Summary
Background
Steroid 21-hydroxylase deficiency is present in 90–95% of all cases with congenital adrenal hyperplasia (CAH), an autosomal recessive disorder. It can present as the severe classical salt wasting (SW) or simple virilising (SV) form, or the milder, nonclassical form. Nine pseudogene-derived point mutations account for about 80% of all defects in the CYP21A2 gene coding the 21-hydroxylase enzyme.
Methods
We have studied nine CYP21A2 point mutations in 61 Macedonian and 24 Serbian patients with different clinical presentations of CAH, using the PCR/ACRS method.
Results
Six different mutations were detected in 71.3% of alleles of the Macedonian patients. The most prevalent mutation was IVS2. Mutations were detected in 85.4% of the SW, 83.4% SV and 47.7% LO alleles. In the Macedonian patients the most common genotype was IVS2/IVS2. Five different mutations were detected in 64.6% of alleles of the Serbian patients. The most prevalent was P30L. Mutations were present in 83.3% SW, 80% SV and 50% of the LO alleles. In the Serbian patients, the P30L/P30L genotype was the most frequent.
Conclusions
Specific CYP21A2 mutations are involved in different clinical forms of CAH. High frequency of P30L was found in both populations. Also, high prevalence of the mild P30L mutation was found in both the Macedonian and Serbian classical SV patients. Our findings support the role of the P30L mutation in pronounced virilisation. An unusual finding is the low frequency of V281L in the Macedonian non-classical patients and its absence in the ones from Serbia.
Keywords: congenital adrenal hyperplasia, CYP21A2 gene, steroid 21-hydroxylase
Kratak sadržaj
Uvod
Nedostatak enzima steroid 21-hidroksilaze je prisutan u 90–95% slučajeva kongenitalne adrenalne hiperplazije (CAH), autozomalne recesivne bolesti. Ona se može pojaviti kao klasična forma sa gubitkom soli (SW) ili jednostavna viriliziračka (SV) forma i kao slabija neklasična forma (LO). Devet tačkastih mutacija transferiranih sa pseudogena CYP21A1P sačinjavaju oko 80% svih defekata u genu CYP21A2, koji kodira sintezu enzima 21-hidroksilaze.
Metode
Analizirali smo devet tačkastih mutacija u genu CYP21A2 kod 61 makedonskog i 24 srpskih pacijenata sa različitom kliničkom prezentacijom CAH, koristeći metodu PCR/ACRS.
Rezultati
Kod makedonskih pacijenata detektovano je 6 različitih mutacija u 71,3% alela. Najvišu prevalencu je imala IVS2 mutacija. Mutacije su detektovane u 85,4% SW alela, 83,4% SV i 47,7% LO alela. IVS2/IVS2 genotip je imao najvišu učestalost kod makedonskih pacijenata. Srpski pacijenti su imali 5 različitih mutacija detektovanih u 64,6% alela. Najvišu prevalencu je imala P30L mutacija. Mutacije su detektovane u 83,3% SW alela, 80% SV i 50% LO alela. P30L/P30L genotip je imao najvišu učestalost kod srpskih pacijenata.
Zaključak
Specifične CYP21A2 mutacije su prisutne u različitim kliničkim formama CAH. Kod obe populacije je otkrivena visoka frekvencija mutacije P30L. Takođe, visoka prevalenca slabije mutacije P30L pronađena je kod makedonskih i srpskih pacijenata sa klasičnim SV fenotipom. Naši rezultati potkrepljuju ulogu mutacije P30L kod jako izražene virilizacije. Neočekivani rezultat je niska frekvencija mutacije V281L kod makedonskih i njeno potpuno odsustvo kod srpskih pacijenata sa neklasičnim fenotipom.
Introduction
Congenital adrenal hyperplasia (CAH) is one of the most frequent errors of metabolism, causing impaired adrenal cortisol and aldosterone production with increased androgen secretion (1, 2). Steroid 21-hydroxylase deficiency (21OHD, MIM 201910) is the most common form, accounting for 90–95% of all cases of CAH. It is caused by mutations in the 21-hydroxylase gene (CYP21A2 – cytochrome P450, family 21, subfamily A, polypeptide 2) located 30 kb far from its pseudogene (CYP21A1P) on chromosome 6p21.3 (1). The high degree of sequence similarity (96–98%) between CYP21A2 and CYP21A1P permits two types of recombination events: unequal crossing-over during meiosis, which results in large deletions/duplications of CYP21A2, and gene conversion events that transfer deleterious mutations present in the pseudogene to CYP21A2 (3–7).
