Summary
Background
Severe sepsis and/or trauma complicated by multiple organ dysfunction syndrome are the leading causes of death in critically ill patients. The aim of this prospective single-centre study was to assess the prognostic value and daily trend of interleukin-6 (IL-6), neutrophil CD64 expression, C-reactive protein (CRP) and lipopolysaccharide-binding protein (LBP) regarding outcome in critically ill patients with severe trauma and/or severe sepsis. Outcome measure was hospital mortality.
Methods
One hundred and two critically ill patients admitted to the intensive care unit of a tertiary university hospital were enrolled in this prospective study. Blood samples were collected on admission (day 1), days 2 and 3.
Results
CD64 index was 1.6-fold higher on day 1 and 1.78-fold higher on day 2 in non-survivors (p<0.05). The area under the curve (AUC) for the CD64 index on day 1 for outcome was 0.727. At a cut-off level of 2.80 sensitivity was 75% and specificity was 65%. Patients with CD64 index level on day 1 higher than 2.80 had 2.4-fold higher probability of dying. Odds ratio is 2.40; 95% CI 0.60–9.67.
Conclusions
CD64 index on day 1 is a fairly good predictor of outcome. AUCs for IL-6, CRP and LBP were < 0.55, suggesting these biomarkers failed to predict outcome.
Keywords: biomarkers, critical care, leukocytes, outcome, prognosis
Kratak sadržaj
Uvod
Teška sepsa i/ili trauma kod koje se kao komplikacija javlja sindrom multiple organske disfunkcije je vodeći uzrok smrti kod kritično obolelih. Cilj ove prospektivne studije je bio da se proceni prognostička vrednost i utvrdi kinetika inter-leukina-6 (IL-6), ekspresije CD64 na neutrofilima, C-reaktivnog proteina (CRP) i proteina koji vezuje lipopolisaharid (lipopolysaccharide-binding protein – LBP) u smislu ishoda kod kritično obolelih sa teškom traumom i/ili teškom sepsom. Mera ishoda je bio hospitalni mortalitet.
Metode
Sto dva kritično obolela pacijenta primljena u jedini-cu intenzivne terapije tercijarne univerzitetske bolnice su obu-hvaćena prospektivnom studijom. Uzorci krvi su sakupljani na dan prijema (dan 1), a zatim drugog i trećeg dana.
Rezultati
CD64 indeks je bio 1,6 puta viši prvog dana i 1,78 puta viši drugog dana kod umrlih (p<0,05). Vrednost AUC/ROC za CD64 indeks prvog dana u smislu predikcije ishoda je bila 0,727. Pri cut-off vrednosti od 2,80 senzitivnost je bila 75%, specifičnost 65% a OR 2,40 (95% CI 0,60–9,67). Pacijenti sa nivoom CD64 indeksa prvog dana višim od 2,80 imali su 2,4 puta veću verovatnoću smrtnog ishoda.
Zaključak
Vrednost CD64 indeksa prvog dana je dobar prediktor ishoda. AUC/ROC vrednosti za IL-6, CRP i LBP su bile < 0,55, pa se ovi biomarkeri nisu pokazali dobrim pre-diktorima ishoda.
Introduction
Severe sepsis and/or trauma complicated by multiple organ dysfunction syndrome (MODS) are the leading causes of death in critically ill patients. Major determinant of outcome is intensity of insult as well as immuno-inflammatory response (1). Genetic predisposition (2) and high level of variability in circulating levels of various mediators (3–5) contribute to the complexity of the inflammatory response.
Interleukin (IL-6) is a proinflammatory cytokine with a pivotal role in acute-phase protein synthesis induction. Consequently, peak plasma levels of IL-6 are observed earlier than the highest levels of acute-phase proteins. Two of numerous acute-phase proteins, lipopolysaccharide-binding protein (LBP) and the well-known C-reactive protein (CRP), are interesting in terms of being markers of infection. LBP is involved in innate immune response with the specific role of binding to lipopolysaccharide. This complex then interacts with CD14 receptor and Toll-like receptor (TLR)-4 on monocytes/macrophages resulting in cytokine cascade initiation. LBP has two interesting features. First, although it binds to lipopolysaccharide from Gram-negative bacteria, this acute-phase protein is also elevated in Gram-positive sepsis. Second, the half-life of LBP is longer in comparison with cytokines induced by it (6). CRP plays an important role in immune response to insult, notably enhancing phagocytosis by binding to polysaccharides of microbes, and evolutionary is conserved and stable. Its peak concentration in plasma is usually 12 hours after LBP. Liver is mainly responsible for CRP synthesis (7). Quantitative neutrophil CD64 (high affinity Fcγ receptor) expression starts from less than 2000 sites per cell and becomes up-regulated in patients with systemic inflammatory response syndrome (8, 9). Although these biomarkers are not novel, in literature we found conflicting findings for each of them regarding outcome in critically ill patients.
The aim of this prospective single-centre study was to assess the prognostic value and daily trend of IL-6, neutrophil CD64, CRP and LBP regarding outcome in critically ill patients with severe trauma and/or severe sepsis. Outcome measure was hospital mortality.
