Summary
Background
We compared factors of inflammation – high sensitivity C-reactive protein (hsCRP) and pentraxin-3 (PTX3), and we explored their relationship with coronary artery disease (CAD). Also, we tested the usefulness of hsCRP and PTX3 in the risk assessment of coronary stenosis development and the diagnostic ability of these biomarkers to detect disease severity.
Methods
The study group consisted of 93 CAD patients undergoing coronary angiography. Patients were divided into CAD(0), representing subclinical stenosis, and CAD (1–3), representing significant stenosis in one, two or three vessels.
Results
We determined the concentration of lipid status parameters, hsCRP and PTX3. We found significantly lower PTX3 and hsCRP concentrations in CAD(0) than in CAD(1–3) group. Concentration of PTX3 showed an increasing trend with the increasing number of vessels affected. The area under ROC curve (AUC) for the combinations of hsCRP and PTX3 with lipid parameters had useful accuracy for detecting CAD(1–3) patients (AUC=0.770, p<0.001).
Conclusion
PTX3 is a promising independent diagnostic marker for identifying patients with CAD, and a useful indicator of disease progression. In all the analyses PTX3 showed better performance than hsCRP. A combination of PTX3, hsCRP with the lipid status parameters provides risk stratification of the development of coronary stenosis and better classification than their individual application.
Keywords: high sensitivity C-reactive protein, pentraxin-3, coronary artery disease, diagnostic accuracy
Kratak sadržaj
Uvod
Visokoosetljivi C-reaktivni protein (hsCRP), kao marker hronične inflamacije, može biti korišćen u proceni rizika od kardiovaskularnih bolesti. Cilj rada je bio da se uporede markeri inflamacije hsCRP i pentraksin-3 (PTX3) i da se ispita njihova veza sa koronarnom arterijskom bolešću (KAB). Takođe, analiziran je značaj hsCRP i PTX3 u proceni rizika za koronarnu stenozu i dijagnostička sposobnost ovih biomarkera da utvrde težinu bolesti.
Metode
Istraživanjem je obuhvaćeno 93 pacijenta koji su podvrgnuti koronarnoj angiografiji. Pacijenti sa subkliničkom stenozom na koronarnim krvnim sudovima svrstani su u KAB(0) grupu, dok su pacijenti sa značajnom stenozom na jednom, dva ili tri krvna suda svrstani u KAB(1–3) grupu. Određivane su koncentracije parametara lipidnog statusa, hsCRP i PTX3.
Rezultati
Prosečne koncentracije PTX3 i hsCRP kod pacijenata u KAB(0) grupi bile su značajno niže u poređenju sa koncentracijama istih parametara u KAB(1–3) grupi. Koncentracija PTX3 pokazala je značajnu pozitivnu korelaciju sa porastom broja krvnih sudova zahvaćenih stenozom. Površina ispod receiver operating characteristic krive (AUC) za kombinaciju hsCRP i PTX3 sa lipidnim parametrima pokazala je zadovoljavajuću tačnost za detektovanje KAB(1–3) pacijenata (AUC 0,770, p<0,001).
Zaključak
Određivanje PTX3 pomaže u identifikaciji osoba sa KAB i može biti korisno u proceni progresije bolesti. U svim analizama PTX3 je, kao biomarker, pokazao bolje dijagnostičke karakteristike u poređenju sa hsCRP. Kombinacija PTX3, hsCRP i parametara lipidnog statusa, u poređenju sa njihovom pojedinačnom upotrebom, obezbeđuje bolju klasifikaciju pacijenata u zavisnosti od težine KAB.
