Summary
Background
Cortical stab injury (CSI) induces changes in the activity, expression and cellular distribution of specific ectonucleotidases at the injury site. Also, several experimentally induced neuropathologies are associated with changes in soluble ectonucleotidase activities in the plasma and serum, whilst various insults to the brain alter purine compounds levels in cerebrospinal fluid, but also in serum, indicating that insults to the brain may induce alterations in nucleotides release and rate of their hydrolysis in the vascular system. Since adenine nucleotides and adenosine regulate diverse cellular functions in the vascular system, including vascular tone, platelet aggregation and inflammatory responses of lymphocytes and macrophages, alterations of ectonucleotidase activities in the vascular system may be relevant for the clinical outcome of the primary insult.
Methods
We explored ectonucleotidase activities using specific enzyme assays and determined adenine nucleotides concentrations by the UPLC method in the rat serum after cortical stab injury.
Results
At 4-h post-injury, ATP and AMP hydrolysis increased by about 60% and 40%, respectively, while phosphodiesterase activity remained unchanged. Also, at 4-h post-injury a marked decrease in ATP concentration and more than 2-fold increase in AMP concentration were recorded.
Conclusions
CSI induces rapid up-regulation of nucleotide catabolizing soluble ectonucleotidases in rat serum, which leads to the observed shift in serum nucleotide levels. The results obtained imply that ectonucleotidases and adenine nucleotides participate in the communication between the brain and the vascular system in physiological and pathological conditions and thereby may be involved in the development of various human neuropathologies.
Keywords: ectonucleotidases, extracellular adenine nucleotides, ATP, brain injury, rat serum
Kratak sadržaj
Uvod
Ubodna povreda mozga dovodi do promena u aktivnosti, ekspresiji i ćelijskoj distribuciji određenih ektonukleotidaza na mestu povrede. Pored toga, neke eksperimentalno izazvane neuropatologije povezane su sa promenama solubilnih ektonukleotidaznih aktivnosti u plazmi i serumu, dok različite povrede mozga menjaju nivoe purina u cerebrospinalnoj tečnosti, ali i u serumu, ukazujući da povreda mozga može da dovede do promena u oslobađanju nukleotida i u stepenu njihove hidrolize u vaskularnom sistemu. Kako adeninski nukleotidi i adenozin regulišu raznovrsne ćelijske funkcije u vaskularnom sistemu, uključujući vaskularni tonus, agregaciju trombocita i zapaljenski odgovor limfocita i makrofaga, promene ektonukleotidaznih aktivnosti u vaskularnom sistemu mogu biti značajne za klinički ishod primarne povrede.
Metode
Primenom specifičnih enzimskih eseja pratili smo ektonukleotidazne aktivnosti, a upotrebom UPLC metode odredili smo koncentracije adeninskih nukleotida u serumu pacova nakon ubodne povrede korteksa.
Rezultati
Četiri sata nakon povrede, ATP- i AMP-hidrolizujuće aktivnosti bile su povišene za približno 60%, odnosno 40%, dok je fosfodiesterazna aktivnost ostala nepromenjena. Pored toga, 4 h nakon povrede zabeleženo je značajno povećanje koncentracije ATP i više nego dvostruko povećanje nivoa AMP.
Zaključak
Ubodna povreda mozga dovodi do naglog porasta hidrolizujućih aktivnosti solubilnih ektonukleotidaza u serumu pacova, što vodi uočenoj promeni nivoa nukleotida. Dobijeni rezultati pokazuju da ektonukleotidaze i adeninski nukleotidi posreduju u komunikaciji između mozga i vaskularnog sistema, kako u fiziološkim tako i u patološkim uslovima, te stoga mogu biti uključeni u razvoj različitih neuropatologija kod čoveka.
Introduction
Besides having well-known roles in cellular energy metabolism, adenosine 5′-triphosphate (ATP) is now recognized as an extracellular signaling molecule (1). In the central nervous system (CNS), extracellular ATP exists normally at nanomolar and low micromolar concentrations, when it operates as an excitatory neurotransmitter (2) or gliotransmitter (3), by acting at two families of P2 purinoreceptors (4). However, pathological conditions, such as brain trauma and ischemia, are associated with massive release of ATP in the extracellular milieu (5–7), when it operates as a paracrine factor to activate multiple cellular and biochemical responses to injury, such as activation of astrocytes and microglia (8, 9). Extracellular ATP is sequentially hydrolyzed to adenosine, a neuroprotective and neuromodulatory agent, which suppresses further release of ATP and other excitatory neurotransmitters (10) by acting at P1 receptors (11). Thus, the enzymes catalyzing the conversion of ATP to adenosine have a central role in the modulation and control of ATP and adenosine actions in (patho)physiological conditions.