Materials and Methods
Patients
This prospective study was conducted in a tertiary university hospital (Military Medical Academy, Belgrade, Serbia). Approval in concordance with the Declaration of Helsinki was obtained from the local ethics committee and informed consent from the patient or a first-degree relative. One hundred and two critically ill patients, admitted to the surgical intensive care unit (SICU) from August 2010 until March 2012 were enrolled. Blood samples were collected on admission (day 1), day 2 and day 3 from all patients with either severe sepsis or severe trauma with and without secondary sepsis. Sequential Organ Failure Assessment (SOFA) score (10), Simplified Acute Physiology Score (SAPS) II (11) and Acute Physiology and Chronic Health Evaluation (APACHE) II score (12) were calculated and recorded within the first 24 hours after admission to the SICU.
Sepsis patients were enrolled if they had fulfilled the current diagnostic criteria for severe sepsis (sepsis-induced tissue hypoperfusion or organ dysfunction) (13). For patients with blunt and/or penetrating trauma, Injury Severity Score – ISS (determined using Abbreviated Injury Scale – AIS) was calculated and recorded.
Sampling and analysis
Patient’s venous blood was drawn by trained, qualified phlebotomists. Two blood samples were taken from each patient: one serum tube (with clot activator) and one EDTA sample tube. Serum specimens were allowed to clot for 30 minutes in a vertical position and then centrifuged at 1300 RCF for 10 minutes. Quantitative measurements of CRP, IL-6 and LBP were performed from serum specimens, while neutrophil CD64 assay was measured from EDTA anticoagulated whole blood. All laboratory tests were performed within 3 hours of the patient draw time.
IL-6 and LBP measurements
Quantitative measurements of IL-6 and LBP were performed on a Siemens Immulite 2000 by solid-phase, enzyme-labeled, chemiluminescent immunometric assays. The instrument uses beads coated with appropriate monoclonal murine antibodies. The sample was incubated with an alkaline phosphatase-labeled reagent. Four washing cycles allowed no residual unbound label. Light was emitted when the chemiluminescent substrate reacted with alkaline phosphatase. The amount of emitted light was proportional to the IL-6 (LBP) concentration (14, 15).
Neutrophil CD64 assay
Quantitative measurement of neutrophil CD64 expression was done by an Abbott Cell-Dyne Sapphire hematology analyzer with the Trillium Diagnostic Leuko 64 assay kit. The principle of the assay is flow cytometry. The assay contains a mixture of monoclonal antibodies to two different epitopes of CD64 (clones 22 and 32.2) and monoclonal antibody to CD 163 (clone Mac2-158). Fifty μL of reagent A and 50 μL EDTA whole blood were mixed, incubated for 10 min. at room temperature and loaded into the analyzer. The results were calculated and reported as CD64 index by the Leuko64 QuantiCALC software, which used cluster-finding algorithms to locate lymphocytes, monocytes and neutrophils (16).
CRP measurement
Quantitative measurements of C-Reactive Protein were performed using the Siemens Dimension RxL Max analyzer, by particle enhanced turbidimetric immunoassay (PETIA). Anti-CRP coated particles of the reagent in the presence of CRP in the sample aggregate, causing an increase in turbidity. The increase in turbidity (at 340 nm) is proportional to CRP concentration in the sample.
Statistical analysis
All results are reported as mean ± SD. Statistical analysis of the results was performed with Mann-Whitney U – Wilcoxon Rank Sum W test, Pearson’s Chi-Square test and Student’s t-test. Receiver operator curves were generated to determine cut-off values for optimal sensitivity and specificity of the IL-6, CRP, LBP and CD64 levels for outcome prediction. The prognostic accuracy of biomarkers was expressed as the area under the ROC curve (AUC) and the 95% confidence interval (CI). The AUC defines the probability for correct discrimination between survivors and non-survivors. Statistical Package SPSS for Windows (version 7.2; Chicago, Illinois, USA) was used. Differences between groups were considered to be significant at p<0.05 and highly significant at p<0.01.
The expected minimal difference of biomarker values between survivors and non-survivors was estimated to be 30%. With the test power of 0.85 (85%) and the alpha probability of 0.05, the calculated number of patients was at least 96 (total sample size). For this purpose, a Chi-Square test model was performed by using the statistical software package GPower 3.1.
Results
Demographic data of the patients are shown in Table I. Profiles of IL-6, CD64, CRP and LBP from day 1 to day 3 are shown in Table II.
Table I.
Demographic data.
| Total no. of patients | 102 |
|
| |
| Age (median, range) | 60 (from 19 to 87 yrs) |
|
| |
| Sex, n (%) | |
| male | 55 (53.9%) |
| female | 47 (46.1%) |
|
| |
| Simplified Acute Physiology Score II – SAPS II score, mean ± SD | 56.82 ± 9.83 |
|
| |
| Acute Physiology and Chronic Health Evaluation II – APACHE II score, mean ± SD | 21.87 ± 4.21 |
|
| |
| Sequential Organ Failure Assessment – SOFA score, mean ± SD | 7.56 ± 2.30 |
|
| |
| Reason for ICU admission, n (%) | |
| Severe trauma (ISS 28.73 ± 9.40) | 20 (19.7%) |
| Severe trauma and secondary sepsis | 19 (18.6%) |
| Severe sepsis due to | |
| peritonitis | 39 (38.2%) |
| pancreatitis | 22 (21.6%) |
| other causes | 2 (1.90%) |
|
| |
| Blood cultures, n (%) | |
| Gram-positive | 16 (15.7%) |
| Gram-negative | 3 (2.95%) |
| Mixed | 46 (45.1%) |
| Fungi | 3 (2.95%) |
| Sterile | 34 (33.3%) |
|
| |
| Mortality, n (%) | 54 (52.9%) |
Table II.
