancreatic β-cells required to maintain glucose homeostasis.31 Insulin resistance and various degrees of glucose intolerance typically precede the development of T2DM for several years with no clinically apparent symptoms. Although early metabolomics studies have shown a link between dyslipidemia and insulin resistance,32 the exact mechanism of progression from insulin resistance to diabetes appears to involve a much more complex interplay between lipolysis, inflammatory mediators, and a host of other intermediates involved in carbohydrate and amino acid metabolism. Elucidating these metabolic associations may enable the development of improved biomarkers for earlier T2DM risk assessment and prevention.
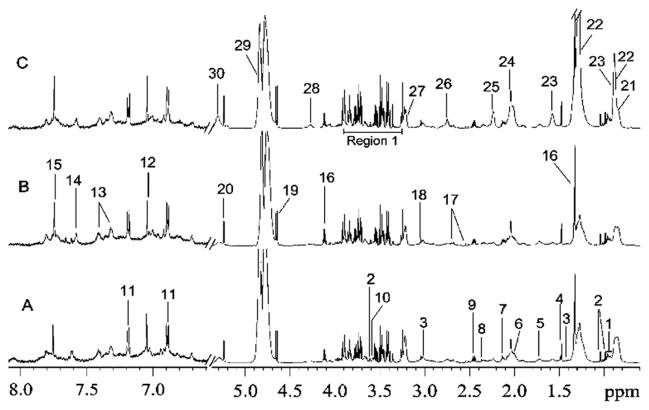
Current methods for measuring insulin resistance rely on estimates of combined hepatic and muscle insulin sensitivity obtained from an oral glucose tolerance test. While an insulin sensitivity threshold to discriminate diabetic from nondiabetic patients exists,33 an index for identifying prediabetic and insulin resistant subjects has yet to be proposed. Recent studies have correlated circulating metabolite levels with degree of insulin sensitivity in an effort to better distinguish between the metabolic phenotypes of glucose intolerance progression. Lucio and colleagues used Fourier transform ion cyclotron resonance MS to discover metabolic fingerprints in plasma of non-diabetic individuals with a range of insulin sensitivities.34 This work detected the greatest number of metabolic differences in arachidonic acid, steroid hormone, bile acid, and linoleic acid pathways. The study was able to differentiate between insulin sensitive and insulin resistant profiles and identified a distinct metabolic transition state. Another investigation involving 231 participants with normal glucose tolerance, impaired glucose regulation, and T2DM used 1H–1H correlation spectroscopy 2D NMR to analyze metabolites in serum samples.35 Fig. 2 shows metabolites identified in the NMR spectra for each of the three conditions. The most prominent metabolic dysfunction in impaired glucose regulation and T2DM samples was observed in pathways related to choline, glucose, lipid, TCA, and amino acid metabolism. A global analysis of human plasma using UHPLC-MS/MS and GC-MS found levels of α-hydroxybutyrate were inversely associated with insulin resistance and impaired glucose regulation; this biomarker was capable of separating insulin resistant, median, and insulin sensitive subjects with 76% accuracy.36 α-Hydroxybutyrate is involved in both elevated GSH synthesis in the liver and in the propanoate pathway, which showed significant dysregulation in diabetic mouse aorta.37 These studies demonstrate the utility of metabolomics in uncovering the underlying biochemical processes involved in progression of insulin resistance to T2DM and are expected to be applied to DCs.
Fig. 2.
1H NMR spectra of human serum samples with (A) normal glucose tolerance, (B) impaired glucose regulation and (C) T2DM (the region at δ6.5–8.0 was expanded for 32 times). The area labeled “region 1” in spectra C contains signals from glucose, lipid and amino acids. Keys: 1, leucine/isolucine; 2, valine; 3, lysine; 4, alanine; 5, arginine, leucine, lysine; 6, proline; 7, methionine; 8, glutamate; 9, glutamine; 10, threonine; 11, tyrosine; 12, methylhistidine; 13, phenylalanine; 14, 3-methylhistidine; 15, histidine; 16, lactate; 17, citrate; 18, creatinine; 19, β-glucose; 20, α-glucose; 21, HDL; 22, LDL; 23, VLDL; 24, glycoprotein; 25, lipid; 26, lipid; 27, choline; 28, glyceryl of lipid; 29, water; 30, unsaturated lipid. From Zhang, et al., Human Serum Metabonomic Analysis Reveals Progression Axes for Glucose Intolerance and Insulin Resistance Statuses, J. Proteome Res., 2009, 8(11), 5188–5195. Used with permission.
Metabolic signatures of diabetes
Metabolite profiling has become a particularly useful tool for observing outcomes in prospective and longitudinal clinical trials. In the Framingham Heart Study, an ongoing experiment which aims at identifying risk factors for cardiovascular disease, LC-MS was used to evaluate plasma samples from individuals who developed new-onset diabetes within the follow-up period.38 Fasting levels of branched-chain and aromatic amino acids isoleucine, leucine, valine, tyrosine, and phenylalanine showed significant elevation up to 12 years before the development of clinical diabetes, representing a fivefold greater risk for the top quartile of study participants. MS-based signatures in human serum have also implicated elevated low carbon number lipids in the pathogenesis of diabetes.39,40 Our recent work on metabolic pathway dysfunction using global and isotope tagging strategies reported elevations in all three branched-chain amino acids and an 18.8% alteration in glycerolipid metabolism in diabetic mouse aorta.37 While the relationship between elevated amino acids and insulin resistance was first postulated over 40 years ago,41 longitudinal metabolomics investigations have provided further insight into the mechanism of this process. Current hypotheses suggest a joint effect of both lipids and amino acids in promoting mitochondrial deficiency and disruption of insulin signaling in skeletal muscle,39,42,43 but further studies are needed to determine whether these compounds are only biomarkers or are causal agents of metabolic dysfunction.
Vascular complications in diabetes
Vascular complications represent the major contributor to diminished quality of life and increased mortality rate in patients with both T1DM and T2DM. While rigorous glycemic control has been shown to reduce the risk of these disorders, a recent study reported that the microvascular complications neuropathy and retinopathy were present in 50% and 31%, respectively, of newly-diagnosed T2DM patients with tight glycemic control.44 The Diabetes Control and Complications Trial/Cohort found a prevalence of 47%, 17%, and 14% for retinopathy, nephropathy, and cardiovascular disease, respectively, in T1DM individuals with conventional clinical management.17 Clearly, traditional glycemic control strategies and vascular therapeutic measures are necessary but insufficient to improve the clinical outcomes of diabetes.
The current scope of the field of diabetes metabolomics has shifted from an all-encompassing view of the diabetic “metabolic syndrome” toward a complication-specific approach which attempts to study the relationships between biochemistry and pathology of diabetic vascular complications. For example, a self-organizing map constructed from biochemical measurements of urine and serum obtained from T1DM patients in the FinnDiane cohort elucidated five distinct metabolic phenotypes, each associated with a particular clinical outcome and risk of premature death.45 The greatest risk of death (>10 fold) was correlated with a metabolic profile that shared features with diabetic kidney disease and metabolic syndrome, including high triglycerides, low HDL cholesterol, and high urine albumin. Although this study focused on known biomarkers using classical biochemical techniques, important statistical patterns relating adverse metabolic phenotype to increased risk of DCs and mortality were elucidated.
Macrovascular complications
Cardiovascular disorders (CVD), the most common cause of death worldwide, are closely related to diabetes both in terms of risk factors and metabolic phenotype. The prevalence of diabetes in some CVD such as heart failure is as high as 40%, and individuals with diabetes have a 2–6 fold higher risk of developing CVD as the nondiabetic population.46 The major types of CVD seen in diabetes are accelerated atherosclerosis, myocardial infarction, stroke, and cardiac dysfunction. Underlying risk factors of both CVD and diabetes include obesity, high blood pressure, and elevated cholesterol and triglyceride levels; these elements are closely linked to hyperglycemia and hyperlipidemia, a combination which promotes inflammation and oxidative stress.
