Summary
Background
C-reactive protein (CRP) has been proposed as a risk marker and risk factor of cardiovascular disease. There have been a number of clinical reports suggesting that supplementation with L-carnitine can modulate systemic inflammation and lower circulating CRP concentrations, but the results have not been consistent.
Methods
A comprehensive literature search in Medline, Scopus and Cochrane Central Register of Controlled Trials was performed in December 2012 to identify clinical trials investigating the impact of oral L-carnitine supplementation on serum/plasma CRP concentration. A random effect method was used to calculate the combined effect size.
Results
Six studies comprising 541 cases and 546 controls met the inclusion criteria. Meta-analysis of included trials revealed a significant reduction of circulating CRP concentrations in subjects under L-carnitine intervention compared to the control treatment. The calculated combined weighted mean reduction in CRP concentrations was −0.39 mg/L [95% CI (−0.62 – −0.16)]. This effect size estimate was found to be robust and remained unaffected by the removal of each single study.
Conclusions
The overall findings of the present meta-analysis support the clinically relevant benefit of L-carnitine supplementation in lowering the circulating levels of CRP.
Keywords: carnitine, nutrition, coronary artery disease, atherosclerosis, systematic review, clinical trial
Kratak sadržaj
Uvod
C-reaktivni protein (CRP) predložen je kao marker rizika i faktor rizika za kardiovaskularnu bolest. Postoji izvestan broj kliničkih izveštaja koji su pokazali da suplementacija L-karnitinom može modulisati sistemsku inflamaciju i sniziti koncentracije CRP-a u cirkulaciji, ali njihovi rezultati nisu usaglašeni.
Metode
U decembru 2012, sprovedena je sveobuhvatna pretraga literature u bazama Medline, Scopus i Cochrane Central Register of Controlled Trials radi identifikacije kliničkih ispitivanja koja su istraživala uticaj oralne suplementacije L-karnitinom na koncentraciju CRP-a u serumu/plazmi. Za izračunavanje kombinovanih dimenzija efekta korišćena je metoda nasumičnog efekta.
Rezultati
Šest studija koje su obuhvatile 541 pacijenta i 546 kontrolnih subjekata ispunilo je kriterijume za uključenje u ovu studiju. Metaanalizom obuhvaćenih ispitivanja je otkriveno značajno sniženje koncentracija CRP-a u cirkulaciji kod subjekata podvrgnutih intervenciji L-karnitinom u poređenju s kontrolom. Izračunato kombinovano srednje sniženje koncentracija CRP-a iznosilo je −0,39 mg/L [95% CI(−0,62 – −0,16)]. Ovakva procena dimenzija efekta označena je kao robusna i ostala je nepromenjena i posle odstranjivanja svake pojedinačne studije.
Zaključak
Ukupni rezultat predstavljene metaanalize svedoči u prilog klinički relevantne koristi od suplementacije L-karnitinom za snižavanje nivoa CRP u cirkulaciji.
Introduction
Atherosclerosis is the cornerstone of the pathogenesis of cardiovascular disease (CVD) and its complications. Recent advances in basic research have provided compelling clues to the pivotal role of inflammation in the development and progression of atherosclerosis (1). In parallel, there is a large body of evidence indicating a heightened state of systemic inflammation in patients with CVD. Nearly all the known risk factors for CVD such as dyslipidemia, hypertension, diabetes, obesity and infection are influential in triggering the inflammatory response during atherosclerosis (2). However, the central role pertains to the oxidatively modified lipoproteins, in particular low-density lipoprotein (LDL). Given the established role of inflammation in all stages of atherogenesis, antiinflammatory therapy has been suggested as a promising approach to lower the concentrations of atherogenic inflammatory mediators and cover the substantial residual risk following conventional lipid lowering therapy (2).
