Summary
Background
Prediabetes is characterized by isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), and combined IFG/IGT. This study aimed to establish the prevalence of prediabetes and examine possible contributory factors in a cohort of obese adolescents.
Methods
In this prospective study, we recruited 85 obese patients from the Obesity Clinic at the University Children’s Hospital and 17 normal weight controls. All patients were of Caucasian origin, 60 males/42 females, aged 7.4–18.3 years, with at least Tanner 2 stage of puberty.
Results
Depending on criteria we used, insulin resistance was confirmed in 62–100% of obese patients, predominantly in the group with BMI SDS > 3. oGTT revealed isolated impaired fasting glucose (IFG) in 13.9%, impaired glucose tolerance (IGT) in 20.8% and combined IFG and IGT only in 2.8% of the obese patients. Patients in the prediabetes group were older (14±2.4 vs 12.8±2.5 p=0.04) and had higher glucose levels (p<0.001) during the whole oGTT compared to normal glucose tolerance (NGT) group. There was no difference between groups in respect to family history, BMI, lipids and fasting insulin. Insulinogenic index, WBISI and HOMA%B were significantly lower in the prediabetes group compared to the NGT group (p=0.07, 0.01 and 0.04 respectively). HbA1c level was measured in 58% of patients and was significantly higher in the prediabetes group (5.4±0.3 vs 5.7±0.4, p=0.002).
Conclusion
Prediabetes occurrence was fairly high in our obese adolescents. Further studies should establish what would be the most appropriate screening test to diagnose these patients at risk for type 2 diabetes and initiate treatment without delay.
Keywords: adolescents, children, impaired glucose tolerance, obesity, prediabetes
Kratak sadržaj
Uvod
Stanja kao što su izolovana povišena glikemija našte ili poremećaj tolerancije glukoze i kombinacija ova dva poremećaja glukoze mogu se definisati kao predijabetes. Cilj nam je bio ispitati prevalencu predijabetesa i odrediti potencijalne pridružene faktore u grupi gojaznih adolescenata.
Metode
Prospektivna studija, sprovedena u Univerzitetskoj dečjoj klinici, uključila je 85 gojaznih ispitanika i 17 normalno uhranjenih pacijenata. Svi pacijenti su bili bele rase, 60 muškog, 42 ženskog pola, uzrasta 7,4–18,3 godina, sa prisutnim znacima puberteta.
Rezultati
Zavisno od kriterijuma koje smo koristili, insulinska rezistencija je postojala kod 62–100% gojaznih pacijenata, posebno izražena u grupi pacijenata sa ITM SDS > 3. Nakon oGTT-a, dijagnoza izolovane povišene glikemije našte je postavljena kod 13,9% pacijenata, poremećaj tolerancije glukoze kod 20,8%, a udruženost ova dva poremećaja kod samo 2,8 gojaznih pacijenata. Pacijenti sa predijabetesom su bili stariji (14±2,4 vs 12,8±2,5 p=0,04) i imali više nivoe glukoze tokom celog testa (p<0,001). Nije nađena statistički značajna razlika u odnosu na porodičnu anamnezu za tip 2 dijabetesa, ITM, vrednosti lipida i insulina našte. Insulinogeni, ukupni indeks senzitivnosti, kao i HOMA%B bili su značajno niži u predijabetesnoj grupi u poređenju sa grupom koja je imala normalnu toleranciju glukoze (p=0,07, 0,01 i 0,04). HbA1c je određen kod 58% pacijenata i bio je značajno viši u predijabetesnoj grupi (5,4±0,3 vs 5,7±0,4, p=0,002).
Zaključak
Dijagnoza pre-dijabetesa je postavljena u visokom procentu kod naših gojaznih adolescenata. Neophodna su dalja ispitivanja koja bi pokazala koji je najbolji test za rano prepoznavanje i lečenje ovih pacijenata sa rizikom za tip 2 dijabetesa.
