Abstract
Objective
Although HAART effectively suppresses viral replication, it fails to eradicate latent viral reservoirs. The ‘shock and kill’ strategy involves the activation of HIV from latent reservoirs and targeting them for eradication. Our goal was to develop new approaches for activating HIV from latent reservoirs.
Design
We investigated capacity of Ingenol B (IngB), a newly modified derivative of Ingenol ester that was originally isolated from a Brazilian plant in Amazon, for its capacity and mechanisms of HIV reactivation.
Methods
Reactivation of HIV-1 by IngB was evaluated in J-Lat A1 cell culture model of HIV latency as well as in purified primary CD4+ T cells from long-term HAART-treated virologically-suppressed HIV-infected individuals. The underlining molecular mechanisms of viral reactivation were investigated using flow cytometry, RT-qPCR and chromatin immunoprecipitation.
Results
IngB is highly effective in reactivating HIV in J-Lat A1 cells with relatively low cellular toxicity. It is also able to reactivate latent HIV in purified CD4+ T cells from HAART-treated HIV-positive individuals ex vivo. Our data show that IngB may reactivate HIV expression by both activating protein kinase C (PKC)δ–nuclear factor kappalight-chain-enhancer of activated B cells (NF-κB) pathway and directly inducing NF-κB protein expression. Importantly, IngB has a synergistic effect with JQ1, a BET bromodomain inhibitor, in latent HIV reactivation.
Conclusions
IngB is a new promising compound to activate latent HIV reservoirs. Our data suggest that formulating novel derivatives from Ingenol esters may be an innovative approach to develop new lead compounds to reactivate latent HIV.
Keywords: HIV latency, Ingenol ester, JQ1, NF-κB, protein kinase C
Introduction
With the advent of HAART, significant advances have been made in controlling HIV-1 (HIV) infection and improving health of HIV-infected patients. However, HAART fails to eradicate latent HIV reservoirs, and interruption of HAART leads to a rapid rebound of viral loads in both peripheral blood and gut-associated lymphoid tissue [1–4]. Therefore, new strategies are needed for eliminating latent HIV pool.
Recent studies suggest that quiescent memory CD4+ T cells constitute most of the long-lived viral reservoirs [5–9]. Histone modification-mediated chromosomal suppression of HIV long terminal repeat (LTR) was reported to be critical for establishing HIV latency [10–15]. Histone deacetylases (HDACs) and histone methyltransferases are recruited to HIV LTR and are involved in establishing HIV latency [10,11,16–18]. Disruption of HDACs or enhancer of zeste homolog 2 binding to HIV LTR resulted in reactivation of latent HIV-1 in vivo as well as in vitro [10,16,19–22]. Resting CD4+ T cells harbor low levels of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and nuclear factor of activated T-cells which may support the establishment of HIV latency [23]. Under most circumstances, in resting CD4+ T cells, p-TEFb is restricted in a transcriptionally inactive complex with hexamethylene bis-acetamide inducible 1/bromodomain-containing protein 4/7SK small nuclear RNA for establishing viral latency [24–29]. Therefore, compounds that can disrupt binding or inhibit enzyme activity of HIV-1 transcriptional repressors, such as suberanilohydroxamic acid (SAHA), hexamethylene bisacetamide (HMBA) or a BET bromodomain inhibitor JQ1, or activate NF-κB signaling, such as prostratin, have been considered for inducing reactivation of HIV from latency [30]. A recent study reported reactivation of latent HIV-1 with a single dose of SAHA administration in HIV-infected patients on HAART [31]. Although SAHA induced viral reactivation in patients, identification of novel compounds is important to achieve effective reactivation of latent HIV in the future [31,32]. A new group of compounds, Ingenol derivatives, have been shown to regulate HIV expression in vitro by either activating or repressing HIV transcription [33–35]. It is interesting to note that Ingenol esters are structurally analogous to phorbol esters, which are known to reactivate latent HIV reservoirs [36,37].
In this study, we found that a newly modified Ingenol ester compound originally isolated from Euphorbia tirucalli, Ingenol B (IngB), effectively promoted reactivation of HIV LTR-induced gene expression through activation of protein kinase C (PKC)δ–Serine 664–NF-κB pathway in the HIV latency culture model of J-Lat A1 cell line and in purified CD4+ T cells from HIV-infected individuals under long-term HAART. We also found that IngB had minimal cellular toxicity and higher potency for HIV reactivation than SAHA, JQ1, or prostratin in vitro. In addition, IngB had synergistic effects on HIV reactivation in combination with JQ1 or HMBA in vitro. In summary, formulation of derivatives from Ingenol family may provide a novel opportunity for HIV reactivation from latent viral reservoirs and potentially supporting viral eradication efforts.
Materials and methods
Cell culture
The J-Lat A1 cells (harboring HIV LTR and green fluorescent protein (GFP) gene under the HIV LTR control) or U1 cells (harboring latent HIV genome) were cultured with RPMI1640 medium with 10% fetal bovine serum at 37°C. For reactivation of HIV LTR, cells were treated with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich), SAHA (Santa Cruz Biotechnology, Inc.). JQ1 (Biovision Incorporated, USA), IngB (patent submitted from KyoLab), Prostratin (Sigma-Aldrich), HMBA (Sigma-Aldrich) or TNF-α (BD Biosciences, USA) for 24 h. HIV reactivation was measured by GFP flow cytometry and the data were analyzed using FlowJo Software for J-Lat cells or p24 ELISA for U1 cells. Cell viability was evaluated using Live/Dead dye.
Primary CD4+ T-cell isolation
Peripheral blood samples were collected from HIV-infected patients (n = 7, all men, age ranged from 40 to 66 years) receiving antiretroviral therapy (ART) for more than 5 years. These individuals had CD4+ T-cell numbers ranging from 347 to 1403 cells/ µl and plasma viral loads were below 50 copies/ml as measured by qPCR. Patient samples were obtained under informed written consent and a protocol approved by the UC Davis institutional review board. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll Hystopaque as previously described [1]. The CD4+ T cells were further purified with EasySep kit from STEMCELL Technologies Inc. (Vancouver, British Columbia, Canada). The purified CD4+ T cells were plated at a density of 1 × 106 cells and treated with 200 ng/ml PMA and 2 µmol/l ionomycin, 3 nmol/l IngB, 500 nmol/l SAHA, or 2 µmol/l JQ1 for 6 or 48 h.
To measure changes in the cell activation status in CD4+ and CD8+ T-cell subsets, PBMCs were isolated from healthy donors and 2 × 106 cells were incubated with DMSO, 200 ng/ml PMA and 2 µmol/l ionomycin, 3nmol/l IngB, 500nmol/l SAHA, or 2 µmol/l JQ1 for 24 or 72 h, and immune-stained with anti-CD3, anti-CD4, anti-CD8, anti-CD38, anti-CD69, or anti-human leukocyte antigens (HLA)-DR antibodies (Biolegend, San Diego, California, USA) for 20 min at 4°C. Cells were fixed and analyzed by flow cytometry (FlowJo). In addition, PBMCs were treated with similar regimens for 24–72 h and cytokine was analyzed with ELISA (supernatants) or reverse transcription-quantitative PCR (cells) (Biolegend).
