Summary
Background
This study investigated the effects of a nutritionally relevant intake of eicosapentaenoic (EPA) and docosahexaenoic (DHA) fatty acids derived from oily fish or a fish oil supplement on selected cardiovascular risk factors in average middle-aged individuals.
Methods
Thirty-three participants were randomized to receive salmon (oily fish) providing 274 mg EPA + 671 mg DHA/day or a commercial fish oil supplement providing 396 mg EPA + 250 mg DHA/day in a cross-over trial over an 8-week period separated by a 6-month washout period. Blood samples were collected before and after each intervention and lipids, inflammatory and oxidative stress parameters were determined.
Results
Plasma levels of EPA, DHA and total n-3 fatty acids significantly increased after both interventions. A decreasing trend in triglycerides was more pronounced with salmon than with the fish oil supplement, but the changes noticed were not significant. Although there were no relevant changes in inflammatory marker concentrations at the end of both interventions, significant negative correlations were noticed between total plasma n-3 fatty acids and soluble intercellular adhesion molecule and C-reactive protein throughout the whole intervention period (p<0.05). Among the oxidative stress parameters, intervention with salmon showed a prooxidative effect through a superoxide anion increase (p=0.025). A relevant positive correlation was also found between its concentration and total plasma n-3 fatty acids (p<0.05). Other oxidative stress markers were not significantly influenced by the dietary interventions applied.
Conclusions
Following two sets of recommendations for n-3 fatty acids intake aimed at the general public had only a moderate effect on the selected cardiovascular risk factors in average healthy middle-aged subjects over a short-term period.
Keywords: n-3 fatty acids, fish and fish oil supplements, circulating inflammatory markers, oxidative stress, cardiovascular disease
Kratak sadržaj
Uvod
U ovoj studiji praćeni su efekti preporuka za unos eikozapentaenske (EPA) i dokozoheksaenske kiseline (DHA), iz dva različita izvora, lososa i suplementa sa ribljim uljem, na odabrane faktore kardiovaskularnog rizika u prosečnoj populaciji srednjih godina.
Metode
Trideset i tri ispitanika su po slučajnom izboru konzumirali losos koji je obezbeđivao 274 mg EPA + 671 mg DHA/dan ili komercijalni suplement ribljeg ulja koji je obezbeđivao dnevno 396 mg EPA + 250 mg DHA tokom 8 nedelja. Nakon perioda od 6 meseci ispitanicima su zamenjene intervencije (ukrštena studija). Uzorci krvi su sakupljani pre i posle svake intervencije, a zatim su određivani lipidni, inflamatorni parametri kao i markeri oksidativnog stresa.
Rezultati
Koncentracije EPA, DHA i ukupnih n-3 masnih kiselina u plazmi su značajno povećane posle obe intervencije. Nije bilo statistički značajnih promena u lipidnim parametrima, iako je zabeleženo veće smanjenje nivoa triglicerida posle intervencije lososom u poređenju sa suplementom. Nisu uočene značajne promene u koncentraciji inflamatornih markera, ali je utvrđena značajna negativna korelacija između ukupnih n-3 masnih kiselina plazme i rastvorljivog intracelularnog adhezionog molekula i C-reaktivnog proteina tokom ukupnog trajanja studije (p<0,05). Od parametara oksidativnog stresa, intervencija lososom je imala umeren prooksidativni efekat usled povećanja superoksidnog anjona (p=0,025). Između ovog parametra i ukupnih n-3 masnih kiselina nađena je statistički značajna pozitivna korelacija. Ostali markeri oksidativnog stresa nisu se značajno menjali.
Zaključak
Preporučene vrednosti za unos n-3 masnih kiselina pokazale su umeren efekat na parametre kardiovaskularnog rizika u prosečnoj populaciji srednjih godina u kratkom periodu trajanja studije.
Introduction
It is widely accepted that n-3 polyunsaturated fatty acids (PUFA), especially long chain (LC) eicosapentaenoic (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3), have several physiological roles in the human organism that can explain their positive impact on cardiovascular events. LC n-3 PUFA are present mainly in oily fish and commercially available supplements (as concentrated pharmaceutical preparations), and dietary guidelines recommend use of these fatty acids for the purpose of primary and secondary cardiovascular prevention. There is documented evidence that cardioprotective effects of n-3 PUFA may be mediated by their antiinflammatory properties, lipid-modulating effects, as well as antithrombotic and vasodilatory effects (1, 2). In numerous human studies, low dietary intake of n-3 fatty acids had no effect on inflammatory markers in healthy individuals (3); however, in several studies the concentration of soluble intercellular adhesion molecule (sICAM-1), soluble vascular cell adhesion molecule (sVCAM-1), and C-reactive protein (CRP) was affected, which was dependent on the dose and duration of the study (4, 5). Although LC n-3 PUFA play a role in the modification of lipid and lipoprotein metabolism, it is obvious that these effects become evident at higher daily doses of EPA and DHA (1). The influence of diet on prooxidative and antioxidative processes is still an intriguing area, due to lack of proper indicators of increased oxidation in vivo. There is no clear evidence whether dietary or supplemental n-3 PUFA can reduce oxidative stress, although some experimental data support the notion that a high intake of PUFA may increase in vivo lipid peroxidation (6).
Despite sometimes conflicting results of individual clinical trials as well as meta-analyses of n-3 fatty acids impact on cardiovascular outcomes, the current evidence is sufficient to encourage an intake of 500–1000 mg of EPA+DHA daily in primary and secondary prevention of cardiovascular disease, either in the form of two fatty fish meals per week, or in the form of fish oil supplements (7, 8). Regardless the so-far collected information from fish and fish oil supplement clinical trials, there is still a need for more controlled studies in specific populations and with specific clinical targets. So far, few studies have been conducted on the effect of n-3 fatty acids in the Serbian population, especially in average healthy middle-aged individuals which are characterized by one or more cardiovascular risk factors and a low level of fish consumption (9–11). These facts suggest this population is in need of improvement of dietary habits, either by increasing fish intake or by incorporating fish oil supplements in regular diet.
