This phase II trial of the AE37 vaccine shows no benefit in the intention-to-treat population. However, the trial confirms the vaccine to be safe and capable of stimulating an immune response. Prespecified subgroup analyses show the vaccine may reduce the recurrence rate in triple-negative breast cancer patients, suggesting an appropriate patient population for further clinical investigation.
Keywords: immunotherapy, vaccine, breast cancer, triple-negative breast cancer, HER2
Abstract
Background
AE37 is the Ii-Key hybrid of the MHC class II peptide, AE36 (HER2 aa:776–790). Phase I studies showed AE37 administered with granulocyte macrophage colony-stimulating factor (GM-CSF) to be safe and highly immunogenic. A prospective, randomized, multicenter phase II adjuvant trial was conducted to evaluate the vaccine's efficacy.
Methods
Clinically disease-free node-positive and high-risk node-negative breast cancer patients with tumors expressing any degree of HER2 [immunohistochemistry (IHC) 1–3+] were enrolled. Patients were randomized to AE37 + GM-CSF versus GM-CSF alone. Toxicity was monitored. Clinical recurrences were documented and disease-free survival (DFS) analyzed.
Results
The trial enrolled 298 patients; 153 received AE37 + GM-CSF and 145 received GM-CSF alone. The groups were well matched for clinicopathologic characteristics. Toxicities have been minimal. At the time of the primary analysis, the recurrence rate in the vaccinated group was 12.4% versus 13.8% in the control group [relative risk reduction 12%, HR 0.885, 95% confidence interval (CI) 0.472–1.659, P = 0.70]. The Kaplan–Meier estimated 5-year DFS rate was 80.8% in vaccinated versus 79.5% in control patients. In planned subset analyses of patients with IHC 1+/2+ HER2-expressing tumors, 5-year DFS was 77.2% in vaccinated patients (n = 76) versus 65.7% in control patients (n = 78) (P = 0.21). In patients with triple-negative breast cancer (HER2 IHC 1+/2+ and hormone receptor negative) DFS was 77.7% in vaccinated patients (n = 25) versus 49.0% in control patients (n = 25) (P = 0.12).
Conclusion
The overall intention-to-treat analysis demonstrates no benefit to vaccination. However, the results confirm that the vaccine is safe and suggest that vaccination may have clinical benefit in patients with low HER2-expressing tumors, specifically TNBC. Further evaluation in a randomized trial enrolling TNBC patients is warranted.
introduction
There is significant interest in using immunotherapeutic strategies, including vaccines, to treat cancer patients. In breast cancer, HER2 is the most studied antigen, and several HER2-derived peptide vaccines have been shown to elicit immune responses. The most studied is E75, a major histocompatibility class (MHC) I vaccine which stimulates a CD8+ T-cell response [1]. While CD8+ T-cell-eliciting vaccines elicit cytolytic activity against target antigen-expressing tumors, there is concern regarding the durability of the vaccine-specific immune response. In early trials evaluating E75, waning peptide-specific immunity was noted over time; not unexpected given that CD8+ T cells cannot sustain a prolonged memory response in the absence of continued antigen exposure and stimulation by antigen-presenting cells [2]. One strategy to enhance the immune response and maintain long-term immunity is to vaccinate in combination with an MHC class II epitope that stimulates a CD4+ helper T-cell response [3, 4].
Several MHC class II epitopes from the HER2 protein have been described including AE36 [5–8]. Class II epitopes have lower binding affinity compared with class I epitopes. To address this, a novel method to increase antigen-specific stimulation of CD4+ helper T cells has been investigated. AE37 is a hybrid peptide generated by the covalent linkage of the Ii-Key peptide (LRMK), to the AE36 amino-terminus (reviewed by Sears et al. [9]). This Ii-key moiety enhances epitope charging resulting in improved antigen presentation [10–12]. Preclinical work evaluating a series of Ii-Key hybrid peptides showed them to be more effective than native peptides in stimulating peptide-specific CD4+ T cells. Furthermore, hybrid peptide-induced CD4+ T cells provided stronger helper effect to HER2-specific CD8+ T cells [13].