Cell viability and proliferation measurements
Cells were placed in 96-well plates and treated with compounds for HIV reactivation. After 24 or 72 h of incubation at 37°C, cell viability was measured using MTT (3-[4,5–dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay (Roche Laboratories). Cell proliferation was used as a measure of cell activation and was detected by determining BrdU incorporation in the S-phase of cell replication using ELISA (EMD-Millipore, QIA58).
Immunoblot analysis
One million J-Lat A1 cells or PBMCs from healthy donors were incubated with IngB for 6 h. Whole cell protein extracts were prepared with radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich). Expression of the isoforms of PKC protein or NF-κB/p65 was evaluated using the PKC Isoform Sampler Antibody Kit (Cell Signaling Technology, 9960S) and anti-NF-κB/p65 (Abcam). The level of phosphorylation of PKC was determined using anti-Phospho-Ser664-PKC (EMD-Millipore).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed as previously described [38,39]. Briefly, 1 × 106 J-Lat A1 cells were fixed in 1% formaldehyde. The chromatin was sonicated into fragments of 200–1500 nucleotides long and subjected to immunoprecipitation. After incubating with 50 µl of protein A agarose beads, the immunocomplexes were washed, the chromatin was eluted and reverse cross-linked at 65°C overnight. DNA was extracted with Qiagen PCR purification kit. The upstream primer sequence is 5′-AGCTTGCTACAAGGGACTTTCC-3′, and the downstream primer sequence is 5′-ACCCAGTACAGGCAAAAAGCAG-3′. Quantitative real-time PCR was performed using Agilent Brilliant Ultra-Fast SYBR Green QPCR reagent.
HIV RNA quantification
Total RNA was extracted with the Qiagen RNeasy Kit. Ten microliter and 450 ng RNA were assayed for HIV RNA using TaqMan Fast Virus 1-Step Master Mix (Applied Biosystems at Life Technologies), well conserved primers (HXB2 positions 533–543, 626–643) and fluorescent probe (559–584) from LTR region [40]. Reverse transcription and amplification was performed in a ViiA7 Thermocycler. External genomic HIV RNA standards were prepared from NL4–3 virions obtained from viral stocks expanded in tissue culture in vitro and validated via the Abbot Real Time assay. HIV RNA copy numbers were normalized to RNA input. Non-reverse transcription controls were performed using TaqMan Fast Advanced Mastermix (Applied Biosystems at Life Technologies), and HIV DNA co-purified on RNeasy columns was quantified with the same external standards. Final values for HIV RNA expression levels were adjusted by subtraction of HIV DNA copy numbers from total reverse transcription (RT)-PCR readouts.
Statistical analysis
Means and standard errors (SEs) were calculated for all data points from at least three independent experiments in triplicates. Statistical significance was determined using the Student’s t test, in which P values less than 0.05 were considered significant.
Results
Ingenol B induces reactivation of HIV LTR-regulated GFP expression in J-Lat A1 cell model of HIV latency
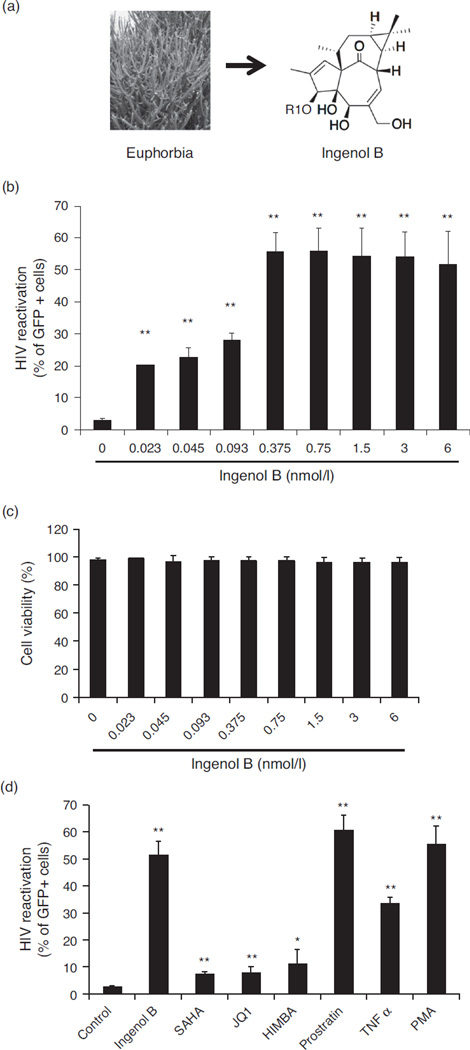
An Ingenol ester compound was initially extracted from E. tirucalli by KYOLAB Laboratories and was found to share the similar core structure with other Ingenol esters (Fig. 1a). The natural Ingenol was modified to produce a new derivative called Ingenol-3-hexoanante (IngB). Ingenol esters are structurally analogous to phorbol esters, which are known to reactivate HIV from latency. However, there is limited information regarding the effects of Ingenol against viral latency. Therefore, we investigated the ability of IngB to reactivate latent HIV. We opted to evaluate the potency of IngB in the J-Lat A1 cells, a well studied HIV latency cell culture model [41,42]. The J-Lat A1 cells harbor GFP gene under the control of HIV-1 LTR and provide an opportunity to measure activity of compounds that can induce GFP expression by re-activating HIV LTR. Treatment with IngB effectively induced GFP expression in about 50% of the J-Lat A1 cells (Fig. 1b). The ability of IngB to reactivate latent HIV in J-Lat A1 cells was evaluated at concentrations ranging from 23 pmol/l to 6 nmol/l. It effectively activated GFP expression through HIV LTR in more than 50% of the cells at concentrations at 375 pmol/l and above, and it was able to activate GFP expression in 20% of the cells even at the 23 pmol/l concentration. Flow cytometric analysis using live/dead dye showed that IngB caused minimal cellular toxicity on J-Lat A1 cells and had minor effects on cell proliferation at 3–24 nmol/l concentrations (Fig. 1c). Our data showed that IngB is an effective activator of HIV from latency without exerting detectable cellular cytotoxicity.
Fig. 1. Ingenol B is a potent activator of HIV LTR in J-Lat A1 cell line model for HIV latency.