However, apart from gene deletions and large gene conversions, nine such pseudogene-derived mutations account for about 95% of all affected CYP21A2 alleles in different ethnic groups, while ~5% are de novo mutations which did not arise in the pseudogene (1). Defects of the CYP21A2 gene lead to various degrees of impaired cortisol and aldosterone synthesis and to androgen excess. Deficiency of both cortisol and aldosterone synthesis results in salt-wasting (SW) CAH, usually presenting early after birth as salt-wasting crises and ambiguous external genitalia in girls. In the simple virilising (SV) form of CAH, presenting with early precocious pubarche and a variable degree of virilisation of the external genitalia in girls, only the cortisol synthesis is deficient. In the nonclassical, late-onset (LO) form of CAH, the phenotypic manifestations of androgen excess are variable, occurring either in childhood, but later than in the SV form, or found in adult women referred for hirsutism, menstrual irregularity, and/or decreased fertility; sometimes no signs of CAH are present. Men are often asymptomatic, further enhancing the difficulty to evaluate the incidence of this mild form of CAH (8–10).
Mutations are divided into three groups according to residual enzyme activity, depending on the nature of the mutations and in vitro studies in the case of missense mutations (11–13). The first group consists of mutations abolishing enzyme activity that are thus associated with the SW form, such as large rearrangements, nonsense, frameshift, or missense mutations. The second group, found in patients with the SV form, consists mainly of the missense I172N mutation (14) with very low residual 21-hydroxylase activity, but sufficient to prevent neonatal SW (15, 16). The third group includes mutations such as P30L (17) and V281L (15) that produce enzymes retaining 20–70% of the normal activity. There is a good relationship between the genotype and clinical presentation, but a combination of CYP21A2 mutations can cause different phenotypes. The less severely mutated allele determines the patient’s phenotype (10, 18, 19).
The severe classical form occurs in one in 10,000 – 15,000 Caucasians (1). The milder non-classical CAH occurs in approximately one in 1,700 in the general population (20). Based on newborn screening data, the carrier frequency of CAH in the general population is estimated to be 1 in 55 (21).
Materials and Methods
Patients
We studied 61 Macedonian and 24 Serbian patients with clinical and laboratory signs of CAH evaluated at the Department of Endocrinology and Genetics, University Children’s Clinic, Skopje, Republic of Macedonia and the Mother and Child Health Care Institute, Belgrade, Serbia, respectively. All patients had elevated plasma 17-hydroxyprogesterone and were classified according to standard criteria (22). Of the Macedonian patients, 24 had the SW form, 15 the SV, and 22 the LO form of the disease. Among Serbian patients, 6 had the SW form, 5 the SV and 13 the LO form of the disease. SW patients had onset of dehydration and/or shock associated with hyperkalemia and hyponatremia. Females had ambiguous genitalia. The diagnosis was made within 2 months after birth (average 23.3±19.6 days). The patients with the SV form were diagnosed at the age of 2–14 years (average 4.8±3 years) due to signs of androgen excess, after corticotropin stimulation. LO patients were characterized by normal external genitalia with hirsutism/oligomenorrhea in girls and by precocious pubarche and elevated 17-hydroxyprogesterone levels, 60 min after stimulation with ACTH in both sexes. The diagnosis was made at the average age of 8.9±4.3 years.
Molecular analysis
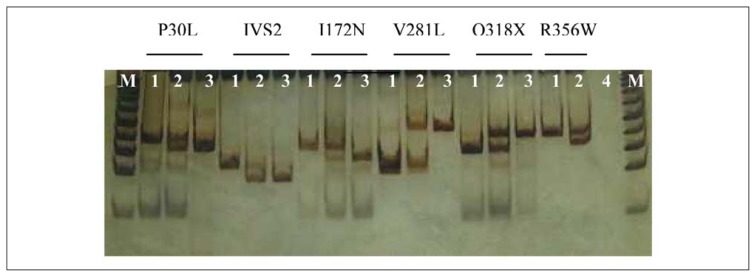
DNA samples of all subjects were obtained from peripheral blood lymphocytes using the standard proteinase K-phenol-chloroform method (23). Direct molecular screening of nine common pseudogene-derived point mutations: P30L, IVS2, 8 bp deletion in exon 3 (G110 8nt), I172N, exon 6 cluster (I236N, V237E, M239K), F306+T, V281L, Q318X and R356W were performed with the PCR/ACRS method, followed by restriction enzyme digestion, as previously described (24). Normal and mutated alleles were distinguished by the size of the restriction fragments, using electrophoresis on 12% polyacryl-amide gels and visualised by silver staining (25–27) (Figure 1). The digestion with the restriction enzyme allowed not only mutation detection, but also determination of the zygosity of the individual mutations.