Our group conducted the first in-human phase I trial of AE37 + GM-CSF in breast cancer patients. The trial showed the vaccine safe and able to induce peptide-specific immune responses [14]. Subsequently, we have conducted a prospective, randomized, multicenter phase II trial investigating AE37 + GM-CSF administered in the adjuvant setting to node-positive and high-risk node-negative breast cancer patients with tumors expressing any degree of HER2 [immunohistochemistry (IHC) 1–3+]. The study's primary objective was to determine whether vaccination could reduce the recurrence rate. Here, we report the primary analysis.
methods
patient characteristics and clinical protocol
The study was a prospective, randomized, single-blinded, multicenter trial (NCT00524277) conducted under an investigational new drug application (BB-IND#12229). The protocol was approved by the Institutional Review Board at all 16 participating sites. Eligible patients had node-positive or high-risk node-negative breast cancer expressing some degree of HER2 [IHC 1–3+ and/or fluorescence in situ hybridization (FISH) ratio >1.2]. High-risk node-negative was defined as tumors that were ≥T2, grade 3, hormone receptor-negative, HER2 3+ by IHC or FISH amplified >2.0 (before CAP/ASCO guideline changes), had lymphovascular invasion, or had isolated tumor cells (N0(i+)) in the sentinel lymph node(s). Enrollment occurred 1–6 months after completing standard therapy with surgery, chemotherapy, and if indicated, radiation; patients receiving endocrine therapy continued on their prescribed regimen. The trial's objective was to determine whether there are differences in the recurrence rate for patients administered AE37 + GM-CSF versus those receiving GM-CSF alone. The protocol called for the primary analysis after 39 events defined as recurrences, second malignancies, or death from any cause.
vaccine and vaccination series
The AE37 peptide (LRMK-GVGSPYVSRLLGICL) was produced in good manufacturing practices grade and purified to >95%. Sterility, endotoxin, and general safety testing were carried out by the manufacturer. Lyophilized peptide was reconstituted in 0.5 ml of sterile saline at a dose of 500 µg. The peptide was mixed with GM-CSF and sterile saline added to a final 1 ml volume. Initially, 62.5 µg of GM-CSF was utilized based on data from the phase I trial [14]. However, because the local reactions in the initial 26 patients (12 in the AE37 + GM-CSF arm and 14 in the GM-CSF-only arm) were not as robust, the GM-CSF dose was increased to 125 µg. For immunoadjuvant-only patients, GM-CSF was diluted to a final 1 ml volume with sterile saline.
The primary vaccination series (PVS) consisted of six inoculations given every 21–28 days. The 1.0 ml inoculation (vaccine or GM-CSF alone) was split with 0.5 ml given intradermally at two sites 5 cm apart in the same lymph node draining area (upper thigh). Four booster inoculations were administered in the same extremity as the primary series 12, 18, 24, and 30 months from the enrollment date.
in vivo immune monitoring
In vivo immune responses were assessed using a delayed-type hypersensitivity (DTH) reaction. One hundred microgram of AE37 in 0.5 ml normal saline, 100 µg of AE36 in 0.5 ml normal saline, and 0.5 ml of normal saline (volume control) were injected intradermally prevaccination and 1 month after PVS completion. The DTH was measured in two dimensions 48–72 h after injection using the sensitive ballpoint-pen method [15]. Data were recorded as the orthogonal mean.
ex vivo immune monitoring
Ex vivo immune responses were assessed by radioactive 3H-thymidine incorporation assays to assess peptide-specific T-cell proliferation on all patients. Briefly, peripheral blood mononuclear cells (PBMCs) were cultured in the absence and presence of the AE36 and AE37 peptides. On the 4th day, wells were pulsed with 1 µCi/well of radioactive 3H-thymidine. The following day, proliferative activity was determined by measuring the amount of thymidine incorporation determined as counts per minute (cpm). Results are expressed as delta cpm, i.e. cpm in the presence of peptide minus cpm with medium alone.
Additionally, in a subset of patients (n = 97), interferon-gamma (IFN-γ) was assessed by a standard enzyme-linked immunospot (ELISPOT) as previously described [16]. Assays were performed comparing the vaccinated patients (n = 53) versus control patients (n = 44) at baseline and multiple postvaccine time points. Levels of regulatory T cells (Treg) were also assessed in this subset and compared between groups. Treg were analyzed by flow cytometry, defined as CD4 + CD25hiCD127low/neg, and expressed as the percent of all CD4+ T cells.
clinical recurrences of disease
Patients were evaluated for disease recurrence per standard screening dictated by their oncologists. Patients were considered to have recurrent disease if the recurrence was biopsy-proven or if they were treated for recurrence.