Atherosclerosis and coronary artery disease
Atherosclerosis is a chronic condition in which inflammatory processes in endothelial cells cause binding of macrophages and T cells to the blood vessel wall. The compromised endothelium is more prone to absorbing small low-density lipoprotein (LDL) particles from the blood stream, which subsequently become oxidized in the sub-endothelial space.17 When macrophages take in oxidized LDL (oxLDL), they become foam cells and accumulate at the site of the damaged endothelium. Aggregations of foam cells and monocytes combined with calcified smooth muscle cells are known as atherosclerotic plaques and appear as fatty streaks in the arterial lumen. Foam cells are unstable and eventually rupture, releasing extracellular matrix material, cholesterol, and other components into the blood. If this material obstructs blood flow to the heart or brain, a myocardial infarction or stroke event, respectively, occurs.
Current techniques for assessing vascular wall integrity and diagnosing atherosclerosis rely on radiation and invasive procedures such as angiography.47 Atherosclerosis can remain asymptomatic for decades before the onset of a sudden vascular occlusion. Metabolomic fingerprinting experiments have recently yielded new insights into the pathophysiology of atherosclerotic lesion formation as well as biomarkers which may afford physicians with better diagnostic and prognostic indicators of clinical outcome. The Framingham Heart Study first exposed the relationship between major coronary events and a decreased ratio of high-density lipoprotein (HDL) to LDL,48 and over the years other investigations have further characterized the role of lipoprotein abnormalities in atherosclerosis. A proton NMR technique first demonstrated the simultaneous determination of multiple lipoprotein subclasses in plasma, taking advantage of the fact that methyl lipids on the lipoparticle core and surface are subject to different magnetic environments.49 This technique was subsequently utilized in the Insulin Resistance Atherosclerosis Study, which found that compositional and size changes in HDL, LDL, and very low-density lipoprotein (VLDL) particles preceded the onset of diabetes in nondiabetic individuals over a 5.2 year follow-up period.50 1H NMR was also utilized in the discovery of morphological abnormalities and abnormal lipid distribution in small HDL in diabetic patients, which was not responsive to pharmacological therapy.51 However, another 1H NMR study indicated modest improvements in total HDL levels resulted after 16 weeks of treatment with rosiglitazone, an insulin sensitizer, in patients with both coronary heart disease and diabetes.52
Lipolysis and oxidative stress
In addition to elevated LDL and oxLDL cholesterol content, enhanced lipolysis of triglyceride-rich lipoprotein has also been implicated in vascular inflammation and atherosclerosis. Partially lipolyzed fatty acid particles are capable of more easily entering the subendothelial space of blood vessels and being phagocytosed by macrophages without prior oxidation, leading to accelerated foam cell formation.53 Lipotoxicity, the build-up of unoxidized fatty acids and reactive lipid species in sites other than adipose tissue, results in cell dysfunction and can lead to apoptosis of heart cells.54 Furthermore, fatty acid oxidation is known to inhibit glucose metabolism and β-cell insulin release. During fatty acid oxidation, mitochondrial respiration rate increases and consequently a larger proportion of electrons are transferred to complex 2 of the electron transport chain during oxidative phosphorylation.55 This disruption in redox balance contributes to the production of ROS and oxidative stress. Analysis of endothelial cells exposed to high glucose showed significant changes to thiol containing metabolites and a perturbation of the cysteine-GSH pathway, which detoxifies ROS.56 Additional work examining carbonyl metabolites by isotope labeling and LC-MS showed a general elevation in oxidized metabolites.57
The fields of metabolomics and lipidomics have recently taken a considerable interest in the contribution of lipid metabolism to diabetic cardiomyopathy and macrovascular oxidative damage.58 Metabolic profiles have been utilized to distinguish between serum samples of diabetic individuals with high blood pressure, coronary heart disease, nonalcoholic fatty liver disease, or some combination of these three complications.59 Two of the metabolites most strongly correlated with these complications, dodecanoic and decanoic acid, are involved in fatty acid metabolism, and the ketogenic amino acid leucine (further discussed below) was found at higher concentrations in the high blood pressure and nonalcoholic fatty liver disease samples, which were associated with more abnormal lipid metabolism than coronary heart disease. Abnormalities in short-chain fatty acid metabolism, including the propanoate and butanoate pathways, have also been observed in diabetic vascular tissue.36 Short-chain fatty acids are known to prevent diet-induced obesity in free fatty acid receptor deficient mice60 and alter glucose and lipid metabolism.61 The exact relationship between these pathways and macrovascular dysfunction remains unknown.
Amino acid and carbohydrate biomarkers of macrovascular dysfunction
Amino acids play critical roles in cellular function including protein and lipid synthesis, cellular signaling, and insulin regulation; disruptions in amino acid metabolism, particularly of the branched-chain amino acids, have been associated with diabetes.62 However, only recently has a link between altered amino acid levels and future risk of cardiovascular disease been investigated.63 Using the diabetes mellitus-amino acid score derived from data from the Framingham Heart Study, which includes tyrosine, phenylalanine, isoleucine, valine, and leucine, a targeted LC-triple quadrupole-MS investigation revealed that individuals with plasma amino acid levels in the top quartile of the score 12 years before follow-up were associated with a nearly five-fold greater risk for inducible myocardial ischemia and atherosclerosis.63 Elevations in the branched-chain amino acids in aortic tissue in a mouse model of T2DM have been reported as well, including significant changes in 12.5% of the compounds involved in valine, leucine, and isoleucine degradation.37 Compounds involved in the metabolism of pyridoxal phosphate, the biologically active form of Vitamin B6, showed significant downregulation. Pyridoxal phosphate is an important cofactor in transamination reactions, and its deficiency may exacerbate insulin resistance by preventing effective amino acid catabolism. This study employed a deuterium isobaric amine reactive tag to enhance MS sensitivity and increase multiplexing of amine metabolites. This tag was also used to investigate changes to amine metabolism in human aortic endothelial cells under high and low glucose conditions.64 Of 31 targeted amines, the levels of 21 were significantly increased after 7 days exposure to high glucose. Notably, the elevation of the ROS scavengers proline, hypotaurine, and cystathionine indicated a potential antioxidant response as early as 3 hours after treatment with high glucose. As amino acids often show poor ionization efficiency and chromatographic resolution, amine labelling was essential for improving sensitivity and enabling the detection of these metabolites.
The myocardium is an insulin-responsive tissue which uses glucose, lactate, and fatty acids as primary energy sources.52 Circulating levels of compounds involved in oxidative metabolism of these substrates therefore indicates the efficiency of myocardial extraction and utilization of fuel. In response to stress, the heart increases anaerobic respiration by extracting more glucose from blood, which produces more lactate. Extracellular lactate is associated with insulin resistance and was found to inhibit glucose metabolism in ischemic rat myocardium.65,66 An NMR-based metabolomics profiling method was used to measure circulating metabolites in patients with T2DM and coronary heart disease following administration of rosiglitazone; positron emission tomography was also employed to assess myocardial glucose uptake.52 Lactate concentration was found to be inversely associated with glucose extraction, but was decreased 16% by rosiglitazone treatment. Glutamine, which improves plasma insulin secretion in diabetes,67 was increased by rosiglitazone. Although these findings indicate modest improvements in insulin sensitivity, other analytes investigated in the study, including LDL particles, showed no significant changes following rosiglitazone administration. Further investigation of myocardial fatty acid oxidation during diabetes is warranted and could potentially yield therapeutic insight.
Microvascular complications
Although macrovascular complications are responsible for the greatest number of deaths from diabetes, the microvascular complications nephropathy, neuropathy, and retinopathy significantly contribute to the morbidity of the disease. Because endothelial cells of the microvasculature are insulin independent (in contrast to muscle cells), hyperglycemia leads to an unregulated influx of glucose into tissues supplied by microvessels.68,69 As such, microvascular damage arises from some biochemical mechanisms distinct from those involved in macrovascular dysfunction. Hyperglycemia is directly responsible for many of the physiological changes seen in diabetes such as alterations to microvascular contractility and blood flow, as well as underlying biochemical changes that contribute to its pathogenesis.70 Metabolomics has identified unique biomarkers of each of the major microvascular complications to establish a better understanding of the process by which hyperglycemia leads to microvascular disorders.