CRP is a 206 amino acid pentraxin-like acute-phase protein which is synthesized by hepatocytes in response to inflammation (3). CRP could be regarded as one of the best known biomarkers of systemic inflammation. Elevated circulating levels of CRP have been suggested to serve as an independent and strong predictor of CVD and atherothrombotic events (4). Recent evidence has suggested that CRP is not only a CVD risk marker, but also has a direct role in the development of vascular damage and CVD outcomes. Findings of the JUPITER (Justification for the Use of Statins in Primary Prevention: an Intervention Evaluating Rosuvastatin) trial have revealed a tight association between the degree of CRP reduction and corresponding decrement in CVD risk (5). Therefore, reduction of CRP concentrations is regarded as an effective approach for both primary and secondary prevention of CVD and its complications. Heretofore, only a few therapeutic options have been identified for the purpose of serum CRP reduction. Among these, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase – known as statins – have been the most effective class of drugs (6, 7). According to the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline for assessment of cardiovascular risk in asymptomatic adults, statin therapy is indicated for persons with LDL-C levels <2.59 mmol/L but elevated CRP. Nevertheless, successful CRP reduction may not be achieved in patients who are refractory to the effects of statins or those who are intolerant of statins. Therefore, it would be ideal to introduce novel agents, preferably of natural origin, that could lower CRP, and on the other hand have a wide safety margin that allows their chronic supplementation. Such safe CRP-lowering agents may also be used as an adjunct to statins in order to achieve stronger reductions in CRP levels.
Carnitine (L-β-hydroxy-γ-N-trimethylaminobutyric acid) is a vitamin-like nonprotein nutraceutical primarily biosynthesized in the liver and kidney from the amino acids lysine and methionine (8). Due to its chiral structure, carnitine has two stereoisomeric forms: D and L. However, only the L isomer is known to be essential for human and animal health and possess biological activity, while the other isomer is biologically inert. The main physiologic role of L-carnitine is involvement in fat and energy metabolism by mediating the transport of long-chain free fatty acids across the mitochondrial membrane for β-oxidation (9). Aside from this leading task, L-carnitine supplementation has been reported to be associated with several health benefits such as regulation of carbohydrate metabolism and insulin sensitivity, mitigation of lipid peroxidation and oxidative stress, and enhancement of the immune system and spermatogenesis (10–14). Carnitine is also endowed with several cardioprotective properties (15–17). Several clinical trials have indicated the favorable impact of L-carnitine supplementation in the modulation of CVD incidence and mortality (16–18). However, it remains unclarified if mitigation of systemic inflammation plays a role in these beneficial properties of L-carnitine.
Thus far, there have been scattered reports in different patient groups on the impact of oral supplementation with L-carnitine and its analogues on the circulating levels of CRP (19–26). While the overall balance in the findings of conducted trials favors the efficacy of L-carnitine, some negative reports make the judgment inconclusive. This controversy in findings necessitates conducting a systematic review of literature and a meta-analysis of the published studies to clarify if oral consumption of L-carnitine could lower the circulating levels of CRP.
Methods
Search strategy
This study was designed according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (27). A systematic literature search for English-language articles was performed in the following databases from inception through December 2012: PubMed-Medline (http://www.ncbi.nlm.nih.gov/pubmed) and SCOPUS (http://www.scopus.com) and the Cochrane Database of Systematic Review published (http://www.cochrane.org). Databases were searched using the following terms: (carnitine OR L-carnitine OR acetylL-carnitine OR »acetyl L-carnitine« OR propionyl-L-carnitine OR »propionyl L-carnitine«) AND (C-reactive protein OR CRP OR hsCRP OR hs-CRP). The wild-card term »*« was used to increase the sensitivity of the search strategy.
Study selection
Studies were included if they fulfilled all of the following criteria: (1) an intervention study using L-carnitine or its analogues as main or adjunctive therapy, (2) controlled design: drug- or placebo-controlled parallel or cross-over randomized trial, (3) study design consisted of random allocation of study participants to L-carnitine or control treatment, and (4) reported mean/median and SD/SE/IQR of serum/plasma CRP in both intervention and treatment groups at baseline as well as at the end of trial. Studies were excluded if they were: (1) a review article or meeting/conference paper; (2) were not of a clinical design; or (3) administered L-carnitine via the intravenous route.
Data extraction
Aside from baseline and post-trial CRP concentrations, data on the study location, publication year, population size, type of intervention, administered daily dose of carnitine, duration of supplementation, control group allocation, age, gender, smoking habit and serum/plasma levels of glucose, total cholesterol, LDL-cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglycerides were extracted from all retrieved articles.
Assessment of risk of bias in included studies
A methodological quality assessment of the included studies was carried out by employing the Jadad score level-of-evidence rating for randomized controlled trials (28). Jadad scale ranges from score 0 to 5, with higher scores indicative of better quality. The items for quality assessment in the Jadad scale include randomization, blinding, and description of withdrawals and dropouts. Using this scale, the overall quality of a trial could be classified as low (Jadad score of ≤3) or high (Jadad score of 4 or 5).