statistical analysis
The study was designed to detect a 0.45 hazard ratio (HR) corresponding to an improvement in 2-year disease-free survival (DFS) from 85% for GM-CSF-only control patients to 93% for AE37 + GM-CSF vaccinated patients. Enrolled patients were stratified by site and by nodal status after which they were randomly assigned using computerized tables to two treatment groups in a 1:1 allocation ratio using an institution-balancing algorithm. Per the statistical analysis plan, this intention-to-treat (ITT) (all randomized patients) primary analysis was performed after 39 events. Prespecified subgroup analyses of DFS were conducted for baseline patient and disease characteristics. Clinicopathologic data were compared between groups. Median and range were used to summarize age, and the groups were compared using analysis of variance techniques. Baseline categorical variables were summarized with frequencies and proportions, and groups were compared using a two-sided Fisher's exact test. DTH data are presented as means ± standard errors and compared using a paired Student's t-test. Immunologic assay results were compared by Wilcoxon (two-tailed) t-test. DFS was calculated from the date of randomization to the date of recurrence or death due to any cause, and otherwise was censored on the date of last contact. DFS was analyzed using Kaplan–Meier techniques, and groups were compared using the log-rank test. Cox proportional hazards models were used to estimate HRs and 95% confidence intervals (CIs) to estimate the relative risk of recurrence or death between arms. P values <0.05 were considered significant.
results
patients
The trial enrolled 298 patients (Figure 1). There were no differences with respect to clinicopathologic characteristics between groups (Table 1).
Figure 1.
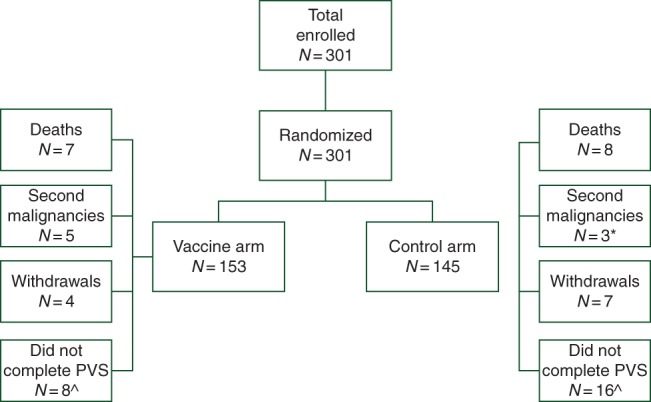
Consort diagram. Flow of patients through the study. Asterisk indicates that one of three patients with a second malignancy in the control arm withdrew and is included in the seven withdrawals. Circumfex symbol indicates that the number of patients that did not complete the primary vaccination series (PVS) includes patients that withdrew, met the primary end point (recurrence, second malignancy, or death from any cause), or chose not to continue on study before completing the PVS.
Table 1.
Clinicopathologic characteristics by treatment group
| Characteristic | No. (%) of vaccinated patients (N = 153) | No. (%) of controls (N = 145) | P value |
|---|---|---|---|
| Median age (years) (range) | 49 (26–76) | 50 (27–77) | 0.52 |
| T stage | 0.67a | ||
| T0/is | 5 (3%) | 5 (3%) | |
| T1 | 62 (41%) | 50 (34%) | |
| T2 | 58 (38%) | 68 (47%) | |
| T3 | 20 (13%) | 17 (12%) | |
| T4 | 5 (3%) | 4 (3%) | |
| Tx | 3 (2%) | 1 (1%) | |
| Nodal status | >0.99a | ||
| Positive | 98 (64%) | 95 (66%) | |
| Negative | 53 (35%) | 50 (34%) | |
| Unknown | 2 (1%) | 0 | |
| Histology | >0.99 | ||
| Ductal | 143 (93%) | 135 (93%) | |
| Lobular | 9 (6%) | 9 (6%) | |
| Other | 1 (1%) | 1 (1%) | |
| Grade | 0.49 | ||
| Moderate/well differentiated | 75 (49%) | 65 (45%) | |
| Poorly differentiated | 78 (51%) | 80 (55%) | |
| Hormone receptor status | >0.99 | ||
| Positive | 94 (61%) | 90 (62%) | |
| Negative | 59 (39%) | 55 (38%) | |
| HER2 status | 0.49 | ||
| Positive | 77 (50%) | 67 (46%) | |
| Negative | 76 (50%) | 78 (54%) | |
| Surgery | 0.19a | ||
| Lumpectomy | 63 (41%) | 48 (33%) | |
| Mastectomy | 90 (59%) | 95 (66%) | |
| Unknown | 0 | 2 (1%) | |
| Postmastectomy radiation | >0.99 | ||
| Yes | 58 (64%) | 62 (65%) | |
| No | 32 (36%) | 33 (35%) | |
| Chemotherapy | 0.10 | ||
| Yes | 149 (97%) | 135 (93%) | |
| No | 4 (3%) | 10 (7%) | |
| Endocrine therapy in hormone receptor-positive patients | >0.99 | ||
| Yes | 90 (96%) | 86 (96%) | |
| No | 4 (4%) | 4 (4%) | |
| Trastuzumab use in HER2-positive patients | 0.19 | ||
| Yes | 74 (96%) | 60 (90%) | |
| No | 3 (4%) | 7 (10%) |
aUnknown values were not included in statistical analyses.