Introduction
It is well known that immunological and inflammatory processes play a fundamental role in atherogenesis (1), from the endothelial injury to acute clinical manifestations (2). With the growing number of coronary artery disease (CAD) population, the aim is to find a biomarker, or the best combination of markers, which could help in identifying patients prone to developing severe coronary stenosis, in early stages of the atherosclerotic process. A variety of inflammatory and other biochemical markers potentially related to atherogenesis has been identified. These markers include acute-phase reactants, pro-inflammatory cytokines (interleukin-1, interleukin-6, tumor necrosis factor-α), anti-inflammatory cytokines (interleukin-1 receptor antagonist, interleukin-10), matrix metalloproteinases (MMPs), cellular adhesion molecules, soluble CD40 ligand (sCD40L), leukocyte myeloperoxidase. Some of those markers have been shown to be significantly associated with CAD (MMPs, sCD40L, interleukin-1 receptor antagonist), but they are not related to the severity of coronary stenosis. Science is still searching for a marker that will have better diagnostic abilities than those previously examined, which will be able to predict coronary stenosis and allow avoiding unnecessary angiography in patients.
There are a few systemic inflammatory markers that are recognized as established measures of cardiovascular risk prediction (3, 4). C-reactive protein (CRP), an acute phase reactant and a member of the pentraxin superfamily, is one of the most actively studied biomarkers. CRP is widely accepted as a biomarker of systemic inflammation and routinely used as a marker of infection and inflammation in clinical practice. Slightly elevated CRP concentrations may indicate a low level of chronic inflammatory reaction in patients at risk of developing cardiovascular disease (CVD). High-sensitivity assays were developed with improved sensitivity and precision at low concentrations of CRP with the aim to detect subclinical inflammation. The association between elevated high sensitivity serum C-reactive protein (hsCRP) level and increased risk of developing cardiovascular events has been well established in many studies (5–7). However, CRP response is triggered by many disorders unrelated to CVD.
Pentraxin-3 (PTX3) is the prototypic member of a long pentraxin family and has C-terminal sequence homology with short pentraxin CRP (8). However, PTX3 and CRP are produced by different tissues and may be involved in different pathophysiological mechanisms (9). Whereas hepatocytes are the main source of CRP, PTX3 is produced at sites of inflammation by a wide range of different cell types, including endothelial cells, fibroblasts, monocytes, macrophages, dendritic cells, adipocytes (9). PTX3 is produced locally in atherosclerotic lesions (10) and was reported to accumulate during atherosclerotic progression in a murine model (11).
Studies showed that deficiency of PTX3 is associated with increased heart damage with a greater noreflow area and increased inflammatory response (12, 13). More recently, deficiency of PTX3 in apolipoprotein E knockout mice was shown to be associated with increased atherosclerosis, macrophage accumulation within the plaque, and a more pronounced inflammatory profile in the vascular wall (11). Although these observations indicate a cardiovascular protective effect of PTX3, they also suggest the possibility that the increased levels of PTX3 in subjects with CVD may reflect a protective physiological response that correlates with the severity of the disease.
In this cross-sectional study we compared two familiar factors of inflammation – hsCRP and PTX3, and we examined their association with the extent of coronary stenosis in patients with coronary artery disease (CAD). Also, we tested the usefulness of hsCRP and PTX3 in the risk assessment of significant coronary stenosis development using a multi-marker approach, as well as the diagnostic ability of these two biomarkers for detection of disease severity.
Material and Methods
Subjects
The study group consisted of 93 CAD patients who were undergoing coronary angiography. All patients were treated at the Institute for Cardiovascular Diseases located in the Clinical Centre of Serbia in Belgrade. Two cardiologists unaware that the patients were enrolled in the study reviewed all the angiograms.
