Summary
Background
In the past decades, the obesity epidemic in children of all ages has been an important research field for detecting the metabolic causes and consequences of obesity, the major focus being on insulin and adipocytokine levels. Metabolic work-up in obese children is recommended in the age group as young as 2–6 years. There is evidence that birth weight can be a factor causing obesity later in life accompanied by metabolic complications.
Methods
Insulin, leptin, and adiponectin levels were analyzed in 269 obese children and 60 controls, as well as 110 newborn children with different birth weight and different length of gestation, using standard methods.
Results
In 53.6% of the obese children, complications of obesity such as diabetes mellitus, obesity, hyperlipidemia, heart attack or stroke were found in family members. The peak insulinemia on OGTT was significantly higher in the pubertal compared to the prepubertal group (110.5± 75.9 μU/mL versus 72.2±62.7 μU/mL) (p<0.005). Glucose intolerance was confirmed in 24%. The leptin level was significantly higher and the adiponectin level was lower in pubertal obese children compared to the prepubertal children and controls (p<0.05). In newborns the leptin and adiponectin levels were in correlation with anthropometric parameters: body weight (BW), body length (BL), BW/BL, BMI, and the pondered index (p<0.05).
Conclusion
Obese children have high insulinemia in all ages, reaching its peak towards puberty. The leptin and adiponectin levels might be indicators of the metabolic syndrome. Our findings in newborns might influence the nutritional approach in the future in order to prevent complications of obesity.
Keywords: adiponectin, children, leptin, insulinemia, metabolic setup, obesity
Kratak sadržaj
Uvod
Epidemija gojaznosti kod dece svih uzrasta u toku poslednjih decenija je plodno polje za istraživanje uzroka i posledica gojaznosti sa glavnim fokusom na uticaju insulinemije i adipocitokina. Metabolička obrada se preporučuje čak i u najmlađoj grupi uzrasta 2–6 godina. Postoje podaci da težina na rođenju može biti faktor pojave i komplikacija gojaznosti kasnije u životu.
Metode
Standardnim testovima analizirani su nivoi insulina, leptina i adiponektina kod 269 gojazne i 60 zdrave dece kao i kod 110 novorođenčadi različite težine na rođenju i različite dužine gestacije.
Rezultati
Faktori rizika za komplikacije gojaznosti kao što su dijabetes melitus, gojaznost, hiperlipidemija, infarkt ili cerebralni insult su nađeni kod 53,6% rođaka gojazne dece. Pik insulinemija na OGTT-u je bila značajno viša u pubertetskoj u poređenju sa pretpubertetskom grupom, 110,5±75,9 μU/mL prema 72,2±62,7 μU/mL (p<0,005). Glikozna intolerancija je potvrđena kod 24% ispitanika. Nivo leptina je signifikantno viši kod gojazne dece a adiponektin je niži u poređenju sa pretpubertetskom decom i kontrolnom grupom (p<0,05). Kod novorođenčadi nivoi leptina i adiponektina su u korelaciji sa antropometrijskim parametrima: težinom, visinom, odnosom težina/visina, indeksom telesne mase i ponderalnim indeksom (p<0,05 i <0,01).
Zaključak
Gojazna deca imaju visoku vrednost insulina u svim uzrastima i ona dostiže najviše vrednosti u pubertetu. Nivoi leptina i adiponektina mogu biti uključeni u indikatore metaboličkog sindroma. Naši nalazi kod novorođenčadi mogli bi uticati na nutritivni pristup ovoj grupi u budućnosti sa ciljem prevencije komplikacija gojaznosti.