The primary aim of this study was to investigate the relationship between the recommended intake of n-3 fatty acids from oily fish or fish oil supplements and selected cardiovascular risk markers in healthy Serbian adults that included blood lipid parameters, circulating inflammatory markers and oxidative stress parameters. The secondary aim was to explore the differences between the effects of two generally-accepted food-based dietary recommendations for LC n-3 PUFA intake from two dietary sources. In order to avoid variations between participants, we implemented a randomized cross-over trial.
Materials and Methods
Subjects
Thirty-five volunteers without any known medical condition (17 female and 18 male), aged between 44 and 64, body mass index (BMI) <30 kg/m2 and blood pressure <140/90 mmHg, were enrolled into the intervention study. Exclusion criteria were as follows: recent weight changes (± 3 kg); consumption of fish oil, calcium or vitamin D supplements in the last three months; use of any regular medications known to affect plasma lipid levels or nonsteroidal antiinflammatory drugs such as aspirin; more than 10 cigarettes per day; an allergy or intolerance to fish. Further exclusion criteria were concentrated on participants who habitually consumed more than one fish meal per week and were drinking more than three standard alcoholic drinks per day. The criterion for dyslipidemic status was low-density lipoprotein cholesterol (LDL-C) concentration above 3.36 mmol/L and/or triglyceride (TG) concentration more than 1.69 mmol/L according to the National Cholesterol Education Program Guidelines (12). The baseline data collected at the time of the first clinical visit are shown in Table I.
Table I.
Characteristics of the study population at baseline*
| Characteristics | Whole group (n=35) |
|---|---|
| Gender (female/male) | 17/18 |
| Age (range, years) | 55 (46–64) |
| Smokers, n (%) | 9 (26%) |
| Body weight (kg) | 79.5±14.0 |
| BMI (kg/m2) | 26.1±3.4 |
| Overweight, n (%) | 19 (54%) |
| Total cholesterol (mmol/L) | 6.55±0.93 |
| LDL cholesterol (mmol/L) | 4.28±0.89 |
| HDL cholesterol (mmol/L) | 1.47±0.36 |
| Triglycerides (mmol/L) | 1.77±0.94 |
| Dyslipidemic, n (%) | 25 (71%) |
| Fasting Glucose (mmol/L) | 5.1±0.57 |
| Blood pressure (mm Hg) | |
| Systolic | 129.4±10 |
| Diastolic | 83.0±7.5 |
| sICAM-1 (ng/mL) | 246 (155–375) |
| sVCAM-1 (ng/mL) | 508 (343–819) |
| CRP (mg/L) | 1.2 (0.3–3.5) |
| (mmol/min/L) | 110 (51–205) |
| MDA (mmol/L) | 0.6 (0.2–1.1) |
| TAS (mmol/L) | 0.7 (0.4–1.3) |
Values are presented as means ± SD or median (10th–90th percentile).
BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; sICAM-1, soluble intercellular adhesion molecule; sVCAM-1, soluble vascular cell adhesion molecule; CRP, C-reactive protein; , superoxide anion; MDA, malondialdehyde; TAS, total antioxidant status.
The study was approved by the Clinical Research Ethics Committee of the Faculty of Pharmacy, University of Belgrade, Serbia. Informed consent was obtained from all subjects before starting experimental procedures and the study followed the Helsinki guidelines.
Intervention
Dietary intervention was designed as a randomized, cross-over trial. Participants were randomly assigned at the beginning of the trial to one of the two treatment orders. Participants were randomized to either: Group 1: eight weeks of salmon consumption followed by a six-month washout period followed by eight weeks of fish oil supplementation; Group 2: eight weeks of fish oil supplementation followed by a six-month washout period followed by eight weeks of salmon consumption. Participants were asked not to consume any additional oily fish during the study period, but were otherwise encouraged to follow their normal dietary habits. During the salmon intervention, participants received 150 g of cold smoked skinned fillet of Norwegian Atlantic farmed salmon two times per week (by Squadra, d.o.o., Belgrade, Serbia). Fish oil capsules used contained 1000 mg of fish oil/capsule in the form of triglycerides (Pharmanova d.o.o., Belgrade, Serbia) and were typical of commercially available dietary supplements given in the amount that was most frequently recommended by the producers. The whole trial included four clinical visits, one before and one after each intervention period. The following assays were performed at all four visits. To monitor changes in energy intake, participants completed a 3-day food diary at two baseline points during the intervention. Dietary data were analyzed by using CRON-O-Meter v0.9.6. software. The mean energy and macronutrient daily intake measured at two baseline points is presented in Table II together with daily n-3 PUFA consumption from salmon and fish oil.
Table II.
Estimated daily energy intake and daily n-3 PUFA intake from salmon and capsules.
| Energy (kcal) | 2357±561 | |
| Protein (g) | 109±6.5 | |
| Carbohydrates (g) | 295±31 | |
| Fat (g) | 76±17 | |
| n-3 fatty acid intake (mg/day) | ||
| Salmon | Capsules | |
| EPA | 274 | 396 |
| DHA | 671 | 250 |
| EPA+DHA | 945 | 646 |
| Total n-3 FAs | 1206 | 704 |
Values for energy and macronutrients are presented as means ± SD PUFA, polyunsaturated fatty acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Biochemical analyses
Lipid status parameters (total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C) and TG) were measured in serum, using an ILAB 300 plus analyzer (Instrumentation Laboratory, Milan, Italy), employing commercial kits. Atherogenic index of plasma (AIP) was calculated as TG/HDL-C ratio.
Fatty acid analysis
Fish lipids for further fatty acid analysis were extracted according to the method of Folch et al. (13). Total lipids from plasma were extracted with chloroform/methanol according to Folch, modified by Kates (14). Fish, supplement and plasma lipids were then transesterified with hydrochloric acid (HCl) in methanol according to the method described by Ichihara and Fukubayashi (15) and fatty acid methyl esters were obtained (FAMEs). FAMEs were quantified using an Agilent Technologies 7890A Gas Chromatograph with a flame ionization detector (Agilent Technologies, Santa Clara, CA, USA). Separation of the FAMEs was performed on a 112–88A7, HP-88 capillary column (100 m × 0.25 mm × 0.2 μm) using He as a carrier gas at a flow rate of 105 mL/min. The samples were injected at a starting oven temperature of 175 °C; injector temperature was 250 °C and detector temperature was 280 °C.