Introduction
Prediabetes is a state of altered glucose homeostasis associated with a high risk of progression to type 2 diabetes in adults and children (1, 2). This condition is characterized by isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), and combined IFG/IGT (2). The prevalence of prediabetes varies depending on the population studied. In the US it could be from 4.1–4.5% in children recruited from the community, up to 25% in an obesity clinic (3). In the last 20 years we have observed a dramatic increase in the percentage of adolescents with IGT, from 1.76% in 1998 to 23% in 2008 (4). Based on data from the 1999–2000 and 2001–2002 National Health and Nutrition Examination Surveys (NHANES), the most recent estimate for the prevalence of IFG among U.S. adolescents is 11% (5, 6). In contrast, more recent data from the pilot STOP-T2DM, a school based study, reported an unexpectedly high percentage (40.5%) of youth with IFG (7). Thus, a substantial number of youngsters in the United States have IFG. The prevalence of impaired glucose regulation in Serbia was reported to be 15.9% among patients in an obesity clinic (8). This increase reflects the obesity epidemic and is more common in those with family history of type 2 diabetes (9). However, the pathophysiology of prediabetes and its progression to type 2 diabetes in children are not well understood. Studies in pediatrics using different methodologies have shown conflicting results (1, 9–11). Obese children and adolescents with IGT were reported to have higher BMI and worse fasting indices of insulin resistance compared with those with NGT, but insulin secretion was estimated to be similar between the two groups. Also, it was recently suggested that HbA1c (5.7–6.4%) could be used as a diagnostic criterion for prediabetes in the adult population (2). In view of the fact that puberty increases IR, we wanted to screen for prediabetes in a group of pubertal children from our obesity clinic (12).
Methods
The study population consisted of 102 patients: a study group of 85 obese patients and 17 normal weight controls. All patients were of Caucasian origin, 60 M/42 F, aged 7.4–18.3 yrs (mean 13.4±2.6, median 13.4). Obese patients were recruited from the Pediatric Obesity Clinic at the University Children’s Hospital in Belgrade, a tertiary-care center. The study was conducted between 2010 and 2013. The main inclusion criteria were obesity (defined as BMI > 97th percentile) and puberty. Patients with chronic diseases, syndromic or secondary obesity, including previously diagnosed T2DM or hypothyroidism, were excluded from this study. The study was approved by the University Children’s Hospital Ethics Board and informed consent was obtained. A detailed medical and family history was captured for all subjects, including family history of type 2 diabetes or maternal history of gestational diabetes and presence of complications secondary to obesity (hypertension, fatty liver). Physical examination included measurements of height and weight, evaluation for the presence of acanthosis nigricans, and assessment of pubertal stage (on the basis of breast development in girls and testicular volume in boys), according to the criteria of Marshall and Tanner (13, 14). Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. Children with BMI values greater than the 97th percentile for age and gender were classified as obese (5). To compare BMI values across different ages and by gender, the BMI SDS was calculated with the Centers for Disease Control and Prevention 2000 reference (15).
After a 12-h overnight fast, blood samples were obtained for laboratory evaluation of fasting glucose and insulin, triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, liver enzymes, CRP and HbA1c. A standard (1.75 g/kg body weight (up to 75 g)) oral glucose tolerance test (oGTT) was performed in 72 patients (84.7%). Blood samples for measurements of plasma glucose and insulin were obtained at baseline (fasting) and every 30 min for 120 min after an oral glucose load. Normal glucose regulation was defined as fasting glucose < 5.6 mmol/L and 120 min glucose < 7.8 mmol/L. Impaired fasting glucose (IFG) was defined as a fasting glucose level of 5.6–6.9 mmol/l and IGT was defined as a 120-min glucose level of 7.8–11.0 mmol/L. The term prediabetes was used for all subjects with IFG and/or IGT (16). Pubertal development was assessed according to the Tanner stages and categorized into 2 groups: early puberty (Tanner stages II and III) and late puberty/postpubertal (Tanner stages IV and V). The gold standard methods for measuring insulin sensitivity and pancreatic β-cell function are the hyperinsulinemic–euglycemic clamp and hyperglycemic clamp, respectively (17). However, because these procedures are invasive and labor-intensive, we used simple surrogate measures that have been shown to correlate with the clamp procedures (18–21).