toxicity
Local and systemic toxicities were mild during the PVS (Figure 2). For patients receiving AE37+ GM-CSF, maximum local toxicities were grade 1 (72%) or grade 2 (28%). Maximum systemic toxicities were grade 0 (36%), grade 1 (53%), grade 2 (11%), or grade 3 (1%). The toxicities were comparable for patients receiving GM-CSF only.
Figure 2.
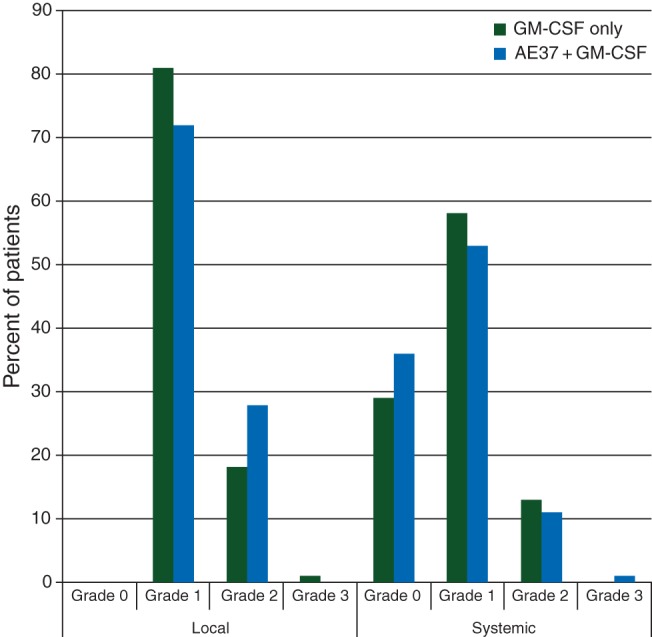
Maximum toxicity. The maximum local and systemic toxicity experienced by patients administered the AE37 + GM-CSF vaccine were comparable with those experienced by patients receiving GM-CSF alone.
immunologic response
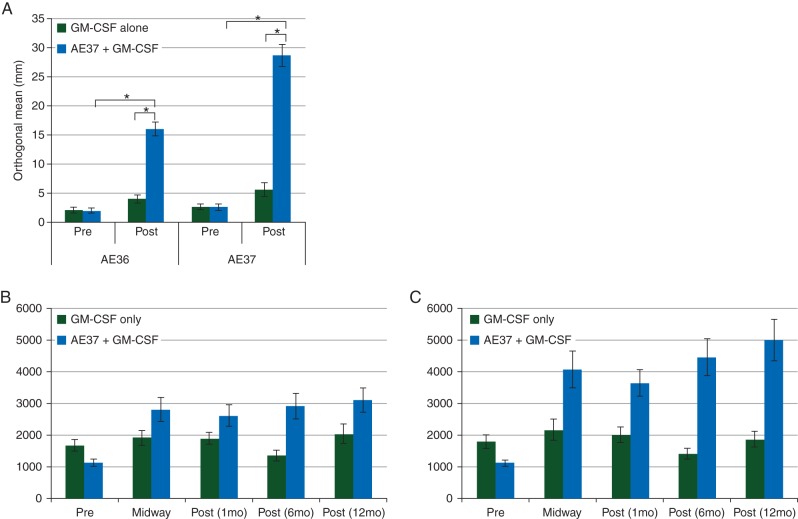
In vivo immunologic responses were evaluated using a DTH reaction to both AE36 and AE37. For vaccinated patients, there were statistically significant increases in postvaccination DTH reactions compared with prevaccination DTH reactions for both peptides (Figure 3A). Ex vivo immunologic responses were determined measuring AE36- and AE37-specific proliferation of T-cell prevaccination, midway through the PVS, and then 1, 6, and 12 months after PVS completion. As shown in Figure 3B (AE36) and C (AE37), T-cell proliferation to both AE36 and AE37 in patients receiving the vaccine was greater than that in patients receiving GM-CSF only.