Extracellular hydrolysis of ATP to adenosine is mediated by a chain of ectonucleotidase enzymes, which includes members of the ecton-ucleoside triphosphate diphosphohydrolase family (NTPDases1-8) and ecto-nucleotide pyrophosphatase/phosphodiesterase family (NPP1-3), which hydrolyze ATP, either to adenosine 5′-diphosphate (ADP) or directly to adenosine 5′-monophosphate (AMP) and ecto-5′-nucleotidase (eN), which catalyzes the final degradation of AMP to adenosine. Ectonucleotidases are membrane-bound ecto-enzymes present in all mammalian cells and tissues (12), but they also exist in a soluble form in the extracellular fluids (13–18), where they arise from shedding of the membrane-bound enzymes (13, 19, 20) or from release via microvesicles or exosomes (21).
It has been previously shown that cortical stab injury (CSI) induces significant changes in the activity, expression and cellular distribution of NTPDase1 and eN at the injury site (22–24). It has been shown as well that experimentally induced neuropathologies are associated with significant alterations in soluble ectonucleotidases activity at the periphery, i.e. in the plasma and serum (16, 25, 28). Namely, variations in soluble ectonucleotidase activities were observed in rats with experimentally induced demyelination (25), autoimmune encephalomyelitis (26) and seizures (27, 28). These findings indicate that insults to the brain, probably due to disturbed cerebral blood flow, oxidative metabolism or compromised blood–brain barrier, may induce alterations in nucleotides release and rate of their hydrolysis in the vascular system. Since adenine nucleotides and adenosine regulate diverse cellular functions in the vascular system, including vascular tone, platelet aggregation and inflammatory responses of lymphocytes and macrophages (29), alterations of ectonucleotidase activities in the vascular system may be relevant for the clinical outcomes of the primary insult. Therefore, in this study, we explored alterations in soluble ectonucleotidase activities and serum levels of adenine nucleotides and nucleosides in a rat model of cortical stab injury.
Materials and Methods
Animals
Adult male rats of the Wistar strain (3-month-old, 250–300 g body weight at the time of surgery) were used in the study. The animals were maintained 3/cage at constant temperature on a 12 h light/dark cycle with free access to food and water. The study was performed in accordance with EU Directive 2010/63/EU for Animal Experiments, whereas all the experimental protocols were approved by the Institutional Animal Care Committee. Maximum efforts were made to minimize the number of animals used and their suffering.
Surgical procedure
Twenty-five animals were used in the study. Twenty animals were randomly divided into two groups. Animals of the first group were anesthetized with ether and positioned in a stereotaxic frame. Cortical stab injury (CSI) was inflicted by inserting a 1 mm wide dental drill through the skull, 1.5 mm below the underlying cortical region on the left side (2 mm lateral from the midline, 2 mm posterior to Bregma), as previously described (22). The wound was sterile closed. Animals from another group were anesthetized, positioned in the stereotaxic frame and after making the incision along the midline leaving dura intact, the wound was sutured (sham surgery group). All the animals were placed in a heated room and monitored while recovering from anesthesia. In both CSI and sham groups, animals were allowed to recover for 4 h and 24 h after the surgery (5 animals/group). Another five age-matched animals were used as intact controls.
Isolation of serum
For enzymatic assays, serum samples were prepared as previously described by our group (17). Blood was drawn after decapitation and was allowed to clot at room temperature for 30 min. Blood was centrifuged in plastic tubes at 2000 × g for 5 min. The resultant serum samples were kept separately at −80 °C. Serum protein content was determined by the method of Bradford (30).
For ultra performance liquid chromatography assays (UPLC) serum samples were prepared as previously described (17). Briefly, serum protein content was precipitated by adding 0.6 mol/L perchloric acid (PCA) and by subsequent centrifugation at 14 000 × g for 10 min. Supernatants were neutralized by 4.0 N NaOH and clarified with second centrifugation at 14 000 × g for 15 min. Resultant supernatants were kept at −80 °C until use.