Profiles of IL-6, CD64, CRP and LBP from day 1 to day 3.
| Parameter (Mean±SD) | Day 1 | Day 2 | Day 3 | p |
|---|---|---|---|---|
| IL-6 (pg/mL) | 595.74±278.08 | 212.30±128.17 | 151.98±115.84 | Z=–2.595; p<0.01 (day 1 vs day 2) |
| CD64 index | 4.54±3.25 | 4.47±2.77 | 3.99±2.25 | Z=−1.965; p<0.05 (day 2 vs day 3) |
| CRP (mg/L) | 120.82±55.83 | 143.43±71.55 | 125.04±57.76 | n.s. |
| LBP (μg/mL) | 36.80±17.34 | 34.90±15.37 | 37.33±17.03 | n.s. |
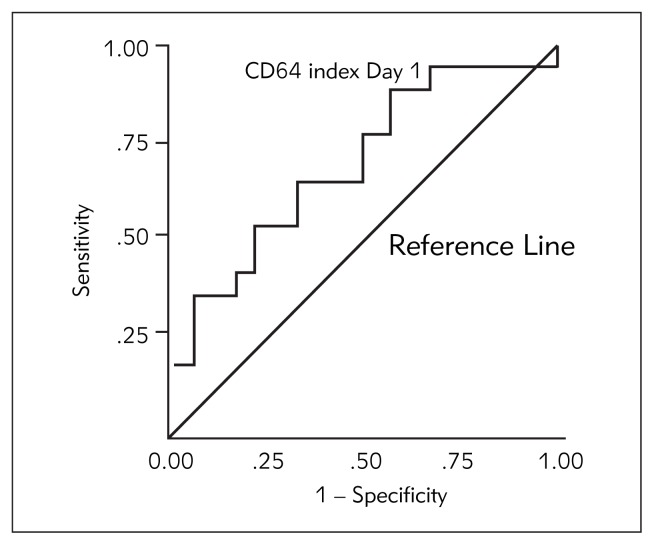
Of all the investigated parameters, there was a statistically significant difference only in the CD64 index between survivors and non-survivors on day 1 (t=−2.036; p<0.05) and day 2 (t=−2.391; p<0.05) of the study. CD64 index was 1.6-fold higher on day 1 and 1.78-fold higher on day 2 in non-survivors. This was confirmed with the Mann-Whitney U test: for CD64 on day 1 Z=1.947; p<0.05, and on day 2 Z=1.752; p<0.05. Receiver operator curves were generated to determine cut-off values for optimal sensitivity and specificity for the CD64 index levels on day 1 for outcome. The area under the curve (AUC) for the CD64 index on day 1 plots for outcome was 0.727; p<0.05 (Figure 1). CD64 index on day 1 is a fairly good predictor of outcome. At a cut-off level of 2.80, sensitivity was 75% and specificity was 65%. Patients with CD64 index level on day 1 higher than 2.80 had 2.4-fold higher probability of death than those with lower values. Odds ratio is 2.40; 95% CI 0.60 – 9.67. The area under the curve (AUC) for IL-6, CRP and LBP was < 0.55, so these biomarkers failed to predict outcome.
Figure 1.
Receiver operator curve for the CD64 index levels on day 1.
We also investigated all the parameters from day 1 to day 3. As far as IL-6 is concerned, there is a statistically highly significant difference between day 1 and day 2 (Wilcoxon Signed Ranks Test; Z=−2.595; p<0.01). A similar trend was observed in CD64: there is a statistically significant difference between day 2 and day 3 (Wilcoxon Signed Ranks Test; Z=−1.965; p<0.05). Profiles of IL-6, CD64, CRP and LBP from day 1 to day 3 according to outcome are shown in Table III and Figures 2–5.
Table III.
Profiles of IL-6, CD64, CRP and LBP from day 1 to day 3 according to outcome.
