Pemetrexed-based treatment regimens should be considered when selecting chemotherapy for patients with RET-rearranged non-small cell lung cancers. We demonstrate that pemetrexed-based therapies are active in patients with RET-rearranged lung cancers, with an overall response rate of 45% and a median time to progression of 20 months. Clinical benefit with pemetrexed-based therapies in RET-rearranged lung cancers was durable and comparable with that observed in ROS1- and ALK-rearranged lung cancers.
Keywords: pemetrexed, RET rearrangement, non-small-cell lung cancer
Abstract
Background
RET rearrangements are targetable, oncogenic lung cancer drivers. While previous series have shown durable clinical benefit with pemetrexed-based therapies in ALK- and ROS1-rearranged lung cancers, the benefits of pemetrexed-based treatments in patients with RET-rearranged lung cancers relative to other genomic subsets have not previously been explored.
Patients and methods
A retrospective review of patients with pathologically confirmed stage IIIB/IV lung adenocarcinomas and evidence of a RET, ROS1, or ALK rearrangement, or a KRAS mutation was conducted. Patients were eligible if they received treatment with pemetrexed alone or in combination. The primary outcome of progression-free survival (PFS), and secondary outcomes of overall response rate (ORR, RECIST v1.1), time to progression (TTP), and time to treatment discontinuation were compared between RET-rearranged and groups of ROS1-rearranged, ALK-rearranged, and KRAS-mutant lung cancers.
Results
We evaluated 104 patients. Patients with RET-rearranged lung cancers (n = 18) had a median PFS of 19 months [95% confidence interval (CI) 12–not reached (NR)] that was comparable with patients with ROS1- (23 months, 95% CI 14–NR, n = 10) and ALK-rearranged (19 months, 95% CI 15–36, n = 36) lung cancers, and significantly improved compared with patients with KRAS-mutant lung cancers (6 months, 95% CI 5–9, P < 0.001, n = 40). ORR (45%), median TTP (20 months, 95% CI 17–NR), and median time to treatment discontinuation (21 months, 95% CI 6–NR) in patients with RET-rearranged lung cancers were not significantly different compared with patients with ALK- and ROS1-rearranged lung cancers, and improved compared with patients with KRAS-mutant lung cancers.
Conclusion
Durable benefits with pemetrexed-based therapies in RET-rearranged lung cancers are comparable with ALK- and ROS1-rearranged lung cancers. When selecting therapies for patients with RET-rearranged lung cancers, pemetrexed-containing regimens should be considered.
introduction
Targeted therapy has reshaped the therapeutic landscape for patients with lung cancers [1]. In EGFR-mutant and ALK-rearranged lung cancers, tyrosine kinase inhibition results in dramatic improvements in response rate, quality of life, and progression-free survival (PFS) compared with standard chemotherapy across multiple phase III trials [2, 3]. Additional agents that can be used apart from targeted therapy are needed in all patients.
While immune checkpoint inhibitors represent an exciting class of drugs [4, 5], emerging data points to the possibility of more pronounced benefit in patients with a substantial smoking history, and tumors with a high mutational burden or that harbor-specific neoantigens [6, 7]. The relative utility of immune-directed therapy for tumors harboring genomic alterations that are more common in never smokers, or for tumors that are mutationally less complex remains in question. For these patients, standard chemotherapies continue to represent an important weapon in the medical oncologist's arsenal.
RET rearrangements are found in 1%–2% of unselected non-small-cell lung cancers (NSCLCs) and share many structural and clinical features with ALK- and ROS1-rearranged lung cancers [8, 9]. These rearrangements maintain an intact tyrosine kinase domain fused to a variety of upstream gene partners and are more commonly found in lung adenocarcinomas from patients with minimal or no tobacco exposure [10].