Enzyme assays
Soluble ectonucleotidase activities were assayed using a modification of the method of Oses et al. (16) as previously described (26). For ATP and ADP hydrolysis, the reaction mixture contained (in mmol/L): 112.5 Tris-HCl, pH 8.0, 0.5 EDTA, 5 MgCl2 and 0.5 mg of serum proteins in the final volume of 200 μL. For AMP hydrolysis, the reaction mixture contained (in mmol/L): 112.5 Tris-HCl, pH 8.0, 10 MgCl2 and 0.5 mg of serum proteins. Reaction was initiated by the addition of ATP, ADP or AMP in the final concentration of 0.5 mmol/L and allowed to proceed for 40 min at 37 °C. The reaction was stopped by the addition of 3 mol/L PCA and by transferring the test tubes on ice. Level of non-enzymatic hydrolysis was determined for each sample by adding an aliquot of serum after the reaction was stopped with PCA. All the samples were centrifuged at 5000 × g for 5 min at 4 °C to eliminate precipitated protein and the supernatant was used for the colorimetric determination of inorganic phosphate (Pi) by the malachite green method (31). Rate of nucleotide hydrolysis was expressed as mean specific activity (nmol Pi/mg/min) ± SEM, from n=2 determinations performed in quintuplicate in five different serum samples for each time and surgical procedure group.
Soluble nucleotide pyrophosphatase/phosphodiesterase activity was assessed by measuring phosphodiesterase activity using p-nitrophenyl thymidine 5′-monophosphate (p-Nph-5′-TMP) as a substrate (17). Briefly, reaction mixture, containing (in mmol/L) 0.5 p-Nph-5′-TMP in 100 Tris-HCl, pH 8.9, was incubated with 1 mg of serum protein, for 5 min at 37 °C, in the final volume of 200 μL. The reaction was stopped by the addition of 0.2 mol/L NaOH and the amount of p-nitrophenol (p-Nph) liberated as a result of the enzyme activity was measured at 400 nm using an extinction coefficient of 27.4 × 10−3 (L mol−1 cm−1). Level of non-enzymatic hydrolysis was determined for each sample by adding serum in the standard reaction mixture after the reaction was stopped with NaOH. Rate of phosphodiesterase activity was expressed as mean specific activity (nmol p-Nph/mg/min) ± SEM from n=4 determinations performed in triplicate in five different serum samples for each time and surgical procedure group.
Ultra performance liquid chromatography (UPLC)
For the UPLC assay, an aliquot of each sample (2 μL) was injected to the UPLC system and separated by ACQUITY UPLC™ BEH C18 100 mm × 2.1 mm column (Waters, Milford, USA), kept at 40 °C with 4 mmol/L tetrabutylammonium hydroxide in 4 mmol/L phosphate buffer (A) and methanol (B), in the ratio A:B = 3:1, as a mobile phase. Under these conditions adenine nucleotides were readily separated and detected by a TUV detector at 254 nm. Compounds of interest, ATP, ADP, AMP, adenosine were identified and quantified by using corresponding freshly prepared ultra pure standards of known concentrations to determine retention times for each compound and for the construction of calibration curves. Analyte concentrations are given in μmol/L serum.
Data analysis
Results are expressed as mean ± SEM of n independent experiments. Comparison between different groups (CSI vs. intact control and CSI vs. sham) was made by Student’s t test for independent samples, followed by Tukey test for multiple comparisons among means. Differences between CSI and sham or CSI and intact control group were considered significant at level p<0.05.