Introduction
Obesity in children has been a growing problem around the world during the last decades. It affects children of all ages, even infants and babies (1). The prevalence of obesity in children in the United States is steadily increasing. Approximately 20–25% of children are either overweight or obese, and the prevalence is even greater than in some minority groups, including Pima Indians, Mexican Americans, and African Americans (2, 3). Most of the countries in Europe also report high obesity rates in children (4–6). The American Association of Paediatrics recommends screening for early obesity in the age group of 2–6 years (1). Family habits and lifestyle have an influence on weight and family interventions are aimed to treat childhood obesity (7). Complications caused by early obesity that appear later in life, including diabetes mellitus (DM) type 2, hypertension, dyslipidemia and cardiovascular problems, are common and severe in obese children, as well as in a significant number of immediate family members (8–12). Although many studies in adults have pointed to the major metabolic changes characterizing obesity and defining the metabolic syndrome, studies in children, especially at a younger age, are still few and limited by the small number of patients. Therefore, even the definition of metabolic syndrome in children is still controversial (13–15). Hyperinsulinemia and insulin resistance have been confirmed as the central events driving the metabolic impairment in obese children (16–19). In addition, several hormones which regulate energy metabolism are secreted by the adipose tissue, among which adiponectin and leptin are the main adipokines regulating insulin sensitivity (19–22). Leptin has been found to be increased in obese children; however, data on the correlation of leptin levels with the age of children and their BMI has been controversial (23). Low adiponectin levels have been reported in obese children, but mostly in the adolescent age. Data in young children are very few and inconsistent (24, 25). On the other hand, the concept of metabolic programming has been recognized for several years and focused on the long-term detrimental effects of fetal undernutrition and low birth weight progressing towards obesity later in life. Production of adipokines, predominantly leptin and adiponectin as hormones produced by the adipose tissue, takes place predominantly in the second trimester of pregnancy, playing a role in the lipid and glucose metabolism. Since leptin is secreted by adipocytes in the late stages of differentiation, while adiponectin is secreted only by fully differentiated adipose cells, they can be used as a marker for adipose tissue development and the amount of adipose tissue. Prenatal growth and gestational age play a crucial role in adipose tissue maturation and accumulation, modifying leptin and adiponectin secretion and metabolic functions. The inadequately developed fat tissue in premature infants and infants with intrauterine growth restriction leads to impaired glucose metabolism and insulin resistance. The fact that children with low birth weight are prone to complicated obesity later in life drives the hypothesis of prenatal metabolic programming in these children and provides the opportunity for novel nutritional interventional methods in this group (26–28). Several studies in obese children of different age have been conducted in the Republic of Macedonia. Among others, the risk factors in families of obese children, peak insulin levels on OGTT, the lipid profile, and leptin and adiponectin levels in children of various ages as well as in newborns with different birth weights (BW) have been studied. The results of these studies give some new insight in the metabolic setup and major risk factors in obese children.
Material and Methods
All analyses were conducted according to the Declaration of Helsinki, following the informed consent of the parents and assent of children during the initial diagnostic work-up of obesity.
Ethical approval for the analysis of leptin and adiponectin, as well as the study in newborns was obtained from the Ethical Committee for Clinical Studies at the Medical Faculty in Skopje, Republic of Macedonia.
Patients
A total of 269 obese and overweight children at the age of 3–16 years were included. The pubertal group consisted of 159 pubertal children (89 boys) with the average age of 12.6±1.9 years (range 9–16 years) and 110 prepubertal children (54 boys) with the average age of 6.5±2.2 years (range 3–9 years). Sixty age matched nonobese children were used as a control.
Analysis of newborns included 110 newborns (56 males); 74 term and 36 preterm: 58 small for gestational age (SGA) and 52 appropriate for gestational age (AGA).
Methods
Self-reported family risk factors were recorded using questionnaires. BMI was calculated according to the standard formula. OGTT was performed according to the recommendations of the ADA and peak glycemia >7.8 mmol/L of at least one glucose value during the test was defined as impaired glucose tolerance (IGT). Insulin levels were measured with the immunoturbidimetry method. The HOMA index was calculated using the standard formula. Leptin and adiponectin levels were measured with ELISA (Human DRG Instruments GmbH, Germany). In 32 children with impaired glucose sensitivity a continuous glucose measurement system (CGMS) was used for three days according to the manufacturer’s recommendations (Medtronic MiniMed.Inc). Blood samples in newborns were taken three days after birth, once metabolic stability had been achieved upon the initial stress caused by the delivery.
STATISTICA and ANOVA/MANOVA were used for the comparison of biochemical parameters between groups and multiple regression analysis.