| Parameter mean±SD | Day 1 | Day 2 | Day 3 | |||
|---|---|---|---|---|---|---|
| survivors | non-survivors | survivors | non-survivors | survivors | non-survivors | |
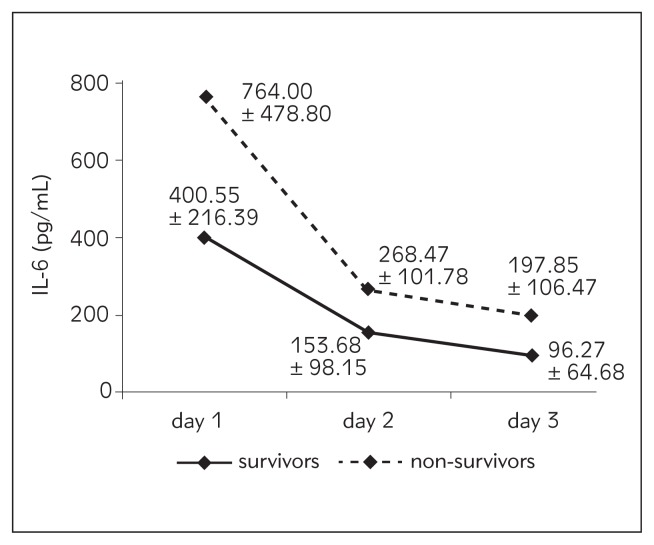
| IL-6 (pg/mL) | 400.55±216.39 | 764.00±478.80 | 153.68±98.15 | 268.47±101.78 | 96.27±64.68 | 197.85±106.47 |
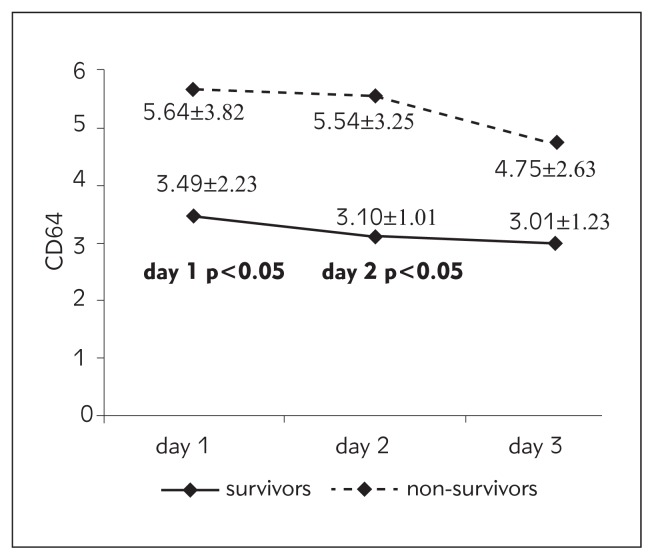
| CD64 index | 3.49±2.23 | 5.64±3.82* | 3.10±1.01 | 5.54±3.25* | 3.01±1.23 | 4.75±2.63 |
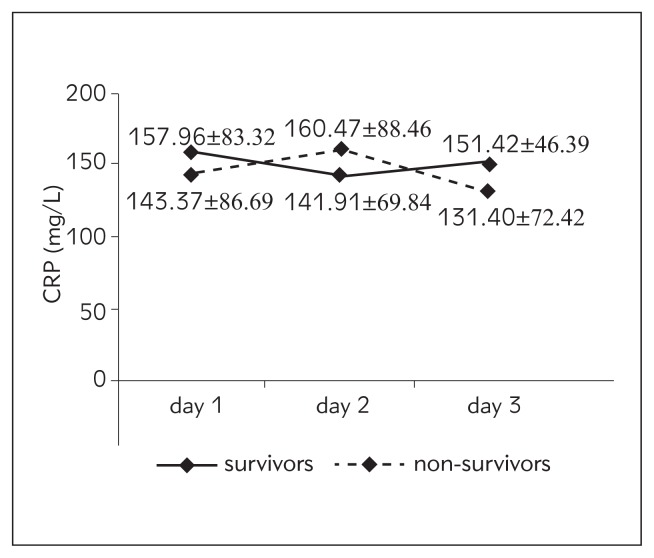
| CRP (mg/L) | 157.96±83.32 | 143.37±86.69 | 141.91±69.84 | 160.47±88.46 | 151.42±46.39 | 131.40±72.42 |
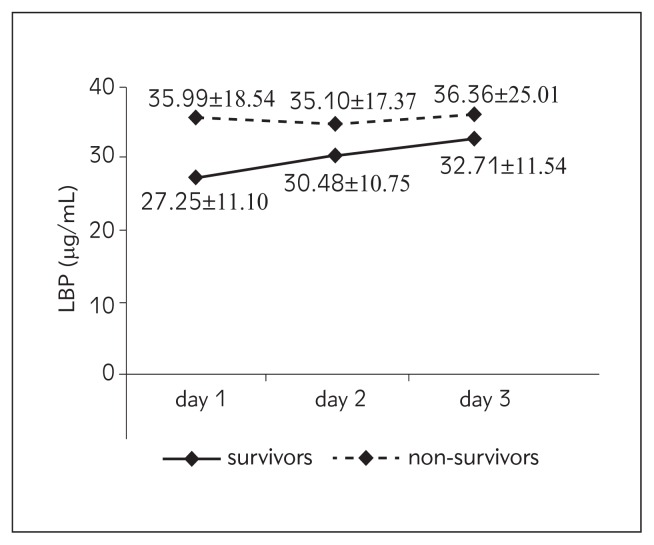
| LBP (μg/mL) | 27.25±11.10 | 35.99±18.54 | 30.48±10.75 | 35.10±17.37 | 32.71±11.54 | 36.36±25.01 |
survivors vs. non-survivors, p<0.05
Figure 2.
Values of IL-6 from day 1 to day 3 according to outcome.
Figure 3.
Values of CD64 index from day 1 to day 3 according to outcome.