Nephropathy
Diabetic nephropathy, or diabetic kidney disease, is defined as a progressive decline in glomerular filtration rate and increase in urinary albumin excretion, often accompanied by high blood pressure.71 Hypertrophy of the proximal tubule component of the diabetic kidney eventually results in mesangial expansion and glomerular basement membrane thickening, which lead to reabsorption of urinary filtrate and fibrosis. Diabetic nephropathy culminates in end-stage renal disease if left untreated, a condition affecting approximately one-third to one-fifth of all diabetic patients.72 The most dangerous consequence of diabetic nephropathy is its associated risk of cardiovascular mortality, which was found to be 8 to 10 times higher than that of individuals with normal renal function.73
Diabetic nephropathy is currently diagnosed based on the rate of urinary excretion of the low-molecular-weight protein, albumin.71 Microalbuminuria, an excretion rate of 30–300 mg per day, has been shown to be an early clinical marker of progression to nephropathy in both T1DM and T2DM patients. Metabolomic investigations of nephropathy were conducted quite recently, and results of long-term follow-up studies are only now beginning to emerge. Already, these metabolomics approaches have yielded important information including the role of uremic solutes, mitochondrial dysfunction, and carbonyl and oxidative stress in the pathophysiology of diabetic nephropathy.
Uremic solutes
Uremic solutes are metabolic end products normally removed by the kidneys, but which are present in excess in the blood during impaired kidney function. Uremic solutes include compounds from a variety of different chemical classes including amino acids, nucleotides, carbonyls, and phenols. It is unclear whether these metabolites are responsible for or are a result of renal impairment. Additionally, because many uremic solutes are bound to serum proteins and are poorly hemodialyzed, their accumulation is difficult to control. Toxic effects of uremic salts include metabolic acidosis, insulin resistance, and altered protein function.74
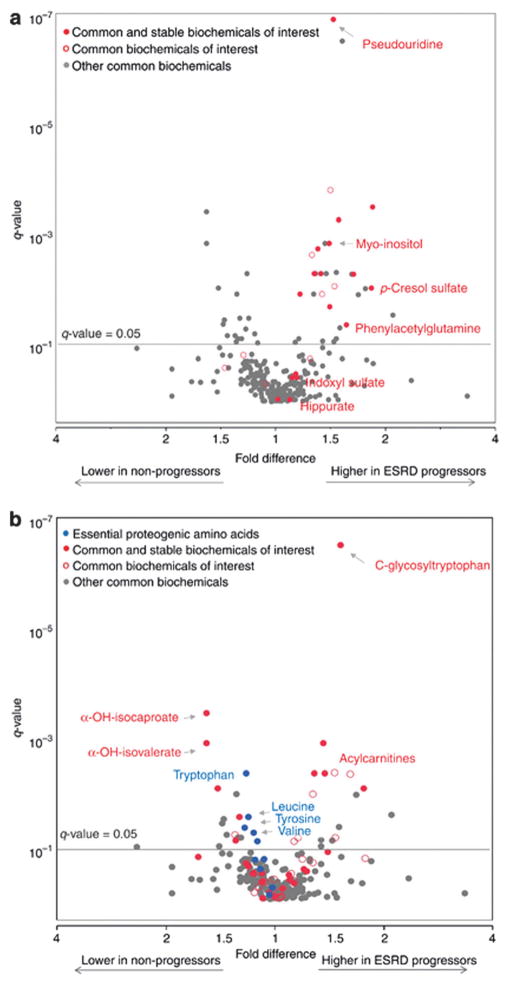
Plasma metabolomics has been utilized to assess the effect of uremic solute concentrations on progression to end-stage renal disease in diabetic patients. In data from the Joslin Kidney Study cohort, sixteen known uremic solutes were found to be elevated in baseline plasma of individuals who progressed to end-stage renal disease over an 8–12 year follow-up period (Fig. 3).75 Two of these metabolites, pseudouridine and myoinositol, were also identified by Dunn and colleagues as novel metabolic markers of heart failure.76 Pseudouridine is a modified nucleoside which is associated with cell turnover and may be involved in cardiac remodeling. Myoinositol/glucose imbalance contributes to kidney fibrosis, altered cellular potassium and sodium flux, and abnormal phospholipid metabolism.75 The identification of these metabolites in both end-stage renal disease and heart failure patients is unsurprising given the role of diminished renal function in the pathophysiology in cardiac dysfunction—often called the “cardiorenal syndrome.”76
Fig. 3.
Volcano plot of common metabolites measured in plasma from T2DM patients with end-stage renal disease. Association with progression to end-stage renal disease is demonstrated as a fold difference (x-axis) and significance is presented as q-value (y-axis). Uremic solutes comprise metabolites of interest in (a) and amino acids are metabolites of interest in (b). Uremic solutes are not displayed in (b). Common and stable metabolites of interest are represented as red circles (
 ), common metabolites that are not stable over time are represented as red empty circles (
), common metabolites that are not stable over time are represented as red empty circles (
 ), and all other common metabolites are represented as gray circles (
), and all other common metabolites are represented as gray circles (
 ). Blue circles represent essential amino acids (
). Blue circles represent essential amino acids (
 ). Adapted from Niewczas, et al., Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study, Kidney Int., 2014, 85(5), 1214–1224. Used with permission.
). Adapted from Niewczas, et al., Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study, Kidney Int., 2014, 85(5), 1214–1224. Used with permission.
Other uremic solutes which have gained considerable attention as markers of renal function are the phenyl compounds indoxyl sulfate and p-cresol sulfate, end products of gut microbial degradation of tryptophan and tyrosine, respectively.77 These and other amino acid-derived microbial metabolites inhibit endothelial wound repair and exacerbate cardiovascular complications in diabetic nephropathy.75 A study using triple quadrupole-MS/MS indicated that a >2-fold elevation of urinary indoxyl sulfate in streptozotocin-induced diabetic mice was returned to baseline concentrations following administration of rosiglitazone.78 A preliminary report found that treatment with synbiotics, a combination of pre- and probiotics, reduced serum levels of p-cresol sulfate in hemodialysis patients, suggesting that microbial therapy may be of benefit in removing protein-bound serum toxins.79
MS-based metabolomics in both the FinnDiane and in the Joslin Kidney Study cohorts has revealed a link between acylcarnitines and progression to kidney disease or end-stage renal disease in diabetic patients (Fig. 3).75,80 Acylcarnitines are derived from either amino acids or lipids and are involved in transporting these metabolites into mitochondria for β-oxidation.81 Normally excreted in urine, elevation of acylcarnitines in serum is a marker of impaired kidney function. Furthermore, both branched-chain amino acid-derived and fatty acid-derived carnitines have been implicated in obesity and insulin resistance.81 The elevation of acylcarnitines in renal impairment may suggest a role of organic ion transport and mitochondrial metabolic dysfunction.