(a) Ingenol B is a modified compound isolated from a Brazilian plant, Euphorbia tirucalli. (b) Ingenol B reactivated latent HIV-1 in J-Lat A1 cells. Increased doses of Ingenol B, from 23 pmol/l to 6 nmol/l, were added in J-Lat A1 cells for 24 h. Latent HIV-1 reactivation was measured by detecting GFP-expressing cells by flow cytometry. (**) P < 0.01. (c) Cell viability during Ingenol B treatment. Increased doses of Ingenol B, from 23 pmol/l to 6 nmol/l, were added to J-Lat A1 cells for 24 h and cell viability was measured using live/dead dye by flow cytometry. (d) Ingenol B has higher potential of HIV LTR reactivation in J-Lat cells compared to SAHA or JQ1. The HIV LTR driven GFP expression in J-Lat A1 cells was determined by flow cytometry at 24 h of treatment with Ingenol B and other compounds. The dosage for the treatment was: Ingenol B 375 pmol/l, PMA 5 ng/ml, TNF-α 25 TNF ng/ml, HMBA 5 mmol/l, JQ1 2 µmol/l, prostratin 10 µmol/l, SAHA 500 nmol/l. HIV LTR reactivation was analyzed in comparison to untreated J-Lat cell controls. TNF, tumor necrosis factor. (*) P < 0.05, (**) P < 0.01.
Ingenol B has higher potency of HIV reactivation compared to other known compounds in vitro
Eliminating latent reservoir is challenging since it is very stable and is established very early during HIV infection [43–46]. Because of the high potency of IngB for HIV reactivation, we sought to examine the ability of IngB to reactivate latent HIV in comparison to other compounds previously known to reactivate latent HIV from T-cell reservoirs. These included SAHA, tumor necrosis factor (TNF)-α, prostratin, HMBA and a BET bromodomain inhibitor JQ1. SAHA has been used in clinical trials for reactivation of latent HIV in patients on ART [25,31,47– 49]. The J-Lat A1 cells were treated with 500 nmol/l SAHA or 375 pmol/l IngB for 24 h. We found that SAHA reactivated about 5.5% of latent cells, whereas IngB reactivated more than 50% of latent J-Lat cells (Fig. 1d). Recently, a bromodomain inhibitor for treatment of male infertility, JQ1, was shown to be a potential candidate for HIV eradication strategy [49–52]. Therefore, we examined the effect of IngB in combination with these previously tested compounds for latent HIV reactivation in J-Lat A1 cells. Treatment with 5 ng/ml PMA served as a positive control in the assay. As shown in Fig. 1d, JQ1 treatment reactivated 7% of latent J-Lat A1 cells and it had potency very similar to that of SAHA [51]. IngB had higher potential of HIV reactivation than SAHA, JQ1, HMBA and TNF-α, except for 10 µmol/l prostratin. SAHA or JQ1 activated 5–10% of J-Lat A1 cells, whereas IngB activated more than 50% of J-Lat A1 cells. IngB was effective at lower concentrations compared to SAHA or JQ1. Similar results were also seen in U1 cells (data not shown). In summary, our data showed that IngB is a highly potent compound for reactivation of latent HIV in the J-Lat cell line model of HIV latency.
Ingenol B is synergistic with other known compounds for reactivation of latent HIV reservoirs
Because of the high potency of IngB for HIV reactivation, we sought to examine the ability of IngB to reactivate latent HIV synergistically in combination with other compounds previously known to reactive HIV from latency. Establishment and maintenance of HIV latency are regulated by multiple cellular and molecular pathways. Therefore, it is reasonable to assume that a single drug may not effectively reactivate all the latent HIV reservoirs in patients and may warrant a multimodal approach for HIV eradication as proposed recently [53,54].
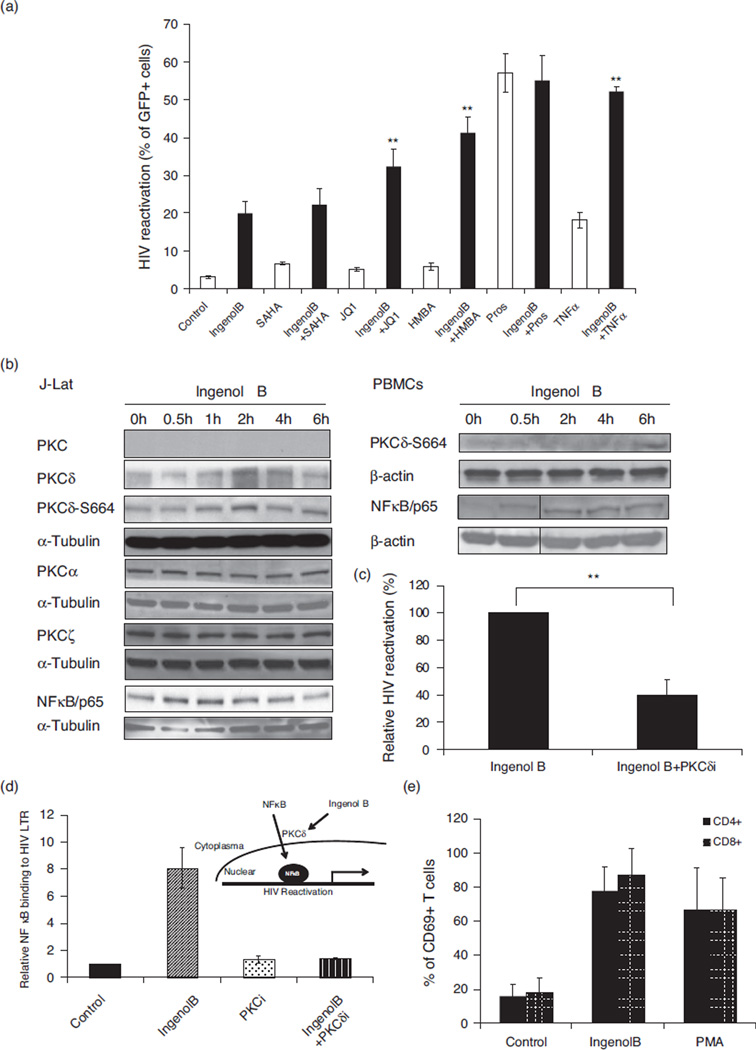
Since high concentration of IngB may mask potential synergistic effects of other compounds, we opted to use lower concentration (46 pmol/l) of IngB at which it can reactivate about 20% of latent J-Lat cells. As shown in Fig. 2a, there was no synergistic effect on latent HIV reactivation in presence of prostratin or SAHA. However, IngB was able to synergistically reactivate HIV from latency with the addition of HMBA, TNF-α, or JQ1. Lack of synergistic effect between IngB and prostratin suggested that IngB regulated HIV reactivation may occur through PKC–NF-κB pathway. As a member of the Ingenol ester family, IngB can modulate PKC signaling and probably may utilize the PKC–NF-κB signaling in reactivation of HIV from latency [37]. To test this hypothesis, we measured changes in PKC expression by western blot analysis using antibodies specific for four PKC superfamilies (Fig. 2b, left panel). We found that, among the four species of PKCs, expression of PKCμ is barely detected, whereas expression of PKCα and PKCζ was not altered in J-Lat A1 cells in the presence of IngB. Only PKCδ level was up-regulated, with a peak expression level at 2–4 h following IngB treatment. Further, phosphorylation of PKCδ following IngB treatment was clearly demonstrated by antiphospho-Ser664-PKC antibody with western blot. PKCδ was phosphorylated at Ser664 as early as 1 h following IngB treatment, peaked at 2 h and returned to baseline at 6 h (Fig. 2b, left panel). Similarly, IngB treatment led to PKCδ phosphorylation in PBMCs from healthy HIV-negative donors (Fig. 2b, right panel). Ingenol was previously reported to upregulate NF-κB protein expression [55]. To examine whether IngB modulated NF-κB protein expression, we performed western blot analysis using anti-NF-κB/p65 antibody. A rapid increase in the expression of NF-κB/p65 was seen following the treatment with IngB (30min–2 h) (Fig. 2c, left panel). Similarly, increased expression of NF-κB/p65 was detected in the PBMCs from HIV-negative healthy individuals following the IngB treatment (Fig. 2b, right panel). Further, addition of PKCδ inhibitor to J-Lat A1 cells inhibited the magnitude of IngB-induced HIV reactivation from latency (Fig. 2c).