Results
Ectonucleotidase enzyme assays
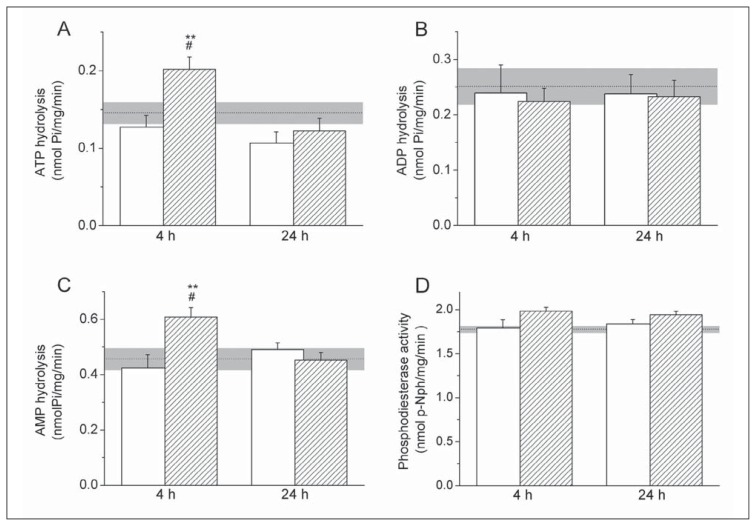
Rates of soluble ectonucleotidase activities following CSI were assessed using enzyme-specific assays. NTPDase activity was assessed by determining the rates of ATP (Figure 1A) and ADP (Figure 1B) hydrolysis, whereas eN activity was assessed by determining the rate of AMP hydrolysis in serum samples (Figure 1C). The rates of soluble ATP, ADP and AMP hydrolysis in intact control animals were 0.14±0.01 nmol Pi/mg/min, 0.25±0.03 nmol Pi/mg/min and 0.45±0.04 nmolPi/mg/min, respectively. Rates of nucleotide hydrolysis significantly changed at 4-h post-injury, when almost 60% increase in ATP hydrolysis (p<0.005) and 40% increase in AMP hydrolysis (p<0.005) were observed in respect to the corresponding sham controls, whereas at 24-h post-injury both ATP and AMP hydrolysis returned to the control values. The rate of ADP hydrolysis remained unchanged after the injury.
Figure 1.
Soluble ectonucleotidase activity after the injury.
A–C: Soluble nucleotide hydrolysis was assayed in the presence of 0.5 mmol/L ATP (A), ADP (B) and AMP (C), whilst nucleotide phosphodiesterase activity (D) was assessed using 0.5 mmol/L p-Nph-5′-TMP as a substrate. Serum samples were obtained from CSI (shaded bars) and sham group (white bars) at different post-injury times (4-h and 24-h). Bars represent mean activity (nmol Pi/mg/min) ± SEM (A–C), and (nmol p-nitrophenol/mg/min) ± SEM (D) from n=2 determinations in five different samples for each time and surgery group, assayed in quintuplicate (A–C) and triplicate (D). Dot line indicates mean activity in serum samples obtained from intact control animals ± SEM (gray areas). Statistical significance was determined in respect to intact control group (# p<0.05) and sham group (**p<0.005) in serum samples obtained from CSI animals at different post-injury times.
Soluble nucleotide pyrophosphatase/phosphodiesterase activity in the serum was determined using p-nitrophenyl thymidine 5′-monophosphate as a substrate (Figure 1D). NPP activity in the serum samples of intact control animals was 1.78±0.04 nmol p-Nph/mg/min. NPP activity in CSI group did not vary in respect to sham control group during the 24-h post-injury period.
Determination of adenine nucleotide and purine concentrations by UPLC
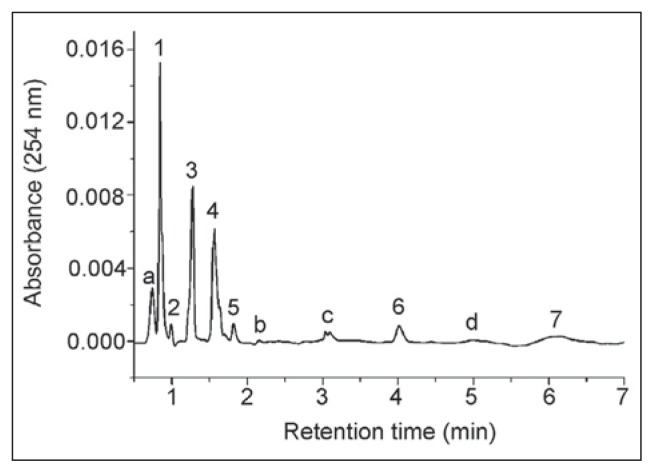
Serum samples were analyzed by the UPLC system with a TUV detector and the representative chromatogram of intact control samples is shown in Figure 2. Eight chromatographic peaks were assigned: (1) inosine (0.93 min), (2) xanthine (1.15 min), (3) adenosine (1.24 min), (4) hypoxanthine (1.51 min), (5) AMP (1.74 min), (6) ADP (4.01 min) and (7) ATP (6.12 min). Peak d was assigned to GTP by co-elution of relevant standard solution, even though its amount could not be determined. Peaks a, b and c require further investigation to be identified. Results obtained by quantitative analysis of chromatographic data are shown in Table I. Basal levels of ATP, ADP and AMP were in the submicromolar range, whereas concentrations of adenosine and its downstream metabolites –inosine, hypoxanthine and xanthine, were about one order of magnitude higher. Analyte concentrations in intact control serum samples have shown a quite narrow range of individual variability and were comparable with those reported in the literature (32, 33).