Insulin resistance was estimated by the homeostatic model assessment (HOMA-IR), insulin sensitivity by the ratio of fasting glucose to fasting insulin (FGIR), the quantitative insulin sensitivity check index (QUICKI) and whole body insulin sensitivity (WBISI) (21–23). A low QUICKI was reported to predict the development of diabetes in adults (21). Pancreatic β-cell function was anticipated by the HOMA-derived β-cell function (HOMA%B) and insulinogenic index (IGI), calculated as the ratio of the increase in the insulin level to the increase in the glucose level during the first 30 min of the oral glucose tolerance test (23, 21).
The calculations were as follows: HOMA-IR= (fasting insulin (μU/mL) fasting glucose (mmol/L)/22.5); insulin resistance was defined as HOMA-IR>2; FGIR in mmol/L × 22.5 for glucose and U/mL for insulin; QUICKI=1/[log (fasting insulin (U/mL))+log (fasting glucose × 22.5 (mmol/L))]; impaired insulin sensitivity was defined as QUIC-KI<0.339 (21). Whole body insulin sensitivity (WBISI) =10,000/[fasting insulin (lU/mL) · fasting glucose × 22.5 (mmol/L)] · (mean insulin (lU/mL) over 2 h · mean glucose × 22.5 (mmol/L) over 2 h), HOMA %B=20 fasting insulin (μU/mL)/(fasting glucose (mmol/L)-3.5); as reported by Matthews et al. (23).
Serum glucose was measured by the hexokinase method using an automated analyzer (Dimension RxLMax, Siemens, USA). Total cholesterol, triglycerides, LDL and HDL cholesterol concentrations were measured by an enzymatic colorimetric method on an automated analyzer (Dimension RxLMax, Siemens, USA). Serum insulin concentrations were measured by an immunometric assay with the CMIA method (Chemiluminescent Microparticle Immunoassay, Architect and 1000 Abbott Diagnostics). HbA1C levels were measured by the TINIA – turbidimetric inhibition immunoassay (Dimension RxLMax, Siemens, USA) (normal range: 4.8–6.0%).
Statistical analysis
Pearson’s test, Kruskal Wallis χ2 test or Fisher’s exact test were used for the analysis of differences in discrete variables, as appropriate, and analysis of variance (ANOVA) was used for continuous variables. Correlations between continuous variables were analyzed with Pearson’s correlation. Probability values of less than 0.05 were considered to be significant, and values are expressed as frequencies or means ± SD unless otherwise stated.
Results
Patient characteristics
Baseline data are presented in Table I. Based on the degree of obesity, obese patients were divided into Group 1 (moderate obesity, BMI SDS between 1.5 and 3) and Group 2 (severe obesity, BMI SDS > 3). Groups were comparable and did not differ by age, gender and pubertal stage. Patients in Group 2 became obese earlier in life than patients in Group 1 (5.5 vs 6.3), although it was not significant. Waist circumference (107.6±12.3 vs 97.8±11.2, p=0.008) and CRP (3.1±1.3 vs 6.3±5.0, p=0.001) were significantly higher in the more obese patients. Fasting glucose was not different between the groups, but more obese patients had higher fasting insulin (25.2±20.6 vs 17.2±7.4, p=0.004) and HOMA-IR values (5.4±4.4 vs 3.7±1.7, p=0.01).
Table I.
Baseline characteristics according to the degree of obesity.