Fig. 2. Ingenol B reactivates latent HIV LTR-regulated expression through protein kinase Cd–S664–NF-kB signaling.
(a) A single compound or a combination of compounds was added to J-Lat A1 cells for 24 h. HIV-1 LTR-driven GFP expression in the cells was measured by flow cytometry as shown in Fig. 1. Combined treatments included Ingenol B at 46 pmol/l. (**) P < 0.01, compared to single compound. (b) Activation of PKCδ and expression of PKC isoforms in J-Lat cells or human PBMCs from healthy donors. J-Lat A1 cells or human PBMCs were treated with 6 nmol/l Ingenol B for 6 h. The cells were collected and lyzed in RIPA buffer. Western blot analysis was performed to detect expression of isoforms of PKC, expression of NF-κB/p65, or Ser664 phosphorylation at PKCδ with indicated antibodies. (c) PKCδ inhibitor suppresses Ingenol B-induced HIV reactivation. J-Lat A1 cells were treated with 3 nmol/l Ingenol B in the presence of 5 µmol/l PKCδ inhibitor and examined for GFP expression by flow cytometry. (**) P < 0.01. (d) Ingenol B promotes NF-κB/p65 binding to HIV LTR through activation of PKCδ. J-Lat A1 cells were treated with 6 nmol/l Ingenol B with or without 5 µmol/l PKCδ inhibitor Millipore). The cells were collected and ChIP-qPCR assay was performed to detect NF-κB/p65 binding to HIV LTR. Relative NF-κB/p65 binding to HIV LTR was expressed after normalized to untreated controls. (e) Activation of CD69 expression in PBMC following Ingenol B treatment. The CD69 expression was analyzed in CD4+ and CD8+ T-cell subsets in PBMC from HIV-negative controls that were treated with PMA or Ingenol B for 24 h. PMA-treated cells served as the positive control. PKC, protein kinase C.
Protein kinase C-induced gene expression is mediated through activation of NF-κB and its binding to promoter region of the cellular genes [56–58]. To examine whether IngB reactivates HIV latency by promoting PKCδ-induced NF-κB binding to HIV LTR, ChIP-qPCR assays were performed on J-Lat A1 cells in the presence of 6 nmol/l IngB with or without PKCδ inhibitor. As shown in Fig. 2d, IngB treatment led to an eight-fold increase in NF-κB/p65 binding to HIV LTR region. This was completely abolished upon the addition of PKCδ-specific inhibitor. Therefore, our findings indicate that IngB regulation of HIV expression is probably through up-regulating PKCδ–NF-κB/p65 pathway.
We evaluated the effect of IngB on the activation of NF-κB in primary CD4+ T cells purified from HIV-negative healthy donors. We measured changes in the cell surface expression of CD69, a marker of cell activation and proliferation, in PBMCs and isolated CD4+ T cells following the treatment with IngB. Since CD69 gene contains three known NF-κB-binding sites in its promoter region, it can serve as a surrogate marker for in-vivo PKCδ-induced NF-κB activation [59]. Up-regulation of CD69 expression can occur in primary CD4+ T cells stimulated with PKC–NF-κB activators including PMA and prostratin [60]. As shown in Fig. 2e, in IngB-treated PBMCs, more than 80% of the CD4+ or CD8+ T cells had cell surface expression of CD69 as compared to untreated controls, with only about 20–30% of CD4+ or CD8+ T cells expressing CD69. The PMA treatment resulted in induction of CD69 expression in 80% of the CD4+ T cells and served as a positive control in the study. These findings suggested that IngB activates PKCδ–NF-κB signaling not only in J-Lat A1 cell lines but also in primary CD4+ T cells. Taken together, our data showed that IngB might reactivate latent HIV through PKCδ–S664–NF-κB signaling pathway.
Ingenol B causes minimal cell activation and cyotoxicity
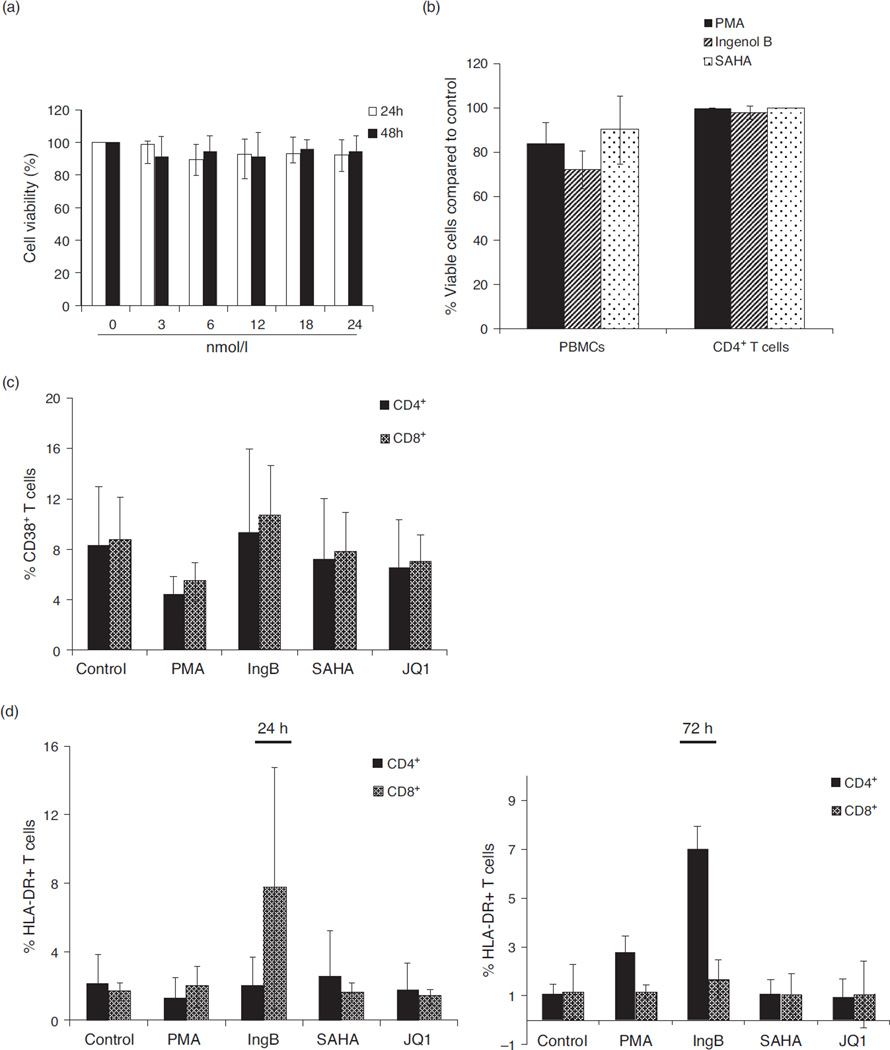
To determine whether IngB caused any cytotoxic effects on cells, we measured cell viability of J-Lat A1, PBMCs, or purified CD4+ T cells following IngB treatment. Minimal cell toxicity was noted in these IngB-treated cells by MTT assay (Fig. 3a, b). This finding is similar to those reported for SAHA treatment.