The oven temperature was programmed to increase from 175 °C to 220 °C at 5 °C/min. Fatty acids were identified by their retention time with reference fatty acid standards (SupelcoTM FAME Mix, USA).
Measurements of inflammatory markers
Serum ICAM-1, VCAM-1 and CRP levels were determined using commercially available ELISA kits (R&D Systems, Inc, USA).
Oxidative stress and antioxidative defense parameters
The rate of superoxide anion (O2−) generation was measured using the rate of nitro blue tetrazolium (NBT) reduction (16). Plasma malondialdehyde (MDA) concentrations were measured using the thiobarbituric acid-reactive substances (TBARS) assay employing the molar absorption coefficient of 1.56 × 105 M−1cm−1 and spectrophotometry at 535 nm as previously described by Girotti et al. (17). Total antioxidant status (TAS) was determined using an automated method developed by Erel (18). The method is based on the decoloration of 2.2′-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid radical cation (ABTS) by antioxidants present in serum. The color change was measured using an ILab 300 Plus auto analyzer. The reaction rate is calibrated with Trolox (water-soluble analogue of vitamin E, 6-hydroxy-2, 5,7, 8-tetra-methylchroman-2-carboxylic acid) and the TAS value of the samples tested is expressed as mmol Trolox equivalent/L. The intra-assay and inter-assay coefficients of variance were 4.3% and 8.8 %, respectively.
Statistical analysis
Data were evaluated by mixed model ANOVA. All variables were tested for normal distribution of the data. A large number of the test variables were not normally distributed; therefore, non-parametric tests were used in the statistical analysis, since data were skewed (Mann–Whitney tests, Spearman correlation). Normally distributed data were expressed as means ±SD or otherwise as medians and 10th and 90th percentiles or medians and interquartile range. To determine if there was a difference in outcome between fish and fish oil supplements, the model fitted treatment and period as fixed effects and participants and visits as random effects, as described in our previous report (19). Least square means and confidence intervals were calculated for the differences between Salmon-wk 0, Fish oil-wk 0, Salmon-wk 8 and Fish oil-wk 8, as well as for the net difference between the interventions, (Salmon wk 8 – Salmon wk 0) – (Fish oil wk 8 –Fish oil wk 0). Exact values of p<0.05 were considered statistically significant. Analysis was carried out in PASW (SPSS) version 18 (Chicago, IL, USA).
Results
Subjects
Only two participants withdrew from the study. Thirty-three participants completed two phases of the intervention with measurable outcomes at all visits. The reason for withdrawal was poor compliance within the study group. The baseline characteristics considering BMI, blood pressure, glucose level, energy and macronutrient intake did not change during the whole study period. The dietary analysis obtained from the 3-day food diary showed that the mean energy intake was slightly over the dietary reference recommendation (20). All subjects were middle-aged (mean age 55 years) and had one or two cardiovascular risk factors at the beginning of the study (54% overweight, 71% dyslipidemic). There were no significant differences in lipid parameters between groups at baseline and after the washout phase. In spite of the higher level of TG at baseline in salmon group compared to fish oil group (2.01 mmol/L vs 1.76 mmol/L), this difference was not significant. During the salmon intervention participants received 274 mg EPA and 671 mg DHA daily, while intervention with fish oil supplements provided daily amounts of 396 mg EPA and 250 mg DHA.
Plasma fatty acids and serum lipids
In Table III, changes in plasma fatty acid composition and serum lipids after 8 weeks of each intervention are shown. Measurements of plasma fatty acid concentration allow the evaluation of the net changes associated with treatments. The initial plasma concentration of fatty acids was not different between groups at baseline and after the washout phase. Basal level of plasma n-3 fatty acids, EPA and DHA was 4.7%, 0.52% and 3.07% respectively in salmon group, and 4.8%, 0.54% and 3.50% in fish oil group. The daily consumption of total n-3 fatty acids was higher from salmon when compared with the level consumed from fish oil supplements (1206 mg/day vs 704 mg/day). Also, daily intake of DHA was higher from salmon, and EPA intake was higher from fish oil supplements. After 8 weeks, there was a significant increase in the level of EPA, DHA and total n-3 fatty acids in plasma of both groups. The percentage values of EPA increased by 135% in salmon group (p<0.0001) and 152% in fish oil group (p<0.0001), whereas DHA increased by 145% in salmon (p<0.0001) and 121% in fish oil group (p<0.010). The increase in plasma total n-3 fatty acids observed after 8 weeks of salmon consumption was greater when compared with fish oil (45% vs 27%) (p<0.05). After 8 weeks of dietary intervention with salmon and fish oil, there were no statistically significant changes in the analyzed lipid parameters in either group. TG were the only lipid components that were noticeably influenced, but only after salmon intervention. Although a 15% decrease in TG was detected in salmon group, resulting in 11.7% AIP decrease, both changes were not statistically significant.
Table III.
