Summary
Newborn blood-spot screening to detect potentially treatable disorders is widely practiced across the globe. However, there are great variations in practice, both in terms of disorders covered, screening technologies, disease definition, information provision, parental informed consent, and storage and disposal of residual specimens, partly reflecting the degree to which screening is the subject of explicit legislation (and thus public and media pressure) or is embedded in a general health care system and managed at an executive level. It is generally accepted that disorders to be screened for should comply with the ten Wilson and Jungner criteria, but the way that compliance is assessed ranges from broadly-based opinion surveys to detailed analysis of quantitative data. Consequently, even countries with comparable levels of economic development and health care show large differences in the number of disorders screened for. There are several areas on which there are no generally accepted guidelines: how should parents be informed about screening and to what extent should they be encouraged to regard screening as an option to choose to refuse? Is DNA mutation analysis acceptable as part of a screening protocol? How soon should the blood samples be destroyed once screening has been completed? As technology advances and the potential scope of screening expands at both the metabolite and genome level, challenging policy issues will have to be faced.
Keywords: newborn screening, cystic fibrosis, medium chain acyl-CoA dehydrogenase deficiency, carrier detection, consent to testing
Kratak sadržaj
Testiranje uzoraka krvi iz pete kod novorođenčadi radi otkrivanja potencijalno izlečivih bolesti primenjuje se širom planete. Međutim, prakse testiranja se umnogome razlikuju, kako u pogledu poremećaja koji se mogu otkriti, tehnologija testiranja, definicije bolesti, pružanja informacija, informisanog pristanka roditelja, tako i što se tiče čuvanja i eliminisanja preostalih uzoraka, što donekle odražava u kojoj je meri ova oblast testiranja predmet zakonske regulative (a time i javnog i medijskog pritiska) ili je ugrađena u zdravstveni sistem i njom se upravlja na izvršnom nivou. U načelu je prihvaćeno da poremećaji koji se mogu otkriti testiranjem treba da ispunjavaju deset kriterijuma Wilsona i Jungnera, ali postoje razni načini pomoću kojih se to utvrđuje, od uopštenih anketa do detaljne analize kvantitativnih podataka. Usled toga, broj poremećaja obuhvaćenih testiranjem veoma se razlikuje čak i u zemljama sa uporedivim nivoima ekonomskog razvoja i zdravstvene nege. Postoji nekoliko oblasti za koje nema opšteprihvaćenih smernica: kako informisati roditelje o testiranju i koliko ih treba podsticati da testiranje posmatraju kao opciju koju, ako žele, mogu odbiti? Da li je analiza DNK mutacija prihvatljiva kao deo protokola za testiranje? Koliko brzo posle završetka testiranja treba uništiti uzorke krvi? Pošto se tehnologija stalno unapređuje a potencijalni obim testiranja se širi na nivou metabolita kao i genoma, biće potrebno suočiti se s problematičnim pitanjima vezanim za praksu testiranja.
Introduction
The scope of newborn screening has expanded greatly in the 52 years since Robert Guthrie published his microbial assay for phenylalanine and the use of dried blood spots in screening for phenylketonuria. Though Guthrie and others went on to develop blood-spot screening methods for further disorders none were taken up very widely until the development of immunoassays and the ability to screen for congenital hypothyroidism, now the most widely practiced screen worldwide. Immunoassay-based screens for other disorders followed, cystic fibrosis and congenital adrenal hyperplasia in particular, but none have achieved general acceptance. More recently, tandem-mass-spectrometry (MS-MS), with its ability to measure a wide range of metabolites simultaneously, has opened up a further range of disorders, many of which are very rare but can be incorporated with screening for phenylketonuria at little additional cost. These developments have accentuated the differences in approach in different countries. Thus, in large parts of the USA screening covers over 50 conditions (1) though this total is inflated counting sickle-associated haemoglobin variants as separate diseases. European countries with equally well-developed medical systems have very different screening policies: in 2009, the maximum number of conditions covered was 30 and some countries were screening for four or less (2). There was little consensus regarding information provision, informed consent, storage and disposal of residual specimens, screening technologies and disease definition. The reasons for these differences are not always obvious though clearly funding issues and the availability of an adequate clinical infrastructure must play a part.
Which Diseases?
All formal policy reviews have acknowledged the ten »principles« enunciated by Wilson and Jungner in 1968 (3). These are couched largely in qualitative terms: the condition sought should be »an important health problem«, »there should be a suitable test which should be acceptable to the population« and there should be an accepted treatment. Such criteria have no clear end-points and ultimately require qualitative judgments. Nevertheless, the UK National Screening Committee has until very recently emphasized the importance of having firm numerical data and evidence from a high-quality randomized controlled trial before accepting a new condition (4). However, it is recognized that for clinically heterogeneous and relatively rare disorders, such as those now readily accessible through MS-MS screening, a degree of compromise is required. Medium-chain acyl-CoA dehydrogenase deficiency was accepted on the basis of a large-scale pilot study (5) and preliminary studies on five additional disorders detectable by MS-MS are underway.