Reactive carbonyl species are a special type of uremic toxin which leads to carbonyl stress via the generation of advanced lipoxidation end products (ALEs) and AGEs. Carbonyl stress contributes to a variety of pathological and inflammatory processes including macrophage recruitment, nitric oxide inhibition, and alterations in protein structure and function.82 In cases of renal failure, AGEs and ALEs have been shown to crosslink collagen residues in the glomerular mesangium, leading to changes in cell-matrix interactions and tissue stiffness.83 Plasma from the Natural History of Diabetic Nephropathy Study analyzed by LC-QQQ-MS showed that the methylglyoxal-derived AGEs carboxyethyl lysine, carboxymethyl lysine, and methylglyoxal hydroimidazolone increased prediction of glomerular basement membrane thickening in fast-progressing T1DM patients.84 Reactive intermediates/carbonyls malonate, imidazolone, malondialdehyde, methylglyoxal, and glyoxylic acid all showed increases in urine in a streptozotocin-induced diabetic mouse model, but only glyoxylic acid was reversed by rosiglitazone.78 Global metabolic profiling in the FinnDiane study using GC-MS and LC-FT-MS also reported elevations in deoxyfructose, a product of deoxyglucosone detoxification resulting from diabetic stress, in individuals with diabetic kidney disease.80 Increases in the AGE precursor fructoselysine as well as malondialdehyde and imidazolone (fold changes 7.11, 1.51, and 2.81, respectively) were seen in aortic tissue from a db/db mouse model, suggesting that carbonyl stress may have a role in the cardiorenal syndrome.37
Oxidative stress and mitochondrial dysfunction
Mitochondria are essential to the development and progression of hyperglycemia-induced oxidative stress. Relative abundances of serum and urine TCA metabolites have been proposed as a means to assess mitochondrial function in diabetic nephropathy. Recent work investigating the evolution of nephropathy in db/db mice over 16 weeks found significantly higher levels of citrate, malate, and α-ketoglutarate in serum at week 6 which showed a sharp decline 2 weeks later, suggesting downregulation of glycolysis and development of insulin resistance.72 In contrast, the portion of the TCA metabolites excreted in the urine increased after week 8. Citrate and malate levels in urine relative to serum were shown to be the strongest indicator of kidney function. Elevations in urinary TCA metabolites were also reported in a GC-MS study using diabetic mice.85 Of note, only fumarate levels were restored by NADPH oxidase inhibition in this experiment; fumarate is involved in the regulation of transcription growth factor-β production in renal cells, and its accumulation may be linked to poor clinical outcomes in patients with diabetic nephropathy.86 In clinical studies of T1DM and T2DM patients with chronic kidney disease, GC-MS was used to quantify urine and serum metabolites. 12 of the 13 differentially expressed compounds have a role in mitochondrial metabolism, and most of the species detectable in both biofluids were decreased in the urine samples.87 The same work found that mitochondrial protein and DNA were markedly reduced in the kidney sections and urine, respectively, of diabetic patients, suggesting a decrease in overall mitochondrial mass.
Another mechanism by which hyperglycemia induces oxidative damage in renal cells is the activation of the purine oxidoreductase enzyme xanthine oxidase, which catalyzes the conversion of hypoxanthine to xanthine and xanthine to uric acid; both reactions produce the ROS hydrogen peroxide. 1H NMR-based analysis of serum, urine, and renal extracts from streptozotocin-induced diabetic rats revealed dramatic increases in uric acid and its oxidized form allantoin, whose production is driven by ROS.88 Both metabolites are involved in the development of microalbuminuria and inflammation. Dysregulated purine metabolism and excessive generation of allantoin was also recently implicated in the pathogenesis of ischemic retinopathy (see Retinopathy section), and may hold potential as a therapeutic route for multiple diabetic complications.
Neuropathy
Diabetic neuropathy, the most common complication of diabetes, is characterized by damage to any of the body’s pain and nerve fibers, most often peripheral sensory nerves. Resulting complications of diabetic neuropathy include numbness, burning, a “pins and needles” sensation, vision impairment, loss of balance, atrophy, and cardiac diseases.89 Numbness and loss of reflexes typically first manifest in the feet and hands, as longer nerve fibers exhibit an earlier loss of conduction velocity.17 Individuals with diabetic neuropathy are therefore especially prone to developing ulcers and foot injuries.
The pathophysiology of diabetic neuropathy involves both dysfunction in the blood vessels which supply neurons as well as hyperglycemia-induced injury to nerve cells themselves. Excessive glucose metabolism promotes capillary basement membrane thickening and changes in vascular tone which lead to hypoxia and subsequent neuronal ischemia.17 Glucose is the primary fuel source for peripheral nervous tissue, which is insulin-independent.90 Increased glucose metabolism via the five standard biochemical pathways of DCs has been implicated in neuronal dysfunction.89 For an in-depth discussion on the other biochemical mechanisms of diabetic neuropathic progression, the reader is directed to the 2008 Feldman review.89
Mitochondrial dysfunction
The generation of ROS by the mitochondrial electron transport chain was once thought to be the main process responsible for vascular damage in diabetes.9 However, recent work suggests that in certain tissues with downregulated glycolytic and TCA cycle metabolism, mitochondrial production of ROS may be diminished and other pathogenic biochemical processes may predominate.90 Metabolomics investigations have recently made important contributions to the understanding of neuropathy, which appears to involve a complex combination of reactive nitrogen species (RNS) and ROS generation, aberrant mitochondrial metabolism, and inflammation. An LC/MS/MS study using a db/db mouse model of T2DM demonstrated significant decreases in four of the five measured glycolytic metabolites in sural and sciatic nerves, including a 75% decrease in phosphoenolpyruvate, but no change was observed in dorsal root ganglia glycolytic content.90 The same work also reported diminished citrate and isocitrate levels in all three nerve types, but found no change in other TCA intermediates. Aconitase, the TCA cycle enzyme which catalyzes the conversion of citrate to isocitrate, was associated with a nearly 2-fold decrease in activity in the sciatic nerve of diabetic vs. control mice, indicating ROS inhibition. Aconitase activity in the dorsal root ganglia of diabetic mice was downregulated to a lesser extent. These findings were corroborated by Freeman, et al., who described a neuronal proximal-to-distal increase in oxidative phosphorylation, polyol pathway upregulation, and mitochondrial dysfunction in three types of nervous tissue in diabetic rats using GC- and LC-MS.91 However, a recent investigation of a triple antioxidant therapy regimen on cardiovascular autonomic neuropathy showed no benefit on disease progression and other therapeutic avenues should be explored.11
Lipoxidation
Dyslipidemia is an early risk factor for diabetic neuropathy. Oxidized lipoproteins, altered dihydroxy/epoxygenated fatty acid ratio, and elevated triglyceride levels have been correlated with inflammation, allodynia, and accelerated disease course.92,93 The mechanisms of neuronal lipotoxicity appear to be closely related to oxidative stress. For example, plasma lipids measured by immunocytochemical methods were significantly oxidized in mice which were fed a high-fat diet and subsequently developed neuropathy.92 Oxidized LDLs were found to increase mitochondrial superoxide production in dorsal root ganglia via NADPH oxidase activation, and oxidative stress generated by hyperglycemia and dyslipidemia was shown to be additive. In an LC-MS/MS investigation of bioactive lipids in plasma and spinal cord of T1DM rats, levels of epoxygenated fatty acids produced from linoleic, arachidonic, eicosapentaenoic, and docosahexaenoic acids by cytochrome P450 enzymes were decreased.93 Epoxygenated fatty acids play important roles in modulating vascular tone and downregulating cyclooxygenase-2 (COX-2), which is responsible for nociceptive and inflammatory processes.94 COX-2 is also associated with insulin resistance and increased oxidative stress in high-fat fed rats.95 Elevation of epoxygenated fatty acid levels by soluble epoxide hydrolase inhibition resulted in attenuation of pain in the rodent model and may be a therapeutic strategy for improving lipid metabolism in human patients.
Nitrosative stress
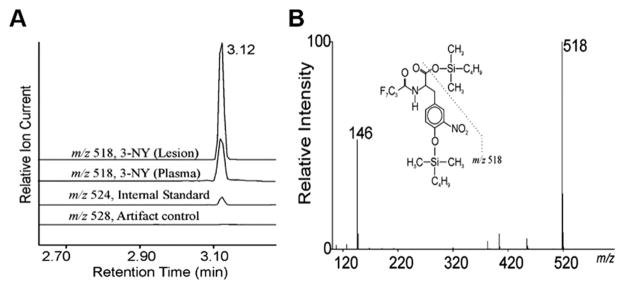
The generation of the reactive nitrogen species peroxynitrite from nitric oxide and superoxide contributes to biochemical damage in diabetes by depleting stores of nitric oxide, which is an important vasodilator, as well as by altering the function of lipids and proteins through oxidation.96 One of the major products of protein nitration is 3-nitrotyrosine residues. Isotope dilution GC-MS showed that 3-nitrotyrosine content of HDL in aortic atherosclerotic lesions is 6-fold higher than in circulating HDL in patients with coronary artery disease (Fig. 4).96 Research has indicated that nitrosative stress may also be relevant to diabetic neuropathy through activation of the PARP pathway. In the dyslipidemia-induced mouse model of neuropathy, 3-nitrotyrosine was elevated 75%, 60%, and 5.6-fold in plasma, LDL cholesterol, and sciatic nerve, respectively.92 Another study found 32% and 54% increases of 3-nitrotyrosine in sciatic nerve and spinal cord, respectively, of streptozotocin-induced diabetic rats.97 3-nitrotyrosine accumulation was inversely correlated with motor and sensory nerve conduction velocities and myelin thickness. These results implicate peroxynitrite formation via PARP activation as a convincing pathological mechanism for diabetic neuropathy.