Figure 2.
Representative UPLC chromatogram of serum of intact control samples.
Aliquots (2 μL) of protein-free samples were separated by ACQUITY UPLC™ BEH C18 100 mm × 2.1 mm column (Waters) and detected by TUV detector at 254 nm. Analytes of interest were identified and quantified by using freshly prepared ultra pure standards of known concentrations. The identity of chromatographic peaks was confirmed by co-injection of genuine samples of the nucleotides, nucleosides and oxypurines. Peaks: (1) – Inosine; (2) – Xanthine; (3) – Adenosine; (4) – Hypoxanthine; (5) – AMP; (6) – ADP; (7) – ATP. For peaks a, b, c and d, see text.
Table I.
Adenine nucleotides and purine compounds level in serum.
| Concentration (μmol/L serum) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | ATP | ADP | AMP | Ado | Ino | Hypo | Xanth | |
| Intact control | 0.25±0.02 | 0.31 ±0.11 | 0.39±0.04 | 2.76±0.26 | 2.63±0.12 | 3.41±0.04 | 3.71±0.12 | |
| 4-h | Sham | 0.20±0.03 | 0.34±0.07 | 0.58±0.13 | 2.39±0.32 | 2.96±0.11 | 3.47±0.41 | 3.46±0.31 |
| CSI | 0.16±0.01# | 0.24±0.03 | 0.96±0.21# | 2.51±0.42 | 2.29±0.16* | 3.58±0.47 | 4.67±0.15**## | |
| 24-h | Sham | 0.30±0.05 | 0.28±0.04 | 0.51±0.17 | 2.15±0.40 | 2.75±0.12 | 3.17±0.48 | 3.49±0.05 |
| CSI | 0.34±0.07 | 0.28±0.02 | 0.46±0.04 | 1.87±0.27 | 2.58±0.05 | 3.12±0.18 | 3.09±0.11 | |
UPLC measurements of adenine nucleotides ATP, ADP and AMP, adenosine (Ado), inosine (Ino), hypoxanthine (Hypo) and xanthine (Xanth) concentrations in serum of intact control animals and animals subjected to CSI or sham surgery at different post-injury times. Data are mean concentrations (μmol/l serum) from n=2 determinations in 5 serum samples per time and surgery group. Different from intact control group,
p<0.05;
p<0.001; different from sham group,
p<0.05;
p<0.001.
Concentrations of analytes in serum samples at different post-injury times are shown in Table I. At 4-h post-injury, about 20% decrease in ATP concentration (p<0.05), together with more than 2-fold increase in AMP concentration were observed in respect to control group. At the same time point, about 25% decrease in inosine (p<0.05) and corresponding increase in xanthine concentration (p< 0.001) in respect to sham control were detected. At 24-h post-injury concentrations of tested analytes returned to control level.
Discussion
Reciprocal interactions between the brain and the vascular system in health and disease have drawn considerable attention, mostly concerning the identity of signaling molecules and networks that mediate this bi-directional relationship (34, 35). Among many endogenous signaling factors, extracellular adenine nucleotides and nucleosides are now considered as true mediators between the two systems (35). Several groups reported that insult to the brain alters purine compound levels, not just in the cerebrospinal fluid (36, 37), but also in serum (32, 38). Changes in the rate of soluble (21, 25, 26) and membrane-bound ectonucleotidase activities (39) were also observed in the vascular system of humans and animals with different neuropathologies. Therefore, the aim of the present study was to explore the serum levels of adenine nucleotides and nucleosides as well as the activities of soluble ectonucleotidases after brain injury, in order to elucidate their potential role in the relationship between the nervous and the vascular system under physiological and pathological conditions.