Figure 4.
Values of CRP from day 1 to day 3 according to outcome.
Figure 5.
Values of LBP from day 1 to day 3 according to outcome.
Discussion
We chose to investigate IL-6, CRP and LBP because of their functional connection, and CD64 because it is still rather new and not routinely determined. In our study, IL-6 was not a reliable predictor of outcome, but the daily trend of this potent proinflammatory mediator was interesting: overall, there was a trend of decrease with a statistically highly significant difference between day 1 and day 2. Mean value of IL-6 was highest on day 1, 595.74 pg/mL; on day 2 it was 212.3 pg/mL, and it was lowest on day 3, 151.98 pg/mL. The decrease in IL-6 circulatory values on day 2 was much more pronounced in the non-survivors. A similar trend of IL-6 decrease from admission to day 3 and day 7 was found in the study of Miguel-Bayarri et al. (17) in a cohort of 81 septic patients. In their multivariate analysis with mortality as the dependent variable, they found that IL-6 was significant on day 3 (OR 2.6) with an AUC/ROC of 0.86. Contrary to our study, Pettila et al. (18) found in 61 critically ill patients with suspected sepsis a trend of IL-6 increase (1000 pg/mL – day 1 and 2000 pg/mL – day 2) in non-survivors; but in survivors the trend was the same as in our study (426 pg/mL – day 1, 162 pg/mL – day 2). In this study, IL-6 showed good discriminative power in the prediction of hospital mortality only on day 2 with an AUC (95% CI) of 0.79. There are several studies in which the daily trend of inflammatory mediators was not assessed, but their predictive capacity regarding outcome was based on one sample taken at the time of patient inclusion in the study. In two of them (19, 20), IL-6 showed moderate predictive value regarding mortality in critically ill septic patients with an AUC (95% CI) between 0.67 and 0.71. Contrary to that, Cheval et al. found that IL-6 was neither a predictor of infection nor 28-day mortality in 60 critically ill patients, as we did in our study (21). It seems that IL-6 did not meet the high expectations regarding predictive value. One possible explanation is that this cytokine may occur in relative asynchrony with other mediators and/clinical manifestations in the critically ill.
In our study, there was a difference in the daily trend of CRP between survivors and non-survivors but it did not reach statistical significance. CRP was not a reliable predictor of outcome. Overall, mean values were higher in survivors except for day 2. In the study of Miguel-Bayarri et al. (17) there was a somewhat different trend. CRP was higher in survivors on day 1, lower on day 3 and statistically significantly lower on day 7. Similar to our results, Pettila et al. (18) found higher CRP values in survivors on day 1 and in non--survivors on day 2; the difference was not statistically significant, and the predictive value regarding outcome was low with the AUC (95% CI) 0.38 and 0.53 respectively. Contrary to our results, Devran et al. (22) found that CRP was not a good predictor of outcome on day 1 (AUC 0.57) but its discriminative power in prediction of mortality increased on day 3 with an AUC of 0.72 and they concluded that CRP is a valuable predictor of mortality in patients with severe sepsis due to respiratory disease in the ICU. In a large Portuguese study, the authors found that CRP on day 3 is useful in the identification of community-acquired sepsis patients with poor outcome (23). Our patient population was rather different than in these two studies. Results from several studies (19–21) concur with ours, showing a lack of correlation of CRP with outcome and its poor predictive value regarding mortality.
In our study of LBP daily trend, overall there was a trend of increase in both survivors and non-survivors from day 1 to day 3. Mean values were higher in non-survivors, but this difference did not reach statistical significance. Like CRP, LBP was not a reliable predictor of outcome in our patient population. Most of the studies focused on this biomarker and outcome in critically ill patients concur with our results. Tschaikowsky et al. (24) studied 60 patients with postoperative sepsis and serially measured plasma levels of LBP for more than 2 weeks. LBP failed to discriminate survivors from non-survivors (AUC < 0.55) similarly to our results. Reinhart et al. (25) conducted a large study in which 327 critically ill patients were enrolled. Serum LBP concentrations were serially measured daily during the first week after admission to the ICU, and were similar on admission in survivors and non-survivors and did not discriminate ICU mortality. Authors concluded that the correlation of LBP concentrations with outcome is weak and that use of this biomarker is not warranted in this patient population. Another study of 68 ICU patients with systemic inflammatory response syndrome (SIRS), sepsis or septic shock showed similar results (26). On the other hand, contrary to our results, Villar et al. (27) concluded in their study that serial LBP serum measurements may offer a clinically useful biomarker regarding outcome. They investigated the time-course of LBP serum levels in 180 patients with severe sepsis: at study entry, at 48 hours and at day 7. LBP serum levels were similar in survivors and non-survivors at study entry (117 μg/mL vs. 129 μg/mL), but they found a significant difference at 48 hours (77.2 μg/mL vs. 121 μg/mL) and at day 7 (64 μg/mL vs. 89 μg/mL); the increase in LBP levels at 48 hours was associated with higher mortality (odds ratio 3.97). Interestingly, levels of LBP were 2- to 3-fold higher in this study than in others, including our study. We studied a different patient population from surgical ICU, most of them with accidental and/or surgical trauma, while Villar studied medical patients with sepsis. It is well known that acute phase proteins, like CRP and LBP, can be elevated during unspecific response to trauma regardless of its origin.