Figure 1.
ACRS/PCR mutational analysis of the CYP21A2 gene on 12% PAGE, line 1 – normal, line 2 – heterozygote, line 3 –homozygote, M – marker (50 bp).
Ethics
The research was conducted in accordance with the Declaration of Helsinki ethical guidelines, and approved by the institutions where it was conducted.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences 21 for Windows (SPSS Inc., Chicago, Illinois, USA). The distribution and association of the detected mutations with the clinical form of the disease were compared using chi-square tests. P value less than 0.05 was considered statistically significant. Descriptive method was used for statistical analysis of the numerical parameters.
Results
Six different mutations were detected in 71.3% (87/122) of alleles in the Macedonian patients. The most prevalent IVS2 mutation was present in 43 alleles (35.2%), followed by the P30L in 22 (18%), Q318X in 13 (10.7%), V281L in 5 (4.1%), I172N in 4 (3.3%) and R356W in 3 alleles (2.5%). Mutations were revealed in 85.4% of the SW, 83.4% of the SV and 47.7% of the LO alleles. The distribution of the detected mutations among the Macedonian patients with different clinical presentations of CAH is shown in Table I. In 36 (59%) of the Macedonian patients the complete genotype was obtained, with good correlation to phenotype (Table II). Of them, 30 patients (49.2%) were homozygous for one mutation and six patients (9.8%) were compound heterozygous with different mutations on each allele. The most common genotype was IVS2/IVS2 (37.3%). Fifteen (24.6%) of the Macedonian patients were heterozygotes and ten (16.4%) harboured none of the tested mutations.
Table I.
Detected mutant alleles in the Macedonian and Serbian patients with different clinical forms of 21-hydroxylase deficiency.
| Macedonian patients | Serbian patients | |||||
|---|---|---|---|---|---|---|
| Mutations | SW (n=48) | SV (n=30) | LO (n=44) | SW (n=12) | SV (n=10) | LO (n=26) |
| IVS2 | 33 (68.8%) | 9 (30%) | 1 (2.3%) | 7 (58.3%) | 2 (20%) | 1 (3.8%) |
| Q318X | 7 (14.6%) | 1 (3.4%) | 5 (11.4%) | 1 (8.3%) | / | 3 (11.5%) |
| R356W | 2 (4.2%) | 1 (3.4%) | / | 1 (8.3%) | / | 1 (3.8%) |
| V281L | 1 (2.1%) | 2 (6.7%) | 2 (4.5%) | / | / | / |
| P30L | / | 9 (30%) | 13 (29.5%) | 2 (16.7%) | 4 (40%) | 9 (34.6%) |
| I172N | / | 4 (13.4%) | / | / | 2 (20%) | / |
| Total alleles | 41*/48 (85.4%) | 25*/30 (83.4%) | 21/44 (47.7%) | 10*/12 (83.3%) | 8/10 (80%) | 13*/26 (50%) |
There was more than one mutation on the same allele; n – number of the analysed alleles.
Table II.
Genotype-phenotype correlation in the Macedonian patients based on the severity of the CYP21A2 defect.