Previous series have shown durable clinical benefit with pemetrexed-based therapies in ALK- and ROS1-rearranged lung cancers. In the PROFILE 1014 prospective phase III study of crizotinib versus cisplatin and pemetrexed in ALK-rearranged lung cancers, the overall response rate (ORR) was 45% and the median PFS was 7 months in patients that received a chemotherapy doublet [11]. In the EUROS1 cohort of ROS1-rearranged lung cancers, treatment with pemetrexed monotherapy and combination therapy resulted in an ORR of 58% and a median PFS of 7 months [12].
The efficacy of pemetrexed-based systemic therapy in RET-rearranged lung cancers has not previously been explored.
methods
study design and eligibility
We conducted a retrospective review of records of patients treated at Memorial Sloan Kettering Cancer Center between 2007 and 2014 via an institutional review board-approved waiver of authorization. Patients were eligible for inclusion if they fulfilled the following criteria: pathologic evidence lung cancer, advanced (stage IIIB/IV) disease, documented evidence of a recurrent gene rearrangement involving RET, ROS1, or ALK or a mutation in KRAS, and treatment with pemetrexed for advanced disease. While a large number of patients with KRAS-mutant lung cancers were identified during this period, we chose to analyze a subpopulation of patients as a control group for this study, and the first 40 consecutive cases of KRAS-mutant lung cancer patients identified during this period were selected. Subjects treated with pemetrexed monotherapy or combination therapy (platinum or nonplatinum doublet with or without bevacizumab) were included in this analysis. A history of pemetrexed-based chemotherapy given with radiation therapy, targeted therapy, or immune-directed therapy was exclusionary.
molecular profiling
Molecular diagnostic testing was performed as part of a prospective, institutional program: the Memorial Sloan Kettering (MSK) Lung Cancers Mutational Analysis Program or LC-MAP [13]. Screening was initially performed via break-apart fluorescence in situ hybridization tests for RET, ROS1, and ALK, sizing assays, and mass spectrometry multiplex mutation hotspot testing (Sequenom, San Diego, CA). Molecular profiling later migrated to broad, hybrid capture-based next-generation sequencing of 410 cancer-related genes with the MSK-Integrated Mutation Profiling of Actionable Cancer Targets Illumina HiSeq platform [14]. Whenever possible, and if sufficient tissue was available, next generation was performed to confirm the presence of a recurrent gene rearrangement.
radiologic review
A dedicated radiologist performed a review of computed tomography images. Imaging was reviewed at the following specific time points when available: immediately before pemetrexed-based therapy and after discontinuation of a prior therapy, during therapy, and after the patient's last dose of pemetrexed-based therapy. Radiologic response to therapy was classified as a complete response (CR), partial response (PR), stable disease (SD), or progression of disease (PD) via the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [15]. Patients were evaluable for response if baseline imaging and one or more on-treatment scans were available.
statistical analysis
The primary end point of this study was PFS. Secondary end points included ORR, disease control rate (DCR = CR + PR + SD), time to progression (TTP), and overall survival (OS). Time to treatment discontinuation was examined as an exploratory end point, chosen based on the fact that radiologic progression via RECIST did not always trigger therapy discontinuation in patients with continued clinical benefit.
PFS, TTP, and time to treatment discontinuation were calculated using Kaplan–Meier estimates from the date of initiation of pemetrexed-based therapy until radiologic progression (RECIST v1.1) or death, radiologic progression, or the last date of pemetrexed-based therapy administration, respectively. For PFS and TTP, patients who were alive with no evidence of progression on imaging were censored at the date of last follow-up, and patients who were alive but without repeat on-treatment imaging were censored at the end of pemetrexed treatment. For TTP, patients who died were censored at the end of pemetrexed treatment. For time to treatment discontinuation, patients were censored if they stopped pemetrexed treatment for a reason other than clinical progression, including toxicity. OS was calculated from the date of diagnosis of metastatic disease until death, and patients who were still alive at the end of the study were censored at the date of last follow-up.