Plasma n-3 FA composition (% of total fatty acids) and serum lipids at baseline and post-intervention according to study group.
| Salmon | Fish oil capsules | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | After | Pa | Baseline | After | Pb | Pc | |
| EPA (20:5n-3) | 0.52±0.39 | 1.22±0.99 | <0.0001 | 0.54±0.39 | 1.37±0.72 | <0.0001 | 0.643 |
| DHA (22:6n-3) | 3.07±1.26 | 4.45±1.19 | <0.0001 | 3.50±1.25 | 4.22±1.18 | 0.010 | 0.581 |
| EPA+DHA | 3.59±1.55 | 5.70±2.01 | <0.0001 | 4.04±1.55 | 5.59±1.70 | 0.006 | 0.496 |
| Total n-3 FAs | 4.7±1.60 | 6.8±2.20 | <0.0001 | 4.8±2.10 | 6.1±2.20 | 0.011 | 0.826 |
| Total cholesterol | 6.57±1.01 | 6.38±0.91 | 0.345 | 6.40±0.84 | 6.69±1.08 | 0.151 | 0.361 |
| LDL (mmol/L) | 4.29±0.88 | 4.24±0.85 | 0.754 | 4.23±0.80 | 4.46±0.86 | 0.234 | 0.870 |
| HDL (mmol/L) | 1.43±0.42 | 1.37±0.35 | 0.778 | 1.37±0.30 | 1.38±0.36 | 0.805 | 0.906 |
| TG (mmol/L) | 2.01±1.17 | 1.70±0.83 | 0.380 | 1.76±0.86 | 1.77±0.70 | 0.784 | 0.528 |
| AIP | 1.62±1.20 | 1.43±0.97 | 0.686 | 1.43±0.94 | 1.33±0.71 | 0.849 | 0.483 |
Values are expressed as mean ± SD.
Significance in mean values from baseline after salmon consumption.
Significance in mean values from baseline after fish oil capsule intervention.
Significance in mean values between groups at baseline.
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; AIP, atherogenic index of plasma.
Inflammatory markers
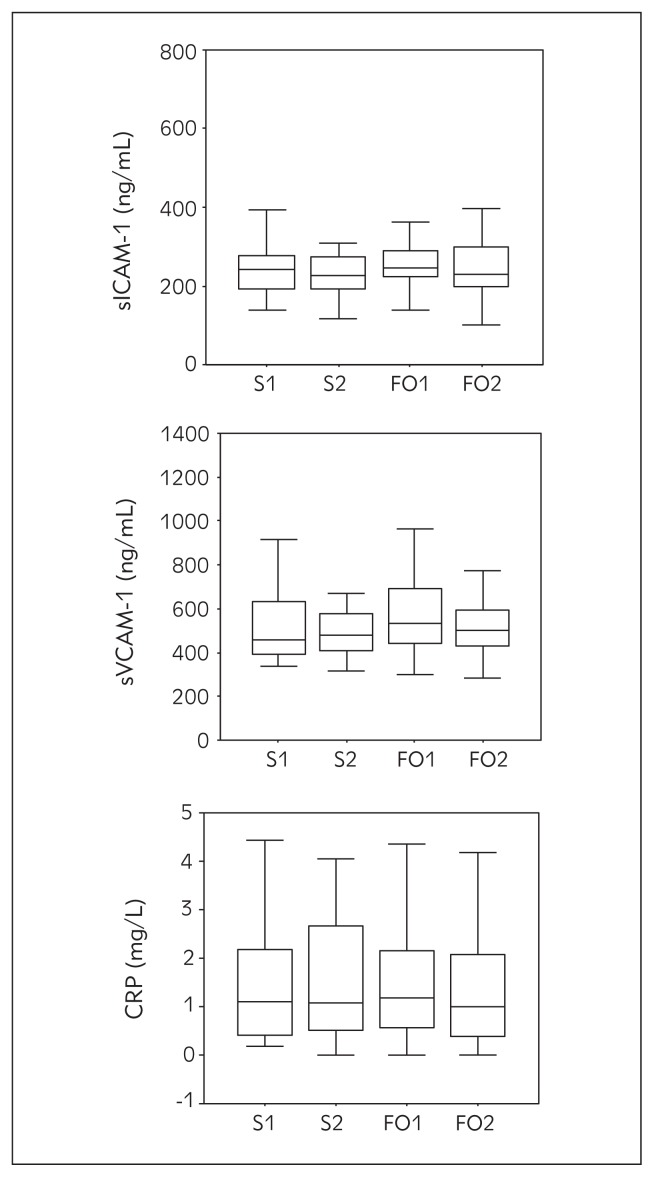
Serum concentrations of the inflammatory markers sICAM-1, sVCAM-1 and CRP are presented in Figure 1. No significant changes were noticed in either of the intervention groups, but some trends could be detected. sICAM-1 levels slightly decreased after both interventions, while serum levels of sVCAM-1 and CRP were slightly higher after salmon, and lower after fish oil intervention, compared with the baseline values.
Figure 1.
sICAM-1, sVCAM-1 and CRP concentrations at baseline and after 8 wks of dietary intervention with salmon and fish oil capsules (points: S1–salmon before, S2–salmon after, FO1–fish oil before, FO2–fish oil after). Values are expressed as median and interquartile range. The difference in relation to baseline was significant at p<0.05 (*).