| Genotype allele 1/allele 2 | No. of patients | SW n=24 |
SV n=15 |
LO n=22 |
p |
|---|---|---|---|---|---|
| severe/severe | 23 | 19 | 4 | / | 0.00* |
| moderate/moderate | 1 | / | 1 | / | / |
| mild/severe | 4 | / | 3 | 1 | 1.00 |
| mild/moderate | 1 | / | 1 | / | / |
| mild/mild | 7 | / | 3 | 4 | 1.00 |
| Total | 36 | 19 | 12 | 5 | / |
Statistically significant (p < 0.05)
Five different mutations were detected in 64.6% (31/48) of alleles in the Serbian patients. The most prevalent P30L mutation was present in 15 alleles (31.3%), followed by 10 IVS2 (20.8%) mutated alleles, 4 Q318X (8.3%), 2 R356W (4.2%) and 2 I172N (4.2%) alleles Mutations were found in 83.3% of the SW, 80% of the SV and 50% of the LO alleles. The distribution of the detected mutations among the Serbian patients with different clinical presentations of CAH is shown in Table I. The complete genotype was obtained in 14 (58.3%) of the Serbian patients, with good correlation with phenotype (Table III). Nine of them (37.5%) were homozygous for one mutation and five (20.8%) were compound heterozygous with different mutations on each allele. The most common genotype was P30L/P30L (23.5%). Three of the patients (12.5%) were heterozygotes and 7 (29.2%) harboured none of the tested mutations.
Table III.
Genotype-phenotype correlation in the Serbian patients based on the severity of the CYP21A2 defect.
| Genotype allele 1/allele 2 | No. of patients | SW n=6 |
SV n=5 |
LO n=13 |
p |
|---|---|---|---|---|---|
| severe/severe | 6 | 5 | / | 2 | 1.00 |
| moderate/moderate | 1 | / | 1 | / | / |
| mild/severe | 3 | / | 2 | / | 1.00 |
| mild/mild | 4 | / | 1 | 3 | 1.00 |
| Total | 14 | 5 | 4 | 5 | / |
Statistically significant (p < 0.05)
Discussion
We observed an interesting pattern of the mutational spectrum of CYP21A2 in our patients; this is the first report in these two Balkan countries. Out of 122 alleles in the Macedonian and 48 alleles in the Serbian patients, we identified mutations in 87 (71.3%) and 31 (64.6%) alleles, respectively. Among the nine common mutations analyzed, IVS2 was the most prevalent mutation in the Macedonian, as opposed to P30L in the Serbian patients. The distribution of the detected mutations in our study was slightly different from those previously reported in large populations (19).
No 8 base-pair deletion, E6 cluster or F306+t were detected in the analysed patients.
We found that specific CYP21A2 mutations are involved in different clinical forms of CAH. IVS2 splicing mutation with less than 1% residual 21-hydroxylase activity was the most prevalent among the Macedonian SW patients (68.8%) with significantly higher frequency than the one in the SV and LO patients (p<0.05). Also, the IVS2 mutation frequency in the SV patients is significantly higher than the one in LO patients (p<0.05). However, the IVS2 mutation was the most prevalent among Serbian (58.3%) SW patients, without any statistically significant difference between the different clinical presentations.
Although IVS2 is usually associated with the SW phenotype, in our SV patients high IVS2 frequency was found in the heterozygote state. However, compound heterozygotes for two different CYP21A2 mutations usually have a phenotype compatible with the presence of the milder gene defects (10, 18). It can explain why our patients with severe and moderate or mild mutations presented with a milder phenotype. We found that the P30L frequency was relatively high in our LO patients, when compared to other studies (19). Also, high prevalence of the mild P30L mutation with more than 30% of enzyme activity was found in our classical SV patients. Our findings support the role of the P30L mutation in pronounced virilisation (17). An unusual finding is the lower frequency of V281L in the Macedonian nonclassical patients compared to other European populations, as well as its complete absence in our Serbian nonclassical patients (28–30). Finally, according to the fact that the severe nonsense Q318X mutation abolishes the 21-hydroxylase activity, homozygosity for Q318X in two of our patients with the late-onset phenotype could be attributed to the tendency of cytochrome P450 enzymes to be ‘promiscuous’ enzymes that bind many different substrates and catalyze a wide variety of hydroxylations, so the adrenal expression of such an enzyme could account for the cryptic 21-hydroxylase activity (31). Variability in the phenotypic expression in some of our NCAH patients strengthens the concept that the genotype is predictive of the phenotype and may be conditioned by the presence of mechanisms other than genetic heterogeneity at the CYP21A2 locus (32, 33) or the involvement of other genes in the androgen sensitivity, the salt balance or the extraadrenal 21-hydroxylase activity (34).