| Obesity | Normal weight (<1.5 SDS) | Kruskal–Wallis χ2 test | ||
|---|---|---|---|---|
|
| ||||
| Moderate (1.5–3 BMI SDS) | Severe (> 3 SDS) | |||
|
| ||||
| N | 44 | 41 | 17 | |
|
| ||||
| Age (years) | ||||
| Mean (SD) | 13.5 (2.4) | 12.9 (2.8) | 4 (2.24) | 0.315 |
| Range | 7.4–18.3 | 8–17.9 | 11–17.8 | |
|
| ||||
| Gender | ||||
| Male | 24 (54.5%) | 26 (63.4 %) | 10 (58.8%) | Pearson χ2; p=0.080 |
| Female | 20 (45.5%) | 15 (36.6%) | 7 (41.2%) | |
|
| ||||
| Birth Weight (kg) | ||||
| Mean (SD) | 3.5 (0.6) | 3.3 (0.5) | 3.5 (0.5) | 0.2307 |
| Range | 1.6–4.9 | 2.2–4.7 | 2.4–4.0 | |
|
| ||||
| Age at obesity start (years) | ||||
| Mean (SD) | 6.3 (4.6) | 5.5 (4.6) | na | 0.5302 |
| Range | 1–13 | 1–16 | ||
|
| ||||
| FH of T2D | 61% | 51% | NA | Fisher Exact Test; 0.2622 |
|
| ||||
| Waist Circumference (cm) | ||||
| Mean (SD) | 97.8 (11.2) | 107.6 (12.3)* | NA | 0.008* |
| Range | 80–120 | 87–131 | ||
|
| ||||
| BMI (kg/m2) | ||||
| Mean (SD) | 29.3 (3.3) | 36.1 (5.2)* | 21.9 (2.9) * | <0.001* |
| Range | 22.5–34.78 | 27.4–50 | 17.6–28 | |
|
| ||||
| BMI SDS | ||||
| Mean (SD) | 2.3 (0.4) | 3.8 (0.6)* | 0.7 (0.7)* | <0.001* |
| Range | 1.5–2.88 | 3.0–5.6 | −1.3–1.4 | |
|
| ||||
| Puberty | ||||
| Early (T 2–3) | 22 | 22 | 8 | Pearson χ2; 0.8872 |
| Late (T 4–5) | 22 | 19 | 9 | |
|
| ||||
| Fasting glucose (mmol/L) | ||||
| Mean (SD) | 4.86 (0.7) | 4.79 (0.65) | 4.82 (0.55) | 0.6300 |
| Range | 2–6.2 | 3.5–6.7 | 3.7–5.9 | |
|
| ||||
| Fasting insulin (μU/mL) | ||||
| Mean (SD) | 17.2 (7.4) | 25.2 (20.6)* | 11.9 (6.7)* | 0.004* |
| Range | 5.3–39.7 | 9.5–140.4 | 5.5–29.2 | |
|
| ||||
| HOMA–IR | ||||
| Mean (SD) | 3.7 (1.7) | 5.4 (4.4)* | 1.7 (0.9)* | 0.01* |
| Range | 0.9–9.0 | 1.9–29.0 | 0.7–3.7 | |
|
| ||||
| CRP (mg/L) | ||||
| N (%) | 32 (72%) | 34 (83%) | 10 (59%) | 0.001* |
| Mean (SD) | 3.1 (1.3) | 6.3 (5.0)* | 3.3 (1.0) | |
| Range | 1.5–6.3 | 1.2–24.8 | 4–2 | |
NA – not available, na – not applicable, SDS – standard deviation score, FH – family history, T2D – type 2 diabetes, HOMA-IR – homeostatic model assessment, CRP – C reactive protein (NV < 3).
All patients were pubertal, according to the study design. We divided patients into two groups according to their Tanner stage: Early Puberty (Tanner 2–3) and Late Puberty (Tanner 4–5). Those two groups were not different with respect to the obesity indexes, fasting insulin and HOMA values, but patients in the late puberty stage had higher fasting glucose (5.1±0.6 vs 4.6±0.6, p=0.01), compared with those in early puberty.
HOMA-IR values were above 2.0 in 94% of the obese patients, being higher in the more obese patients. Insulin values from oGTT strengthened the insulin resistance: insulin at 120 min > 75 μU/mL in 93%, peak insulin > 150 μU/mL in 62% and sum of insulins > 300 μU/mL in 100% patients. In agreement with that, Quicki was below 0.339 in 86% of the patients, the cut-off reported in adult studies and proved in our control group (0.34±0.2).