All the enrolled patients completed a questionnaire that incorporated numerous risk-related issues. The following factors were recorded: (1) smoking at the time of angiography or at the time when the first symptoms appeared; (2) hypertension (HT) indicated by a systolic blood pressure ≥ 140 mm Hg, a diastolic blood pressure ≥ 90 mm Hg or prescribed anti-hypertensive medication; (3) body mass index (BMI), (4) waist-to-hip ratio (W/H). We excluded patients with a history of recent clinical infection, concurrent major renal, hepatic or malignant disease, surgery or major trauma during the month prior to study entry. Since low-grade inflammation is usually defined when the hsCRP concentration is below 10 mg/L we excluded patients with concentrations of hsCRP ≥ 10 mg/L which indicated a clinically relevant inflammatory condition (14). The patients using statins in the treatment were eliminated from analysis because of their effect on inflammatory parameters. All patients gave informed consent prior to their enrolment in the study. The study was planned according to the ethical guidelines following the Declaration of Helsinki. The institutional review committee approved our study protocol thereby following local biomedical research regulations.
Patients were divided into two groups: CAD(0) representing stenosis less than 50% or subclinical stenosis (43 patients) and CAD(1–3) representing stenosis above 50% in one or more vessels – significant stenosis (50 patients). A further division of the patients was performed according to the extent of CAD. We subdivided CAD(1–3) group into 3 groups: CAD(1) included 14 patients (28%) with significant stenosis of one vessel, CAD(2) included 20 patients (40%) with significant stenosis of two vessels and CAD(3) included 16 patients (32%) with significant stenosis of three vessels.
Methods
Blood samples were obtained from the patients after overnight fasting. Peripheral venous blood was drawn into collection tubes containing ethylene diamine-tetraacetic acid (EDTA) or serum separator gel. Lipid and lipoprotein parameters were determined in EDTA plasma, hsCRP and PTX3 in serum. Aliquots of plasma and serum were stored at −80 °C until assayed. Most of the biochemical markers were measured using an ILAB 600 analyser (Instrumentation Laboratory, Milan, Italy). Total cholesterol (TC) and triglycerides (TG) were assayed using routine enzymatic methods. HDL-C was quantified using the same enzymatic method after precipitation of the plasma with phosphotungstic acid in the presence of magnesium ions (15). The concentration of LDL-C was calculated using the Friedewald formula (16). hsCRP was measured using a latex-enhanced immunoturbidimetric method (Tina-quant CRP Roche, Indianapolis, USA). We excluded individuals with hsCRP ≥ 10 mg/L, a level considered to be indicative of a clinically-relevant inflammatory condition. PTX3 was measured by ELISA (Human Pentraxin3 DuoSet ELISA R&D Systems, Minneapolis, USA).
Statistical analysis
Data are shown as mean ± standard deviation for normally-distributed continuous variables and relative to absolute frequencies for categorical variables. As the distributions of TG, hsCRP and PTX3 were skewed, data were log-transformed for analysis and presented as geometrical means and 95% confidence intervals (CIs). Continuous variables were compared using Student’s t test and ANOVA with the Tukey’s post hoc test for subgroup differences. Categorical variables were analysed using Chi-square tests for contingency tables. Univariate associations were evaluated by Pearson’s linear correlation analysis. Independent association of higher hsCRP and PTX3 with significant stenosis was tested using binary logistic regression analysis. Adjustments were made to correct the influence of hsCRP, traditional non-lipid and lipid risk factors (age, BMI, W/H ratio, TC, LDL-C, HDL-C and TG) on PTX3. Odds ratio (OR) and 95% CI were calculated. Predictive and discriminative abilities of these models were assessed by the Hosmer–Lemeshow goodness of fit test and by the area under the receiver operating characteristic (ROC) curve (AUC), respectively. The former compares the observed frequencies of patients with the event of interest with the expected frequencies based on the values of the estimated probabilities obtained by the logistic regression models. In this test, a high p-value (non-significant) indicates that the model is performing well and has a good fit. The AUC ranges from 0 to 1 and provides a measure of the model’s ability to discriminate between those subjects who experience the outcome of interest and those who do not. An AUC equal to 0.5 means no classification accuracy. Swets (17) suggested the following guidelines for interpretation of the area under ROC curves (AUCs): 0.5–0.7 low accuracy; 0.7–0.9 accuracy useful for some purposes; and >0.9, high accuracy. To compare the AUCs from each of these models, the method described by DeLong et al. was used (18).