Oxidative stress biomarkers
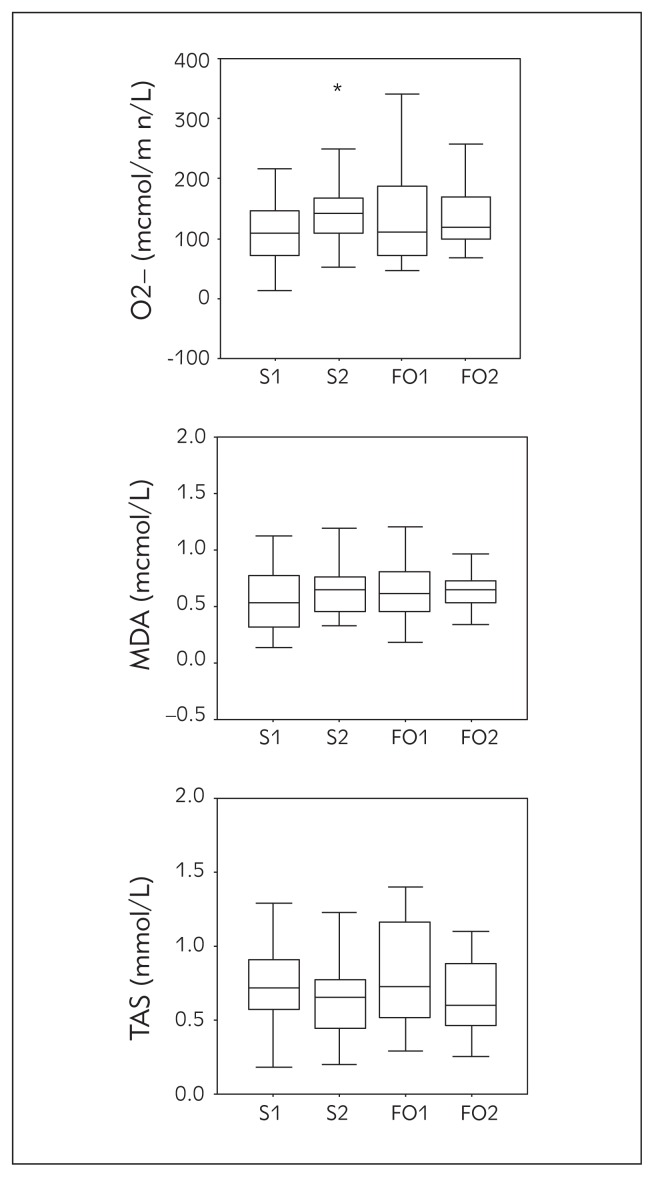
The dynamic of summary changes in all the analyzed oxidative stress parameters ( , MDA, TAS) during n-3 PUFA interventions is presented in Figure 2. We performed an analysis of oxidative stress changes during the intervention period by analyzing level and MDA status as oxidative stress markers. Concentration of increased by 29% in salmon group which was significant (p=0.025), and 9.5% (p=0.218) in fish oil group after the intervention period. The increased levels of MDA noticed after both fish and fish oil dietary intervention were not significant (p=0.188 and p=0.869, respectively). Non-enzymatic antioxidative protection, i.e. TAS concentration was lowered slightly but not significantly after both interventions compared to the baseline values (p=0.307 for salmon and p=0.246 for fish oil).
Figure 2.
, MDA and TAS concentrations at baseline and after 8 wks of dietary interventions with salmon and fish oil capsules (points: S1–salmon before, S2–salmon after, FO1–fish oil before, FO2–fish oil after). Values are expressed as median and interquartile range. The difference in relation to baseline was significant at p<0.05 (*).
Correlation coefficients between the plasma level of n-3 fatty acids and serum lipids, inflammatory and oxidative stress parameters were determined (Table IV). The present study revealed a statistically significant negative correlation between plasma n-3 fatty acids and sICAM-1 and CRP (p<0.05). On the other hand, a significant positive correlation was obtained between total n-3 fatty acids and (p<0.05).
Table IV.
Correlation coefficients between plasma level of n-3 FAs (n=140) and inflammatory markers and oxidative stress parameters before and after 8 weeks of each intervention with salmon and fish oil during the study period.
| TC | HDL | LDL | TG | sVCAM-1 | sICAM-1 | CRP | MDA | TAS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| EPA | −0.119 | 0.057 | −0.152 | −0.145 | 0.063 | −0.048 | −0.143* | 0.009 | −0.066 | 0.177 |
| DHA | −0.109 | 0.065 | −0.098 | −0.118 | −0.050 | −0.077 | −0.225* | 0.139 | −0.015 | 0.027 |
| EPA+DHA | −0.123 | 0.078 | −0.118 | −0.141 | −0.024 | −0.146 | −0.231* | 0.105 | −0.025 | 0.095 |
| Total n-3 FAs | −0.121 | 0.053 | −0.128 | −0.104 | −0.066 | −0.186* | −0.227* | 0.176* | −0.037 | 0.127 |
Marked correlations are significant at p<0.05.
TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; sVCAM-1, soluble vascular cell adhesion molecule; sICAM-1, soluble intercellular adhesion molecule; CRP, C-reactive protein; , superoxide anion; MDA, malondialdehyde; TAS, total antioxidant status.
Discussion
This investigation analyzed the possibility of modulating diverse cardiovascular risk factors with nutritionally relevant doses of LC n-3 PUFA from two different dietary sources in dyslipidemic middle-aged subjects. Although some beneficial trends were shown, the results of the present study suggested that following two sets of recommendations for LC n-3 PUFA intake had only a moderate effect on the lipid status, inflammatory or oxidative stress parameters.
The average middle-aged Serbian population is characterized by one or more cardiovascular risk factors as was proven in several previous reports (9, 11). According to the baseline values for serum lipids, BMI, blood pressure and glucose levels, the chosen group of participants represented a healthy middle-aged Serbian population with moderately altered lipid profile and increased BMI as risk factors. Subjects received salmon (fatty fish) and used commercial fish oil capsules during the cross-over intervention, with no energy and macronutrient changes under the free lifestyle conditions. Intervention was conducted taking into consideration two sets of dietary recommendations for LC n-3 PUFA intake for preventive purposes: two fish meals (preferably oily fish) per week for primary prevention of cardiovascular disease (7, 8); intake of a fish oil supplement in the quantity that is the median of the recommended daily intake given for these products by the producers (2 g/day). The fish oil capsules used in the study were chosen to ensure the best bioavailability of LC n-3 PUFA comparable to their bioavailability from fish (TG-binding form) (21, 22), with the most common ratio of EPA and DHA among n-3 supplements available on the Serbian market (3/2). No matching for the total amount of dietary n-3 PUFA, EPA, and DHA was done in order to increase the ecological validity of the study. Daily intake of EPA + DHA from salmon (945 mg) and from FOC (646 mg) was in accordance with the general AHA recommendations. A cross-over was used as a fairly unexplored study design in this kind of investigation to compare two sets of dietary recommendations for n-3 fatty acids intake coming from fish and a fish oil supplement.