It is hard to find an adequate biomarker of the immune response with good predictive value regarding outcome because both proinflammatory and anti-inflammatory responses are concomitantly present in the immune response to insult (28). Neutrophil CD64 is one of many activation-related antigenic changes manifested by neutrophils during the normal patho-physiological acute inflammatory or innate immune response. CD64 appears to be a marker of neutrophil activation or systemic acute inflammatory response as its expression starts from less than 1000 to 2000 sites per cell (resting state) and becomes up-regulated in a graded fashion depending upon the intensity of stimulation (within 4 to 6 hours it can reach more than 10-fold higher levels) contrary to monocytes with a constitutively expressed CD64 antigen (29). Neutrophil CD64 index is designed so that normal inactivated neutrophils yield values of < 1.00 and blood samples from individuals with documented infection or sepsis typically show values > 1.50.
Of all the biomarkers investigated in our study, CD64 index showed the best performance regarding mortality prediction. There was a statistically significant difference in CD64 index between survivors and non-survivors on day 1 and day 2 of the study. CD64 index was 1.6-fold higher on day 1 and 1.78-fold higher on day 2 in non-survivors. Receiver operator curves were generated to determine cut-off values for optimal sensitivity and specificity for the CD64 index levels on day 1 for outcome (Figure 1). The area under the curve (AUC) for the CD64 index on day 1 plots for outcome was 0.727; p<0.05 (Figure 1). We concluded that CD64 index on day 1 has good discriminative power in the prediction of hospital mortality. At a cut-off level of 2.80, sensitivity was 75% and specificity was 65%. Patients with CD64 index level on day 1 higher than 2.80 had 2.4-fold higher probability of death than those with lower values. Odds ratio is 2.40; 95% CI 0.60–9.67.
Several studies focused on neutrophil CD64 and outcome in critically ill patients concur with our results. Hsu et al. enrolled 66 critically ill patients in their study (30) and they showed that CD64 expression was significantly higher in non-survivors than in survivors and that this biomarker was a significant predictor of mortality with an AUC of 0.70. Disseminated intravascular coagulation (DIC) is frequent in critically ill patients. Song et al. investigated the association of neutrophil CD64 expression with severity and prognosis of DIC (31). They enrolled 94 patients with suspected DIC and their results demonstrated that neutrophil CD64 expression was significantly increased in a 28-day non-survival group and that the 28-day survival rate showed a stronger association with the neutrophil CD64 expression than with the DIC score. Prognostic value of this biomarker in terms of 28-day mortality was very good with an AUC of 0.81. In another study, authors investigated neutrophil CD64 expression as an early marker of severity and outcome in 47 critically ill septic patients (32). They found that CD64 expression was associated with the 28-day mortality (OR=1.3, p=0.01); ROC curve analysis showed that CD64 was a good predictor of outcome with an AUC of 0.75. In their patient population, an increase of CD64 expression on neutrophils by 1000 units increases 1.3-fold the probability of death. Contrary to our results, some authors showed a correlation between survival and higher CD64 expression. Cid et et al. (33) included 132 patients with fever > 38 °C during the previous 24 hours from the emergency department in their study. They found that survivors showed a higher CD64 index when compared with non-survivors. ROC curve analysis for predicting outcome showed an AUC of 0.71 with the cut-off value of 1.5, with sensitivity of 85% and rather low specificity of 33%. Our patient population was significantly different than in this study. There are two studies (34, 35) with a rather small number of severe septic patients (31 and 20 respectively) demonstrating that survival is associated with increased CD64 antigen expression. In both studies the conclusion is very similar: poor outcome is due to neutrophil inactivation with reduced phagocytic activity during compensatory antiinflammatory response syndrome (CARS). But, there is growing evidence of the role of proinflammatory mediators and acute phase response in developing immune dysfunction (36, 37). This observation may contribute to the explanation of the apparent paradox of immune suppression presence in a patient with manifested hyper-inflammation (38). Clinically, many patients show signs of persisting inflammation and immune-mediated organ damage while simultaneously remaining highly susceptible to secondary infections, suggesting the term complex immune dysfunction syndrome – CIDS (3). The novel investigations of sepsis point out that virtually all immune cells demonstrate immune hypoactivity. For example, neutrophils display a dual state by the concomitant presence of activation and dysfunction features. In the critically ill patients, dysfunction of organs is, to a considerable degree, driven by neutrophils, which are key immune cells (39, 40). They tend to express surface markers of activation (increased levels of CD11b and CD64), but simultaneously they display major impairment of phagocytic capacity and generation of reactive oxygen species (ROS). Mortality rate can rise above 50% in critically ill patients with immune dysfunction despite modern therapy (41), so our goal was to conduct a real-life study regarding biomarkers in an attempt to improve the standard of care for this patient population.
Conclusions
It is evident that there are conflicting findings for each of the investigated biomarkers. It might be due to the relatively small number of patients and/or slightly different patient populations. Unlike IL-6, CRP and LBP, in our study the neutrophil CD64 index showed good discriminative power in the prediction of hospital mortality. However, large multicenter studies are warranted to validate this biomarker in the routine clinical ICU setting.