Footnotes
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocrinol Rev. 2000;21(3):245–91. doi: 10.1210/edrv.21.3.0398. [DOI] [PubMed] [Google Scholar]
- 2.Miller WL, Morel Y. The molecular genetics of 21-hydroxylase deficiency. Annu Rev Genet. 1989;23:371–93. doi: 10.1146/annurev.ge.23.120189.002103. [DOI] [PubMed] [Google Scholar]
- 3.White PC, New MI, Dupont B. Structure of the human steroid 21-hydroxylase gene. Proc Natl Acad Sci USA. 1986;83(14):5111–5. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White PC, Vitek A, Dupont B, New MI. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 1988;85:4436–40. doi: 10.1073/pnas.85.12.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White PC, New MI, Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA. 1984;81:7505–9. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higashi Y, Tanae A, Inoue H, Fujii-Kuriyama Y. Evidence for frequent gene conversion in the steroid 21-hydroxylase-P-450(C21) gene: implications for steroid 21-hydroxylase deficiency. Am J Hum Genet. 1988;42:17–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Tusié-Luna MT, White PC. Gene conversion and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc Natl Acad Sci USA. 1995;92(23):10796–800. doi: 10.1073/pnas.92.23.10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White PC, New MI, Dupont B. Congenital adrenal hyperplasia. New Eng J Med. 1987;316:1519–24. doi: 10.1056/NEJM198706113162406. [DOI] [PubMed] [Google Scholar]
- 9.White PC, New MI. Genetic basis of endocrine disease 2: congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1992;74:6–11. doi: 10.1210/jcem.74.1.1727830. [DOI] [PubMed] [Google Scholar]
- 10.Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna M-T, Lesser M, New MI, White PC. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992;90:584–95. doi: 10.1172/JCI115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lajic S, Levo A, Nikoshkov A, Lundberg Y, Partanen J, Wedell A. A cluster of missense mutations at Arg356 of human steroid 21-hydroxylase may impair redox partner interaction. Hum Genet. 1997;99:704–9. doi: 10.1007/s004390050436. [DOI] [PubMed] [Google Scholar]
- 12.Krone N, Riepe FG, Grötzinger J, Partsch CJ, Sippell WG. Functional characterization of two novel point mutations in the CYP21 gene causing simple virilizing forms of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2005;90:445–54. doi: 10.1210/jc.2004-0813. [DOI] [PubMed] [Google Scholar]
- 13.Menassa R, Tardy V, Despert F, Bouvattier-Morel C, Brossier JP, Cartigny M, Morel Y. p.H62L, a rare mutation of the CYP21 gene identified in two forms of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2008;93:1901–8. doi: 10.1210/jc.2007-2701. [DOI] [PubMed] [Google Scholar]
- 14.Amor M, Parker KL, Globerman H, New MI, White PC. Mutation in the CYP21B gene (Ile-172-Asn) causes steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 1988;85:1600–4. doi: 10.1073/pnas.85.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tusie-Luna MT, Traktman P, White PC. Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem. 1990;265:20916–22. [PubMed] [Google Scholar]
- 16.Hsu LC, Hsu NC, Guzova JA, Guzov VM, Chang SF, Chang BC. The common I172N mutattion causes conformational change of cytochrome P450c21 revealed by systematic mutation, kinetic, and structural studies. J Biol Chem. 1996;271:3306–10. doi: 10.1074/jbc.271.6.3306. [DOI] [PubMed] [Google Scholar]
- 17.Tusie-Luna MT, Speiser PW, Dumic M, New MI, White PC. A mutation (Pro-30 to Leu) in CYP21 represents a potential nonclassic steroid 21-hydroxylase deficiency allele. Mol Endocrinol. 1991;5(5):685–92. doi: 10.1210/mend-5-5-685. [DOI] [PubMed] [Google Scholar]
- 18.Jaaskelainen J, Levo A, Voutilainen R, Partanen J. Population-wide evaluation of disease manifestation in relation to molecular genotype in steroid 21-hydroxylase (CYP21) deficiency: good correlation in well defined population. J Clin Endocrinol Metab. 1997;82(10):3293–7. doi: 10.1210/jcem.82.10.4271. [DOI] [PubMed] [Google Scholar]