The net reclassification index (NRI) was also calculated. NRI summarizes the net proportion of individuals with »correct« reclassification (e.g., those who develop events were up-classified, and those who do not develop events were down-classified) and »incorrect« reclassification (those who develop events who were down-classified, and those who do not develop events who were up-classified) (19). A two-tailed p value of p≤0.05 was considered significant.
Results
Demographic characteristics of the 93 patients are presented in Table I. The prevalence of males was higher in CAD(1–3) patients than in CAD(0) patients. In addition, a higher W/H ratio and prevalence of smokers were also apparent in CAD(1–3) patients. CAD(1–3) patients had higher concentrations of hsCRP and PTX3 than CAD(0) patients.
Table I.
Demographic characteristics and plasma levels of lipids and inflammatory markers in CAD(1–3) and CAD(0) patients.
| Variable | CAD(0) patients | CAD(1–3) patients | P-value |
|---|---|---|---|
| N | 43 | 50 | |
| Male/female, (N) | 21/22 | 38/12 | 0.011 |
| Age, y | 60.43±12.44 | 61.32±10.79 | 0.671 |
| BMI, kg/m2 | 26.32±3.27 | 27.11±3.94 | 0.207 |
| HT, % | 34.9 | 28.0 | 0.559 |
| Smokers, % | 40.9 | 76.0 | 0.010 |
| W/H ratio | 0.91±0.10 | 0.96±0.09 | 0.003 |
| TC, mmol/L | 4.89±1.13 | 5.05±1.87 | 0.542 |
| TG, mmol/L* | 1.41 (1.22–1.63) | 1.57 (1.33–1.86) | 0.174 |
| HDL-C, mmol/L | 1.24±0.32 | 1.08±0.32 | 0.006 |
| LDL-C, mmol/L | 2.94±0.93 | 3.20±1.44 | 0.215 |
| hsCRP, mg/L* | 2.71 (1.81–4.05) | 5.83 (3.53–9.63) | 0.001 |
| PTX3 * | 3.96 (3.13–5.01) | 6.72 (4.58–9.85) | <0.001 |
Continuous variables are presented as mean ±1 SD, and categorical variables are presented as absolute and relative frequencies.
For TG, hs-CRP and PTX3 the geometrical mean and 95% confidence interval (CI) are presented
(BMI – body mass index; HT – hypertension; W/H – waist-to-hip ratio; TC – total cholesterol; TG – triglycerides; HDL-C – HDL-cholesterol; LDL-C – LDL-cholesterol; hsCRP – high-sensitivity C-reactive protein; PTX3 – pentraxin-3).
Demographic characteristics and plasma levels of lipids and inflammatory markers in patients with different extent of coronary stenosis are presented in Table II. Levels of PTX3 and hsCRP in CAD(1–3) patients were significantly different between subgroups with different extents of coronary stenosis (p<0.001 and p=0.001, respectively). Concentration of PTX3 showed an increasing trend with the increasing number of vessels affected. We found significantly higher values of PTX3 in CAD(2) (p=0.008) and CAD(3) (p=0.014) subgroups, compared with the CAD(0) group. CAD(2) and CAD(3) subgroup levels of hsCRP were also significantly greater than in the CAD(0) subgroup (p<0.001 and p<0.025, respectively). In addition, hsCRP levels were significantly higher in CAD(2) than in CAD(1) subgroup (p=0.007).
Table II.