Footnotes
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Surbatovic M, Radakovic S, Jovanovic K, Romic P. New strategies in multiple organ dysfunction syndrome therapy for sepsis. Srp Arh Celok Lek. 2005;133(7–8):3791.83. doi: 10.2298/sarh0508379s. [DOI] [PubMed] [Google Scholar]
- 2.Surbatovic M, Grujic K, Cikota B, Jevtic M, Filipovic N, Romic P, et al. Polymorphisms of genes encoding tumor necrosis factor-alpha, interleukin-10, cluster of differentiation-14 and interleukin-1ra in critically ill patients. J Crit Care. 2010;25(3):542.e1–8. doi: 10.1016/j.jcrc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Morris AC, Simpson AJ, Walsh TS. Hyperinflammation and mediators of immune suppression in critical illness. In: Vincent JL, editor. Annual update in intensive care and emergency medicine. Berlin Heidelberg: Springer-Verlag; 2013. pp. 135–44. [Google Scholar]
- 4.Surbatovic M, Radakovic S. Tumor necrosis factor-alpha levels early in severe acute pancreatitis: is there predictive value regarding severity and outcome? J Clin Gastroenterol. 2013;47(7):637–43. doi: 10.1097/MCG.0b013e31828a6cfc. [DOI] [PubMed] [Google Scholar]
- 5.Surbatovic M, Filipovic N, Radakovic S, Stankovic N, Slavkovic Z. Immune cytokine response in combat casualties: blast or explosive trauma with or without secondary sepsis. Mil Med. 2007;172(2):190–5. doi: 10.7205/milmed.172.2.190. [DOI] [PubMed] [Google Scholar]
- 6.Gaini S, Graesboll Koldkjaer O, Pedersen C, Stenvang Pedersen S. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community acquired infections and sepsis: a prospective study. Crit Care. 2006;10:R53. doi: 10.1186/cc4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Povoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, et al. Early identification of intensive care unit-acquired infections with daily monitoring of C-reactive protein: a prospective observational study. Crit Care. 2006;10:R63. doi: 10.1186/cc4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi SS, Lewis SM, Gant VA, Treacher D, Davis BH, Brown KA. Increased distribution and expression of CD64 on blood polymorphonuclear cells from patients with the systemic inflammatory response syndrome (SIRS) Clin Exp Immunol. 2001;125(2):258–65. doi: 10.1046/j.1365-2249.2001.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med. 2006;130(5):654–61. doi: 10.5858/2006-130-654-NCIAII. [DOI] [PubMed] [Google Scholar]
- 10.Moreno RP, Vincent JL, Matos R, Mendonca A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25(7):686–96. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 11.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 12.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 14.Meszaros K, Aberle S, White M, Parent JB. Immuno-reactivity and bioactivity of lipopolysaccharide binding protein in normal and heat-inactivated sera. Infect Immun. 1995;63(1):363–5. doi: 10.1128/iai.63.1.363-365.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hack CE, De Groot ER, Felt-Bersma RJF, Nuijens JH, Strack Van Schijndel RJM, Eerenberg-Belmer AJM, Thijs LG, Aarden LA. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74(5):1704–10. [PubMed] [Google Scholar]
- 16.Van der Meer W, van Dun L, Klein Gunnewiek J, Roemer B, Scott CS. Simultaneous determination of membrane CD64 and HLA-DR expression by blood neutrophils and monocytes using the monoclonal antibody fluorescence capability of routine haematology analyser. J Immunol Meth. 2006;311:207–19. doi: 10.1016/j.jim.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Miguel-Bayarri V, Casanoves-Laparra EB, Pallas-Beneyto L, Sancho-Chinesta S, Martin-Osorio LF, Tormo-Calandin C, et al. Prognostic value of the biomarkers procalcitonin, interleukin-6 and C-reactive protein in severe sepsis. Med Intensiva. 2012;36(8):556–62. doi: 10.1016/j.medin.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Pettila V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28(9):1220–5. doi: 10.1007/s00134-002-1416-1. [DOI] [PubMed] [Google Scholar]
- 19.Suares-Santamaria M, Santolaria F, Perez-Ramirez A, Aleman-Valls MR, Martinez-Riera A, Gonzales-Reimers E, et al. Prognostic value of inflammatory markers (notably cytokines and procalcitonin), nutritional assessment, and organ function in patients with sepsis. Eur Cytokine Netw. 2010;21(1):19–26. doi: 10.1684/ecn.2009.0185. [DOI] [PubMed] [Google Scholar]
- 20.Fraunberger P, Wang Y, Holler E, Parhofer KG, Nagel D, Walli AK, et al. Prognostic value of interleukin 6, procalcitonin and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock. 2006;26(1):10–12. doi: 10.1097/01.shk.0000215319.06866.bd. [DOI] [PubMed] [Google Scholar]
- 21.Cheval C, Timsit JF, Garrouste-Orgeas M, Assicot M, De Jonghe B, Misset B, et al. Procalcitonin (PCT) is useful in predicting the bacterial origin of an acute circulatory failure in critically ill patients. Intensive Care Med. 2000;26(Suppl 2):S153–S158. doi: 10.1007/BF02900729. [DOI] [PubMed] [Google Scholar]