Fig. 3. Ingenol B causes minimal T-cell activation and cell toxicity.
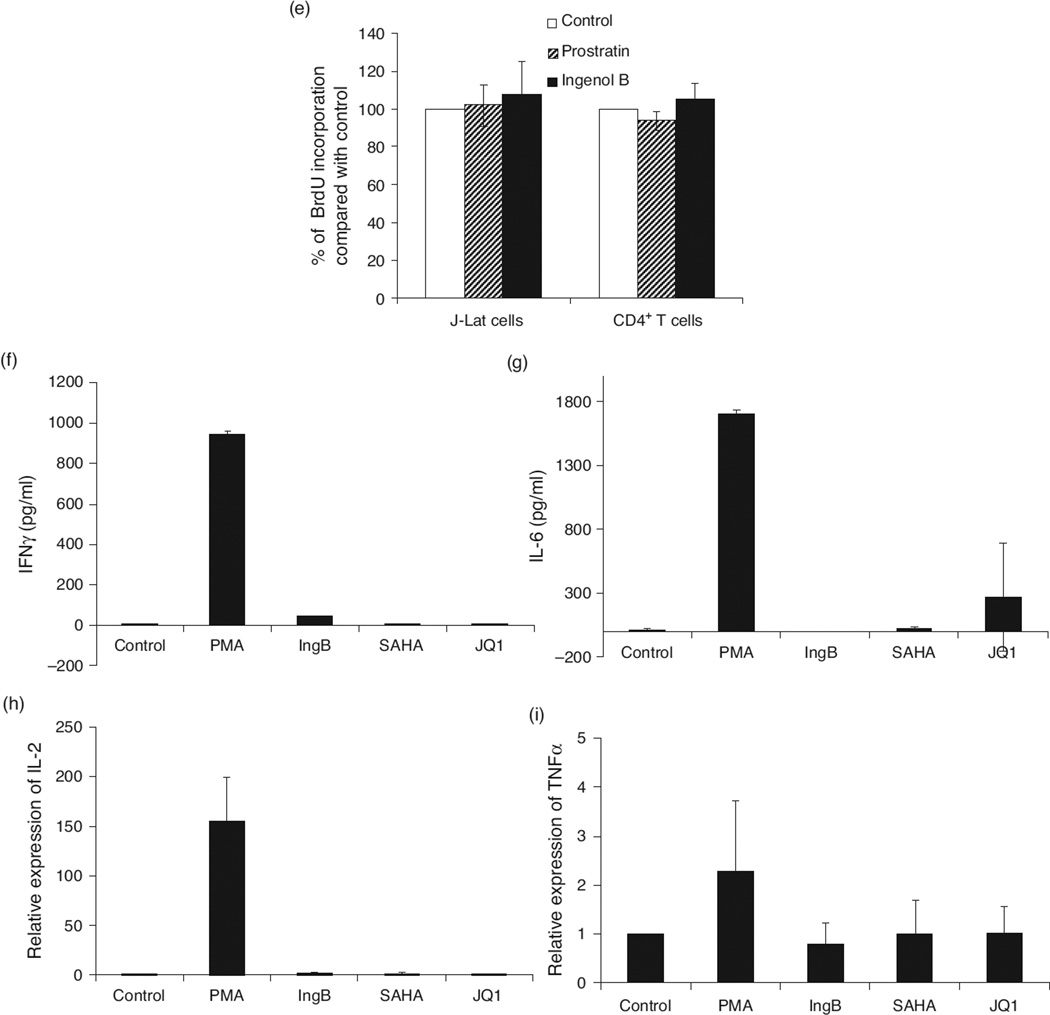
(a) Lack of cellular toxicity in J-Lat cells during Ingenol B treatment. J-Lat A1 cells were treated with Ingenol B from 3 to 24 nmol/l for 24 or 48 h, and cell proliferation and viability were measured by MTT. (b) Ingenol B has minimal cell toxicity in PBMCs or isolated CD4+ T cells. Human PBMCs or CD4+ T cells were isolated from peripheral blood of healthy HIV-negative donors and treated with 5 ng/ml PMA, 500 nmol/l SAHA, or 3 nmol/l Ingenol B for 20 h, and evaluated for cell proliferation/toxicity by MTT. (c) PBMCs were treated with specified agents for 24 h and examined for CD38 expression on CD4+ and CD8+ T cells by flow cytometry. (d) PBMCs were treated with indicated agents for 24 or 72 h and examined for HLA-DR expression on CD4+ or CD8+ T cells by flow cytometry. (e) J-Lat A1 cells and CD4+ T cells were treated with BrdU with or without Ingenol B or prostratin for 24 h, and BrdU ELISAs were performed with anti-BrdU antibody for quantification of BrdU incorporation. (f–i) Expression of IFNγ, IL-6, IL-2, or TNF-α in PBMCs from healthy donors (n = 3) following treatment with 3 nmol/l Ingenol B for 24 h. The expression of IFNγ and IL-6 proteins was detected by ELISA, and RNA expression of IL-2 and TNF-α was detected by RT-qPCR after normalizing the values with glyceraldehyde 3-phosphate dehydrogenase housekeeping gene and untreated controls. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
To investigate whether IngB caused nonspecific T-cell activation, we measured expression of cell activation markers including HLA-DR and CD38 in CD4+ and CD8+ T-cell subsets from IngB-treated PBMC samples of HIV-negative controls. Compounds causing massive global T-cell activation tend to exert cytotoxicity and may not be suitable for clinical use [61]. Our data showed that IngB did not cause demonstrable up-regulation of expression of CD38 in T-cell subsets (Fig. 3c). However, IngB treatment caused a minor increase of HLA-DR expression in CD4+ T cells and a major increase of CD69 expression in CD4+ and CD8+ T cells (Figs. 2e and 3d). Our findings raised a concern about potential side effects of this compound. Therefore, we investigated cells in the S-phase of cell cycle using BrdU incorporation assay. However, IngB treatment of J-Lat A1 cells and isolated CD4+ T cells from PBMCs did not result in significant changes, which suggested that IngB treatment did not lead to any substantial changes in cell cycle progression or cell proliferation (Fig. 3e). Since cytokine release may induce T-cell activation or be toxic to T cells, we examined levels of several cytokines after IngB treatment [62]. Although IngB induced a low level of interferon (IFN)γ protein production at 24 h, it was about 20-fold lower than the level produced following PMA stimulation (Fig. 3f, h), and it was undetectable after 48 h (data not shown). Both SAHA and JQ1 barely stimulated IFNγ expression, but induced production of interleukin (IL)-6 in two of the three samples, whereas IngB did not have a detectable effect on the expression of IL-6. There was lack of induction of the expression of IL-2 and TNF-α following the treatment with SAHA, JQ1, or IngB. In summary, IngB did not cause massive CD4+ T-cell activation or cytoxicity, and may serve as a suitable candidate for activating HIV from latent reservoirs.