Comparisons of ORR and DCR were made between RET-rearranged lung cancer patients, and the three control groups, ROS1-rearranged, ALK-rearranged, and KRAS-mutant lung cancers using Fisher's exact test. Comparisons of PFS, TTP, time to treatment discontinuation, and OS were performed among subgroups using the log-rank test. Multivariate analysis was carried out using Cox regression models and logistic regression. Clinical features, therapy line, and type of therapy were compared using Fisher's exact test and the Kruskal–Wallis test. All statistical tests were two-sided, and P < 0.05 was considered significant. Statistical analyses were carried out using R 3.2.0 (R Development Core Team) including the ‘survival’ package.
results
clinicopathologic features and pemetrexed administration
One hundred four patients with advanced non-small-cell lung carcinomas who received pemetrexed-based therapies were evaluated on this study. RET-rearranged lung cancer patients had a median age of 63 (range 39–79 years) with a slight female predominance (56%, n = 10/18). All patients had metastatic disease at diagnosis. The baseline characteristics of patients with RET-rearranged lung cancers (n = 18) were compared with patients with ROS1-rearranged (n = 10), ALK-rearranged (n = 36), and KRAS-mutant (n = 40) lung cancers as summarized in Table 1. All patients had lung adenocarcinomas. RET, ROS1, and ALK rearrangements, and KRAS mutations were mutually exclusive in individual patient samples and did not co-occur with other major lung cancer drivers. As expected, patients with KRAS-mutant lung cancers had a higher median pack-year history of cigarette smoking (P < 0.001).
Table 1.
Clinicopathologic features and systemic therapy details
| RET (N = 18) | ROS1 (N = 10) | ALK (N = 36) | KRAS (N = 40) | P value | |
|---|---|---|---|---|---|
| Median age (range) | 63 (39–79) | 50 (18–61) | 55 (30–80) | 61 (30–81) | 0.01 |
| Sex | |||||
| M | 44% (n = 8) | 60% (n = 6) | 47% (n = 17) | 35% (n = 14) | 0.48 |
| F | 56% (n = 10) | 40% (n = 4) | 53% (n = 19) | 65% (n = 26) | |
| Pack years | |||||
| Median (range) | 0 (0–48) | 0 (0–12) | 0 (0–74) | 36 (0–93) | <0.001 |
| Adenocarcinoma | 100% (n = 18) | 100% (n = 10) | 100% (n = 36) | 100% (n = 40) | – |
| Line of therapy | |||||
| First-line therapy | 78% (n = 14) | 100% (n = 10) | 67% (n = 24) | 65% (n = 26) | P = 0.12 |
| ≥Second-line therapy | 22% (n = 4) | 0% (n = 0) | 33% (n = 12) | 35% (n = 14) | |
| Monotherapy | 6% (n = 1) | 0% (n = 0) | 11% (n = 4) | 20% (n = 8) | P = 0.16 |
| Combination | 94% (n = 17) | 100% (n = 10) | 89% (n = 32) | 80% (n = 32) | |
| Platinum | 88% (n = 15/17) | 100% (n = 10/10) | 78% (n = 25/32) | 69% (n = 22/32) | P = 0.15 |
| Nonplatinum | 12% (n = 2/17) | 0% (n = 0/10) | 22% (n = 7/32) | 31% (n = 10/32) | |
| Bevacizumab-containing | 71% (n = 12/17) | 80% (n = 8/10) | 75% (n = 24/32) | 69% (n = 22/32) | P = 0.90 |
| No bevacizumab | 29% (n = 5/17) | 20% (n = 2/10) | 25% (n = 8/32) | 31% (n = 10/32) | |
| Maintenance therapy | 59% (n = 10/17) | 70% (n = 7/10) | 63% (n = 20/32) | 53% (n = 17/32) | P = 0.80 |
| No maintenance | 41% (n = 7/17) | 30% (n = 3/10) | 37% (n = 12/32) | 47% (n = 15/32) | |
| Number of pemetrexed cycles, median (range) | 7 (2–30) | 13 (3–38) | 10 (2–50) | 8 (1–30) | P = 0.28 |
| Dose reduction | 20% (n = 3/15) | 10% (n = 1/10) | 17% (n = 5/29) | 18% (n = 7/40) | P = 0.98 |
| No dose reduction | 80% (n = 12/15) | 90% (n = 9/10) | 83% (n = 24/29) | 82% (n = 33/40) | |
The clinicopathologic features and pemetrexed-based systemic therapy details of patients with advanced non-small-cell lung cancers harboring RET, ROS1, or ALK rearrangements, or KRAS mutations are summarized and compared between groups.