Fig. 4.
Detection by mass spectrometry of 3-nitrotyrosine in HDL isolated from plasma and atherosclerotic human aortic tissue harvested at surgery. (A) Amino acids derived from HDL were isolated on a C18 solid phase column, derivatized, and analyzed by isotope dilution negative ion electron capture GC/MS with selected monitoring of ions of m/z 518 (for 3-nitrotyrosine), m/z 524 (for 3-nitro[13C6]tyrosine), or m/z 528 (for 3-nitro[13C9, 15N]tyrosine derived from l-[13C9, 15N]tyrosine). (B) Full scan mass spectrum of the t-butyl dimethylsilyl derivative of authentic 3-nitrotyrosine. Note that the major fragment ion has an m/z of 518. From Pennathur, et al., Human Atherosclerotic Intima and Blood of Patients with Established Coronary Artery Disease Contain High Density Lipoprotein Damaged by Reactive Nitrogen Species, J. Biol. Chem., 2004, 279(41), 42977–42983. Used with permission.
Retinopathy
Diabetic retinopathy, which consists of changes in the retinal microvasculature resulting in some degree of retinal lesions and vision impairment, is the leading cause of blindness among adults worldwide and affects the majority of diabetic patients after 20 years of disease.17 Early diabetic retinopathy, known as nonproliferative retinopathy, is characterized by weakening of the blood-retinal barrier, thickening of the vascular basement membrane, and the death of pericytes, the contractile cells which surround retinal endothelial cells.17 Prolonged hypoxic conditions due to changes in vascular tone and permeability can lead to proliferative retinopathy, during which fragile new blood vessels grow and eventually begin to hemorrhage into the part of the retina known as the macula. The combination of fibrous neovascularization and macular swelling caused by fluid accumulation results in vision loss and may even lead to retinal detachment.98
The pathophysiology of diabetic retinopathy appears to be closely linked to that of neuropathy, and immunocytochemical studies have shown that diabetic retinopathy is initiated by damage to nonvascular retinal nerve cells before the development of vascular dysfunction.99 Like neuropathy, many of the biochemical mechanisms with proposed involvement in retinopathy are directly related to chronic hyperglycemia. Alterations in polyol metabolism and the production of AGEs, inflammatory compounds, and ROS have been identified as pathogenic agents, but therapies including aldose reductase inhibitors, antioxidants, and anti-inflammatory drugs have shown limited efficacy in clinical trials.
Glycolysis and oxidative stress
Changes in glucose utilization and redox status in diabetic eyes contribute to the progression of retinopathy by affecting osmolarity, promoting oxidative damage, and altering neurotransmission. Very early research using gravimetric and fluorimetric microassays implicated osmotic stress due to the production of sorbitol from glucose via aldose reductase (the polyol pathway) in retinopathy.100 1H NMR-based metabolomics of vitreous fluid from patients with proliferative diabetic retinopathy uncovered further evidence supporting the role of activated polyol metabolism in this complication.101 Depletions in galactitol, the reduction product of galactose via aldose reductase, were reported in diabetic samples. Because aldose reductase normally has a higher affinity for galactose than for glucose, a decrease in galactitol production could be attributed to favored generation of sorbitol during hyperglycemia.102 Sorbitol is impermeable to the cell membrane, and studies have shown a compensatory decline in intracellular taurine and myo-inositol in retinal epithelial cells in response to its accumulation.103 Depletion of these metabolites is linked to other metabolic consequences including abnormal sodium and potassium fluxes.104,105
The significance of glycolytic dysfunction and a weakened antioxidant defense system in diabetic retinopathy pathogenesis has also been substantiated by both biochemical and metabolomic methods. Large abundances in lactate production in human diabetic vitreous fluid were found by NMR,101 and spectrofluorometric measurements of streptozotocin-induced diabetic rat lenses revealed elevations in glycolytic intermediates up to dihydroxyacetone phosphate.106 Such symptoms of anaerobic respiration point to hypoxia and microvascular dysfunction as pathogenic agents. Lactate generation may be further stimulated by increased tissue acidosis and inflammation. Other results from these experiments include depletions in the key antioxidants ascorbic acid and free cytosolic NADPH in diabetic vitreous and lens, respectively. A mass spectrometry-based experiment using diabetic vitreous samples identified compromised purine metabolism resulting from xanthine oxidase overactivity, yielding high levels of the oxidative stress biomarker allantoin (Fig. 5, see also Nephropathy section).107 These current metabolomics findings combined with conventional biochemical studies support a mechanism for retinopathy which involves diabetes-induced inhibition of glycolysis, increased polyol pathway flux, and oxidative stress.
Fig. 5.
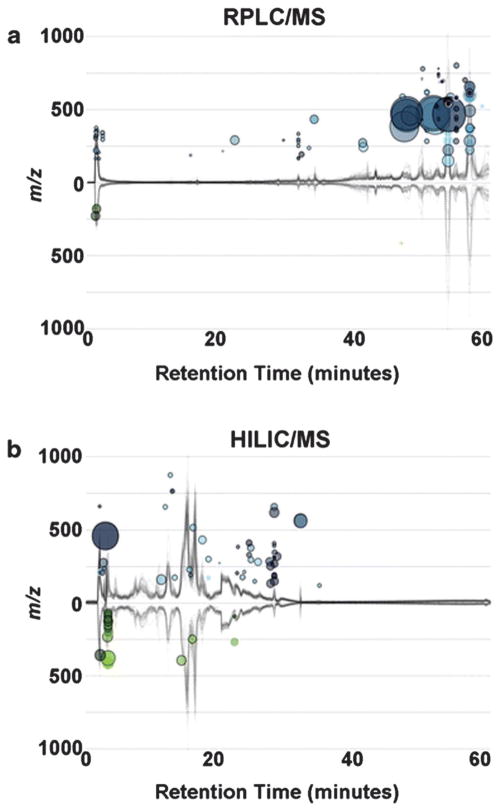
Global liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) metabolomics. Cloud plots generated by XCMS online showing dysregulated features between control and diabetic samples (two-tailed Mann–Whitney test) for (a) RPLC-MS analysis, control (n = 11), proliferative diabetic retinopathy (n = 9) top plot and (b) HILIC-MS analysis, control (n = 11), proliferative diabetic retinopathy (n = 7), lower plot. Total ion chromatograms for each sample can be seen on the plot; features whose intensity are increased in diabetic retinopathy vitreous are shown on the upper part of the plot as blue circles and features whose intensity decreases are shown on the bottom part of the plot as green circles. Larger and brighter circles (features) correspond to larger fold changes and lower p-values respectively. Adapted from L. P. Paris, et al., Metabolomics, 2016, 12, 15. Used with permission.