Ingenol B is able to activate HIV expression in purified primary CD4+ T cells from HAART-treated HIV-positive individuals
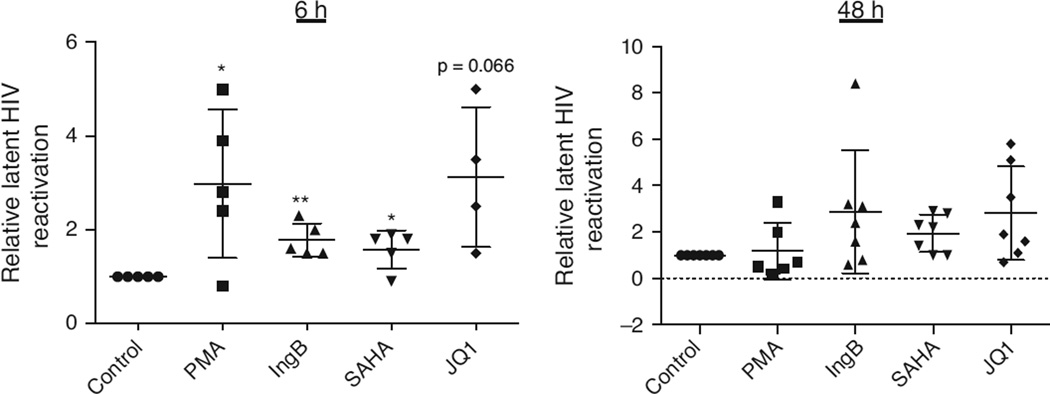
We sought to determine the efficacy of IngB to reactivate HIV in purified CD4+ T cells from long-term HAART-treated HIV-positive individuals. Purified CD4+ T cells from these patients were treated for 6 or 48 h with 3 nmol/l IngB, 500 nmol/l SAHA, or 2 µmol/l JQ1, and cellular HIV reactivation was examined by HIV RNA expression with RT-qPCR. Our data indicated that, at 6 h after treatment, both IngB and JQ1 were able to reactivate latent HIV from all the CD4+ T cells isolated from patients, SAHA reactivated latent HIV except one sample; at 48 h after treatment, both IngB and SAHA were able to reactivate latent HIV-4 in seven samples, whereas JQ1 reactivated latent HIV in five of the seven samples (Fig. 4). Thus, IngB is able to reactivate latent HIV from CD4+ T cells from HIV-infected patients and is comparable to SAHA or JQ1 in its potency for HIV reactivation. These findings were consistent with our results from J-Lat A1 cell cultures in vitro.
Fig. 4. Ingenol B reactivates latent HIV in primary CD4+ T cells from long-term HAART-treated HIV-positive individuals.
Primary CD4+ T cells were purified from PBMCs isolated from cohorts of HAART-treated virologically suppressed HIV-infected individuals, and incubated with 200 ng/ml PMA and 2 µmol/l inomycin, 3 nmol/l Ingenol B, 500 nmol/l SAHA or 2 µmol/l JQ1 for 6 or 48 h. Cells were collected and RNAs were extracted by Qiagen RNeasy kit. RT-qPCR was performed for HIV reactivation analysis after normalized with expression of internal control (18S rRNA). The value was expressed relative to control treatment. (*) P < 0.05, (**) P < 0.01.
Structural similarity of Ingenol ester to phorbol ester can be used for new formulations for HIV reactivation
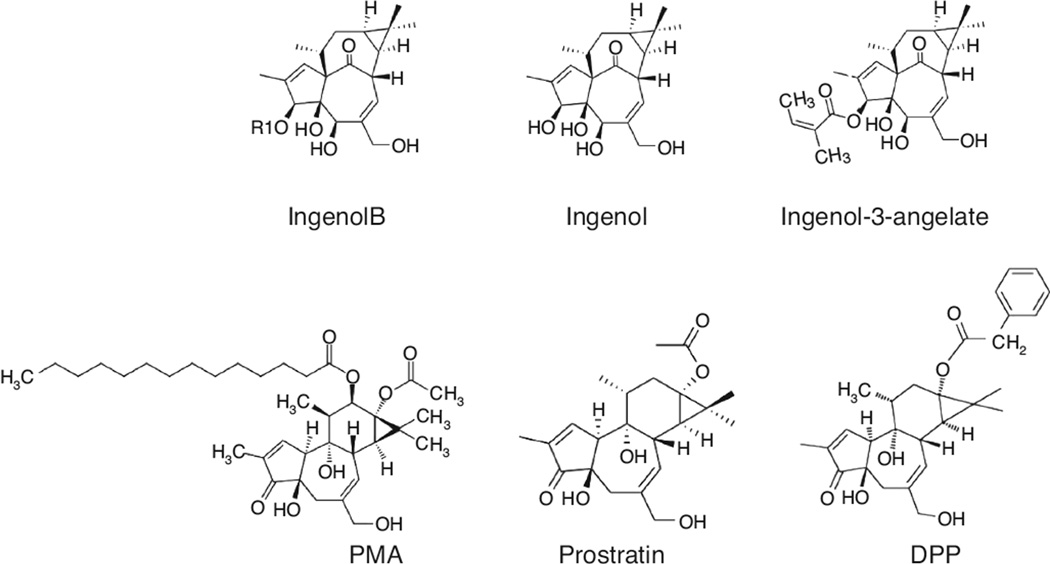
Ingenol ester is structurally analogous to phorbol esters [36,37] (Fig. 5). Phorbol esters, such as PMA, are highly potent to reactivate latent HIV, but toxic to T cells and may promote tumor formation. Prostratin or 12-Deoxyphorbol 13-phenylacetate does not have similar tumorigenic effects [63–65]. Interestingly, only PKC agonists, including prostratin and possibly IngB, showed broad activity in reactivation of latent HIV [66]. Therefore, our data indicate that formulating compounds from Ingenol esters could be a new direction to develop or formulate potent and PKC–NF-κB pathway-specific small molecules for HIV eradication [67].