Statistical analysis
Meta-analysis was conducted using the Cochrane Program Review Manager version 5.1. Blood CRP levels were collated in mg/L. A multiplication by 0.0259, 0.0113 and 0.0555 was used to convert cholesterol (total cholesterol, HDL-C or LDL-C), triglyceride and glucose levels expressed in mg/dL into mmol/L, respectively. Standard deviations at one time point were calculated with the formula SD = SEM × square root n (SEM: standard error of the mean, n: number of participants). Standard deviations (SDs) of the mean difference were calculated using the formula: square root [(SDpretreatment)2 + (SDposttreatment)2 − (2R × SDpretreatment × SDpost-treatment)], assuming a correlation coefficient (R) = 0.5 (29). For studies in which serum/plasma CRP levels were determined in multiple intervals, data from the last time point was used as the post-trial value in analyses.
For parallel and cross-over trials, net changes in measurements were calculated as follows: (measure at end of follow-up in the L-carnitine group − measure at baseline in the L-carnitine group) − (measure at end of follow-up in the control group − measure at baseline in the control group). Due to the inter-study heterogeneities regarding design, underlying disease and age of recruited participants, and L-carnitine dosage and supplementation duration, quantitative data synthesis was performed using a random effect approach with the inverse variance weighting method. In order to evaluate the influence of each study on the overall effect size, a sensitivity analysis was conducted using the one-study remove approach (30).
Results
Summary of included studies
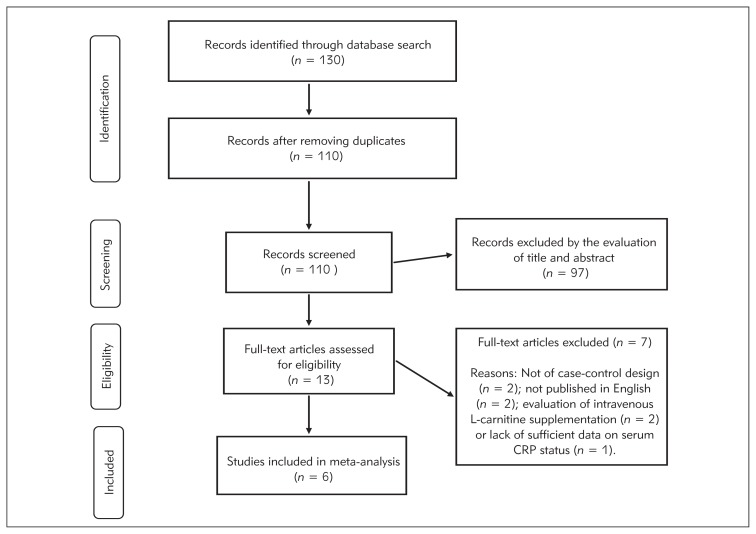
Following the database search and removal of duplicate articles, a total of 110 articles were identified and subjected to initial screening. Thirteen articles were provisionally selected for further full-text evaluation. Out of these 13 publications, 6 met the inclusion criteria and were used for data extraction (19–24). The reasons for the exclusion of the remaining 7 articles were being not original (n=2) (31, 32), investigation of intravenous L-carnitine (n=2) (18, 33), publication in languages other than English (n=2) (34, 35), and lack of sufficient data on serum CRP status (n=1) (36) (Figure 1).
Figure 1.
Flow diagram of studies through review.
CRP measurement
Measurement of serum CRP was carried out using either the particle-enhanced immunoturbidimetry (19–21) or enzyme-linked immunosorbent assay (22–24).
Study characteristics
The pooled population of included studies comprised 1087 individuals, of which 541 were classified as the case group and 546 as the control group. Three studies were conducted in diabetic or prediabetic patients (19, 20, 22), 2 studies in hemodialysis patients (23, 24) and 1 study in patients with non-alcoholic steatohepatitis (21). All studies were double-blind and placebo controlled apart from that of Shakeri et al. which had an open-label design (25). Duration of L-carnitine supplementation ranged between 8 (22) to 48 (19, 20) weeks. Five of the included studies used L-carnitine as intervention (19–21, 23, 24) while Bloomer et al. administered acetyl L-carnitine arginate (ALCA) (22). Dosage of L-carnitine ranged between 1–2 g/day in all the included trials. In 4 trials, L-carnitine was used as monotherapy (21–24), while 2 studies used L-carnitine supplementation as adjunctive therapy to sibutramine (19) or orlistat (20). Characteristics of included studies are summarized in Table I.