The majority of RET-rearranged lung cancer patients were treated with first-line (78%, n = 14/18) combination therapy (94%, n = 17/18) with a platinum doublet (88%, n = 15/18). A comparison of all four molecular subgroups (Table 1) revealed no significant differences in terms of line of therapy (P = 0.12), single versus combination therapy (P = 0.16), platinum versus nonplatinum combination therapy (P = 0.15), bevacizumab versus nonbevacizumab-containing combination therapy (P = 0.90), maintenance therapy (P = 0.80), and the need for pemetrexed dose reduction (P = 0.98).
response and progression-free survival
A total of 83 patients were evaluable for response. The ORR with pemetrexed-based systemic therapy of 45% (n = 5/11) in RET-rearranged lung cancers was not significantly different from the ORR in ROS1-rearranged (78%, n = 7/9), and ALK-rearranged (50% n = 14/28) lung cancers (P = 0.30). The ORR was numerically improved in RET-rearranged compared with KRAS-mutant (26%, n = 9/35) lung cancers (P = 0.39). DCR was not significantly different between groups (Table 2).
Table 2.
Response to pemetrexed-based therapy
| Patients | ORR (PR) | DCR (PR + SD) |
|---|---|---|
| RET-rearranged | 45% (n = 5/11) | 91% (n = 10/11) |
| ROS1-rearranged | 78% (n = 7/9) | 90% (n = 8/9) |
| ALK-rearranged | 50% (n = 14/28) | 93% (n = 26/28) |
| KRAS-mutant | 26% (n = 9/35) | 86% (n = 30/35) |
| P value | 0.02 | 0.91 |
The overall response rate (ORR) and disease control rate (DCR) with pemetrexed-based systemic therapy in 83 patients with evaluable disease are summarized. These outcomes were compared between patient groups, with the P values reflecting an overall comparison of the four molecular subgroups listed. Only partial responses (PR) and no complete responses were observed.
SD, stable disease.
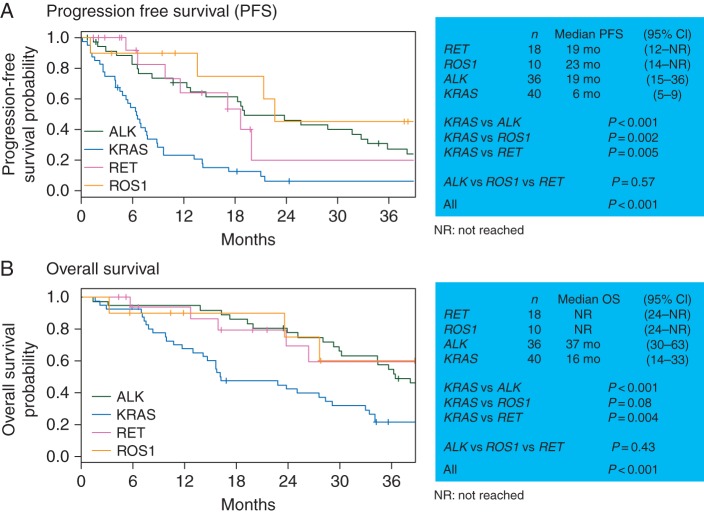
The primary end point of PFS, evaluable in all 104 patients, was significantly different between all four molecular subgroups (P < 0.001, Figure 1A). RET-rearranged lung cancer patients had a median PFS of 19 months [95% confidence interval (CI) 12–not reached (NR)]. Their PFS was not significantly different from the PFS of ROS1-rearranged (median 23 months, 95% CI 14–NR) or ALK-rearranged (median 19 months, 95% CI 15–36) lung cancer patients (P = 0.57). PFS was significantly improved in RET-rearranged (P = 0.005), ROS1-rearranged (P = 0.002), and ALK-rearranged lung cancers (P < 0.001) when each of these individual groups was compared with KRAS-mutant lung cancers (median 6 months, 95% CI 5–9).