The approach in the USA has been quite different, with Federal policy based largely on the results of a survey carried out by the American College of Medical Genetics (6). This canvassed opinions (rather than data) from a wide range of professional and lay personnel in the USA and abroad and commissioned literature reviews on specific diseases from practicing academic clinicians. This resulted in the identification of 29 conditions for which screening should be mandated and an additional 25 conditions that would be identified as part of differential diagnosis. The work was subsequently taken forward and amplified by the Secretary’s Advisory Committee on Hereditary Disorders in Infants and Children (7), forming the basis for both Federal and State legislation. Screening policy is ultimately decided at State level and based on explicit legislation, with significant regional variations in both the number of disorders covered (1) and practices such as how long the blood collecting cards are retained after screening has finished (8). This overt political dimension leaves policy-makers exposed to pressure from groups such as the Save Babies Through Screening Foundation and there is almost a degree of competition as to which State screens for the most disorders. In countries like the UK where screening is offered as part of the National Health Service with no direct legislative basis policy tends to attract less public attention though nevertheless the UK screening programmes for cystic fibrosis and sickle cell diseases were introduced following direct pressure at the political level rather than through the formal advisory channels.
The Wilson and Jungner analysis was based mainly on adult disease and largely neglected the family dimension of newborn screening: the value of genetic information and the way that prompt diagnosis, even of an incurable condition, can ease the family journey. Family impact is the main rationale behind the Welsh screening programme for Duchenne muscular dystrophy which has been well-accepted locally but requires considerable professional input and is not supported in the rest of the UK. Similar considerations apply to other screening programmes. Cystic fibrosis is an obvious example as in the absence of screening diagnosis is often considerably delayed, leading to significantly worse outcomes. The family undergoes a »diagnostic odyssey« and has no warning of the possibility of future children also being affected. Attitudes of at-risk couples towards antenatal diagnosis of cystic fibrosis are ambivalent (9) but overall the introduction of newborn screening has resulted in a significant decrease in the number of babies born with the condition (10). Similar considerations must apply to many other disorders. Even before antenatal diagnosis became possible families with a phenylketonuric child diagnosed by screening tended to limit family size subsequently (11). However, in general policy-makers focus their attention on the value of newborn screening to the baby concerned and have mixed views about wider genetic implications.
Genetic Exceptionalism and Screening Methodology
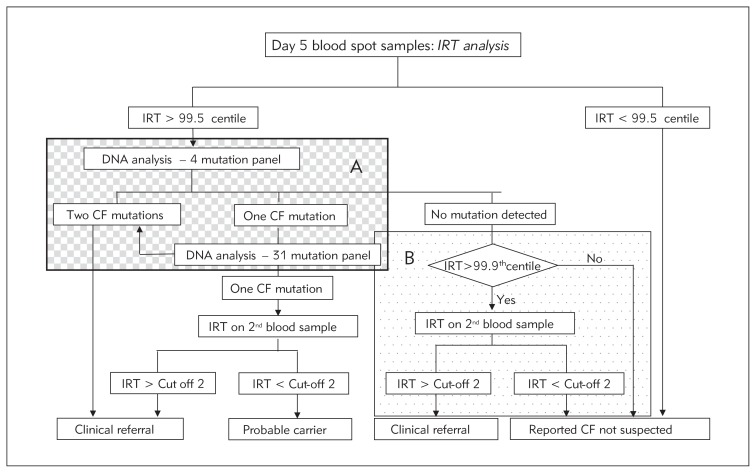
Genetic exceptionalism is the view, strongly held by some civil liberties groups, that genetic information is special and must be treated differently from other types of medical information. DNA analysis may reveal carrier status in individuals with no family history of the disorder concerned and in some countries is prohibited without a specific request from a registered medical practitioner. Such sensitivities are reflected in some of the screening protocols, particularly those for cystic fibrosis. Several screening programmes have retained the original two-step IRT approach and require a second blood sample despite the convenience and greater specificity of analysing the CFTR gene on the initial sample. Programmes that do use DNA analysis vary greatly in the mutation panel used: some restricting it to a few relatively common mutations associated with severe disease, others using a much broader panel with a corresponding increase in the number of unaffected carriers found. Other programmes include a PAP (pancreatitis associated protein) step in an attempt to maintain specificity but minimise or avoid carrier detection (12, 13). The UK protocol attempts to minimise carrier detection but by different means (Figure 1, panel A) while also recognising that a mutation panel optimised for northern European populations has inherent disadvantages for those of other geographical origin (panel B). Complicated protocols of this type place a considerable administrative burden on the laboratory. Travelling in the opposite direction, studies of the potential of exome sequencing as a first-line method of newborn screening and the possibility that genomic data be made available as a resource for parents and doctors throughout infancy and childhood to inform health care have recently been announced (14).