Table I.
Characteristics of the included studies.
| Bloomer et al. (23) | Derosa et al. (20) | Derosa et al. (21) | Hakeshzadeh et al. (24) | Malaguarnera et al. (22) | Shakeri et al. (25) | ||
|---|---|---|---|---|---|---|---|
| Jadad score | 4 | 5 | 5 | 3 | 5 | 2 | |
| Year | 2009 | 2011 | 2010 | 2010 | 2010 | 2010 | |
| Location | USA | Italy | Italy | Iran | Italy | Iran | |
| Underlying disease | Prediabetes | Uncontrolled type 2 diabetes mellitus | Obese patients with uncontrolled type 2 diabetes mellitus | Hemodialysis patients | Patients with nonalcoholic steatohepatitis | Hemodialysis patients with Lp (a) hyperlipoproteinemia | |
| Duration | 8 weeks | 48 weeks | 48 weeks | 12 weeks | 12 weeks | 12 weeks | |
| Control treatment | Placebo | Sibutramine (10 mg/d) | Orlistat (120 mg/d) | Placebo | Placebo | None | |
| Allocation in case group | 3 g/d acetyl L-carnitine arginate (eq 1350 mg acetyl L-carnitine/day + 1200 mg/day arginine) | L-carnitine (2 g/day) + sibutramine (10 mg/day) | L-carnitine (2 g/day) + orlistat (120 mg/day) | L-carnitine (1 g/day) | L-carnitine (2 g/day) | L-carnitine (1 g/day) | |
| N | Case | 14 | 129 | 126 | 18 | 36 | 18 |
| Control | 15 | 125 | 132 | 18 | 38 | 18 | |
| Age | Case | 31±3 | 54±5 | 51 ± 4 | 48 ± 10 | 47.9 ± 5.4 | 54.5±19.0 |
| Control | 35±3 | 51±4 | 53 ± 6 | 52 ± 14 | 47.8 ± 5.8 | 57.0±20.0 | |
| %Males | Case | Ns | 50.4 | 49.2 | 33 | 55.6 | 67 |
| Control | NR | 50.4 | 49.2 | 50 | 52.6 | 61 | |
| Smoking | Case | 0 | 31.8 | 32.6 | 0 | NR | 17 |
| Control | 0 | 33.6 | 36.5 | 0 | NR | 6 | |
| Weight (kg) | Case | 85±4* | 96.9±10.8 | 95.1±10.3 | 59±8 | NR | 65±16 |
| Control | 91±5 | 97.7±11.4 | 94.5±9.6 | 64±9 | NR | 63±11 | |
| BMI (kg/m2) | Case | 28.5±1.9 | 33.9±3.5 | 32.9±2.8 | 24±4 | 26.6±3.7 | 23±4 |
| Control | 31.7±2.1 | 33.4±3.2 | 33.1±2.9 | 24.5±4 | 26.5±3.8 | 23±3 | |
| TC (mmol/L) | Case | 5.6±0.3 | 5.7±0.7 | 5.8±0.6 | NR | 6.3± 0.6 | NR |
| Control | 4.8±0.4 | 5.8±0.7 | 5.7±0.6 | NR | 6.0±0.9 | NR | |
| TG (mmol/L) | Case | NR | 1.2±0.5 | 1.2±0.5 | NR | 3.1±0.9 | NR |
| Control | NR | 1.2±0.5 | 1.2±0.5 | NR | 3.0±1.0 | NR | |
| HDL-C (mmol/L) | Case | 1.3±0.2 | 1.1±0.2 | 1.1±0.2 | NR | 0.9±0.1 | NR |
| Control | 1.2±0.1 | 1.1±0.2 | 1.2±0.2 | NR | 1.0±0.1 | NR | |
| LDL-C (mmol/L) | Case | 3.3±0.3 | 4.1±0.4 | 4.1±0.4 | NR | 4.7±0.8 | NR |
| Control | 3.2±0.2 | 4.1±0.4 | 4.0±0.4 | NR | 4.4±0.9 | NR | |
| FPG (mmol/L) | Case | 6.0±0.3 | 8.1±1.2 | 7.8±1.1 | NR | 6.1±0.7 | NR |
| Control | 6.2±0.3 | 8.0±1.1 | 7.5±0.9 | NR | 6.0±0.7 | NR |
Values are stated as mean ± SD.