Results
Insulinemia and glucose intolerance
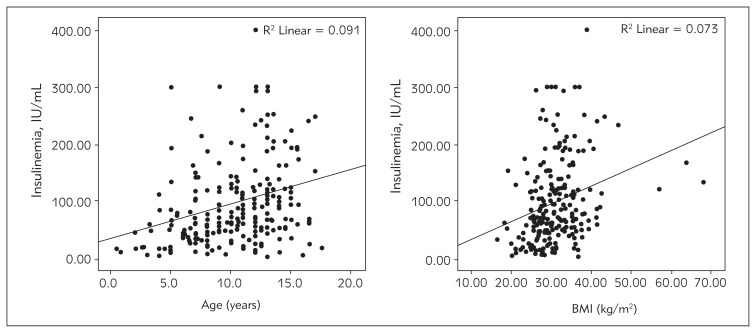
One or more relatives with diabetes, obesity, hyperlipidemia and/or hypertension were detected in 53.6% of the patients. The analysis of insulin, leptin and adiponectin levels in the obese children compared to the age-matched controls is presented in Table I. The BMI was higher in the pubertal group compared to the prepubertal children (32.4±4.9 kg/m2 versus 27.7±4.6 kg/m2) and significantly higher in pubertal girls (p<0.05). In the younger group no gender difference was found. The peak insulinemia was significantly higher in pubertal children and correlated with the BMI (Table II, Figure 1). The HOMA index was also higher in the older group. Impaired glucose tolerance (IGT) was found in 65 children (24%), 45 in the pubertal group (28%), and 20 (18%) in the prepubertal group (p<0.05). Continuous glucose measurement was performed in 32 children with IGT. Unexpected hyper- and hypoglycemia were detected on CGMS in 4 children. Overt diabetes mellitus type 2 developed in one 12-year-old girl within 1 year of the diagnosis of glucose intolerance.
Table I.
Insulin, leptin and adiponectin levels in obese children and healthy controls.
| Obese children (N=269) | Controls (N=60) | p | |
|---|---|---|---|
| Insulinemia (IU/mL) | 88.25±80.34 (3.9–327) | 17.5±3.5 (1.9–25.7) | <0.001 |
| Leptin (ng/mL) | 36.1±13.7 (14.2–72) | 5.7±1.5 (3.4–10.4) | <0.001 |
| Adiponectin (ng/mL) | 10.5±4.8 (2.2–23.7) | 15.2±6.9 (4.2–37.4) | <0.05 |
Table II.
BMI, insulin, leptin and adiponectin levels in prepubertal and pubertal children.
| <9 years (N=110) | >9 years (N=159) | p | |
|---|---|---|---|
| Age | 6.5±2.2 | 12.6±1.9 | <0.001 |
| BMI (kg/m2) | 27.7±4.6 | 32.2±5.7 | <0.05 |
| Insulinemia (IU/mL) | 72.2±62.7 | 110.5±75.8 | <0.005 |
| Leptin (ng/mL) | 32.2±23.1 | 41.4±23.2 | <0.05 |
| Adiponectin (ng/mL) | 18.6±13.5 | 12.8±11.1 | <0.001 |
| HOMA | 2.7±0.9 | 4.1±1.9 | <0.05 |
Figure 1.
Correlation between insulinemia and age, and insulinemia and BMI.
Leptin/adiponectin
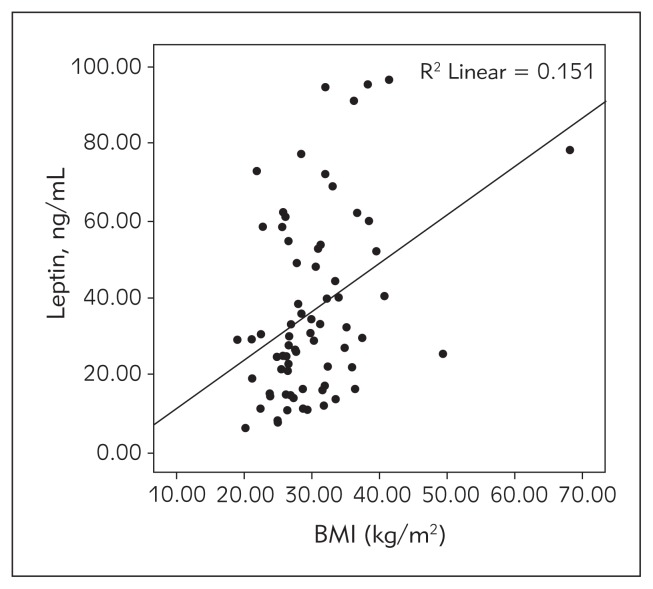
The average level of leptin was higher, while the adiponectin level was significantly lower in the pubertal group (p<0.05 and <0.001 respectively). Leptin levels correlated well with the insulinemia and BMI (Figure 2). It was also in positive correlation with insulinemia.