Neurotransmission and amino acid metabolism
More recently, investigations of diabetic retinopathy pathogenesis have shifted to the influence of retinal amino acid neurotransmitters on neuronal cell death and ischemia. Reduced amplitudes in oscillatory potentials of electroretinograms have been shown to precede the onset of diabetic retinopathy, suggesting that neuronal injury due to altered retinal metabolism may be an early consequence of diabetes.108 In particular, a study using RP-LC and a Pico-Tag amino acid analyzer found elevations in GABA (26–51%) and glycine (33%) in the retinas of streptozotocin-induced diabetic rats, and corresponding oscillatory potential attenuations. Possible explanations for altered electrophysiological responses in retinopathy include excess releases or decreased uptake of these neurotransmitters by retinal neurons.108 Neural excitotoxicity of glutamate has been implicated in the pathogenesis of several disorders related to the central nervous system. The influence of diabetes on glutamatergic neurotransmission, synthesis, and cataplerosis in rat retinas was recently investigated using radioactive labeling.109 The glutamate anaplerotic/cataplerotic cycle between neurons and Müller cells, which remove glutamate from synaptic spaces, was studied by incubating diabetic rat retinas with either 14C CO2 (anaplerosis) or 14C glutamine (cataplerosis). The 14C-labeled metabolites were extracted and separated by ion-exchange chromatography; fractions were quantified by scintillation counting. The rate of glutamate anaplerosis was found to be 42% lower in diabetic retinas vs. controls, whereas that of cataplerosis was 90% lower. High elevations in glutamate (fold change 3.2) and other amino acids were also reported in a recent metabolomics global profiling investigation using RPLC- and HILIC-MS to identify altered metabolites in retinopathy vitreous samples.107 These findings implicate an uncoupling of the glutamate cataplerosis/anaplerosis cycle in diabetes which may be mediated by competition between glutamate and branched-chain amino acids for binding sites on mitochondrial branched-chain amino transferase.110 Other forms of amino acid toxicity in diabetic retinopathy include altered arginine metabolism, which was shown to result in increased urea cycle activity and an uncoupling of the nitric oxide synthase pathway, leading to impaired vasodilation and endothelial damage.107
Tear metabolomics
Somewhat surprisingly, a literature search revealed a number of publications comparing tear proteins in healthy individuals and in diabetic retinopathy patients, but none involving the diabetic retinopathic tear metabolome. Tears are a noninvasive, low cost, easily accessible biofluid and may hold particular value in population screening assays. Although tear metabolite extraction procedures have been described, tear metabolomics remains hindered by low sample volumes and metabolite concentrations as compared to other biofluids.111 However, a global LC-MS/MS approach was recently used to identify 60 tear metabolites,112 including 19 amino acid derivatives, several carnitine species, and other metabolites relevant to diabetic complications. Further improvements in sample collection and analysis methods may someday enable the characterization of the diabetic tear metabolome.
Conclusion and perspective
In the past 15 years, the field of metabolomics has expanded the current understanding of the pathophysiology of diabetic complications far beyond the five standard biochemical pathways which contribute to oxidative stress and inflammation. Branched-chain amino acids, phospholipid metabolism, and the glutamine/glutamate cycle are just a few of the previously unknown pathways and biomarkers of diabetes which have come to light due to advancements in sensitivity, sample preparation, and data processing. Metabolic irregularities in vitamin B6 metabolism and in the propanoate and butanoate pathways were also recently reported for the first time in diabetic vascular tissue.37 Fig. 6 summarizes the pathways involved in DCs which have been elucidated since the advent of metabolomics. Clearly, metabolic abnormalities in diabetes include compounds which play a wide variety of biochemical roles, from insulin signaling to neurotransmission. The evidence for oxidative stress as a key component of DCs remains strong. Despite this, previous clinical failures in antioxidant therapy suggest that deeper examination of how metabolic changes interact with ROS will be critical in developing treatments to DCs. Improving current risk predictors and therapies by correlating these metabolic signatures to specific complications poses the next major challenge in the field.
Fig. 6.
Summary of the metabolic pathways dysregulated in diabetic complications. Pathways which were unknown or understudied prior to the development of metabolomics technologies are outlined in red. Abbreviations: G-6-P, glucose-6-phosphate; Gly-3-P, glyceraldehyde-3-phosphate; DHAP, dihydroxyacetone phosphate; DAG, diacylglycerol; 3-HB, 3-hydroxybutyrate. Adapted from L. A. Filla, et al., J. Proteome Res., 2014, 13, 6121–6134. Used with permission.
As metabolomics technologies continue to increase in sensitivity and throughput, biomarker discovery for predicting the onset and severity is expected to provide critical tools for clinicians. Previous clinical data show that (1) inadequate glycemic control is strongly correlated with macrovascular complications and moderately correlated with microvascular complications and (2) that maintaining that control is very difficult to sustain over long periods of time. Biomarkers which could predict severity of onset will designate which patients are in need of additional support to sustain and maintain tight glycemic control.
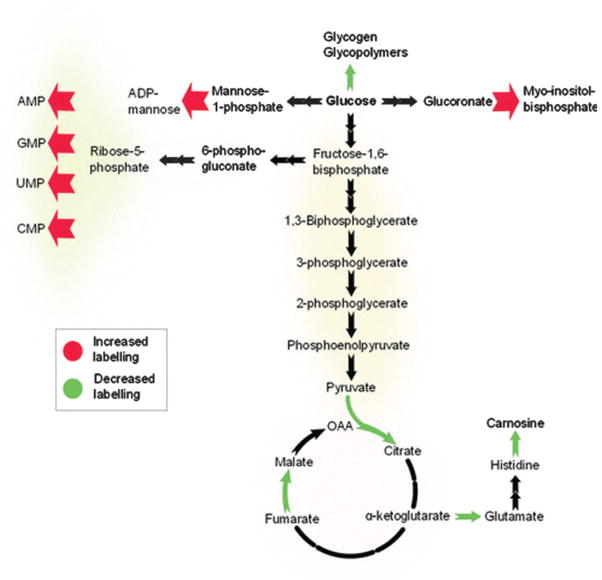
One metabolic aspect that is critically needed but not fully utilized is a catalog of the various pathways which experience increased flux during hyperglycemia. Under normoglycemic conditions, glucose is burned off as CO2 or converted to lactate. Under hyperglycemic conditions, elevated levels of glucose metabolites will undoubtedly rise above the Km of different enzymes and be diverted to new pathways. Metabolic flux analysis, where U–13C glucose is pulsed into the system, would allow for tracking of new pathways (Fig. 7).113 This will prove useful in evaluating the total effects of novel therapeutics.
Fig. 7.
Schematic of the effect of hyperglycemia on pathway activation in human retinal pigment epithelium cells. Cells were cultured in either unlabeled D-glucose (5.5 mM) or D-[U–13C]-glucose (25 mM), and metabolite labeling was assessed using the automated untargeted isotope analysis program geoRge. Adapted from J. Capellades, M. Navarro, S. Samino, M. Garcia-Ramirez, C. Hernandez, R. Simo, M. Vinaixa and O. Yanes, Anal. Chem., 2016, 88, 621–628. Used with permission.