NR: not reported.
The studies by Derosa et al. (20) investigated the efficacy of adjunctive therapy with L-carnitine in diabetic patients (19). In both trials, 12-month supplementation with L-carnitine was not associated with an additional benefit in terms of serum CRP reduction. There were 40% and 35% reductions in serum CRP following treatment with orlistat and sibutramine, respectively, whereas the reduction rates changed to 56% and 42% when L-carnitine was added as an adjunct. In another trial among prediabetic subjects, treatment with ALCA for 8 weeks did not result in a significant alteration in circulating CRP concentrations compared to placebo (22). In contrast to the aforementioned trials among diabetic or prediabetic patients, findings from two studies in end-stage renal disease (ESRD) patients under hemodialysis revealed a significantly improved effect on serum CRP (−41% (23) and −29% (24)) compared to control group (−3% (23) and +13% (24)) following supplementation with L-carnitine. This finding is also supported in patients with nonalcoholic steatohepatitis in whom L-carnitine caused a 42.9% reduction in serum CRP which was significantly greater than that obtained by placebo (14.9%) (21).
Quantitative data synthesis
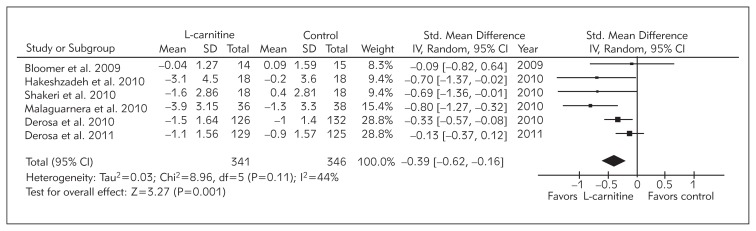
A statistically significant pooled effect size [net change: −0.39 mg/L; 95% CI: −0.62 to −0.16; p = 0.001] was estimated for the impact of L-carnitine supplementation among 541 cases and 546 controls. Using random effect analysis, the overall inter-study heterogeneity was not found to be significant (I2 = 44%; p = 0.11) (Figure 2).
Figure 2.
Net change in serum CRP concentrations associated with L-carnitine supplementation. The overall effect size has been obtained using a random effect model and weighted by inverse variance of each trial.
Sensitivity analysis
Findings of the one-study remove sensitivity analysis revealed that the observed CRP lowering effect of L-carnitine is robust and not dependent on a single study. The pooled effect size remained statistically significant following leaving each study out, as shown in Table II.
Table II.
Leave-one-out sensitivity analysis of pooled effect size.
| C-reactive protein | |||
|---|---|---|---|
| Excluded study | Standardized mean difference (mg/L) | 95% CI | p–value |
| Overall | −0.39 | −0.62 – −0.16 | 0.001 |
| Bloomer et al. 2009 | −0.43 | −0.69 – −0.17 | 0.001 |
| Hakeshzadeh et al. 2010 | −0.36 | −0.61 – −0.11 | 0.004 |
| Malaguarnera et al. 2010 | −0.29 | −0.48 – −0.10 | 0.003 |
| Derosa et al. 2010 | −0.45 | −0.79 – −0.10 | 0.010 |
| Shakeri et al. 2010 | −0.36 | −0.61 – −0.11 | 0.004 |
| Derosa et al. 2011 | −0.48 | −0.72 – −0.24 | <0.0001 |
Discussion
Overall, the findings arising from the present meta-analysis revealed a significant and positive effect of L-carnitine supplementation in reducing the circulating levels of CRP. Of the six included trials, three favored the significant effect of L-carnitine on CRP (21, 23, 24). Two studies which reported negative data on the efficacy of L-carnitine were those which investigated L-carnitine as an adjunctive therapy (19, 20). This disparity in findings may be due to the impact of sibutramine and orlistat which were used in combination with L-carnitine in the above referenced studies. Sibutramine and orlistat have been previously reported to possess antiinflammatory properties and to be capable of reducing serum concentrations of CRP (37, 38). Besides, in both of the aforementioned studies, although the reduction in serum CRP was comparable between the groups after 12 months, the decreasing trend started earlier in the group that received the L-carnitine supplement. Finally, both studies by Derosa et al. (38) were performed among obese individuals who normally bear a heightened state of inflammation. Hence, the anti-obesity effects of orlistat and sibutramine, reflected as reduced weight and BMI, are likely to predominate over any antiinflammatory effect of L-carnitine and could account for a substantial part of the observed antiinflammatory effects. The study by Bloomer et al. is another study which failed to find a significant CRP lowering effect from L-carnitine (23). However, this latter study had the lowest duration of supplementation which could be regarded as a probable reason for not detecting any effect from L-carnitine.