Considering basal purine levels in serum, submicromolar concentrations of nucleotides in respect to approximately ten-times higher levels of nucleosides and oxopurines were determined, suggesting predominance of nucleotide-catabolizing over nucleotide-regenerating pathways in the vascular system in physiological conditions. It was further found that unilateral cortical stab injury induces dynamic changes in the serum levels of adenine nucleotides and purine compounds, probably resulting from sequential activation of the two opposite pathways of purine metabolism. Namely, as early as 4 h post-injury, ATP and inosine concentrations decreased, while AMP and xanthine concentrations increased, suggesting up-regulation of enzymes that mediate ATP hydrolysis in serum early after the injury. Alterations obtained may arise from increased energy demands caused by injury, since increase in plasma levels of purine bases such as xanthine or hypoxanthine are considered as an indicator of energy stress (32). In particular, depletion of extracellular ATP may be related to excessive consumption by vascular cells, while AMP may act as a signaling molecule at P1 receptors (40) to potentiate energy-preserving pathways (37). Variations in serum nucleotide levels at 4-h post-injury were in close correlation with changes in the rate of ectonucleotidase activities, namely, at the same time point, ATP-hydrolysis significantly increased. Since phosphodiesterase activity remained unchanged after the injury, implying that NPP soluble forms are not involved in the increased ATP hydrolysis in serum, it is most likely that the up-regulation of soluble NTPDase1, which hydrolyzes ATP directly to AMP, mostly contributes to the change obtained. Increase in AMP hydrolysis at 4-h post-injury implies up-regulation of soluble eN, which is the only enzyme that converts AMP to adenosine. On the other hand, 24-h post-injury, both purine concentrations and nucleotide-hydrolysing activities in serum returned to the control level, additionally confirming the key role of ectonucleotidases in the regulation of adenine nucleotide levels in blood. Up-regulation of ATP and AMP hydrolysis rates in rat serum were previosly observed in several seizure models (27, 28), implying that injury to the brain generates changes in the catabolism of adenine nucleotides in the vascular system. Interestingly, in our study, the adenosine level in serum remained stable during the whole post-injury period, although increased levels of this tissue-protective and immunosupressive nucleoside (41) were repeatedly found in the cerebrospinal fluid in several in vivo models of brain injury (7, 36). Since the adenosine level in extracellular milieu results from two opposite activities, namely from adenosine-producing eN activity and adenosine-eliminating activity catalyzed by adenosine deaminase, the stable serum adenosine level might reflect a balance between the two activities. Even though adenosine metabolizing enzymes (42) were not the subject of this study, it is worth noting that the serum levels of adenosine metabolites, inosine and xanthine, were significantly altered 4-h after the injury. Beside enzymatic degradation, the stable serum adenosine level could be also maintained by nucleoside transporters (43), which mediate either facilitated or Na+-coupled adenosine transport back to the cells of the vasculature or, through the endothelial wall of the blood–brain barrier, to the brain parenchyma. Anyhow, unchanged serum adenosine level indicates the existence of a stable adenosine pool continually available for the purine recycling pathway (42).
Since ATP acts as a ligand at multiple P2 receptors, alterations in its serum concentration after the injury may affect numerous cellular functions. For instance, at high micromolar concentrations, ATP promotes vasodilatation (4) and triggers proinflammatory effector functions (4, 44) including production of proinflammatory cytokines (45–47).
To summarize the results of the study, brain injury induces dynamic changes in serum purine levels as well as an increase in soluble nucleotide-hydrolysing activities. Early after the injury, enhanced ATP-hydrolysis, probably due to the up-regulation of NTPDase1, produces a decrease in ATP and an increase in AMP levels in blood. Changes in nucleotide concentration and their ratios affect the type and duration of P2 receptor activation and shape many effector functions in the vascular system, including the blood flow and responses of vascular endothelial, immune and blood cells. Thus, the results of our study imply that adenine nucleotides and ectonucleotidases may be the members of a communication network between the brain and the vascular system in physiological and pathological conditions, and thereby involved in the development of various human neuropathologies.
Acknowledgment
This study was supported by the Serbian Ministry of Education, Science and Technological Development Project Nos. 41014 and 172023.