Compliance to the study protocol was confirmed by demonstration of changes in relevant fatty acids in plasma phospholipids, because plasma FA take only several hours or days to reflect the incorporation of dietary n-3 fatty acids (23). Plasma n-3 fatty acid profile was remarkably increased with both salmon and fish oil capsules intervention. Increase in plasma EPA and DHA levels was dose-dependent in both cases and our results are in line with the results obtained in other studies. Cao et al. (24) who supplemented their participants with higher daily doses of EPA (1296 mg) and DHA (864 mg) after 8 weeks of supplementation noticed that the mean percent of EPA in plasma increased by 245%, whereas DHA concentrations increased by 73%. In the study of Harris (25), in which EPA and DHA concentrations from fish and fish oil capsules were matched, participants received 485 mg EPA+DHA during 16 weeks and this intervention produced a 60–80% rise in plasma EPA+DHA. In the studies mentioned the fatty acids from capsules were in the form of ethyl esters, while in our trial the fatty acids from both sources were in the form of triglycerides (21, 22).
Dyslipidemia is a traditional risk factor for atherosclerosis development and it is closely associated with increased endothelial dysfunction. It has been reported that LC n-3 PUFA can increase total cholesterol concentration by 5% to 10% and decrease TG by 20% to 50% (26, 27). Generally, the LC n-3 PUFA are used because of their ability to decrease TG concentrations (28). Participants in our study had slightly increased values of TC, LDL-C and TG at baseline and the recommended doses of fish and fish oil supplements did not result in significant changes in serum lipids, although a positive trend in TG reduction was observed in the salmon group. This result is in accordance with other authors, who reported no relevant changes in cholesterol and TG levels in healthy subjects after a 6-week intervention with 927 mg EPA+DHA (29), and after an 8-week intervention with 1200 mg EPA+DHA (30). Differences that were noticed between the salmon and supplement group in TG reduction potential may be explained by the higher level of TG at baseline in the salmon group compared to the fish oil group (2.01 mmol/L vs 1.76 mmol/L) and with the higher daily intake of total n-3 PUFA from salmon compared to fish oil supplements (1206 mg/d vs 704 mg/d). It seems that the effect on TG may be expected at higher baseline values of TG, but also with much higher doses of n-3 PUFA (1). EFSA health claim for beneficial effects of EPA and DHA on TG reduction specifies that 2 g daily intake is needed for such a result, but this intake cannot be achieved by following current general dietary recommendations. Although the effect of salmon consumption on TG level did not reach statistical significance, the 15% decrease noticed may be of clinical importance.
Based on the fact that endothelial dysfunction is the earliest manifestation of atherosclerosis along with the involvement of inflammation and oxidative stress at all stages of coronary atherosclerosis development, inflammatory biomarkers such as sICAM-1, sVCAM-1 and CRP are of particular interest (31–33). There is some evidence that these parameters may be regulated by fatty acids. In the current study, sICAM-1, sVCAM-1 and CRP concentrations were not affected by the quantities of LC n-3 PUFA used. Lack of direct association between fish or fish oil consumption and sICAM-1 levels is in accordance with other authors who reported no relevant differences in sICAM-1 level after consumption of much higher daily doses of EPA+DHA (34–36). Paulo et al. (37) also showed that sICAM-1 concentration did not change, but sVCAM-1 concentration significantly increased after eight weeks of intervention with oily fish and fish oil diets providing 3003 mg/d of n-3 PUFA by salmon and 1418 mg of n-3 PUFA by fish oil capsules. An increase in sVCAM-1 level was also reported in healthy women taking 6.6 g/day of n-3 PUFA but not in the group taking 2 g/day during 12 weeks (34). Our findings support a possible dose-dependent effect of n-3 PUFA on sVCAM-1 since low levels of n-3 PUFA had no effect on this parameter in our study. Both interventions in our study did not significantly influence plasma CRP concentrations and this is in accordance with the reports of Vega-Lopez et al. (38) and Madsen et al. (39) who did not observe any changes in the CRP serum levels, although some studies have reported a decrease in CRP concentration with low doses of LC n-3 PUFA (40). It is generally accepted that the effects of LC n-3 PUFA on CRP are highly variable (41).
An imbalance between the production of reactive oxygen species and antioxidant defense leads to oxidative stress and oxidative modification of LDL-cholesterol (42). Beneficial effects of reactive oxygen species are found only with physiologic low quantities while their overproduction may cause cell damage. Increased oxidative stress is involved in the development of the atherosclerotic process and is one of the risk factors for cardiovascular disease development. It is well-known that PUFA are prone to peroxidation by free radical products including reactive oxygen species and that the length and number of double bonds have great influence on the FA oxidative capacity (43, 44). On the other hand, other studies have shown that PUFA counteract the oxidative stress (45), so that the role of PUFA in prooxidative/antioxidative balance is still controversial.
In the current study we measured , MDA and TAS concentrations. The superoxide anion is considered to be a key player in oxidative stress. MDA is the lipid peroxidation end product that is one of the most reliable and widely used indices of oxidative stress. TAS has been successfully used to show the body’s ability to counteract oxidative stress since TAS represents many different plasma components with reducing power. The results from our study revealed elevated levels of both oxidative stress status parameters ( and MDA) and reduced protective TAS levels after both interventions compared to baseline. Intervention with salmon has shown a significant prooxidative effect through increase during the study period (p=0.025). The free radical disbalance noticed was probably counteracted with other protective factors, so we did not observe a significant increase in MDA concentration (p=0.188). However, fish oil supplementation did not influence any of the two oxidative stress markers measured in our current study in a significant way (p=0.218 for and p=0.869 for MDA).
Intervention with salmon has shown stronger prooxidative effects during the study period which could be explained with the higher content of n-3 PUFA (6, 46). This effect seems to be in the physiological range considering the values for the analyzed parameters obtained previously in healthy Serbian middle-aged subjects (47) and probably is part of the protective mechanisms which enable antioxidative gene expression activation as a reaction of the defense system in order to lower lipid peroxidation (48).
In the present study, TAS levels slightly decreased after both types of dietary intervention but this decrease was not statistically significant. Increased antioxidant utilization is needed because of the higher level of n-3 PUFA incorporation in the biological membranes after interventions and this could be an explanation for the decrease in measurable antioxidative defense (49).