Findings from the sensitivity analysis implied that the detected significance for the efficacy of L-carnitine is robust and not considerably affected by a single study. It is interesting to note that the weighted pooled estimate for the effect of L-carnitine on circulating levels of CRP is greater than those reported from previous meta-analyses on soy isoflavones (40) and telmisartan (41), but lower than those of fibrates (42) and statins (6).
Further, beside its overreported role as a bio-marker of the risk and severity of atherosclerosis and cardiovascular disorders (43), CRP has been suggested to play an active role in the pathogenesis of CVD (39, 44). Clues to the atherogenic potential of CRP include its interaction with lipoproteins and other components of atheroma which leads to subsequent activation of the complement system and inflammation cascade (44, 45). Furthermore, there have been claims stating that CRP can trigger proinflammatory and proapoptotic responses through complement-independent mechanisms such as overexpression of cytokines, adhesion molecules, etc. (46). Since the significant combined effect size calculated in the present study was mainly derived from studies on hemodialysis patients, the clinical benefits of L-carnitine supplementation might be especially important for patients with chronic renal failure or end-stage renal disease who are on hemodialysis, as CVD is the leading cause of death among these patients (47). Moreover, heightened inflammation, as characterized by elevated CRP levels, has been shown to be associated with CVD and mortality in patients with end-stage renal disease (48).
There are several limitations that need to be taken into account prior to any interpretation of the present results. As the most important limitation, the number of studies included in the quantitative data synthesis was small. The present work was an attempt to define the inclusion criteria in such a way that eligible studies would constitute a homogenous population. Nonetheless, there was still inter-study variability regarding the underlying disease, age, smoking habit and anthropometric indices of the recruited patient populations. It is anticipated that these heterogeneities have been covered, at least in part, by applying the random effect model of analysis. Finally, the prevalence of cardiometabolic risk factors was not uniformly expressed across all the included studies. Therefore, it was not possible to correct the estimated pooled effect for these risk factors.
Conclusion
The overall findings of the present meta-analysis support the clinically relevant benefit of L-carnitine supplementation in lowering the circulating levels of CRP. Conducting future, large-scale, randomized clinical trials is warranted in homogenous populations to verify the findings of this meta-analysis.
Footnotes
Conflict of interest statement
The authors stated that there are no conflicts of interest regarding the publication of this article.
References
- 1.Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 2.Paoletti R, Gotto AM, Jr, Hajjar DP. Inflammation in atherosclerosis and implications for therapy. Circulation. 2004;109:III20–6. doi: 10.1161/01.CIR.0000131514.71167.2e. [DOI] [PubMed] [Google Scholar]
- 3.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 6.Hao PP, Chen YG, Wang XL, Xu F, Wang JL, Zhang Y. A meta-analysis of the effects of statins on serum C-reactive protein in Chinese population with coronary heart disease or hyperlipidemia. Chinese Science Bulletin. 2009;54:4404–10. [Google Scholar]
- 7.He ZF, Liu F, Zhai SD, Wu XA, Wang T, Liang L. Statins therapy for C-reactive protein and carotid intima-media thickness in patients with cerebral infarction: A systematic review. Chinese Journal of Evidence-Based Medicine. 2009;9:873–9. [Google Scholar]
- 8.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Bremer J. Carnitine metabolism and functions. Physiol Rev. 1983;63:142–80. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 10.Foster DW. The role of the carnitine system in human metabolism. Ann N Y Acad Sci. 2004;1033:1–16. doi: 10.1196/annals.1320.001. [DOI] [PubMed] [Google Scholar]