Fig. 5. Core chemical structure of Ingenol B is analogous to phorbol esters.
Ingenols share core structure analogous to phorbol esters.
Discussion
We found that a newly modified Ingenol ester derivative, IngB, is highly potent in reactivation of latent HIV-1. It is more effective than some of the known compounds, including SAHA, HMBA, JQ1, and prostratin, in HIV reactivation in vitro. Importantly, it has limited cellular toxicity in both J-Lat cells and primary human CD4+ T cells. We found that IngB reactivates latent HIV through PKCδ–NF-κB signaling by up-regulating expression of both PKCδ and NF-κB. However, PKCu may also be involved in IngB-induced reactivation of HIV latency since 5 µmol/l of PKC inhibitor could inhibit PKCu (Fig. 2c and d). It is also possible that activation of PKCδ may induce degradation of IκB and promote release of NF-κB from NF-κB–IκB complex, which facilitates nuclear translocation of NF-κB to recruit into HIV LTR. This has been noted in case of activation of other PKCs. Our data show that JQ1 or SAHA alone is not most effective in reactivating latent HIV in vitro. The effect of IngB in combination with JQ1 on reversing HIV latency is of interest. Therefore, future evaluation of combined use of JQ1 with IngB in purified CD4+ T cells from HIV-infected patients on long-term HAART is warranted. IngB does not modulate proliferation of Jurkat T cells or CD4+ T cells, cytokine expression except for the minimal expression of IFNγ. IngB was also shown not to enhance cell proliferation/cell cycle S-phase progression, although IngB can cause increased levels of CD69 expression. We can speculate that higher levels of IngB most probably may not exert serious cytotoxic effects on CD4+ T cells. The value of these characteristics of IngB to clinical application remains to be tested.
Recently, an Ingenol ester compound, PICATO (ingenol mebutate), was approved by the US Food and Drug Administration (FDA) for treatment of skin precancer [68] (Fig. 5). It seems that only PKC agonists, such as prostratin, had broad impact on reactivation of latent HIV in currently available in-vitro or ex-vivo HIV latency models [66]. As a PKC agonist, IngB may share similar property with prostratin. It is possible that new Ingenol compounds can be developed through step-economical synthesis [67,69,70]. This will provide an innovative strategy to seek novel compounds that are highly potent and yet safe PKC–NF-κB activators for HIV cure in the future.
Acknowledgments
The study is supported by NIH grants DK61297 and AI43274 and by a postdoctoral fellowship from CAPES/Brazil (BEX 2951/12–6) to E.A.M. P.K. was supported by the Swiss National Science Foundation (PBZHP3_147260). IngB is an experimental drug developed by Amazonia Fitomedicamentos-patent pending.
Footnotes
Conflicts of interest
There are no conflicts of interest to declare for all authors.
References
- 1.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 2.Lerner P, Guadalupe M, Donovan R, Hung J, Flamm J, Prindiville T, et al. The gut mucosal viral reservoir in HIV-infected patients is not the major source of rebound plasma viremia following interruption of highly active antiretroviral therapy. J Virol. 2011;85:4772–4782. doi: 10.1128/JVI.02409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 4.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 5.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun TW, Chadwick K, Margolick J, Siliciano RF. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J Virol. 1997;71:4436–4444. doi: 10.1128/jvi.71.6.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 10.Jiang G, Espeseth A, Hazuda DJ, Margolis DM. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J Virol. 2007;81:10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman J, Cho WK, Chu CK, Keedy KS, Archin NM, Margolis DM, et al. Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of Zeste 2. J Virol. 2011;85:9078–9089. doi: 10.1128/JVI.00836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.du Chene I, Basyuk E, Lin YL, Triboulet R, Knezevich A, Chable-Bessia C, et al. Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J. 2007;26:424–435. doi: 10.1038/sj.emboj.7601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouchat S, Gatot JS, Kabeya K, Cardona C, Colin L, Herbein G, et al. Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4(+) T cells from HIV-1-infected HAART-treated patients. AIDS. 2012;26:1473–1482. doi: 10.1097/QAD.0b013e32835535f5. [DOI] [PubMed] [Google Scholar]
- 14.Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, Guiguen A, et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5:e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coull JJ, Romerio F, Sun JM, Volker JL, Galvin KM, Davie JR, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keedy KS, Archin NM, Gates AT, Espeseth A, Hazuda DJ, Margolis DM. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J Virol. 2009;83:4749–4756. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Lint C, Quivy V, Demonte D, Chariot A, Vanhulle C, de Walque S, et al. Molecular mechanisms involved in HIV-1 transcriptional latency and reactivation: implications for the development of therapeutic strategies. Bull Mem Acad R Med Belg. 2004;159:176–189. [PubMed] [Google Scholar]
- 19.Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Archin NM, Keedy KS, Espeseth A, Dang H, Hazuda DJ, Margolis DM. Expression of latent human immunodeficiency type 1 is induced by novel and selective histone deacetylase inhibitors. AIDS. 2009;23:1799–1806. doi: 10.1097/QAD.0b013e32832ec1dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ylisastigui L, Coull JJ, Rucker VC, Melander C, Bosch RJ, Brodie SJ, et al. Polyamides reveal a role for repression in latency within resting T cells of HIV-infected donors. J Infect Dis. 2004;190:1429–1437. doi: 10.1086/423822. [DOI] [PubMed] [Google Scholar]
- 23.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 24.Molle D, Maiuri P, Boireau S, Bertrand E, Knezevich A, Marcello A, et al. A real-time view of the TAR:Tat:P-TEFb complex at HIV-1 transcription sites. Retrovirology. 2007;4:36. doi: 10.1186/1742-4690-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhary SK, Archin NM, Margolis DM. Hexamethylbisacetamide and disruption of human immunodeficiency virus type 1 latency in CD4(+) T cells. J Infect Dis. 2008;197:1162–1170. doi: 10.1086/529525. [DOI] [PubMed] [Google Scholar]