Figure 2.
Correlation of leptin with BMI.
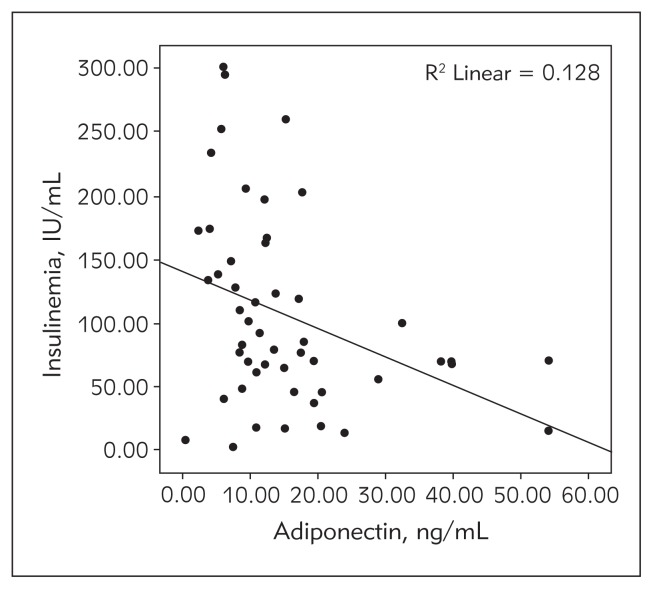
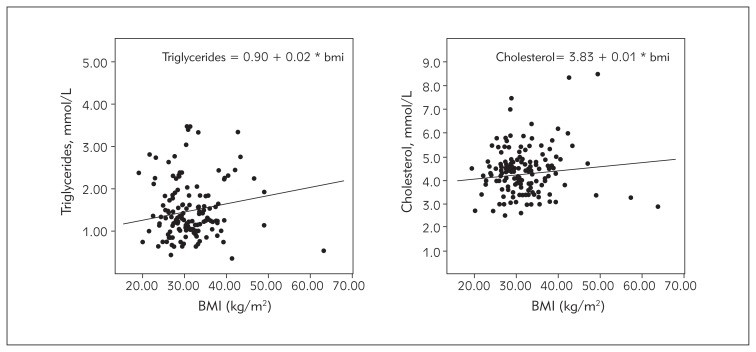
BMI and leptin levels were higher in girls compared to boys (p<0.05). Adiponectin level was in negative correlation with the BMI (Figure 3). It was also in negative correlation with the level of insulin in both boys and girls, especially in the pubertal group. This correlation was significantly weaker in the young obese children, both boys and girls. Adiponectin level was inversely associated with the atherogenic lipid levels in adolescents, even after adjusting for obesity and IR (Figure 4).
Figure 3.
Correlation of adiponectin with insulinemia.
Figure 4.
Influence of the BMI on the lipid profile; triglycerides – left, cholesterol – right.
Metabolic programming
In our study, we assessed the correlation between anthropometric parameters and insulin, leptin and adiponectin levels in 110 healthy preterm and term newborns (Table III). The insulinemia tended to be significantly higher in SGA children. Leptin values were lower in the preterm group due to the low fat tissue, while adiponectin, an unfavorable predictor of later obesity and complications, was lowest in the SGA preterm newborns. However, multiple regression analysis confirmed significantly higher correlation of the leptin and adiponectin levels with BW compared to the gestational age.
Table III.