Figure 1.
The UK screening protocol for cystic fibrosis.
There is usually less anxiety about genetic information obtained at the protein level even though, with haemoglobin variants for example, this may inform directly on the underlying DNA sequence. Indeed, when a baby has received a blood transfusion prior to the screening sample being taken some programmes use DNA analysis rather than waiting several weeks for the infused erythrocytes to be removed. Newborn screening for sickle cell diseases and related haemoglobin abnormalities detects a significant number of carriers: 1 baby in 71 born in England in 2011–12. There are different views on whether this is desirable despite the presence of an antenatal screening programme and, as far as possible, linkage between the two programmes. MS-MS methods capable of detecting individual haemoglobin variants without the corresponding carriers have been developed (15) and it remains to be seen whether this will affect practice.
Disease Definition: What Constitutes a Positive Case?
With improvements in analytical performance the definition of »normal« has tended to be drawn ever more tightly. However, the primary purpose of screening is prevention or amelioration of disease rather than the detection of a biochemical/genetic abnormality as such. Thus, not all children with a persistently increased blood phenylalanine concentration will become mentally impaired: there is fairly good evidence for a threshold of about 600 micromol/L below which dietary treatment is unnecessary (16). This threshold has been adopted in France and Germany but is ignored in many other countries. Similarly with congenital hypothyroidism, improvements in assay sensitivity have resulted in many screening centres adopting lower cut-offs and consequently finding an increased number of cases. However, their gender distribution is markedly different from that in cases diagnosed clinically prior to the start of screening and there is no firm evidence that most of these additional cases benefit from their treatment. The biological level (gene, protein, enzymic activity, metabolic pathway, individual metabolite) at which screening and confirmatory investigations are performed also has an important impact on disease definition. Thus, medium-chain acyl-CoA dehydrogenase deficiency (MCADD) is readily detectable by increased blood-spot octanoylcarnitine on MS-MS screening. In the Netherlands enzyme assay using phenylpropionyl-CoA is regarded as the »gold-standard« confirmatory test. The current UK screening protocol defines MCADD in terms of having two mutations in the MCAD (ACADM) gene and genotyping is central to the clinical follow-up protocol. However, data from the pilot study (5) show that all babies except one with genotypes that have previously been reported from clinically-presenting cases showed urinary hexanoylglycine >5 micromol/mol creatinine on immediate follow-up. This would appear to be a more effective indicator of the risk of metabolic decompensation (which is what we are really wishing to screen for) than mutation analysis and avoids the complication of inadvertent carrier detection.
Information and Consent
Newborn screening is an intrusion into the family at a particularly vulnerable time. Viewed in the context of everything else that can go wrong in pregnancy, childbirth and afterwards, newborn screening seems a fairly insignificant thing to worry about but the importance of having suitable information available if required is universally recognized. The way that this is provided and the amount of detail given vary considerably. The UK National Screening Committee requires that »evidence-based information, explaining the consequences of testing, investigation and treatment, should be made available to potential participants to assist them in making an informed choice«. The emphasis on choice is in keeping with current political philosophy but it could be argued that if parents were truly informed then choosing to have their baby screened would be automatic. Even carefully worded information can leave room for misunderstanding: »We read the form … and our understanding was that the conditions were hereditary. Neither of us had these conditions in our family. We didn’t want to cause baby the pain of having a needle in her heel. We didn’t need the test and wouldn’t have it done. Little did we know! The midwife came and she was lovely, very bubbly, and said ‘come on then let’s get on with it’. We both looked at each other and were afraid to say anything so the baby screamed and we felt bad for doing it. But we will always be very grateful to that midwife. She saved our daughter (with phenylketonuria) to grow up a lovely healthy bright little girl« (17). Should we really be presenting screening as an option which rational parents might like to consider?
Jurisdictions respond to this question in different ways. In some US states screening is compulsory, required by law, so that (in theory at least) every baby gets screened. Other jurisdictions are totally opposed to compulsion. In a majority judgment the Supreme Court of Ireland upheld the right of parents who already had a child with phenylketonuria not to have their newborn baby screened (18). The United Nations Convention on the Rights of the Child states that the best interests of the child must be a top priority in all actions. However, whether screening is truly in the interests of the affected child is sometimes debatable, particularly for disorders where treatment is invasive and the effect is to slow the progression of a debilitating disease rather than prevent it. Most disorders show some degree of heterogeneity and this may make a clear answer impossible: for example, severe propionic acidaemia is almost untreatable, with an inexorable deterioration, but patients with milder variants can survive and go on to lead virtually normal lives if diagnosed and treated early.