Despite no relevant changes in inflammatory markers after the nutritional salmon and fish oil diet, we established significant negative correlations between plasma n-3 PUFA and sICAM-1 or CRP throughout the whole intervention period. This may suggest that n-3 PUFA intake in the recommended range may be beneficial with regard to the expression of sICAM-1/CRP and contribute to decreased inflammation in the vascular endothelium. A relevant positive correlation was found among plasma n-3 PUFA and . We assume that these changes occurred normally as part of the physiological response.
There were some limitations to this study. The small number of participants means that the study was underpowered to detect all statistically significant differences that might explain the trends observed for some results. Also, the intervention period lasted 8 weeks, which may be too short to detect differences between the two n-3 PUFA dietary sources. In conclusion, following the two sets of dietary recommendations for LC n-3 PUFA aimed at the general public had a similar and only moderate effect on lipid levels in average healthy middle-aged Serbian subjects with low fish consumption, primarily reducing the level of TG.
At the same time, the recommended doses did not substantially change the levels of inflammation markers and oxidative stress parameters. Consequently, a long-term follow-up could provide additional insights into the effect of these interventions on selected cardiovascular risk markers in this population.
Acknowledgment
This study was supported by a grant from the National Ministry of Science (III 46001), Belgrade, Serbia. We also thank Squadra, d.o.o., Belgrade, Serbia and Pharmanova d.o.o., Belgrade, Serbia for their interest and support.
List of abbreviations
- AHA
American Heart Association
- ANOVA
analysis of variance
- BMI
body mass index
- CRP
C-reactive protein
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FAME
fatty acid methyl ester
- LC PUFA
long-chain polyunsaturated fatty acid
- MDA
malondialdehyde
superoxide anion
- sICAM-1
soluble intercellular adhesion molecule
- sVCAM-1
soluble vascular cell adhesion molecule
- TAS
total antioxidant status
- TG
triglyceride.
Footnotes
Conflict of interest statement
The authors stated that there are no conflicts of interest regarding the publication of this article.
References
- 1.Vrablik M, Prusikova M, Snejdrlova M, Zlatohlavek L. Omega-3 fatty acids and cardiovascular disease risk: do we understand the relationship? Physiol Res. 2009;58:19–26. doi: 10.33549/physiolres.931860. [DOI] [PubMed] [Google Scholar]
- 2.Yates CM, Calder PC, Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Therapeut. 2014;141:272–82. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Myhrstad MC, Retterstøl K, Telle-Hansen VH, Ottestad I, Halvorsen B, Holven KB, Ulven SM. Effect of marine n-3 fatty acids on circulating inflammatory markers in healthy subjects and subjects with cardiovascular risk factors. Inflamm Res. 2011;60(4):309–19. doi: 10.1007/s00011-010-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miles EA, Thies F, Wallace FA, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of age and dietary fish oil on plasma soluble adhesion molecule concentrations. Clin Sci. 2001;100(1):91–100. [PubMed] [Google Scholar]
- 5.Cazzola R, Russo-Volpe S, Miles EA, Rees D, Banerjee T, Roynette CE, et al. Age- and dose-dependent effects of an eicosapentaenoic acid-rich oil on cardiovascular risk factors in healthy male subjects. Atherosclerosis. 2007;193(1):159–67. doi: 10.1016/j.atherosclerosis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Eritsland J. Safety considerations of polyunsaturated fatty acids. Am J Clin Nutr. 2000;71(1):197–201. doi: 10.1093/ajcn/71.1.197S. [DOI] [PubMed] [Google Scholar]
- 7.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA dietary guidelines: revision 2000: A statement for healthcare professionals from the nutrition committee of the American Heart Association. Circulation. 2000;102:2284–99. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 8.Joint WHO/FAO Expert Consultation, diet, nutrition, and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:89–90. [PubMed] [Google Scholar]
- 9.Đuričić I, Šobajić S, Peruničić-Peković G, Stojanov M, Rašić Z. Consumption of fish oil supplement alters erythrocyte fatty acid composition in overweight, hypercholesterolemic middle-aged Serbians. Nutr Res. 2007;27:529–34. [Google Scholar]
- 10.Vekić J, Kotur-Stevuljević J, Jelic-Ivanović Z, Spasić S, Spasojević-Kalimanovska V, Topić A, et al. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur J Clin Invest. 2007;37:15–23. doi: 10.1111/j.1365-2362.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 11.Serbian Acute Coronary Syndrome Registry – Report No 6. Institute of Public Health of Serbia; Belgrade: 2012. Incidence and mortality of acute coronary syndrome in Serbia in 2011. [Google Scholar]
- 12.The Expert Panel Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane-Stanley GH. Methods for the isolation and purification of total lipids from animal tissue. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Kates M. Techniques in lipidology. Amsterdam: North-Holland Publishing Company; 1972. [Google Scholar]
- 15.Ichihara K, Fukubayashi Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res. 2010;51(3):635–40. doi: 10.1194/jlr.D001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auclar C, Voisin E. Nitroblue tetrazolium reduction. In: Greenworld RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Florida: CRC Press; 1985. pp. 123–32. [Google Scholar]
- 17.Girotti MJ, Khan N, McLellan BA. Early measurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. The Journal of Trauma and Acute Care Surgery. 1991;31(1):32–5. doi: 10.1097/00005373-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–19. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Djuricic ID, Mazic SD, Kotur-Stevuljevic JM, Djordjevic VR, Sobajic SS. Longchain n-3 PUFA dietary recommendations are moderately efficient in optimizing their status in healthy middle-aged subjects with low fish consumption: A cross-over study. Nutr Res. 2014;34:210–18. doi: 10.1016/j.nutres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 21.Beckermann B, Beneke M, Seitz I. Comparative bioavailability of eicosapentaenoic acid and docasahexaenoic acid from triglycerides, free fatty acids and ethyl esters in volunteers. Arzneimittel-Forschung. 1990;40(6):700–4. [PubMed] [Google Scholar]