- 22.Devran O, Karakurt Z, Adiguzel N, Gungor G, Mocin YO, Balci MK, et al. C-reactive protein as a predictor of mortality in patients affected with severe sepsis in intensive care unit. Multidiscip Respir Med. 2012;7(1):47–52. doi: 10.1186/2049-6958-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Povoa P, Teixeira-Pinto AM, Carneiro AH for the Portuguese Community-Acquired Sepsis Study Group (SACiUCI) C-reactive protein, an early marker of community-acquired sepsis resolution: a multi-center prospective observational study. Crit Care. 2011;15:R169. doi: 10.1186/cc10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tschaikowsky K, Hedwig–Geissing M, Schmidt J, Braun GG. Lipopolysaccharide-Binding Protein for Monitoring of Postoperative Sepsis: Complemental to C-Reactive Protein or Redundant? PLoS ONE. 2011;6(8):e23615. doi: 10.1371/journal.pone.0023615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. Lipopolysaccharide binding protein in surgical intensive care unit: A marker of sepsis? Crit Care Med. 2008;36(7):2014–22. doi: 10.1097/CCM.0b013e31817b86e3. [DOI] [PubMed] [Google Scholar]
- 26.Prucha M, Herold I, Zazula R, Dubska L, Dostal M, Hildebrand T, et al. Significance of lipopolysaccharide-binding protein (an acute phase protein) in monitoring critically ill patients. Crit Care. 2003;7:R154. doi: 10.1186/cc2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villar J, Perez-Mendez L, Espinosa E, Flores C, Blanco J, Muriel A, et al. Serum Lipopolysaccharide Binding Protein Levels Predict Severity of Lung Injury and Mortality in Patients with Severe Sepsis. PLoS ONE. 2009;4(8):e6818. doi: 10.1371/journal.pone.0006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12(3):151–70. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann J. Neutrophil CD64 as a sepsis biomarker. Biochem Med. 2011;21(3):282–90. doi: 10.11613/bm.2011.038. [DOI] [PubMed] [Google Scholar]
- 30.Hsu KH, Chan MC, Wang JM, Lin LY, Wu CL. Comparison of Fcg receptor expression on neutrophils with procalcitonin for the diagnosis of sepsis in critically ill patients. Respirology. 2011;16(1):152–60. doi: 10.1111/j.1440-1843.2010.01876.x. [DOI] [PubMed] [Google Scholar]
- 31.Song SH, Kim HK, Park MH, Cho Hl. Neutrophil CD64 expression is associated with severity and prognosis of disseminated intravascular coagulation. Thromb Res. 2008;121(4):499–507. doi: 10.1016/j.thromres.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Livaditi O, Kotanidou A, Psarra A, Dimopoulou I, Sotiropoulou C, Augustatou K, et al. Neutrophil CD64 expression and serum IL-8: Sensitive early markers of severity and outcome in sepsis. Cytokine. 2006;36(5–6):283–90. doi: 10.1016/j.cyto.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Cid J, Garcia-Pardo G, Aguinaco R, Sanchez R, Llorente A. Neutrophil CD64: diagnostic accuracy and prognostic value in patients presenting to the emergency department. Eur J Clin Microbiol Infect Dis. 2011;30(7):845–52. doi: 10.1007/s10096-011-1164-7. [DOI] [PubMed] [Google Scholar]
- 34.Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol. 2008;154(1):87–97. doi: 10.1111/j.1365-2249.2008.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller Kobold AC, Tulleken JE, Zijlstra JG, Sluiter W, Hermans J, Kallenberg CGM, et al. Leukocyte activation in sepsis; correlations with disease state and mortality. Intensive Care Med. 2000;26(7):883–92. doi: 10.1007/s001340051277. [DOI] [PubMed] [Google Scholar]
- 36.Surbatovic M, Veljovic M, Jevdjic J, Popovic N, Djordjevic D, Radakovic S. Immunoinflammatory response in critically ill patients: severe sepsis and/or trauma. Mediators Inflamm. 2013;2013:362793. doi: 10.1155/2013/362793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NF. New insights into the biology of the acute phase response. J Clin Immunol. 1999;19(4):203–14. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- 38.Ward PA. Immunosuppression in sepsis. JAMA. 2011;306(23):2618–9. doi: 10.1001/jama.2011.1831. [DOI] [PubMed] [Google Scholar]
- 39.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157–69. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 40.Raicevic R, Jovicic A, Dimitrijevic R, Surbatovic M, Marenovic T. Septic encephalopathy – prognostic value of the intensity of consciousness disorder to the outcome of sepsis. Vojnosanit Pregl. 2001;58(2):151–6. [PubMed] [Google Scholar]
- 41.Surbatovic M, Jevdjic J, Veljovic M, Popovic N, Djordjevic D, Radakovic S. Immune response in severe infection: could life-saving drugs be potentially harmful? Scientific-World Journal. 2013;2013:961852. doi: 10.1155/2013/961852. [DOI] [PMC free article] [PubMed] [Google Scholar]