Notes and references
- 1.World Health Organization. Global status report on non-communicable diseases. Geneva: 2014. [accessed January 4, 2016]. [Google Scholar]
- 2.Mathers CD, Loncar D. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sas KM, Karnovsky A, Michailidis G, Pennathur S. Diabetes. 2015;64:718–732. doi: 10.2337/db14-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Diabetes. 2009;58:2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll J, Raththagala M, Subasinghe W, Baguzis S, D’Amico Oblak T, Root P, Spence D. Mol BioSyst. 2006;2:305–311. doi: 10.1039/b604362n. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JS, Ku CJ, Karunarathne W, Spence DM. Anal Chem. 2007;79:5133–5138. doi: 10.1021/ac0706271. [DOI] [PubMed] [Google Scholar]
- 7.Chung SSM, Ho ECM, Lam KSL, Chung SK. J Am Soc Nephrol. 2003;14:S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 8.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. ISRN Ophthalmol. 2013;2013:13. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacco F, Brownlee M. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownlee M. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 11.Pop-Busui R, Stevens MJ, Raffel DM, White EA, Mehta M, Plunkett CD, Brown MB, Feldman EL. Diabetologia. 2013;56:1835–1844. doi: 10.1007/s00125-013-2942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schemmel KE, Padiyara RS, D’Souza JJ. J Diabetes Complications. 2010;24:354–360. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Hotta N, Kawamori R, Fukuda M, Shigeta Y. Diabetic Med. 2012;29:1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 15.Nenna A, Nappi F, Avtaar Singh SS, Sutherland FW, Di Domenico F, Chello M, Spadaccio C. Man Res Tech Cardiovasc Med. 2015;4:e26949. doi: 10.5812/cardiovascmed.4(2)2015.26949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS, Whittier FC, Wuerth JP. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 17.Forbes JM, Cooper ME. Mechanisms of Diabetic Complications. 2013. [DOI] [PubMed] [Google Scholar]
- 18.Yanes O, Tautenhahn R, Patti GJ, Siuzdak G. Anal Chem. 2011;83:2152–2161. doi: 10.1021/ac102981k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz MA, Burant CF, Kennedy RT. Anal Chem. 2011;83:3406–3414. doi: 10.1021/ac103313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Dunn WB, Bailey NJC, Johnson HE. Analyst. 2005;130:606–625. doi: 10.1039/b418288j. [DOI] [PubMed] [Google Scholar]
- 22.Sun G, Yang K, Zhao Z, Guan S, Han X, Gross RW. Anal Chem. 2007;79:6629–6640. doi: 10.1021/ac070843+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Want EJ, Cravatt BF, Siuzdak G. Chem Bio Chem. 2005;6:1941–1951. doi: 10.1002/cbic.200500151. [DOI] [PubMed] [Google Scholar]
- 24.Edwards JL, Yuan W. Bioanalysis. 2010;2:953–963. doi: 10.4155/bio.10.40. [DOI] [PubMed] [Google Scholar]
- 25.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 26.Lyons TJ, Basu A. Transl Res. 2012;159:303–312. doi: 10.1016/j.trsl.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A. American Diabetes. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borai A, Livingstone C, Abdelaal F, Bawazeer A, Keti V, Ferns G. Scand J Clin Lab Invest. 2011;71:168–172. doi: 10.3109/00365513.2010.547947. [DOI] [PubMed] [Google Scholar]
- 29.Lippi G, Targher G. Clin Chem Lab Med. 2010;48:609–614. doi: 10.1515/cclm.2010.144. [DOI] [PubMed] [Google Scholar]
- 30.Nikiforova VJ, Giesbertz P, Wiemer J, Bethan B, Looser R, Liebenberg V, Ruiz Noppinger P, Daniel H, Rein D. J Diabetes Res. 2014;2014:9. doi: 10.1155/2014/685204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Würtz P, Mäkinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J, Savolainen MJ, Tammelin T, Viikari JS, Rönnemaa T, Kähönen M, Lehtimäki T, Ripatti S, Raitakari OT, Järvelin MR, Ala-Korpela M. Diabetes. 2012;61:1372–1380. doi: 10.2337/db11-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran A, Jacobs DR, Steinberger J, Steffen LM, Pankow JS, Hong CP, Sinaiko AR. Circulation. 2008;117:2361–2368. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, DeFronzo RA. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 34.Lucio M, Fekete A, Weigert C, Wägele B, Zhao X, Chen J, Fritsche A, Häring HU, Schleicher ED, Xu G, Schmitt-Kopplin P, Lehmann R. PLoS One. 2010;5:e13317. doi: 10.1371/journal.pone.0013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Wang Y, Hao F, Zhou X, Han X, Tang H, Ji L. J Proteome Res. 2009;8:5188–5195. doi: 10.1021/pr900524z. [DOI] [PubMed] [Google Scholar]
- 36.Gall WE, Beebe K, Lawton KA, Adam K-P, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E R. S. G. for the. PLoS One. 2010;5:e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filla LA, Yuan W, Feldman EL, Li S, Edwards JL. J Proteome Res. 2014;13:6121–6134. doi: 10.1021/pr501030e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newgard CB. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. J Clin Endocrinol Metab. 2013;98:E1060–E1065. doi: 10.1210/jc.2012-4132. [DOI] [PubMed] [Google Scholar]
- 41.Felig P, Marliss E, Cahill GF. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 42.Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, Nowotny P, Roth E, Waldhausl W, Roden M. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 43.Anderson SG, Dunn WB, Banerjee M, Brown M, Broadhurst DI, Goodacre R, Cooper GJS, Kell DB, Cruickshank JK. PLoS One. 2014;9:e103217. doi: 10.1371/journal.pone.0103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali A, Iqbal F, Taj A, Iqbal Z, Amin MJ, Iqbal QZ. Pak J Med Sci. 2013;29:899–902. doi: 10.12669/pjms.294.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mäkinen VP, Forsblom C, Thorn LM, Wadén J, Gordin D, Heikkilä O, Hietala K, Kyllönen L, Kytö J, Rosengård-Bärlund M, Saraheimo M, Tolonen N, Parkkonen M, Kaski K, Ala-Korpela M, Groop PH. Diabetes. 2008;57:2480–2487. [Google Scholar]
- 46.Dunn WB, Goodacre R, Neyses L, Mamas M. Bioanalysis. 2011;3:2205–2222. doi: 10.4155/bio.11.223. [DOI] [PubMed] [Google Scholar]
- 47.Teul J, Rupérez FJ, Garcia A, Vaysse J, Balayssac S, Gilard V, Malet-Martino M, Martin-Ventura JL, Blanco-Colio LM, Tuñón J, Egido J, Barbas C. J Proteome Res. 2009;8:5580–5589. doi: 10.1021/pr900668v. [DOI] [PubMed] [Google Scholar]
- 48.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. JAMA J Am Med Assoc. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 49.Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Clin Chem. 1992;38:1632–1638. [PubMed] [Google Scholar]
- 50.Festa A, Williams K, Hanley AJG, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 51.Amigó N, Mallol R, Heras M, Martínez-Hervás S, Blanco-Vaca F, Escolà-Gil JC, Plana N, Yanes Ó, Masana L, Correig X. Sci Rep. 2016;6:19249. doi: 10.1038/srep19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Badeau RM, Honka MJ, Lautamaki R, Stewart M, Kangas AJ, Soininen P, Ala-Korpela M, Nuutila P. Ann Med. 2014;46:18–23. doi: 10.3109/07853890.2013.853369. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz EA, Reaven PD. Biochim Biophys Acta Mol Cell Biol Lipids. 2012;1821:858–866. doi: 10.1016/j.bbalip.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 54.Kusminski C, Shetty S, Orci L, Unger R, Scherer P. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 55.Hue L, Taegtmeyer H. Am J Physiol: Endocrinol Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esch C, Hui DS, Lee R, Edwards JL. Anal Methods. 2015;7:7164–7169. [Google Scholar]
- 57.Yuan W, Edwards JL, Li S. Chem Commun. 2013;49:11080–11082. doi: 10.1039/c3cc45956j. [DOI] [PubMed] [Google Scholar]
- 58.Demarco VG, Ford DA, Henriksen EJ, Aroor AR, Johnson MS, Habibi J, Ma L, Yang M, Albert CJ, Lally JW, Ford CA, Prasannarong M, Hayden MR, Whaley-Connell AT, Sowers JR. Endocrinology. 2013;154:159–171. doi: 10.1210/en.2012-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu T, Xie G, Ni Y, Liu T, Yang M, Wei H, Jia W, Ji G. J Proteome Res. 2015;14:447–456. doi: 10.1021/pr500825y. [DOI] [PubMed] [Google Scholar]