Leptin, adiponectin, and insulin levels in term and preterm newborns.
| Term | Preterm | |||
|---|---|---|---|---|
| AGA | SGA | AGA | SGA | |
| Leptin (ng/mL) | 1.9±0.7a,b | 1.7±0.5c | 1.3±0.4a | 1.1±0.3b,c |
| Adiponectin (ng/mL) | 32.8±25.4a,b,c | 12.7±2.4c | 10.5±5.5a | 7.4±2.1b |
| Insulin (IU/mL) | 3.78±2.79a,b | 5.74±2.99a,b,c | 3.67±3.09c | 4.43±2.12b,c |
Discussion
Our studies confirm clustering of obese patients in families with obesity associated with complications. Obese children who had family members with complications of obesity had higher BMIs and were more prone to high leptin and low adiponectin levels, showing an increased risk for cardiovascular complications later in life. These findings are similar to those reported in other studies in the literature providing evidence that obese children coming from families with risk factors should be closely monitored (9, 29). A significant metabolic difference between the prepubertal and pubertal groups was found in the peak insulinemia and HOMA index suggesting that the risk for glucose intolerance rises during puberty. A major complication of childhood obesity is diabetes mellitus type 2. In the USA, the percentage of children with type 2 diabetes as a consequence of obesity reaches up to 30%, depending on the rate of obesity in different states (30). In Europe, DM2 is rare, however, several cases are reported each year. Glucose intolerance in our study was similar to the one reported by D’Adamo et al. (31). However, taking into consideration that our population consists mostly of white Caucasians, it seems to be higher. Continuous glucose monitoring has rarely been used for the follow-up of glucose tolerance in obese children. In our study it helped detect early progression of impaired glucose tolerance to type 2 diabetes in one girl.
The correlation of insulinemia to BMI is well known, however, in our study this correlation was stronger in female patients who had higher BMIs. Taking into consideration that hyperinsulinemia is associated with hyperandrogenism and PCOS in obese girls, these girls should be under special scrutiny when gaining weight (32). Many studies in the literature confirm that the leptin level is a good marker for insulin resistance and for the metabolic syndrome (18, 33). Our study confirms this fact even for the youngest group of patients (3–9 years).
Obese adolescents have lower adiponectin levels and a more atherogenic lipid profile, associated with increased IR, compared to adolescents with normal BMI. Our finding of lower adiponectin serum levels in obese children, and the correlation between adiponectin and age, BMI, and peak insulinemia are in concordance with several studies in the literature. However, we did not find lower values of adiponectin in obese boys compared to obese girls (24, 25). It seems worthwhile to further investigate the option of applying a simple measurement of serum adiponectin as a screening tool for cardiovascular risks before applying more time-consuming techniques in young obese individuals.
While there is no consensus regarding the diagnosis of metabolic syndrome in children and adolescents, it is evident that each component of the syndrome must be identified as early as possible in order to prevent definitive lesions and chronic disease during childhood and later in life. Furthermore, it has been recognized that childhood obesity predicts metabolic syndrome in adulthood (8, 9, 12, 32–34). Leptin and adiponectin levels can be included as useful indicators for the definition of metabolic syndrome in obese children. Early detection of these metabolic risk factors might direct the therapeutic approach, including diet, physical activity and family therapy.
Our study in newborns showed that leptin and adiponectin levels were positively correlated with all anthropometric parameters: body weight, body length, BW/BL, BMI, and the pondered index (p<0.01). These results indicate that the stage of body growth maturity is positively correlated to adipocytokines involved in fetal growth regulation, as previously reported (27, 36, 37). SGA newborns have the riskiest metabolic profile among all other newborn groups. Thus, increasing the nutritional intake in this group of newborns, which used to be a common practice, might need reassessment as this may enhance the development of obesity associated with complications later in childhood.
Conclusions
The conclusions are as follows:
Family risk factors cluster in families of obese children.
Glucose intolerance is a frequent finding in obese children and should be monitored closely in order to prevent overt DM2.
Obese children show significant early metabolic parameters of obesity such as hyperinsulinemia, high leptin levels and low adiponectin levels.
Significant fetal programming is involved in childhood obesity.
SGA newborns are an especially vulnerable group prone to develop metabolic syndrome later in life.