Demographic characteristics and plasma levels of lipids and inflammatory markers in patients with different stage of coronary stenosis.
| Variable | CAD(0) | CAD(1) | CAD(2) | CAD(3) | P-value |
|---|---|---|---|---|---|
| N | 43 | 14 | 20 | 16 | |
| Age, y | 60.43±12.44 | 61.00±14.01 | 60.44±10.76 | 62.75±8.86 | 0.909 |
| BMI, kg/m2 | 26.32±3.27 | 25.44±3.13 | 27.82±5.28 | 27.69±2.25 | 0.109 |
| W/H ratio | 0.91±0.10 | 0.95±0.10 | 0.96±0.09 | 0.96±0.07 | 0.234 |
| TC, mmol/L | 4.89±0.72 | 4.60±0.72 | 5.02±2.06 | 5.48±2.36 | 0.711 |
| TG, mmol/L* | 1.41 (1.22–1.63) | 1.25 (0.92–1.69) | 1.59 (1.19–2.12) | 1.88 (1.32–2.67) | 0.289 |
| HDL-C, mmol/L | 1.24±0.32 | 1.21±0.27 | 1.05±0.40 | 1.01±0.33 | 0.162 |
| LDL-C, mmol/L | 2.94±0.93 | 2.80±0.58 | 3.20±1.52 | 3.56±1.90 | 0.545 |
| hsCRP, mg/L* | 2.71 (1.81–4.05) | 2.34 (0.81–6.74) | 9.55 (4.25–21.45)a,b *** | 7.01 (2.96–16.66)a, ** | <0.001 |
| PTX3 * | 3.96 (3.13–5.01) | 4.71(1.86–11.90) | 7.62 (3.73–15.56)a, ** | 7.83 (4.04–15,16)a, * | 0.001 |
Continuous variables were compared by ANOVA and categorical variables by Chi-square test.
TG, hsCRP and PTX3 concentrations were presented as geometrical mean and 95% confidence interval (CI) for mean.
P<0.001;
P<0.01;
P<0.05 (Tukey’s post hoc test).
Significantly different from CAD(0) by Tukey’s post hoc test.
Significantly different from CAD(1) by Tukey’s post hoc test.
(BMI – body mass index; HT – hypertension; W/H – waist-to-hip ratio; TC – total cholesterol; TG – triglycerides; HDL-C – HDL-cholesterol; LDL-C – LDL-cholesterol; hsCRP – high-sensitivity C-reactive protein; PTX3 – pentraxin-3)
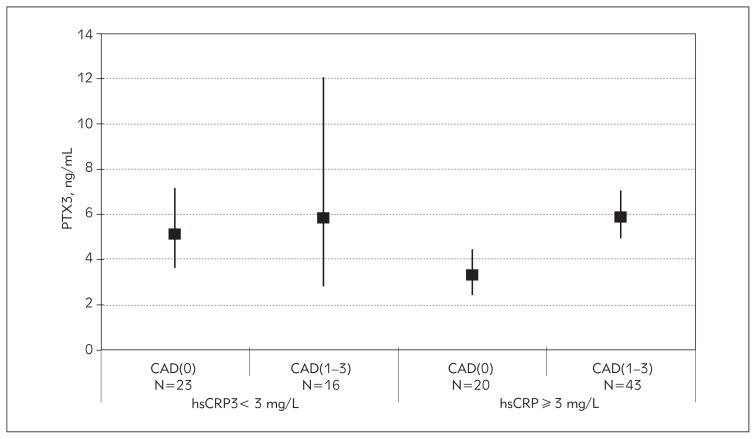
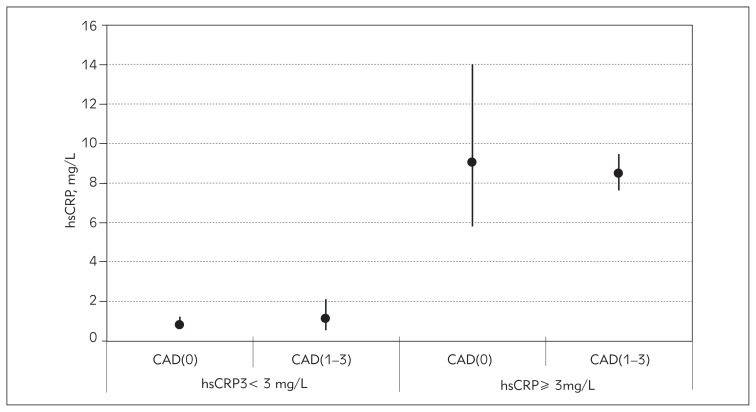
As hsCRP concentrations lower than 3 mg/L define subjects without high risk of CAD and values higher than 3 mg/L define those with high risk for CAD, we divided the patients into two groups according to their hsCRP concentration and each group was additionally separated into CAD(1–3) and CAD(0) patients. PTX3 levels significantly differed between the groups (ANOVA, p=0.003). In the group without high risk defined on the basis of hsCRP concentration, PTX3 means for CAD(0) and CAD(1–3) patients did not differ significantly (p=0.999, post hoc Tukey test). However, in the group of high risk patients, those with significant stenosis had higher PTX3 values compared with those without significant stenosis (p=0.01), Figure 1. There were no significant differences in hsCRP concentrations between patients with and without significant stenosis in the group without high risk (p=0.999) and in the high risk group (p=0.999), Figure 2.