Figure 1.
Survival outcomes with pemetrexed-based therapy. Progression-free survival and overall survival in RET-rearranged lung cancer patients were comparable with ROS1- and ALK-rearranged lung cancer patients and significantly improved compared with KRAS-mutant lung cancer patients.
time to progression and treatment discontinuation
In RET-rearranged lung cancers, median TTP and time to treatment discontinuation were 20 months (95% CI 17 months–NR) and 21 months (95% CI 6–NR), respectively. Similarly, TTP and time to treatment discontinuation were not different between RET-, ROS1-, and ALK-rearranged lung cancers and improved when each of these groups was individually compared with KRAS-mutant lung cancers (supplementary Figure S1, available at Annals of Oncology online).
overall survival
As expected, OS was significantly different between all four molecular subgroups of patients (P < 0.001, Figure 1B). OS was not significantly different between RET-rearranged (median NR, 95% CI 24 months–NR), ROS1-rearranged (median NR, 95% CI 24 months–NR), and ALK-rearranged (median 37 months, 95% CI 30–63) lung cancer patients (P = 0.43). OS was significantly improved in RET-rearranged (P = 0.004) and ALK-rearranged lung cancers (P < 0.001) when each of these groups was compared with KRAS-mutant lung cancers (median 16 months, 95% CI 14–33). A trend in improvement of OS was seen in ROS1-rearranged lung cancers compared with KRAS-mutant lung cancers (P = 0.08). Of note, 67% (n = 12/18) of RET-rearranged, 90% (n = 9/10) of ROS1-rearranged, and 89% (n = 32/36) of ALK-rearranged lung cancers received RET-, ROS1-, and ALK-directed targeted therapy, respectively.
The outcomes of ORR, PFS, TTP, time to treatment discontinuation, and OS were unaffected by smoking history. When analyzed using a multivariate model, KRAS-mutant lung cancers were associated with worse outcomes compared with the three gene rearrangements combined, even after controlling for smoking status (supplementary Table S1, available at Annals of Oncology online).
discussion
This paper represents the first series to demonstrate that RET-rearranged lung cancers are sensitive to pemetrexed-based systemic therapy. We observed an ORR of 45% in comparison to a historical response rate of 30% with platinum doublet chemotherapy. In addition, we previously reported that the ORR with cabozantinib, a multikinase inhibitor with activity against RET, was 38% in the first stage of an ongoing Simon two-stage phase II clinical trial [16, 17]. Disease control was durable, with a median PFS of 19 months, and prolonged TTP and time to treatment discontinuation. A number of factors should be considered as potential contributors to the observed efficacy of pemetrexed in RET-rearranged lung cancers.
First, prolonged survival outcomes may reflect the natural history of these tumors. In ALK-rearranged lung cancers, however, the clinical benefit of pemetrexed-based therapy was directly compared by Camidge et al. to EGFR-mutant lung cancers that are likewise associated with an improved prognosis compared with unselected NSCLCs. Median PFS was 9 months in ALK-rearranged compared with 5.5 months in EGFR-mutant lung cancers, and on multivariate analysis, only the presence of an ALK rearrangement and not an EGFR mutation was associated with improved PFS [hazard ratio (HR) 0.36, 95% CI 0.17–0.73, P = 0.0051] [18]. Future analyses would benefit from a focus on a comparison of outcomes with pemetrexed-based chemotherapy between RET-rearranged and EGFR-mutant lung cancers.