Figure 3.
Immunologic response to vaccination. (A) In vivo immune responses were determined in all patients using a delayed-type hypersensitivity (DTH) reaction. Patients who were vaccinated with AE37 + GM-CSF had a significant increase in their DTH reaction to both the immunizing peptide, AE37, and the wild-type peptide, AE36, postvaccination compared with prevaccination. In vaccinated patients, the average (± standard error) orthogonal mean to AE36 before vaccination was 2.0 ± 0.4 versus 16.0 ± 1.2 mm postvaccination (P < 0.001). The average induration to AE37 before vaccination was 2.6 ± 0.6 versus 28.7 ± 1.9 mm postvaccination (P < 0.001). In addition, the postvaccination response was significantly greater in vaccinated patients than in control patients (AE36: 16.0 ± 1.2 versus 4.0 ± 0.7 mm, P < 0.001; AE37: 28.7 ± 1.9 versus 5.6 ± 1.2 mm, P < 0.001). (B) Ex vivo immune responses were determined in all patients using a 3H-thymidine incorporation assay to measure peptide-specific T-cell proliferation in response to the native peptide AE36. Patients vaccinated with AE37 + GM-CSF demonstrated robust T-cell proliferation at all time points assessed after the initiation of vaccination. (C) T-cell proliferation in response to the vaccinating peptide AE37 was also determined using a 3H-thymidine incorporation assay and vaccinated patients demonstrated robust T-cell proliferation at all time points assessed. *P < 0.001.
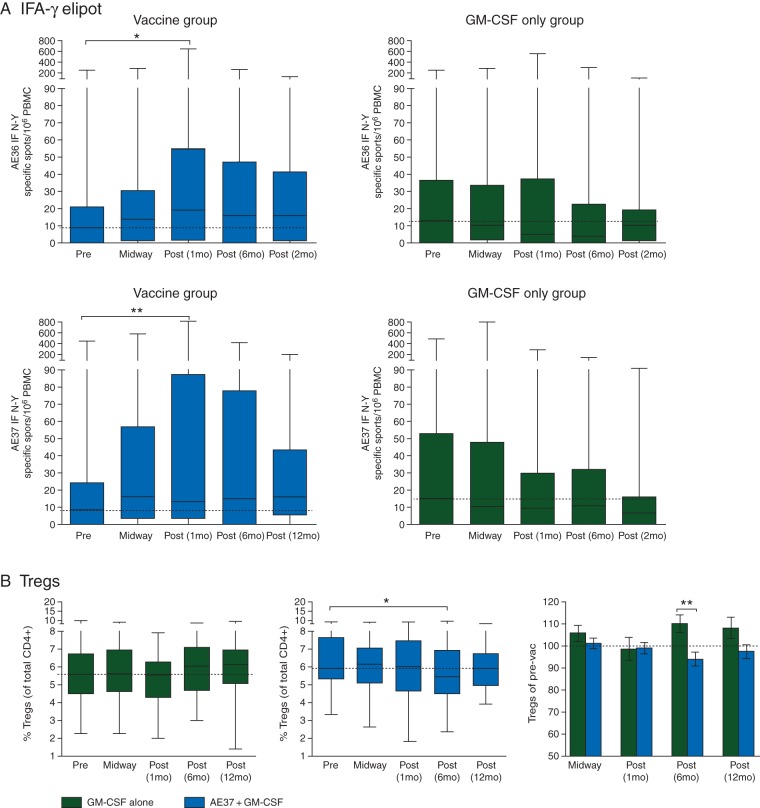
In a subset of patients (n = 97), ELISPOT (Figure 4A) and Treg levels (Figure 4B) were also assessed between vaccinated (n = 53) and control (n = 44) patients. As shown in Figure 4A, there was a statistical increase in peptide-specific IFN-γ release between baseline and 1 month postvaccine to both AE36 and AE37, while no increase postvaccine was seen in the control group. Despite the theoretical concerns of a CD4-eliciting vaccine, Figure 4B demonstrates Treg levels did not increase in the vaccine group and actually decreased statistically at 6 months postvaccine compared with that of baseline and the control group at this time point.
Figure 4.