Figure 1.
Geometrical means of PTX3 in groups of patients at low and high risk for CAD, based on hsCRP level and with additional separation into patients with subclinical [CAD(0)] and significant stenosis [CAD(1–3)].
Figure 2.
Geometrical means of hsCRP in groups of patients at low and high risk for CAD, based on hsCRP level and with additional separation into patients with subclinical [CAD(0)] and significant stenosis [CAD(1–3)].
hsCRP and PTX3 concentrations were not significantly correlated, either in the group with low hsCRP levels (r=0.242, p=0.138), or in the group with high hsCRP levels (r=0.167, p=0.323). PTX3 inversely correlated only with HDL-C (r=−0.142, p=0.047) when all subjects were considered together.
ORs from binary logistic regressions and the corresponding 95% CI for PTX3 and hsCRP are presented in Table III. Both PTX3 (OR=1.133; p<0.001) and hsCRP (OR=1.057; p=0.006) were associated with the presence of significant CAD. The models were built adjusting for conventional lipid and non-lipid risk factors: the first (PTX3 was adjusted for hsCRP, and hsCRP was adjusted for PTX3); the second, adjusting for demographic data (age, BMI and W/H ratio); and the third, adjusting for lipid status parameters (TC, TG, HDL-C and LDL-C) (Table III).
Table III.
Logistic regression analysis for the association of elevated hsCRP and PTX with significant stenosis.
| hsCRP | PTX | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Unadjusted 1 | 1.057 (1.016–1.099) | 0.006 | 1.133 (1.060–1.210) | <0.001 |
| Adjusted: | ||||
| Model 1 | 1.042 (1.001–1.084) | 0.045 | 1.113 (1.042–1.188) | 0.002 |
| Model 2 | 1.052 (1.007–1.099) | 0.023 | 1.133 (1.051–1.221) | 0.001 |
| Model 3 | 1.056 (1.011–1.104) | 0.015 | 1.145 (1.060–1.236) | 0.001 |
hsCRP and PTX3 were entered as continuous variables.
Model 1, PTX3 adjusted for hsCRP and hsCRP adjusted for PTX3; model 2 adjusted for age (years), BMI (kg/m2) and W/H ratio; model 3, adjusted for TC (mmol/L), TG (mmol/L), HDL-C (mmol/L) and LDL-C (mmol/L).
When taking concentrations of PTX3 into account, elevated hsCRP was linked with significant stenosis (p=0.045). The association between elevated PTX3 and significant stenosis remained strong after adjustment for the concentrations of hsCRP (p=0.002).
The adjustment for demographic and lipid factors changed the association of PTX3 and significant stenosis only slightly (Table III). The association between PTX3 and CAD stenosis remained strong, regardless of the confounding variables.