Second, pemetrexed benefit may be reflective of outcomes with chemotherapy in general. Again, looking to the experience in ALK-rearranged lung cancers, a differential effect of pemetrexed in comparison to docetaxel has been reported. The PROFILE 1007 phase III study randomized ALK-rearranged lung cancer patients to second-line therapy with crizotinib versus chemotherapy with either pemetrexed or docetaxel. Both ORR and PFS were improved with pemetrexed relative to docetaxel: ORR 29% (95% CI 21–39) versus 7% (95% CI 2–16), and HR for PFS 0.59 (95% CI 0.43–0.80, crizotinib compared with pemetrexed) versus 0.30 (95% CI 0.21–0.43, crizotinib compared with docetaxel), respectively [2].
As with any retrospective series, a number of limitations need to be taken into consideration. Patients were treated in a single tertiary center, there was no standardized schedule of tumor assessments which may have affected several study end points, and, finally, patients with select driver-positive lung cancers were chosen as control groups that may not have represented the breadth of cancer patients whose tumors do not harbor a RET rearrangement. It is worth pointing out that while outcomes such as median PFS were improved in our series compared with other studies such as PROFILE 1014 and EUROS1, the goal of this study was to compare outcomes between molecular subgroups in this single-center study.
In addition, while the sensitivity of ALK-rearranged lung cancers to pemetrexed has previously been ascribed to lower levels of thymidylate synthase in comparison to tumors that do not harbor ALK fusions [19], the biologic rationale behind the increased activity of pemetrexed-based systemic therapy in RET-rearranged lung cancers will require exploration. Our observation of comparable outcomes with pemetrexed-based therapy between ALK-, ROS1-, and RET-rearranged lung cancers suggests the possibility of shared or related biologic processes that drive this benefit.
conclusion
Pemetrexed-based systemic therapies are active in patients with RET-rearranged lung cancers. Clinical benefit is similar to that observed in ALK-rearranged and ROS1-rearranged lung cancers, and improved compared with KRAS-mutant lung cancers. When selecting between various chemotherapy agents for patients with RET-rearranged lung cancers, a pemetrexed-containing regimen should be considered.
funding
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support (Grant P30 CA008748).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Masters GA, Temin S, Azzoli CG et al. . Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015; 33: 3488–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw AT, Kim D-W, Nakagawa K et al. . Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385–2394. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M, Wu Y-L, Thongprasert S et al. . Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29: 2866–2874. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H, Paz-Ares L, Horn L et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R et al. . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 7.Rizvi NA, Hellmann MD, Snyder A et al. . Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi K, Soda M, Togashi Y et al. . RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012; 18: 378–381. [DOI] [PubMed] [Google Scholar]
- 9.Bergethon K, Shaw AT, Ou SH et al. . ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012; 30: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R, Hu H, Pan Y et al. . RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012; 30: 4352–4359. [DOI] [PubMed] [Google Scholar]
- 11.Solomon BJ, Mok T, Kim D-W et al. . First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371: 2167–2177. [DOI] [PubMed] [Google Scholar]
- 12.Mazières J, Zalcman G, Crinò L et al. . Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol 2015; 33: 992–999. [DOI] [PubMed] [Google Scholar]
- 13.Drilon A, Wang L, Arcila ME et al. . Broad, hybrid capture-based next-generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin Cancer Res 2015; 21: 3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng DT, Mitchell TN, Zehir A et al. . Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015; 17: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 16.Drilon A, Wang L, Hasanovic A et al. . Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013; 3: 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drilon A, Sima CS, Somwar R et al. . Phase II study of cabozantinib for patients with advanced RET-rearranged lung cancers. J Clin Oncol 2015; 33(suppl): abstr 8007. [Google Scholar]
- 18.Camidge DR, Kono SA, Lu X et al. . Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol 2011; 6: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw AT, Varghese AM, Solomon BJ et al. . Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol 2013; 24: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.