Additional immunological responses. (A) IFN-γ ELISPOT assays were performed in a subset of the patients (n = 97) comparing AE37 + GM-CSF vaccinated patients (n = 53) with GM-CSF-only control patients (n = 44) at baseline (prevaccine) and multiple postvaccine time points. Results are expressed as specific spots (subtracting background) per 106 PBMC for both AE36 (top row) and AE37 (bottom row). Results are presented as medians, range, and 50% quartiles. *P < 0.01. (B) Regulatory T cells (Treg) were assessed by flow cytometry (CD4 + CD25hiCD127low/neg) in the same subset and at the same time points. Results are expressed as the %Treg per all CD4+ cells and compared within each treatment group at the given time points as well as between groups. *P < 0.02.
disease-free survival
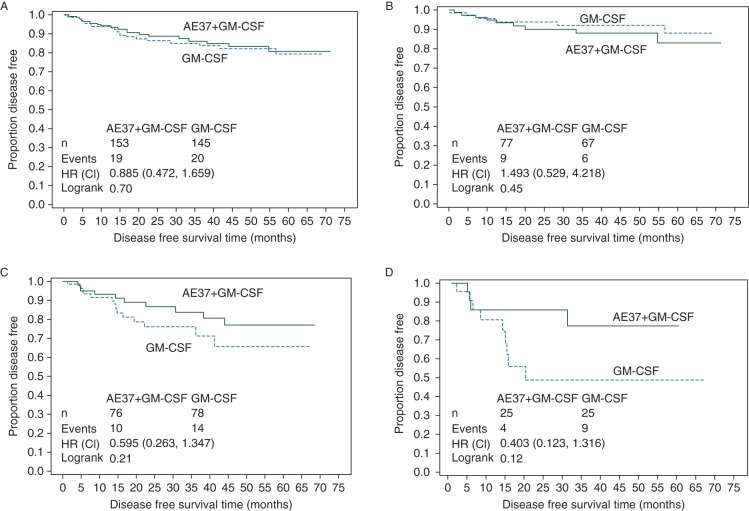
The recurrence rate in the vaccinated group was 12.4% versus 13.8% in the control group (HR 0.885, 95% CI 0.472–1.659, P = 0.70). The Kaplan–Meier estimated DFS rate at 5 years was 80.8% in vaccinated versus 79.5% in control patients after a median follow-up of 25 months (Figure 5A). In planned subset analyses of patients with HER2-positive (i.e. IHC 3+ or FISH > 2.0) tumors, the estimated 5-year DFS rate was 83.2% in vaccinated patients (n = 77) versus 88.0% in control patients (n = 67) (HR 1.493, 95% CI 0.529–4.218, P = 0.45) (Figure 5B). For IHC 1+ or 2+ HER2-expressing tumors, the estimated 5-year DFS was 77.2% in vaccinated patients (n = 76) versus 65.7% in control patients (n = 78) (HR .595, 95% CI 0.263–1.347, P = 0.21) (Figure 5C). In patients with triple-negative breast cancer (TNBC, HER2 IHC 1+ or 2+ and hormone receptor negative), the estimated 5-year DFS rate was 77.7% in vaccinated patients (n = 25) versus 49.0% in control patients (n = 25) (HR 0.403, 95% CI 0.123–1.316, P = 0.12) (Figure 5D).
Figure 5.
Disease-free survival is shown on an intention-to-treat basis for (A) all patients, (B) patients with HER2-overexpressing breast cancer, (C) patients with HER2 1+ and 2+ tumors regardless of hormone receptor status, and (D) triple-negative breast cancer patients (HER2 1+ and 2+ and hormone receptor-negative tumors).
discussion
Here, we report the ITT primary analysis of the randomized phase II trial evaluating AE37 + GM-CSF administered to disease-free, node-positive and high-risk node-negative breast cancer patients in the adjuvant setting. The ITT analysis of the entire randomized study population did not show a reduced recurrence rate in vaccinated patients; therefore, this was a negative trial. However, the study was highly informative. First, the study confirmed that the vaccine is safe and effective in stimulating antigen-specific immune responses. Second, it confirmed the optimal GM-CSF dose to be used and showed that, while the GM-CSF was responsible for the toxicities seen, it was not responsible for the immunologic response. Finally, this study showed that AE37 + GM-CSF may reduce the recurrence rate in select patients, specifically those with TNBC, suggesting an appropriate patient population for further clinical development of this vaccine.