Prediabetes
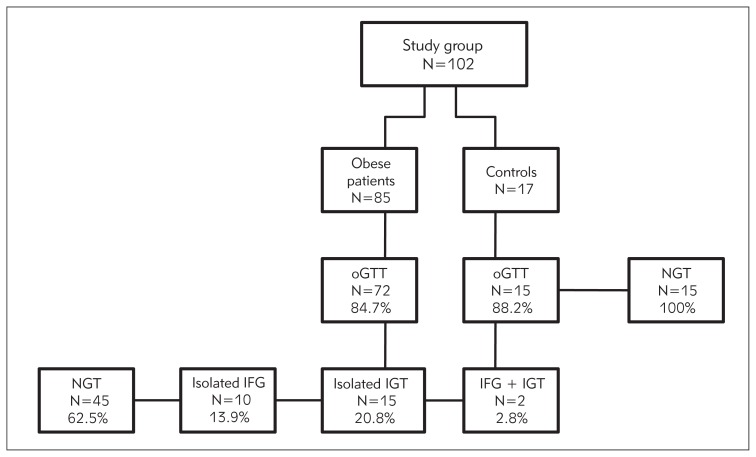
We have completed oGTT in 72 (84.7%) obese patients and 15 (88.2%) patients in the control group. In the obese group 7 patients did not agree to oGTT and in 6 patients (2 in the control group) some data were missing due to technical difficulties.
In the obese group 45 (62.5%) and in the control group 15 (100%) patients had normal glucose tolerance (NGT). Isolated impaired fasting glucose (IFG) was present in 10 (13.9%), impaired glucose tolerance (IGT) in 15 (20.8%) and combined IFG and IGT only in 2 (2.8%) obese patients. None of the patients had silent diabetes. Data are presented in Figure 1.
Figure 1.
Patient flowchart.
NGT – normal glucose tolerance, IFG – impaired fasting glucose, IGT – impaired glucose tolerance
Data for patients with IFG and/or IGT are summarized in the prediabetes group (Table II).
Table II.
Clinical and biochemical characteristics of obese adolescents with normal glucose tolerance (NGT) and prediabetes.
| Normal glucose tolerance mean (SD) | Prediabetes mean (SD) | P value | |
|---|---|---|---|
|
| |||
| N (%) | 45 (62.5%) | 27 (37.5%) | |
|
| |||
| Age (years) | 12.8 (2.5) | 14.0 (2.4) | 0.0435* |
|
| |||
| Gender | |||
| Male | 31 (68.9%) | 13 (48.1%) | 0.0805* |
| Female | 14 (31.1%) | 14 (51.9%) | |
|
| |||
| BMI (kg/m2) | 31.9 (4.8) | 33.6 (6.2) | 0.2919 |
|
| |||
| BMI SDS | 3.0 (1.1) | 2.9 (0.8) | 0.5337 |
|
| |||
| Puberty | |||
| Early (T 2–3) | 17 | 10 | 0.0382* |
| Late (T 4–5) | 28 | 17 | |
|
| |||
| Fasting glucose (mmol/L) | 4.4 (0.6) | 5.3 (2.3) | <0.0005* |
|
| |||
| Glucose 30 min (mmol/L) | 7.0 (1.3) | 8.7 (1.3) | <0.0005* |
|
| |||
| Glucose 60 min (mmol/L) | 6.1 (1.6) | 8.8 (1.9) | <0.0005 |
|
| |||
| Glucose 90 min (mmol/L) | 5.8 (1.2) | 8.0 (1.9) | <0.0005* |
|
| |||
| Glucose 120 min (mmol/L) | 5.4 (1.0) | 7.7 (1.8) | <0.0005* |
|
| |||
| Fasting insulin (μU/mL) | 21.5 (19.7) | 22.6 (10.0) | 0.2145 |
|
| |||
| HOMA | 4.3 (4.1) | 5.3 (2.3) | 0.0070* |
|
| |||
| IGI (μU/mg) | 4.0 (2.9) | 2.1 (1.6) | 0.0009* |
|
| |||
| QUICKI | 0.32 (0.04) | 0.31 (0.04) | 0.0194* |
|
| |||
| WBISI | 3.1 (1.9) | 1.9 (0.9) | 0.004* |
|
| |||
| HOMA %B | 208.7 (63.6) | 176.0 (59.2) | 0.0420* |
|
| |||
| HbA1c | 5.4 (0.3) | 5.8 (0.4) | 0.0014* |
| N | 26 (57.7%) | 16 (59%) | |
SDS – Standard deviation score, IGI – insulinogenic index, HOMA%B – HOMA-derived β–cell function, WBISI – Whole Body Insulin Sensitivity Index.