Footnotes
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Barclay L. Obesity Risk Increases in US Children From Ages 9 Months to 2 Years. Am J Health Promot. 2011;25:190–8. [Google Scholar]
- 2.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 4.Yaemsiri S, Slining MM, Agarwal SK. Perceived weight status, overweight diagnosis, and weight control among US adults: the NHANES 2003–2008 Study. Int J Obes (Lond) 2011;35(8):1063–70. doi: 10.1038/ijo.2010.229. [DOI] [PubMed] [Google Scholar]
- 5.Skinner AC, Skelton JA. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999–2012. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics2014.21. Available at: [DOI] [PubMed] [Google Scholar]
- 6.Dietz HW, Robinon NT. Overweight children and adolescents. The New England Journal of Medicine. 2005;352:2100–9. doi: 10.1056/NEJMcp043052. [DOI] [PubMed] [Google Scholar]
- 7.Wildes JE, Marcus MD, Kalarchian MA, et al. Self-reported binge eating in severe pediatric obesity: impact on weight change in a randomized controlled trial of family-based treatment. Int J Obes (Lond) 2010;34(7):1143–8. doi: 10.1038/ijo.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanVliet M, Heymans MW, von Rosenstiel IA, Brandjes DPM, Beijnen JH, Diamant M. Cardiometabolic Risk Variables in Overweight and Obese Children: A Worldwide Comparison. Cardiovasc Diabetol. 2011;10(106) doi: 10.1186/1475-2840-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiore H, Travis S, Whalen A, Auinger P, Ryan S. Potentially protective factors associated with healthful body mass index in adolescents with obese and nonobese parents: a secondary data analysis of the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc. 2006;106(1):55–64. doi: 10.1016/j.jada.2005.09.046. quiz 76–9. [DOI] [PubMed] [Google Scholar]
- 10.Crowley D, Khoury P, Urbina E, Ippisch H, Kimball T. Cardiovascular Impact of the Pediatric Obesity Epidemic: Higher Left Ventricular Mass is Related to Higher Body Mass Index. J Pediatr. 2011;158(5):709–714.e1. doi: 10.1016/j.jpeds.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Maffeis C, Pinelli L, Brambilla P, Banzato C, Valzolgher L, Ulmi D, et al. Fasting plasma glucose (FPG) and the risk of impaired glucose tolerance in obese children and adolescents. Obesity (Silver Spring) 2010;18(7):1437–42. doi: 10.1038/oby.2009.355. [DOI] [PubMed] [Google Scholar]
- 12.Baker LJ, Olsen WL, Sorensen IAT. Childhood body mass index and the risk of coronary heart disease in adulthood. The New England Journal of Medicine. 2007;357:2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korita I, Bulo A, Langlois, Blaton V. Inflammation markers in patients with cardiovascular disease and metabolic syndrome. J Med Biochem. 2013;32:214–9. [Google Scholar]
- 14.Jessup A, Harrell JS. The metabolic syndrome: look for it in children and adolescents, too! Clin Diabetes. 2005;23:26–32. [Google Scholar]
- 15.Lee S, Bacha F, Gungor N, Arslanian S. Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr. 2008;152:177–84. doi: 10.1016/j.jpeds.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Steinberger J, Steffen I, Jacobs RD, Moran A, Hong CP, Sinaiko RA. Relation of leptin to insulin resistance syndrome in children. Obesity Research. 2003;11:1124–30. doi: 10.1038/oby.2003.153. [DOI] [PubMed] [Google Scholar]
- 17.Washington RL. Metabolic syndrome – no longer an adult only disease. Pediatr. 2008;152:A1. [Google Scholar]
- 18.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4:53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- 19.Makni E, Moalla W, Lac G, Aouichaoui C, Cannon D, Elloumi M, Tabka Z. The Homeostasis Model Assessment-adiponectin (HOMA-AD) is the most sensitive predictor of insulin resistance in obese children. doi: 10.1016/j.ando.2011.12.002. Available at: http://dx.doi.org/10.1016/j.ando.2011.12.002. [DOI] [PubMed]
- 20.Hamidi A, Fakhrzadeh H, Moayyeri A, Heshmat R, Ebrahimpour P, Larijani B. Metabolic syndrome and leptin concentrations in obese children. Indian J Pediatr. 2006;73(7):593–6. doi: 10.1007/BF02759924. [DOI] [PubMed] [Google Scholar]