- 11.Abd-Allah AR, Al-Majed AA, Al-Yahya AA, Fouda SI, Al-Shabana OA. L-Carnitine halts apoptosis and myelo-suppression induced by carboplatin in rat bone marrow cell cultures (BMC) Arch Toxicol. 2005;79:406–13. doi: 10.1007/s00204-004-0643-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Head E, Kuratsune H, Cotman CW, Ames BN. Comparison of the effects of l-carnitine and acetyl-l-carnitine on carnitine levels, ambulatory activity and oxidative stress biomarkers in the brain of old rats. Ann N Y Acad Sci. 2004;1033:117–31. doi: 10.1196/annals.1320.011. [DOI] [PubMed] [Google Scholar]
- 13.Buyse J, Swennen Q, Niewold TA, Klasing KC, Janssens GPJ, Baumgartner M, et al. Dietary l-carnitine supplementation enhances the lipopolysaccharide-induced acute phase protein response in broiler chickens. Veterinary Immunol Immunopathol. 2007;118:154–9. doi: 10.1016/j.vetimm.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 14.De Rosa M, Boggia B, Amalfi B, Zarrilli S, Vita A, Colao A, et al. Correlation between seminal carnitine and functional spermatozoal characteristics in men with semen dysfunction of various origins. Drugs R D. 2005;6:1–9. doi: 10.2165/00126839-200506010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Retter AS. Carnitine and its role in cardiovascular disease. Heart Dis. 1999;1:108–13. [PubMed] [Google Scholar]
- 17.Arsenian MA. Carnitine and its derivatives in cardiovascular disease. Prog Cardiovasc Dis. 1997;40:265–86. doi: 10.1016/s0033-0620(97)80037-0. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C. Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: a review. Ann N Y Acad Sci. 2004;1033:79–91. doi: 10.1196/annals.1320.007. [DOI] [PubMed] [Google Scholar]
- 19.Iliceto S, Scrutinio D, Bruzzi P, D’Ambrosio G, Boni L, Di Biase M, et al. Effects of L-carnitine administration on left ventricular remodelling after acute anterior myocardial infarction: the L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico (CEDIM) Trial. J Am Coll Cardiol. 1995;26:380–7. doi: 10.1016/0735-1097(95)80010-e. [DOI] [PubMed] [Google Scholar]
- 20.Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, et al. Effects of combination of sibutramine and L-carnitine compared with sibutramine monotherapy on inflammatory parameters in diabetic patients. Metabolism. 2011;60:421–9. doi: 10.1016/j.metabol.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Derosa G, Maffioli P, Ferrari I, D’Angelo A, Fogari E, Palumbo I, et al. Orlistat and L-carnitine compared to orlistat alone on insulin resistance in obese diabetic patients. Endocr J. 2010;57:777–86. doi: 10.1507/endocrj.k10e-049. [DOI] [PubMed] [Google Scholar]
- 22.Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Avitabile T, et al. L-carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitis: a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338–45. doi: 10.1038/ajg.2009.719. [DOI] [PubMed] [Google Scholar]
- 23.Bloomer RJ, Fisher-Wellman KH, Tucker PS. Effect of oral acetyl L-carnitine arginate on resting and postprandial blood biomarkers in pre-diabetics. Nutr Metab (Lond) 2009;6:25. doi: 10.1186/1743-7075-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakeshzadeh F, Tabibi H, Ahmadinejad M, Malakoutian T, Hedayati M. Effects of L-Carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren Fail. 2010;32:1109–14. doi: 10.3109/0886022X.2010.510617. [DOI] [PubMed] [Google Scholar]
- 25.Shakeri A, Tabibi H, Hedayati M. Effects of L-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial Int. 2010;14:498–504. doi: 10.1111/j.1542-4758.2010.00476.x. [DOI] [PubMed] [Google Scholar]
- 26.Suchitra MM, Ashalatha VL, Sailaja E, Rao AM, Reddy VS, Bitla AR, et al. The effect of L-carnitine supplementation on lipid parameters, inflammatory and nutritional markers in maintenance hemodialysis patients. Saudi J Kidney Dis Transpl. 2011;22:1155–9. [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions Version 5.0.2. The Cochrane Collboration; 2009. [Google Scholar]
- 30.Sahebkar A. Does PPAR 2 gene Pro12Ala polymorphism affect nonalcoholic fatty liver disease risk? Evidence from a meta-analysis. DNA Cell Biol. 2013;32:188–98. doi: 10.1089/dna.2012.1947. [DOI] [PubMed] [Google Scholar]