- 60.Lin HV, Frassetto A, Kowalik EJ, Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Todesco T, Rao AV, Bosello O, Jenkins DJ. Am J Clin Nutr. 1991;54:860–865. doi: 10.1093/ajcn/54.5.860. [DOI] [PubMed] [Google Scholar]
- 62.Connell T. Metabolites. 2013;3:931–945. doi: 10.3390/metabo3040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engström G, Östling G, Clish C, Wang TJ, Gerszten RE, Melander O. A diabetes-predictive amino acid score and future cardiovascular disease. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan W, Zhang J, Li S, Edwards JL. J Proteome Res. 2011;10:5242–5250. doi: 10.1021/pr200815c. [DOI] [PubMed] [Google Scholar]
- 65.Wurtz P, Tiainen M, Makinen VP, Kangas AJ, Soininen P, Saltevo J, Keinanen-Kiukaanniemi S, Mantyselka P, Lehtimaki T, Laakso M, Jula A, Kahonen M, Vanhala M, Ala-Korpela M. Diabetes Care. 2012;35:1749–1756. doi: 10.2337/dc11-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rovetto MJ, Lamberton WF, Neely JR. Circ Res. 1975;37:742–751. doi: 10.1161/01.res.37.6.742. [DOI] [PubMed] [Google Scholar]
- 67.Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. Am J Clin Nutr. 2009;89:106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Setter SM, Campbell RK, Cahoon CJ. Ann Pharmacother. 2003;37:1858–1866. doi: 10.1345/aph.1D002. [DOI] [PubMed] [Google Scholar]
- 69.Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL. Diabetes. 1993;42:80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 70.Ishii H, Koya D, King GL. J Mol Med. 1998;76:21–31. doi: 10.1007/s001090050187. [DOI] [PubMed] [Google Scholar]
- 71.Jha JC, Jandeleit-Dahm KA, Cooper ME. Adv Chronic Kidney Dis. 2014;21:318–326. doi: 10.1053/j.ackd.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Li M, Wang X, Aa J, Qin W, Zha W, Ge Y, Liu L, Zheng T, Cao B, Shi J, Zhao C, Wang X, Yu X, Wang G, Liu Z. Am J Physiol. 2013;304:F1317–F1324. doi: 10.1152/ajprenal.00536.2012. [DOI] [PubMed] [Google Scholar]
- 73.Couser WG, Remuzzi G, Mendis S, Tonelli M. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 74.Meyer TW, Hostetter TH. N Engl J Med. 2007;357:1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 75.Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J, Karoly ED, Kensicki EM, Berry GT, Bonventre JV, Pennathur S, Meyer TW, Krolewski AS. Kidney Int. 2014;85:1214–1224. doi: 10.1038/ki.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dunn W, Broadhurst D, Deepak S, Buch M, McDowell G, Spasic I, Ellis D, Brooks N, Kell D, Neyses L. Metabolomics. 2007;3:413–426. [Google Scholar]
- 77.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Saha J, Byun J, Schin M, Lorenz M, Kennedy RT, Kretzler M, Feldman EL, Pennathur S, Brosius FC., 3rd Am J Physiol. 2008;295:F1071–1081. doi: 10.1152/ajprenal.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M. Nephrol Dial Transplant. 2011;26:1094–1098. doi: 10.1093/ndt/gfq624. [DOI] [PubMed] [Google Scholar]
- 80.van der Kloet FM, Tempels FW, Ismail N, van der Heijden R, Kasper PT, Rojas-Cherto M, van Doorn R, Spijksma G, Koek M, van der Greef J, Makinen VP, Forsblom C, Holthofer H, Groop PH, Reijmers TH, Hankemeier T. Metabolomics. 2012;8:109–119. doi: 10.1007/s11306-011-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyata T, de Strihou CV, Kurokawa K, Baynes JW. Kidney Int. 1999;55:389–399. doi: 10.1046/j.1523-1755.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 83.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. J Am Soc Nephrol. 2003;14:S254–S258. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 84.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Diabetes Care. 2013;36:3234–3239. doi: 10.2337/dc12-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.You YH, Quach T, Saito R, Pham J, Sharma K. J Am Soc Nephrol. 2016;27:466–481. doi: 10.1681/ASN.2015030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S, Jim B, Ziyadeh FN. Semin Nephrol. 2003;23:532–543. doi: 10.1053/s0270-9295(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 87.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK. J Am Soc Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu J, Wang C, Liu F, Lu Y, Cheng J. Anal Bioanal Chem. 2015;407:2569–2579. doi: 10.1007/s00216-015-8481-0. [DOI] [PubMed] [Google Scholar]
- 89.Edwards JL, Vincent A, Cheng T, Feldman EL. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hinder LM, Vivekanandan-Giri A, McLean LL, Pennathur S, Feldman EL. J Endocrinol. 2013;216:1–11. doi: 10.1530/JOE-12-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freeman OJ, Unwin RD, Dowsey AW, Begley P, Ali S, Hollywood KA, Rustogi N, Petersen RS, Dunn WB, Cooper GJS, Gardiner NJ. Diabetes. 2016;65:228–238. doi: 10.2337/db15-0835. [DOI] [PubMed] [Google Scholar]
- 92.Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, Morisseau C, Haj FG, Hammock BD. Proc Natl Acad Sci U S A. 2012;109:11390–11395. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian YF, Hsia TL, Hsieh CH, Huang DW, Chen CH, Hsieh PS. Life Sci. 2011;89:107–114. doi: 10.1016/j.lfs.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O’Brien K, Geary RL, Heinecke JW. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 97.Drel VR, Lupachyk S, Shevalye H, Vareniuk I, Xu W, Zhang J, Delamere NA, Shahidullah M, Slusher B, Obrosova IG. Endocrinology. 2010;151:2547–2555. doi: 10.1210/en.2009-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frank RN. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 99.Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW. Invest Ophthalmol Visual Sci. 1997;38:36–47. [PubMed] [Google Scholar]
- 100.Gabbay KH. N Engl J Med. 1973;288:831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- 101.Barba I, Garcia-Ramirez M, Hernandez C, Alonso MA, Masmiquel L, Garcia-Dorado D, Simo R. Invest Ophthalmol Visual Sci. 2010;51:4416–4421. doi: 10.1167/iovs.10-5348. [DOI] [PubMed] [Google Scholar]
- 102.Lorenzi M. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stevens MJ, Henry DN, Thomas TP, Killen PD, Greene DA. Am J Physiol: Endocrinol Metab. 1993;265:E428–E438. doi: 10.1152/ajpendo.1993.265.3.E428. [DOI] [PubMed] [Google Scholar]
- 104.MacGregor LC, Rosecan LR, Laties AM, Matschinsky FM. J Biol Chem. 1986;261:4046–4051. [PubMed] [Google Scholar]
- 105.Ottlecz A, Garcia CA, Eichberg J, Fox DA. Curr Eye Res. 1993;12:1111–1121. doi: 10.3109/02713689309033509. [DOI] [PubMed] [Google Scholar]
- 106.Obrosova I, Cao X, Greene DA, Stevens MJ. Diabetologia. 1998;41:1442–1450. doi: 10.1007/s001250051090. [DOI] [PubMed] [Google Scholar]
- 107.Paris LP, Johnson CH, Aguilar E, Usui Y, Cho K, Hoang LT, Feitelberg D, Benton HP, Westenskow PD, Kurihara T, Trombley J, Tsubota K, Ueda S, Wakabayashi Y, Patti GJ, Ivanisevic J, Siuzdak G, Friedlander M. Metabolomics. 2016;12:15. doi: 10.1007/s11306-015-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ishikawa A, Ishiguro SI, Tamai M. Curr Eye Res. 1996;15:63–71. doi: 10.3109/02713689609017612. [DOI] [PubMed] [Google Scholar]
- 109.Gowda K, Zinnanti WJ, LaNoue KF. J Neurochem. 2011;117:309–320. doi: 10.1111/j.1471-4159.2011.07206.x. [DOI] [PubMed] [Google Scholar]
- 110.Hall TR, Wallin R, Reinhart GD, Hutson SM. J Biol Chem. 1993;268:3092–3098. [PubMed] [Google Scholar]
- 111.Pieragostino D, D’Alessandro M, di Ioia M, Di Ilio C, Sacchetta P, Del Boccio P. Proteomics: Clin Appl. 2015;9:169–186. doi: 10.1002/prca.201400084. [DOI] [PubMed] [Google Scholar]
- 112.Chen L, Zhou L, Chan EC, Neo J, Beuerman RW. J Proteome Res. 2011;10:4876–4882. doi: 10.1021/pr2004874. [DOI] [PubMed] [Google Scholar]
- 113.Capellades J, Navarro M, Samino S, Garcia-Ramirez M, Hernandez C, Simo R, Vinaixa M, Yanes O. Anal Chem. 2016;88:621–628. doi: 10.1021/acs.analchem.5b03628. [DOI] [PubMed] [Google Scholar]