The current diversity of national policies relating to newborn screening reflects both practical limitations in health care organization and funding and widely different philosophical approaches to a variety of highly emotive issues. The task of accommodating these different viewpoints within a common policy framework is daunting. Decisions will become more difficult still as screening technology advances, with the use of orbitrap mass spectrometry for example, and the number and variety of disorders readily accessible increases still further.
Footnotes
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Save Babies Through Screening Foundation. www.savebabies.org. accessed 10th March 2014.
- 2.Loeber JG, Burgard P, Cornel MC, Rigter T, Weinreich SS, Rupp K, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1 – From bloodspot to screening result. J Inherit Metab Dis. 2012;35:603–11. doi: 10.1007/s10545-012-9483-0. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JMG, Junger G. Principles and practice of screening for disease. Vol. 34. Geneva: World Health Organisation; Public Health Papers; 1968. [Google Scholar]
- 4.UK National Screening Committee. Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. http://www.screening.nhs.uk/criteria. accessed 14th March 2014.
- 5.Oerton J, Khalid JM, Besley G, Dalton RN, Downing M, Green A, et al. Newborn screening for medium chain acyl-CoA dehydrogenase deficiency in England: prevalence, predictive value and test validity based on 1.5 million screened babies. J Med Screen. 2011;18:173–81. doi: 10.1258/jms.2011.011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Medical Genetics. Newborn screening: towards a uniform screening panel and system. Genetics in Medicine. 2006;8:5. doi: 10.1097/01.gim.0000223891.82390.ad. Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell RR, Lloyd-Puryer MA. Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Semin Perinatol. 2010;34:121–4. doi: 10.1053/j.semperi.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Therrell BL, Hannon WH, Bailey DB, Jr, Goldman EB, Norgaard-Pedersen B, et al. Consideration and recommendations for national guidance regarding the retention and use of residual blood spot specimens after newborn screening. Genet Med. 2011;13:621–4. doi: 10.1097/GIM.0b013e3182147639. [DOI] [PubMed] [Google Scholar]
- 9.Polnay JC, Davidge A, Chin Lyn U, Smyth AR. Parental attitudes: antenatal diagnosis of cystic fibrosis. Arch Dis Child. 2002;87:84–6. doi: 10.1136/adc.87.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scotet V, Audrézet MP, Rousser M, Rault G, Blayau M, De Braekeleer M, Férec C. Impact of public health strategies on the birth prevalence of cystic fibrosis in Brittany, France. Hum Genet. 2003;113:280–5. doi: 10.1007/s00439-003-0962-0. [DOI] [PubMed] [Google Scholar]
- 11.Brookfield JF, Pollitt RJ, Young ID. Family size limitation: a method for demonstrating recessive inheritance. J Med Genet. 1988;25:181–5. doi: 10.1136/jmg.25.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martina C, Cornel MC, Gille JJP, Loeber JG, Vernooijvan Langen AMM, Jeannette Dankert-Roelse J, Bolhuis PA. Improving test properties for neonatal cystic fibrosis screening in the Netherlands before the nationwide start by May 1st 2011. J Inherit Metab Dis. 2012;35:635–40. doi: 10.1007/s10545-012-9452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommerburg O, Lindner M, Muckenthaler M, Kohlmueller D, Leible S, Feneberg R, et al. Initial evaluation of a biochemical cystic fibrosis newborn screening by sequential analysis of immunoreactive trypsinogen and pancreatitis-associated protein (IRT/PAP) as a strategy that does not involve DNA testing in a Northern European population. J Inherit Metab Dis. 2010;(Suppl 2):S263–71. doi: 10.1007/s10545-010-9174-7. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health. News release: NIH program explores the use of genomic sequencing in newborn healthcare. http://www.nichd.nih.gov/news/releases/Pages/090413-newborn-sequencing.aspx. accessed 14th March 2014.
- 15.Turner C, Daniel Y, Dalton RN. Newborn screening for sickle cell disease though use of tandem mass spectrometry. Clinical Chemistry. 2009;55:1243–4. doi: 10.1373/clinchem.2008.120964. [DOI] [PubMed] [Google Scholar]
- 16.Pollitt RJ. Commentary: What degree of hyperphenyl-alaninaemia requires treatment? J Inherit Metab Dis. 2012;35:927–30. doi: 10.1007/s10545-012-9505-y. [DOI] [PubMed] [Google Scholar]
- 17.Newborn Screening Centre. Screening stories – a PKU story (shortened) http://newbornbloodspot.screening.nhs.uk/cms.php?folder=2701. accessed 20th March 2014.
- 18.Supreme Court of Ireland. North Western Health Board-v-H.W. & C.W.. 11.08.2001. www.supremecourt.ie/Judgments.nsf. accessesd 20th March 2014.