- 31.Savica V, Calvani M, Benatti P, Santoro D, Monardo P, Peluso G, et al. Carnitine system in uremic patients: molecular and clinical aspects. Semin Nephrol. 2004;24:464–8. doi: 10.1016/j.semnephrol.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Bellinghieri G, Santoro D, Calvani M, Savica V. Role of carnitine in modulating acute-phase protein synthesis in hemodialysis patients. J Ren Nutr. 2005;15:13–17. doi: 10.1053/j.jrn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Savica V, Santoro D, Mazzaglia G, Ciolino F, Monardo P, Calvani M, et al. L-carnitine infusions may suppress serum C-reactive protein and improve nutritional status in maintenance hemodialysis patients. J Ren Nutr. 2005;15:225–30. doi: 10.1053/j.jrn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Mortazavi M, Asgari S, Ghassami M, Seirafian S, Taheri S, Naini AE, et al. The effect of oral L-carnitine on serum albumin and inflammatory markers levels in patients under peritoneal dialysis: A randomized controlled trial. Journal of Isfahan Medical School. 2011;29:546–54. [Google Scholar]
- 35.Fu RG, Wang L, Zhou JG, Ma F, Liu XD, Ge H, et al. The effect of levocarnitine on nutritional status and lipid metabolism during long-term maintenance hemodialysis. Academic Journal of Xi’an Jiaotong University. 2010;22:203–37. [Google Scholar]
- 36.Duranay M, Akay H, Yilmaz FM, Şeneş M, Tekeli N, Yücel D. Effects of L-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3211–14. doi: 10.1093/ndt/gfl356. [DOI] [PubMed] [Google Scholar]
- 37.Yesilbursa D, Serdar A, Heper Y, Sarac M, Coskun S, Kazazoglu AR, et al. The effect of orlistat-induced weight loss on interleukin-6 and C-reactive protein levels in obese subjects. Acta Cardiol. 2005;60:265–9. doi: 10.2143/AC.60.3.2005002. [DOI] [PubMed] [Google Scholar]
- 38.Derosa G, Maffioli P, Ferrari I, Palumbo I, Randazzo S, D’Angelo A, et al. Variation of inflammatory parameters after sibutramine treatment compared to placebo in type 2 diabetic patients. J Clin Pharm Ther. 2011;36:592–601. doi: 10.1111/j.1365-2710.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- 39.Popović Lj, Lalić K, Vasović O, Drašković Radojković D, Rajković N, Singh S, Stošić Lj, Čivčić M, Škorić Hinić Lj, Petrović Vujić T. C-reactive protein predicts progression of peripheral arterial disease in patients with type 2 diabetes: a 5-year follow-up study. J Med Biochem. 2014;33:347–55. [Google Scholar]
- 40.Dong JY, Wang P, He K, Qin LQ. effect of soy isoflavones on circulating C-reactive protein in post-menopausal women: Meta-analysis of randomized controlled trials. Menopause. 2011;18:1256–62. doi: 10.1097/gme.0b013e31821bfa24. [DOI] [PubMed] [Google Scholar]
- 41.Takagi H, Yamamoto H, Iwata K, Goto SN, Umemoto T. Effects of telmisartan on C-reactive protein levels: a meta-analysis of randomized controlled trials. Int J Cardiol. 2012;156:238–41. doi: 10.1016/j.ijcard.2012.01.104. [DOI] [PubMed] [Google Scholar]
- 42.Ye J, Kiage JN, Arnett DK, Bartolucci AA, Kabagambe EK. Short-term effect of fenofibrate on C-reactive protein: A meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2011;3:24. doi: 10.1186/1758-5996-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 44.Nakou ES, Liberopoulos EN, Milionis HJ, Elisaf MS. The role of C-reactive protein in atherosclerotic cardiovascular disease: an overview. Curr Vasc Pharmacol. 2008;6:258–70. doi: 10.2174/157016108785909733. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson J. CRP-marker or maker of cardiovascular disease? Arterioscler Thromb Vasc Biol. 2005;25:1527–8. doi: 10.1161/01.ATV.0000174796.81443.3f. [DOI] [PubMed] [Google Scholar]
- 46.Yeh ET, Anderson HV, Pasceri V, Willerson JT. C-reactive protein: linking inflammation to cardiovascular complications. Circulation. 2001;104:974–5. doi: 10.1161/01.cir.104.9.974. [DOI] [PubMed] [Google Scholar]
- 47.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S19. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 48.Collins AJ, Shuling L, Ma JZ, Herzog C. Cardiovascular disease in end-stage renal disease patients. Am J Kidney Dis. 2001;38:S26–S9. doi: 10.1053/ajkd.2001.27392. [DOI] [PubMed] [Google Scholar]