Consistent with data from the phase I trials [14, 16], this study confirms the vaccine to be safe with the majority of patients experiencing only grade 1 local and systemic toxicities. The most common local toxicity has been injection site erythema and pruritus; the most common systemic toxicities have been bone pain, influenza-like symptoms, and fatigue. This symptomatology is consistent with that seen in our early phase clinical trial evaluating nelipepimut-S + GM-CSF [2, 17]. In that trial, there was no GM-CSF-only control arm; therefore, attribution of toxicity to the peptide and/or GM-CSF was not possible. In the current study, toxicities in the GM-CSF-only group are comparable with those seen in the vaccinated group, suggesting that toxicities associated with these vaccines are attributable to the immunoadjuvant.
Another important aspect of this study is that we confirmed an optimal GM-CSF dose to be used as adjuvant. The dose and potential detrimental effects of GM-CSF as an immunoadjuvant have been questioned (reviewed by Clive et al. [18]). The current trial began with 500 µg of AE37 plus 62.5 µg of GM-CSF, a dose selected based on the results from the phase I trial [14]. In that trial, the optimal dose of 500 µg of AE37 plus 62.5 µg of GM-CSF was selected because some patients receiving 500 µg of AE37 plus 125 µg of GM-CSF had large local reactions requiring GM-CSF dose reductions to prevent serious skin toxicity, while a dose of 30 µg of GM-CSF was insufficient to produce a robust response. Therefore, a 62.5- µg dose was selected to begin the current trial; however, after the first 26 patients did not experience robust local reactions through the PVS, the decision was made to increase the GM-CSF to 125 µg. This finding may have been related to a change in the GM-CSF formulation from the manufacturer that occurred between the phase I and current study. While this dose has been well tolerated, ∼20% of the patients in the vaccine arm required a dose reduction due to local reactions exceeding 100 mm. Importantly, these patients demonstrated large DTH reactions to the peptide and appear to do better clinically [19].
Although toxicities seen in this trial can be attributed to the GM-CSF, the immune responses cannot, as evidenced by the immune monitoring data. Patients vaccinated with only GM-CSF showed no HER2-specific immunologic responses, while patients vaccinated with AE37 + GM-CSF had robust immune responses against both AE36, the native peptide, and AE37, the immunizing peptide. By showing an immune response against AE36, these data confirm HER2-specific immunity.
A critical aspect of the AE37 vaccine is the modification with the LRMK sequence to facilitate MHC class II molecule loading. Preclinical data showed this increased potency when compared with unmodified class II epitopes [10–12]. We do not have a comparison group vaccinated with just the AE36 peptide. However, the robust in vivo and ex vivo immune responses seen in this trial, together with the phase I data showing activity without adjuvant [14], suggest that this modification does enhance the immune response without increasing regulatory T-cell levels. We have hypothesized that the direct charging ability of Ii-Key/peptide hybrids allows for the activity of this hybrid vaccine in patients with human leukocyte antigen (HLA) alleles with which the native AE36 epitope interacts weakly [14]. This increased ‘promiscuity’ has the potential to be significant clinically as it expands the number of patients that may potentially benefit from vaccination. Unlike MHC class I vaccines that are limited to patients with specific class I alleles, AE37 can be administered to patients regardless of HLA type.
The current data suggest that patients with HER2 3+ tumors do not benefit from AE37vaccination. However, it should be noted that DFS rates in patients with HER2 3+ tumors are excellent in both arms of the trial, a finding that reflects the benefit of trastuzumab therapy in these patients. Our DFS rates are comparable with those seen in the combined analysis of the NSABP B-31 and NCCTG N9831 trials [20]. It should be noted that, although the subset analyses in this trial were prespecified, the number of patients in each subset is small. However, these data do suggest activity in TNBC patients which is the breast cancer subtype that may be most immunogenic. Multiple new trials are assessing immune-modulators like checkpoint inhibitors in TNBC. The AE37 vaccine may enhance/augment the immunologic reaction to this subtype and may show promise alone or in combination with these agents. Additional randomized studies of AE37 are warranted in TNBC patients.
funding
This work was primarily funded by Antigen Express through a grant to GEP via the Henry M. Jackson Foundation. Additional funding was provided by the United States Military Cancer Institute, Department of Surgery, Uniformed Services University of the Health Sciences; and the Department of Clinical Investigation, Walter Reed Army Medical Center. EAM is a R. Lee Clark Fellow of The University of Texas MD Anderson Cancer Center supported by the Jeanne F. Shelby Scholarship Fund. This study was conducted in part at the University of Texas MD Anderson Cancer Center which is supported by the National Institutes of Health Grant CA016672. Funding sources were not involved with the design or conduct of the study; in the collection, management, analysis, or interpretation of data; in the writing of the report; or in the approval or decision to submit the paper for publication.