- 19.Dolžan V, Sólyom J, Fekete G, Kovács J, Rakosnikova V, Voltava F, Lebl J, Pribilincova Z, Baumgartner-Parzer SM, Riedl S, Waldhauser F, Frish H, Stopar-Obreza M, Kržišnik C, Battelino T. Mutational spectrum of steroid 21-hydroxylase and the genotype-phenotype association in the Middle European patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2005;153(1):99–106. doi: 10.1530/eje.1.01944. [DOI] [PubMed] [Google Scholar]
- 20.Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37(4):650–67. [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner-Parzer SM, Nowotny P, Heinze G, Waldhäusl W, Vierhapper H. Carrier frequency of congenital adrenal hyperplasia (21-hydroxylase deficiency) in a Middle European population. J Clin Endocrinol Metab. 2005;90(2):775–8. doi: 10.1210/jc.2004-1728. [DOI] [PubMed] [Google Scholar]
- 22.New MI, Lorenzen F, Lerner AJ, Kohn B, Oberfield SE, Pollack MS, Dupont B, Storner E, Levy DJ, Pang S, Levine LS. Genotyping steroid 21-hydroxylase deficiency: Hormonal reference data. J Clin Endocrinol Metab. 1983;57(2):320–6. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- 23.Efremov GD, Dimovski AJ, Plaseska-Karanfilska D, Simjanovska L, Sukarova E, Koceva S. ICGEB Affiliated Center »Nucleic Acid Based Methods in Human and Veterinary Medicine«. 3rt ed. Skopje; Republic of Macedonia: 1999. Laboratory manual. [Google Scholar]
- 24.Lee HH, Chao HT, Ng HT, Choo KB. Direct molecular diagnosis of CYP21 mutations in congenital adrenal hyperplasia. J Med Gen. 1996;33:371–5. doi: 10.1136/jmg.33.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gužvić M. The history of DNA sequencing. J Med Biochem. 2013;32:301–12. [Google Scholar]
- 26.Nestorov J, Matić G, Elaković I, Tanić N. Gene expression studies: How to obtain accurate and reliable data by quantitative real-time RT PCR. J Med Biochem. 2013;32:325–38. [Google Scholar]
- 27.Radojković D, Kusić J. Silver staining of denaturing gradient gel electrophoresis gels. Clin Chem. 2000;46:883–4. [PubMed] [Google Scholar]
- 28.Dracopoulou-Vabouli M, Maniati CM, Dacou VC. The spectrum of molecular defects of the CYP21 gene in the Hellenic population: variable concordance between genotype and phenotype in the diffrent forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(6):2845–8. doi: 10.1210/jcem.86.6.7574. [DOI] [PubMed] [Google Scholar]
- 29.Torres N, Mello MP, Germano CMR, Elias LLK, Moreira AC, Castro M. Phenotype and genotype correlation of the microconversion from the CYP21A1P to the CYP21A2 gene in congenital adrenal hyperplasia. Brazilian J Medic Biolog Research. 2003;36:1311–8. doi: 10.1590/s0100-879x2003001000006. [DOI] [PubMed] [Google Scholar]
- 30.Kotaška K, Průša R. Frequency of CYP21 gene mutations in Czech patients with steroid 21-hydroxylase deficiency and statistical comparison with other populations. Med Princ Pract. 2003;12:243–7. doi: 10.1159/000072291. [DOI] [PubMed] [Google Scholar]
- 31.Miller WL. Congenital lipoid adrenal hyperplasia: the human gene knockout for the steroidogenic acute regulatory protein. J Molec Endocrinol. 1997;19:227–40. doi: 10.1677/jme.0.0190227. [DOI] [PubMed] [Google Scholar]
- 32.Witchel SF, Bhamidipati DK, Hoffman EP, Cohen JB. Phenotypic heterogeneity associated with the splicing mutation in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1996;81:4081–8. doi: 10.1210/jcem.81.11.8923864. [DOI] [PubMed] [Google Scholar]
- 33.Deneux C, Tardy V, Dib A, Mornet E, Billaud L, Charron D, Morel Y, Kuttenn F. Phenotype-genotype correlation in 56 women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metabol. 2001;86(1):207–13. doi: 10.1210/jcem.86.1.7131. [DOI] [PubMed] [Google Scholar]
- 34.Gomes LG, Huang N, Agrawal V, Mendonca BB, Bachega TASS, Miller WL. The common P450 oxidoreductase variant A503V is not a modifier gene for 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2008;93(7):2913–6. doi: 10.1210/jc.2008-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]