The diagnostic ability of PTX3 and hsCRP for the screening of significant stenosis was investigated by ROC curve analysis (Table IV). hsCRP and PTX3 had low discriminative ability for detection of significant stenosis using Swets’ criteria. A combination of PTX3 with hs-CRP slightly increased the discriminative abilities of hsCRP and PTX3 alone. The potential benefit of adding PTX3 or/and hsCRP to non-lipid (model 2) and lipid (model 3) parameters was tested for discriminating subjects with CAD(1–3) from subjects with CAD(0). The addition of hsCRP or/and PTX3 to model 2 slightly increased the AUC, without statistical significance (from 0.03 to 0.05) and NRI (7% for model 2 with hsCRP and PTX3). On the other hand, the AUC for lipid parameters (model 3) increased significantly after the inclusion of hsCRP and PTX3 combinations in model 3 (AUC=0.69 vs. AUC=0.77; p=0.024) as did the goodness of fit. The AUCs for the combinations of hsCRP and PTX3 with lipid parameters (model 3) were higher than all combinations of those parameters with non-lipid parameters and had useful accuracy for detecting CAD(1–3) patients (AUC=0.770, p<0.001). In addition, NRI increased by 3.2%, 4.3% and 7.0% after inclusion of hsCRP, PTH3 and hsCRP and PTX3, respectively to model 2. Increase in NRI was higher after addition of these parameters to the model 3: 5.9% for addition of hsCRP, 9.1% for addition of PTX3 and 13.5% for addition of hsCRP and PTX3.
Table IV.
The results of ROC analysis for discriminating significant from subclinical coronary stenosis.
| AUC (95% CI) | p | HL chi2 | |
|---|---|---|---|
| hs-CRP | 0.66 (0.52–0.79) | 0.031 | 14.35* |
| PTX3 | 0.67 (0.54–0.81) | 0.017 | 8.41* |
| hsCRP and PTX3 | 0.71 (0.57–0.85) | 0.004 | 11.09* |
| Model 2 | 0.68 (0.54–0.81) | 0.020 | 14.03* |
| Model 2 and hsCRP | 0.71 (0.58–0.84) | 0.006 | 6.85* |
| Model 2 and PTX3 | 0.71 (0.58–0.85) | 0.005 | 33.54 |
| Model 2, hsCRP and PTX3 | 0.74 (0.60–0.87) | 0.002 | 7.25* |
| Model 3 | 0.69 (0.56–0.82) | 0.013 | 8.54* |
| Model 3 and hsCRP | 0.761 (0.63–0.89) | 0.001 | 12.20* |
| Model 3 and PTX3 | 0.74 (0.61–0.88) | 0.002 | 21.73 |
| Model 3, hsCRP and PTX3 | 0.772 (0.64–0.90) | <0.001 | 13.29* |
Hosmer-Lemeshow (HL) tests based on 10 groups.
Significant HL goodness of fit, p>0.05;
significantly different from model 2;
significantly different from model 3.
Discussion
Atherosclerosis is a multifactorial disease whose etiology involves inflammatory, immune and hemostatic factors (18). The role of hsCRP in atherosclerosis and as a marker of inflammation has been intensively studied in the past years (3, 5). PTX3 appeared more recently as a biomarker, and although its role in atherosclerosis has not yet been clarified, studies have shown that PTX3 has tissue-protective and anti-inflammatory properties (9, 21, 22). PTX3 is rapidly induced at the tissue level and released in plasma at sites of myocardial infarction, atherosclerosis, vascular damage, or inflammation (12). PTX3 is a selective marker of vascular activation associated with atherosclerosis (6), and its concentration in plasma reflects local inflammation at the site of atherosclerotic lesion itself (8).