- 21.Diez JJ, Iglesias P. The role of novel adipocyte-derived hormone adiponectin in human disease. European Jour of Endocrinology. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 22.Falorni A, Bini V, Molinari D, Papi F, Celi F, Di Stefano G, Berioli MG, Bacosi ML, Contessa G. Leptin serum levels in normal weight and obese children and adolescents: relationship with age, sex, pubertal development, body mass index and insulin. Inter Jour of Obesity. 1997;21:881–90. doi: 10.1038/sj.ijo.0800485. [DOI] [PubMed] [Google Scholar]
- 23.Hakanen M, Rönnemaa T, Talvia S, Rask-Nissilä L, Koulu M, Viikari J, Bergendhl M, Simell O. Serum leptin concentration poorly reflects growth and energy and nutrient intake in young children. Pediatrics. 2004;113:1273–8. doi: 10.1542/peds.113.5.1273. [DOI] [PubMed] [Google Scholar]
- 24.Panagopoulou P, Galli-Tsinopoulou A, Fleva A, Pavlitou-Tsiontsi E, Vavatsi-Christaki N, Nousia-Arvanitakis S. Adiponectin and insulin resistance in childhood obesity. J Pediatr Gastroenterol Nutr. 2008;47(3):356–62. doi: 10.1097/MPG.0b013e31817fcb67. Available at: [DOI] [PubMed] [Google Scholar]
- 25.Magge SN, Stettler N, Koren D, Levitt Katz LE, Gallagher PR, Mohler ER, III, Rader DJ. Adiponectin Is Associated with Favorable Lipoprotein Profile, Independent of BMI and Insulin Resistance, in Adolescents. Clin Endocrinol Metab. 96(5):1549–54. doi: 10.1210/jc.2010-2364J. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D Barker JP. The malnourished baby and infant. Br Med Bull. 2001;60(1):69–88. doi: 10.1093/bmb/60.1.69. Available at: [DOI] [PubMed] [Google Scholar]
- 27.Palcevska-Kocevska S, Aluloska N, Krstevska M, Shukarova-Angelovska E, Kojik L, Zisovska E, Kocevski D, Kocova M. Correlation of serum adiponectin and leptin concentrations with anthropometric parameters in newborns. SrpArhCelokLek. 2012;140(9–10):595–9. doi: 10.2298/sarh1210595p. [DOI] [PubMed] [Google Scholar]
- 28.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini S. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clinical Nutrition. 2009;90(5):1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124(4):1060–8. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Adamo E, Caprio S. Type 2 Diabetes in Youth: Epidemiology and pathophysiology. Diabetes Care. 2011;34(suppl):S161–165. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin S, Caprio S. Prevalence of Impaired Glucose Tolerance among Children and Adolescents with Marked Obesity. N Engl J Med. 2002;346:802–10. doi: 10.1056/NEJMoa012578. Available at: [DOI] [PubMed] [Google Scholar]
- 32.Sawathiparnich P, Weerakulwattana L, Santiprabhob J, Likitmaskul S. Obese adolescent girls with polycystic ovary syndrome (PCOS) have more severe insulin resistance measured by HOMA-IR score than obese girls without PCOS. J Med Assoc Thai. 2005;88( Suppl 8):S33–7. [PubMed] [Google Scholar]
- 33.Sun SS, Liang R, Huang TT, et al. Childhood obesity predicts adult metabolic syndrome: The Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rappaport EB. Identifying and evaluating the metabolic syndrome in children and adolescents. Ethn Dis. 2007;17(3 suppl 4):S4–1–6. [PubMed] [Google Scholar]
- 35.Evelin AMV, Vissern FLJ, van der Ent CK, Grobee AE, Uiterwaal CSPM. Excess Early Postnatal Weight Gain Leads to Thicker and Stiffer Arteries in Young Children. J Clin Endocrinol Metab. 2013;98(2):794–801. doi: 10.1210/jc.2012-3208. Available at: Epub 2013 Jan 2. [DOI] [PubMed] [Google Scholar]
- 36.Simental-Mendía l, Castañeda-Chacón A, Rodríguez-Morán M, Guerrero-Romero F. Birth-weight, insulin levels, and HOMA-IR in newborns at term. BMC Pediatrics. 2012;12:94. doi: 10.1186/1471-2431-12-94. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siahanidou T, Mandyla H, et al. Circulating levels of adiponectin in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:286–90. doi: 10.1136/adc.2006.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]