Footnotes
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Burnstock G. Introductory overview of purinergic signaling. Fron Biosci (Elite Ed) 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 3.North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch. 2006;452:479–85. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 4.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 5.Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Current Neuropharmacology. 2009;7:160–79. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson CL, Bell MJ, Kochanek PM, Adelson PD, Ruppel RA, Carcillo JA, et al. Increased adenosine in cerebrospinal fluid after severe traumatic brain injury in infants and children: association with severity of injury and excitotoxicity. Crit Care Med. 2001;29(12):2287–93. doi: 10.1097/00003246-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Pearson T, Currie AJ, Etherington LA, Gadalla AE, Damian K, Llaudet E, et al. Plasticity of purine release during cerebral ischemia: clinical implications? J Cell Mol Med. 2003;7(4):362–75. doi: 10.1111/j.1582-4934.2003.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke H, Krügel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–44. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 9.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 10.Stone TW. Adenosine, neurodegeneration and neuroprotection. Neurol Res. 2005;27(2):161–8. doi: 10.1179/016164105X21896. [DOI] [PubMed] [Google Scholar]
- 11.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–16. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto–nucleotidases. Purinergic signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yegutkin GG, Samburski SS, Jalkanen S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 2003;17:1328–30. doi: 10.1096/fj.02-1136fje. [DOI] [PubMed] [Google Scholar]
- 14.Yegutkin GG, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–6. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, Gonzales-Alonso J. Intravascular ADP and soluble nucleotidases contribute to acute prothrombic state during vigorous exercise in humans. J Physiol. 2007;579:553–64. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oses JP, Cardoso CM, Germano RA, Kirst IB, Furstenau CR, Wink MR, et al. Soluble NTPDase: An additional system of nucleotide hydrolysis in rat blood serum. Life Sci. 2004;74:3275–84. doi: 10.1016/j.lfs.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Laketa D, Bjelobaba I, Savic J, Lavrnja I, Stojiljković M, Rakic L, Nedeljkovic N. Biochemical characterization of soluble nucleotide pyrophosphatase/phosphodiesterase activity in rat serum. Mol Cell Biochem. 2010;339:99–106. doi: 10.1007/s11010-009-0373-1. [DOI] [PubMed] [Google Scholar]
- 18.Meerson NR, Delautier D, Durand-Schneider AM, Moreau A, Schilsky ML, Sternlieb I, et al. Identification of B10, an alkaline phosphodiesterase of the apical plasma membrane of hepatocytes and biliary cells, in rat serum: increased levels following bile duct ligation and during the development of cholangiocarcinoma. Hepatology. 1998;27:563–8. doi: 10.1002/hep.510270234. [DOI] [PubMed] [Google Scholar]
- 19.Pettengill M, Robson S, Tresenriter M, Millán JL, Usheva A, Bingham T, et al. Soluble ecto-5′-nucleotidase (5′-NT), alkaline phosphatase, and adenosine deaminase (ADA1) activities in neonatal blood favor elevated extracellular adenosine. J Biol Chem. 2013;288:27315–26. doi: 10.1074/jbc.M113.484212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, Westfall DP. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–9. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- 21.Ceruti S, Colombo L, Magni G, Viganò F, Boccazzi M, Deli MA, et al. Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood-brain barrier. Neurochem Int. 2011;59:259–71. doi: 10.1016/j.neuint.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Nedeljković N, Bjelobaba I, Šubašić S, Lavrnja I, Peković S, Stojkov D, et al. Up-regulation of ectonucleotidase activity after cortical stab injury in rat. Cell Biol Int. 2006;30:541–6. doi: 10.1016/j.cellbi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Nedeljković N, Bjelobaba I, Lavrnja I, Stojkov D, Peković S, Rakić L, Stojiljković M. Early temporal changes in ecto-nucleotidase activity after cortical stab injury in rat. Neurochem Res. 2008;33:873–9. doi: 10.1007/s11064-007-9529-0. [DOI] [PubMed] [Google Scholar]
- 24.Bjelobaba I, Parabucki A, Lavrnja I, Stojkov D, Dačić S, Peković S, et al. Dynamic changes in the expression pattern of ecto-5′-nucleotidase in the rat model of cortical stab injury. J Neurosci Res. 2011;89(6):862–73. doi: 10.1002/jnr.22599. [DOI] [PubMed] [Google Scholar]