- 26.Yik JH, Chen R, Pezda AC, Samford CS, Zhou Q. A human immunodeficiency virus type 1 Tat-like arginine-rich RNA-binding domain is essential for HEXIM1 to inhibit RNA polymerase II transcription through 7SK snRNA-mediated inactivation of P-TEFb. Mol Cell Biol. 2004;24:5094–5105. doi: 10.1128/MCB.24.12.5094-5105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedore SC, Byers SA, Biglione S, Price JP, Maury WJ, Price DH. Manipulation of P-TEFb control machinery by HIV: recruitment of P-TEFb from the large form by Tat and binding of HEXIM1 to TAR. Nucleic Acids Res. 2007;35:4347–4358. doi: 10.1093/nar/gkm443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Chen D, Pierstorff E, Luo K. Transcription elongation factor P-TEFb mediates Tat activation of HIV-1 transcription at multiple stages. EMBO J. 1998;17:3681–3691. doi: 10.1093/emboj/17.13.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barboric M, Yik JH, Czudnochowski N, Yang Z, Chen R, Contreras X, et al. Tat competes with HEXIM1 to increase the active pool of P-TEFb for HIV-1 transcription. Nucleic Acids Res. 2007;35:2003–2012. doi: 10.1093/nar/gkm063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blazkova J, Chun TW, Belay BW, Murray D, Justement JS, Funk EK, et al. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2012;206:765–769. doi: 10.1093/infdis/jis412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara M, Ijichi K, Tokuhisa K, Katsuura K, Shigeta S, Konno K, et al. Mechanism of selective inhibition of human immunodeficiency virus by ingenol triacetate. Antimicrob Agents Chemother. 1996;40:271–273. doi: 10.1128/aac.40.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiwara M, Okamoto M, Ijichi K, Tokuhisa K, Hanasaki Y, Katsuura K, et al. Upregulation of HIV-1 replication in chronically infected cells by ingenol derivatives. Arch Virol. 1998;143:2003–2010. doi: 10.1007/s007050050436. [DOI] [PubMed] [Google Scholar]
- 35.Warrilow D, Gardner J, Darnell GA, Suhrbier A, Harrich D. HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS Res Hum Retroviruses. 2006;22:854–864. doi: 10.1089/aid.2006.22.854. [DOI] [PubMed] [Google Scholar]
- 36.Wender PA, Koehler KF, Sharkey NA, Dell’Aquila ML, Blumberg PM. Analysis of the phorbol ester pharmacophore on protein kinase C as a guide to the rational design of new classes of analogs. Proc Natl Acad Sci U S A. 1986;83:4214–4218. doi: 10.1073/pnas.83.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benhadji KA, Serova M, Ghoul A, Cvitkovic E, Le Tourneau C, Ogbourne SM, et al. Antiproliferative activity of PEP005, a novel ingenol angelate that modulates PKC functions, alone and in combination with cytotoxic agents in human colon cancer cells. Br J Cancer. 2008;99:1808–1815. doi: 10.1038/sj.bjc.6604642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang G, Sancar A. Recruitment of DNA damage checkpoint proteins to damage in transcribed and nontranscribed sequences. Mol Cell Biol. 2006;26:39–49. doi: 10.1128/MCB.26.1.39-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang G, Plo I, Wang T, Rahman M, Cho JH, Yang E, et al. BRCA1-Ku80 protein interaction enhances end-joining fidelity of chromosomal double-strand breaks in the G1 phase of the cell cycle. J Biol Chem. 2013;288:8966–8976. doi: 10.1074/jbc.M112.412650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. J Neurovirol. 2007;13:210–224. doi: 10.1080/13550280701327038. [DOI] [PubMed] [Google Scholar]
- 41.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavigne M, Eskeland R, Azebi S, Saint-Andre V, Jang SM, Batsche E, et al. Interaction of HP1 and Brg1/Brm with the globular domain of histone H3 is required for HP1-mediated repression. PLoS Genet. 2009;5:e1000769. doi: 10.1371/journal.pgen.1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coiras M, Lopez-Huertas MR, Perez-Olmeda M, Alcami J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7:798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- 45.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 46.Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad SciUS A. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Guo J, Wu Y, Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 2013;41:277–287. doi: 10.1093/nar/gks976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, et al. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J Leukoc Biol. 2012;92:1147–1154. doi: 10.1189/jlb.0312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu J, Gaiha GD, John SP, Pertel T, Chin CR, Gao G, et al. Reactivation of latent HIV-1 by inhibition of BRD4. Cell Rep. 2012;2:807–816. doi: 10.1016/j.celrep.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boehm D, Calvanese V, Dar RD, Xing S, Schroeder S, Martins L, et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle. 2013;12:452–462. doi: 10.4161/cc.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolis DM, Hazuda DJ. Combined approaches for HIV cure. Curr Opin HIV AIDS. 2013;8:230–235. doi: 10.1097/COH.0b013e32835ef089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuse S, Calao M, Kabeya K, Guiguen A, Gatot JS, Quivy V, et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS One. 2009;4:e6093. doi: 10.1371/journal.pone.0006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsnes AM, Ersvaer E, Ryningen A, Paulsen K, Hampson P, Lord JM, et al. The protein kinase C agonist PEP005 increases NF-kappaB expression, induces differentiation and increases constitutive chemokine release by primary acute myeloid leukaemia cells. Br J Haematol. 2009;145:761–774. doi: 10.1111/j.1365-2141.2009.07691.x. [DOI] [PubMed] [Google Scholar]
- 56.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Jr, et al. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582–G591. doi: 10.1152/ajpgi.00087.2004. [DOI] [PubMed] [Google Scholar]
- 57.Lin X, O’Mahony A, Mu Y, Geleziunas R, Greene WC. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez-Cabrera M, Munoz E, Blazquez MV, Ursa MA, Santis AG, Sanchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J Biol Chem. 1995;270:21545–21551. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
- 60.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76:8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sung TL, Rice AP. Effects of prostratin on cyclin T1/P-TEFb function and the gene expression profile in primary resting CD4+ T cells. Retrovirology. 2006;3:66. doi: 10.1186/1742-4690-3-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang HC, Xing S, Shan L, O’Connell K, Dinoso J, Shen A, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, Boyd MR, et al. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98:3006–3015. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 64.Wender PA, Kee JM, Warrington JM. Practical synthesis of prostratin, DPP, their analogs, adjuvant leads against latent HIV. Science. 2008;320:649–652. doi: 10.1126/science.1154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burnett JC, Lim KI, Calafi A, Rossi JJ, Schaffer DV, Arkin AP. Combinatorial latency reactivation for HIV-1 subtypes and variants. J Virol. 2010;84:5958–5974. doi: 10.1128/JVI.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013;9:e1003834. doi: 10.1371/journal.ppat.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeChristopher BA, Loy BA, Marsden MD, Schrier AJ, Zack JA, Wender PA. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem. 2012;4:705–710. doi: 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta AK, Paquet M. Ingenol mebutate: a promising treatment for actinic keratoses and nonmelanoma skin cancers. J Cutan Med Surg. 2013;17:173–179. doi: 10.2310/7750.2012.12050. [DOI] [PubMed] [Google Scholar]
- 69.Beans EJ, Fournogerakis D, Gauntlett C, Heumann LV, Kramer R, Marsden MD, et al. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A. 2013;110:11698–11703. doi: 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jorgensen L, McKerrall SJ, Kuttruff CA, Ungeheuer F, Felding J, Baran PS. 14-step synthesis of (+)-ingenol from (+)-3-carene. Science. 2013;341:878–882. doi: 10.1126/science.1241606. [DOI] [PubMed] [Google Scholar]