Patients in the prediabetes group were older (14±2.4 vs 12.8±2.4, p=0.05) and in later stages of puberty, predominantly females (51.9 vs 31.1%, p=0.08) and had higher HOMA values (5.3±2.3 vs 4.3±4.1, p=0.007) compared to the NGT group. There was no difference between the groups for family history, BMI, lipids, fasting insulin, and FGIR. Fasting and all glucose levels during the whole oGTT were significantly higher in the prediabetes group compared to the NGT group (p<0.001) (Table II). IGI, WBISI, Quicki and HOMA%B were significantly lower in the prediabetes group compared to the NGT group (p=0.07, 0.01, 0.02 and 0.04 respectively). HbA1c level was measured in 58% of the patients and was significantly higher in the prediabetes group (5.4±0.3 vs 5.7±0.4, p=0.002) (Table II).
Discussion
The aim was to study obese patients who have an additional risk of insulin resistance in puberty. Therefore, we recruited only obese patients at minimum the Tanner 2 stage of puberty and compared them with normal weight controls. The mean age of our patients was comparable to previous reports but, interestingly, we have male predominance in the obese group (1.4:1) similar to that reported in a Korean group, which differs from the previous reports of female predominance (1.4:1) (8, 24–28).
We found that the group of more obese adolescents was more insulin resistant, which was demonstrated by higher insulin and HOMA values. It was confirmed during the oGTT, because the whole group had insulin sum over 300 (29). We did not detect any patient with silent T2DM and found combined IFG and IGT in only 2 (2.28%) patients. But, isolated IFG or IGT were present in 10 (13.9%) and 15 (20.8%) patients respectively. The reason for this high percentage may be the degree of obesity in our group (mean BMI SDS = 3.0±0.9) accompanied with IR due to puberty (12). Also, insulin sensitivity, measured by the Matsuda index, was lower in the prediabetes group and this is in agreement with previous reports (8). We did not detect a difference in BMI between the prediabetes and NGT groups, in accordance with previous reports that suggested obesity alone is not enough to cause impaired glucose regulation (27). Earlier reports showed decreased IGI and HOMA%B in patients with impaired glucose tolerance (24, 27). In addition, in our group of obese patients glucose levels, IGI and HOMA%B were significantly different between the prediabetes and NGT groups. It may suggest that those patients already have reduced insulin secretion, and are at greater risk to develop type 2 diabetes. The adult literature suggests HbA1c as a screening tool for prediabetes. The advantage of its use in the pediatric population would be: avoidance of fasting, availability of capillary testing and rapid result reporting. Reports from pediatric literature are conflicting; some authors suggested 5.8% for the cutoff, but it was not confirmed in the Caprio studies (26, 30). Although in our study HbA1c was not available for all patients, it was significantly higher in the prediabetes group, suggesting that it might be a good screening criterion in a selected group of adolescents.
The biggest limitation of our study is the small sample size. In this prospective study we recruited patients during 3 consecutive years and this report presents the majority of patients investigated for obesity. Unfortunately, we were not able to obtain all HbA1c data and perform oGTT in all the patients, which might influence our results. The patient population is a highly selected group of obese patients referred to our obesity clinic. The prevalence in the whole population of Serbia cannot be extrapolated.
We report a high percentage of impaired glucose regulation in the obese pubertal patients screened at our obesity clinic. Furthermore, increased insulin resistance but also impaired insulin secretion were verified with oGTT. This demands our timely action in the prevention and treatment of obesity in children. More studies are needed to help us to better understand the pathophysiology of progression from obesity to prediabetes and diabetes.
Abbreviations
- BMI
Body Mass Index
- FGIR
fasting glucose insulin ratio
- IR
insulin resistance
- HOMA IR
homeostatic model assessment
- HOMA %B
HOMA-derived β-cell function
- IFG
impaired fasting glucose tolerance
- IGT
impaired glucose tolerance
- IGI
insulinogenic index
- QUICKI
quantitative insulin sensitivity check index
- SDS
Standard Deviation Score
- T2D
Type 2 Diabetes
- WBISI
whole body insulin sensitivity index.