- 22.Hansen JB, Olsen JO, Wilsgård L, Lyngmo V, Svensson B. Comparative effects of prolonged intake of highly purified fish oils as ethyl ester or triglyceride on lipids, haemostasis and platelet function in normolipaemic men. Eur J Clin Nutr. 1993;47(7):497–507. [PubMed] [Google Scholar]
- 23.Von Schacky C. Use of red blood cell fatty-acid profiles as biomarkers in cardiac disease. Biomarkers. 2009;3(1):25–32. doi: 10.2217/17520363.3.1.25. [DOI] [PubMed] [Google Scholar]
- 24.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–72. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n-3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007;86(6):1621–5. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 26.Harris WS. n-3 Fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65:1645–54. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 27.Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, Beilin LJ. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–94. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 28.Harris WS, Dayspring TD, Moran TJ. Omega-3 fatty acids and cardiovascular disease: new developments and applications. Postgrad Med. 2013;125(6):100–13. doi: 10.3810/pgm.2013.11.2717. [DOI] [PubMed] [Google Scholar]
- 29.Visioli F, Risé P, Barassi MC, Marangoni F, Galli C. Dietary intake of fish vs. formulations leads to higher plasma concentrations of n-3 fatty acids. Lipids. 2003;38(4):415–18. doi: 10.1007/s11745-003-1077-x. [DOI] [PubMed] [Google Scholar]
- 30.Elvevoll EO, Barstad H, Breimo ES, Brox J, Eilertsen KE, Lund T, et al. Enhanced incorporation of n-3 fatty acids from fish compared with fish oils. Lipids. 2006;41(12):1109–14. doi: 10.1007/s11745-006-5060-3. [DOI] [PubMed] [Google Scholar]
- 31.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 32.Korita I, Bulo A, Langlois, Blaton V. Inflammation markers in patients with cardiovascular disease and metabolic syndrome. J Med Biochem. 2013;32:214–9. [Google Scholar]
- 33.Coskun A, Serteser M, Unsal I. Inhibition of cholesterol biosynthesis in hypercholesterolemia – is it right choice. J Med Biochem. 2013;32:16–9. [Google Scholar]
- 34.Eschen O, Christensen JH, De Caterina R, Schmidt EB. Soluble adhesion molecules in healthy subjects: a dose-response study using n-3 fatty acids. Nutr Metab Cardiovas. 2004;14(4):180–5. doi: 10.1016/s0939-4753(04)80002-4. [DOI] [PubMed] [Google Scholar]
- 35.Kew S, Banerjee T, Minihane AM, Finnegan YE, Muggli R, Albers R, et al. Lack of effect of foods enriched with plant- or marine-derived n-3 fatty acids on human immune function. Am J Clin Nutr. 2003;77(5):1287–95. doi: 10.1093/ajcn/77.5.1287. [DOI] [PubMed] [Google Scholar]
- 36.Miles EA, Banerjee T, Dooper MM, M’Rabet L, Graus YM, Calder PC. The influence of different combinations of linolenic acid, stearidonic acid and EPA on immune function in healthy young male subjects. Br J Nutr. 2004;91(6):893–903. doi: 10.1079/BJN20041131. [DOI] [PubMed] [Google Scholar]
- 37.Paulo MC, Andrade AM, Andrade ML, Morais MG, Kiely M, Parra D, et al. Influence of n-3 polyunsaturated fatty acids on soluble cellular adhesion molecules as biomarkers of cardiovascular risk in young healthy subjects. Nutr Metab Cardiovas. 2008;18(10):664–70. doi: 10.1016/j.numecd.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Vega-López S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with omega 3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53(2):236–40. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br J Nutr. 2003;89(4):517–22. doi: 10.1079/BJN2002815. [DOI] [PubMed] [Google Scholar]
- 40.Carrero JJ, Fonollá J, Marti JL, Jiménez J, Boza JJ, López-Huertas E. Intake of fish oil, oleic acid, folic acid, and vitamins B-6 and E for 1 year decreases plasma C-reactive protein and reduces coronary heart disease risk factors in male patients in a cardiac rehabilitation program. J Nutr. 2007;137(2):384–90. doi: 10.1093/jn/137.2.384. [DOI] [PubMed] [Google Scholar]
- 41.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189(1):19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo M, Kotur-Stevuljevic J, Berneis K, Spinas G, Rini GB, Jelic-Ivanovic Z, et al. Atherogenic dyslipidemia and oxidative stress: a new look. Translational Research. 2009;153(5):217–23. doi: 10.1016/j.trsl.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui RA, Harvey K, Stillwell W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem Phys Lipids. 2008;153:47–56. doi: 10.1016/j.chemphyslip.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Mori TA. Dietary n-3 PUFA and CVD: a review of the evidence. Proc Nutr Soc. 2014;73(1):57–64. doi: 10.1017/S0029665113003583. [DOI] [PubMed] [Google Scholar]
- 45.Giordano E, Visioli F. Long-chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostag Leukotr Ess. 2014;90:1–4. doi: 10.1016/j.plefa.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y, Sato M, Kurotani K, Nanri A, Kawai K, Kasai H, et al. PUFAs in serum cholesterol ester and oxidative DNA damage in Japanese men and women. Am J Clin Nutr. 2012;95(5):1209–14. doi: 10.3945/ajcn.111.030817. [DOI] [PubMed] [Google Scholar]
- 47.Stefanović A, Kotur-Stevuljević J, Spasić S, Vekić J, Zeljković A, Spasojević-Kalimanovska V, Jelić-Ivanović Z. HDL 2 Particles are associated with hyperglycaemia, lower PON1 activity and oxidative stress in type 2 diabetes mellitus patients. Clin Biochem. 2010;43(15):1230–5. doi: 10.1016/j.clinbiochem.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt S, Stahl F, Mutz KO, Scheper T, Hahn A, Schuchardt JP. Transcriptome-based identification of antioxidative gene expression after fish oil supplementation in normo- and dyslipidemic men. Nutr Metab. 2012;1:45. doi: 10.1186/1743-7075-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbosa DS, Cecchini R, El Kadri MZ, Marchesan Rodrýguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil – fatty acids. Nutrition. 2003;19(10):837–42. doi: 10.1016/s0899-9007(03)00162-x. [DOI] [PubMed] [Google Scholar]