EAM and GEP had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
disclosure
GEP and SP have partial inventor rights to AE37. If licensed, they are entitled to financial proceeds associated with this license per Federal policy. GEP also consults in the development of the vaccine. EvH is the President of Antigen Express. JS is an uncompensated consultant for Antigen Express. All remaining authors have declared no conflicts of interest.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, Department of Defense or the US Government.
references
- 1.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother 2008; 57: 1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peoples GE, Holmes JP, Hueman MT et al. Combined Clinical Trial Results of a HER2/neu (E75) Vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res 2008; 14: 797–803. [DOI] [PubMed] [Google Scholar]
- 3.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 2005; 54: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knutson KL, Disis ML. Augmenting T helper cell immunity in cancer. Curr Drug Targets Immune Endocr Metabol Disord 2005; 5: 365–371. [DOI] [PubMed] [Google Scholar]
- 5.Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res 1999; 5: 1289–1297. [PubMed] [Google Scholar]
- 6.Salazar LG, Fikes J, Southwood S et al. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res 2003; 9: 5559–5565. [PubMed] [Google Scholar]
- 7.Sotiriadou R, Perez SA, Gritzapis AD et al. Peptide HER2(776–788) represents a naturally processed broad MHC class II-restricted T cell epitope. Br J Cancer 2001; 85: 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuttle TM, Anderson BW, Thompson WE et al. Proliferative and cytokine responses to class II HER-2/neu-associated peptides in breast cancer patients. Clin Cancer Res 1998; 4: 2015–2024. [PubMed] [Google Scholar]
- 9.Sears AK, Perez SA, Clifton GT et al. AE37: a novel T-cell-eliciting vaccine for breast cancer. Expert Opin Biol Ther 2011; 11: 1543–1550. [DOI] [PubMed] [Google Scholar]
- 10.Adams S, Humphreys RE. Invariant chain peptides enhancing or inhibiting the presentation of antigenic peptides by major histocompatibility complex class II molecules. Eur J Immunol 1995; 25: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 11.Gillogly ME, Kallinteris NL, Xu M et al. Ii-Key/HER-2/neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer Immunol Immunother 2004; 53: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphreys RE, Adams S, Koldzic G et al. Increasing the potency of MHC class II-presented epitopes by linkage to Ii-Key peptide. Vaccine 2000; 18: 2693–2697. [DOI] [PubMed] [Google Scholar]
- 13.Sotiriadou NN, Kallinteris NL, Gritzapis AD et al. Ii-Key/HER-2/neu(776–790) hybrid peptides induce more effective immunological responses over the native peptide in lymphocyte cultures from patients with HER-2/neu+ tumors. Cancer Immunol Immunother 2007; 56: 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes JP, Benavides LC, Gates JD et al. Results of the first phase I clinical trial of the novel II-key hybrid preventive HER-2/neu peptide (AE37) vaccine. J Clin Oncol 2008; 26: 3426–3433. [DOI] [PubMed] [Google Scholar]
- 15.Sokal JE. Editorial: Measurement of delayed skin-test responses. N Engl J Med 1975; 293: 501–502. [DOI] [PubMed] [Google Scholar]
- 16.Perez SA, Kallinteris NL, Bisias S et al. Results from a phase I clinical study of the novel Ii-Key/HER-2/neu(776–790) hybrid peptide vaccine in patients with prostate cancer. Clin Cancer Res 2010; 16: 3495–3506. [DOI] [PubMed] [Google Scholar]
- 17.Mittendorf EA, Clifton GT, Holmes JP et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol 2014; 25: 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clive KS, Tyler JA, Clifton GT et al. Use of GM-CSF as an adjuvant with cancer vaccines: beneficial or detrimental? Expert Rev Vaccines 2010; 9: 519–525. [DOI] [PubMed] [Google Scholar]
- 19.Greene JM, Schneble EJ, Litton JK et al. Correlation of robust local reactions prompting GM-CSF dose reduction to clinical response in a phase II trial of the AE37+GM-CSF HER2 peptide vaccine. In Proceedings of the 106th Annual Meeting of the American Association for Cancer Research 2015. (Abstr #CT209), Philadelphia, PA. [Google Scholar]
- 20.Perez EA, Romond EH, Suman VJ et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011; 29: 3366–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]