Footnotes
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32(1):100–5. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomgarden ZT. American College Of Endocrinology Pre-Diabetes Consensus Conference: part one. Diabetes Care. 2008;31(10):2062–9. doi: 10.2337/dc08-zb10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346(11):802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 4.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129(6):1035–41. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 5.Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102(3):E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 6.Giannini C, Weiss R, Cali A, Bonadonna R, Santoro N, Pierpont B, Shaw M, Caprio S. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61(3):606–14. doi: 10.2337/db11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The HEALTHY Study Group. A School-Based Intervention for Diabetes Risk Reduction. N Engl J Med. 2010;363(5):443–53. doi: 10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuković R, Mitrović K, Milenković T, Todorović S, Zdravković D. Type 2 diabetes mellitus and impaired glucose regulation in overweight and obese children and adolescents living in Serbia. Int J Obes. 2012;36(11):1479–81. doi: 10.1038/ijo.2011.273. [DOI] [PubMed] [Google Scholar]
- 9.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28(3):638–44. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss R, Caprio S. The metabolic consequences of childhood obesity. Best Pract Res Clin Endocrinol Metab. 2005;19(3):405–19. doi: 10.1016/j.beem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Weiss R, Taksali S, Tamborlane W, Burgert T, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28( 4):902–09. doi: 10.2337/diacare.28.4.902. [DOI] [PubMed] [Google Scholar]
- 12.Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty. Results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 13.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):12–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Fronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 18.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 19.Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25(11):2081–7. doi: 10.2337/diacare.25.11.2081. [DOI] [PubMed] [Google Scholar]
- 20.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, Tamborlane WV, Caprio S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89(3):1096–101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 21.Hrebicek J, Janout V, Malincikova J, Horakova D, Cizek L. Detection of insulin resistance by simple Quantitative Insulin Sensitivity Check Index (QUICKI) for epidemiological assessment and prevention. J Clin Endocrinol Metab. 2002;87(1):144–7. doi: 10.1210/jcem.87.1.8292. [DOI] [PubMed] [Google Scholar]
- 22.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative Insulin Sensitivity Check Index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Garnett SP, Srinivasan S, Birt SG, Ambler GR, Lawrie EA, Cowell CT, Craig ME. Evaluation of glycaemic status in young people with clinical insulin resistance; fasting glucose, fasting insulin or an oral glucose tolerance test? Clin Endocrinol. 2010;72(4):475–80. doi: 10.1111/j.1365-2265.2009.03677.x. [DOI] [PubMed] [Google Scholar]
- 25.Kiess W, Bottner A, Raile K, Kapellen T, Muller G, Galler A, Paschke R, Wabitsch M. Type 2 diabetes mellitus in children and adolescents: a review from a European perspective. Horm Res. 2003;59( Suppl 1):77–84. doi: 10.1159/000067829. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Park HK, Hwang JS. HbA1c and glucose intolerance in obese children and adolescents. Diabet Med. 2012;29(7):102–5. doi: 10.1111/j.1464-5491.2012.03596.x. [DOI] [PubMed] [Google Scholar]
- 27.Shalitin S, Abrahami M, Lilos P, Phillip M. Insulin resistance and impaired glucose tolerance in obese children and adolescents referred to a tertiary care center in Israel. Int J Obes. 2005;29(6):571–8. doi: 10.1038/sj.ijo.0802919. [DOI] [PubMed] [Google Scholar]
- 28.Wiegand S, Dannemann A, Krude H, Gruters A. Impaired glucose tolerance and type 2 diabetes mellitus: a new field for pediatrics in Europe. Int J Obes (Lond) 2005;29( Suppl 2):S136–42. doi: 10.1038/sj.ijo.0803081. [DOI] [PubMed] [Google Scholar]
- 29.Zannolli R, Rebeggiani A, Chiarelli F, Morgese G. Hyperinsulinism as a marker in obese children. Am J Dis Child. 1993;147(8):837–41. doi: 10.1001/archpedi.1993.02160320039016. [DOI] [PubMed] [Google Scholar]
- 30.Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, Guandalini C, Savoye M, Rose P, Caprio S. Utility of Hemoglobin A1c for Diagnosing Pre-diabetes and Diabetes in Obese Children and Adolescents. Diabetes Care. 2011;34(6):1306–11. doi: 10.2337/dc10-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]