- 25.Spanevello RM, Mazzanti CM, Maldonado PA, Zanin R, Morsch A, Hannel L, et al. Activities of enzymes that hydrolyze adenine nucleotides in platelets from rats experimentally demyelinated with ethidium bromide and treated with interferon-β. Life Sci. 2007;80:1109–14. doi: 10.1016/j.lfs.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 26.Lavrnja I, Bjelobaba I, Stojiljković M, Peković S, Mostarica-Stojković M, Stošić-Grijučić S, et al. Time-course changes in ectonucleotidase activities during experimental autoimmune encephalomyelitis. Neurochem Int. 2009;55:193–8. doi: 10.1016/j.neuint.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Bruno AN, Oses JP, Bonan CD, Walz R, Battastini AM, Sarkis JJ. Increase of nucleotidase activities in rat blood serum after a single convulsive injection of pentylenetetrazol. Neurosci Res. 2002;43:283–8. doi: 10.1016/s0168-0102(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 28.Bruno AN, Oses JP, Amaral O, Coitinho A, Bonan CD, Battastini AM, Sarkis JJ. Changes in nucleotidase hydrolysis in rat blood serum induced by pentylenetetrazol-kindling. Brain Res Mol Brain Res. 2003;114:140–5. doi: 10.1016/s0169-328x(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 29.Bours MJL, Swennen ELR, Di Virgilio F, Cronstein B, Dagnelie PC. Adenosine 5-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A dye binding assay for protein. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 31.Chan K, Delfer TD, Junger KD. A direct colorimetric assay for Ca2+/ATPase activity. Anal Biochem. 1986;157:375–80. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 32.Tavazzi B, Batocchi AP, Amorini AM, Nociti V, D’Urso S, Longo S, et al. Serum metabolic profile in multiple sclerosis patients. Mult Scler Int. 2011;2011:167156. doi: 10.1155/2011/167156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fürstenau CR, Trentin Dda S, Gossenheimer AN, Ramos DB, Casali EA, Barreto-Chaves ML, Sarkis JJ. Ecto-nucleotidase activities are altered in serum and platelets of L-NAME-treated rats. Blood Cells Mol Dis. 2008;41:223–9. doi: 10.1016/j.bcmd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Di Virgilio FD, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Cristofori L, Tavazzi B, Gambin R, Vagnozzi R, Signoretti S, Amorini AM, et al. Biochemical analysis of the cerebrospinal fluid: evidence for catastrophic energy failure and oxidative damage preceding brain death in severe head injury: a case report. Clin Biochem. 2005;38:97–100. doi: 10.1016/j.clinbiochem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Tavazzi B, Signoretti S, Lazzarino G, Amorini AM, Delfini R, Cimatti M, et al. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery. 2005;56:582–9. doi: 10.1227/01.neu.0000156715.04900.e6. [DOI] [PubMed] [Google Scholar]
- 38.Amorini AM, Petzold A, Tavazzi B, Eikelenboom J, Keir G, Belli A, Giovannoni G, Di Pietro V, Polman C, D’Urso S, Vagnozzi R, Uitdehaag B, Lazzarino G. Increase in uric acid and purine compounds in biological fluids of multiple sclerosis patient. Clin Biochem. 2009;42:1001–6. doi: 10.1016/j.clinbiochem.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Laketa D, Bjelobaba I, Stojkov D, Lavrnja I, Parabucki A, Stojiljković M, Nedeljković N. Brain cortical injury induces changes in peripheral lymphocytes ectonucleotidase activities. Arch Biol Sci. 2013;65:33–43. [Google Scholar]
- 40.Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287:5301–9. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–9. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–94. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Löffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–13. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 44.Goepfert C, Imai M, Brouard S, Csizmadia E, Kaczmarek E, Robson SC. CD39 modulates endothelial cell activation and apoptosis. Mol Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- 45.Marković D, Đorđević V. Apoptosis regulation by inhibitors of programmed cell death. J Med Biochem. 2013;32:207–13. [Google Scholar]
- 46.Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell N, Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1β and IL-1α from murine macrophages. J Immunol. 2003;170:3029–36. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 47.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–32. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]