In this study, we found significantly lower PTX3 and hsCRP concentrations in CAD(0) compared to CAD(1–3) patients. We also found statistically higher concentrations of PTX3 and hsCRP in CAD(2) and CAD(3) subgroups, when compared with the group of patients without significant stenosis. These findings regarding hsCRP confirmed the results of Memon et al. from a 2006 study on a similar but larger group of patients undergoing coronary angiography for suspected CAD (23). Our results of the relationship between hsCRP level and the progression of coronary artery stenosis were to be expected and in agreement with previous studies [Arthurs et al., 2008 (24), Kadoglou et al., 2008 (25), Sadat et al., 2009 (26)]. The cause of increased serum PTX3 levels in patients with significant stenosis is still unknown. PTX3 is predominantly released by inflamed tissue in response to the proinflammatory cytokines tumor necrosis factor α and interleukin-1β, microbial components, or biochemical substances (such as HDL and oxidized LDL). Therefore, the elevated serum PTX3 levels may reflect local inflammation. PTX3 may also be released from circulating neutrophils (27), but under conditions of massive leukocyte activation, the release of PTX3 acts locally as a negative feedback mediator, dampening leukocyte recruitment. This pathway underlies the regulatory role of PTX3 in inflammation (21).
We found a significant negative correlation of PTX3 with HDL-C, similar to the results obtained in the study of Zanetti et al. (6) on a group of patients with metabolic syndrome and subclinical atherosclerosis. This negative correlation shows that the anti-inflammatory role of PTX3 is doubtful, in regard to the established protective effects of HDL-C against CAD. Low HDL-c is a strong predictor for CVD, and whether PTX3 in this inverse correlation with HDL-C increases the impact of dyslipidemia on cardiovascular risk, or increased PTX3 have an anti-inflammatory role, needs to be examined in the future. Haybar et al. (30) showed that in the presence of PTX3, HDL-C is no longer protective against CAD. Miyamoto et al. (9) found positive correlation between PTX3 and HDL-C in hemodialysis patients and explained their results with HDL-C stimulating PTX3 mRNA expression and protein release in endothelial cells, leading to an assumption about the involvement of PTX3 in the atheroprotective effects of HDL-C. The distinction in results can be attributable to the different groups of patients involved in studies, or the complex relationships between PTX3, lipid parameters and stage of atherosclerosis in different pathologies. Norata et al. (12) and Inoue et al. (28) suggest that the increase of pentraxins in atherosclerosis could not be regarded as a harmful response but rather a further attempt to protect our body. Accumulated evidence from in vivo studies indicates that, although the predominant role of PTX3 is host-protective, the multi-functional properties of PTX3 make it somewhat host-destructive as well.
We found a difference in PTX3 levels between CAD(0) and CAD(1–3) patients in the group without high risk as well as in the group with high risk, defined on the basis of hsCRP, though the levels of PTX3 were very similar in CAD(1–3) patients in both groups (Figure 1). Our results indicate that PTX3 is more influenced by stenosis than CRP. hsCRP alone had insufficient specificity for CAD because some patients with CAD(1–3) had low hsCRP concentrations, and vice versa, patients with high hsCRP had no stenosis. The use of PTX3 in the group of patients identified to be at high risk would lead to better reclassification of patients into groups with and without significant stenosis, if this marker is measured simultaneously with hsCRP. In the group with high risk based on hsCRP, CAD(0) patients still had low average PTX3 concentrations. It can be assumed that PTX3 could be more successful for identifying CAD(0) individuals who are classified in the high risk group based on hsCRP level, and this opens up the possibility for further research to determine cut-off values for PTX3 in a larger group of patients.
We found no correlation between PTX3 and hsCRP in those groups. Studies of Ogawa et al. (29) and Jylhava et al. (2) also showed no correlation between PTX3 and hsCRP.
We assessed whether measurement of PTX3 had any potential in predicting risk for development of significant coronary stenosis. We found a more significant link between levels of circulating PTX3 and coronary stenosis, than those observed for hsCRP, based on the odds ratio. This difference between PTX3 and CRP may be explained with the release of PTX3 locally, at the site of stenosis, compared to liver-restricted release of CRP, according to Hollan et al. (27), and Jylhava et al. (2).
Our analysis indicated that the influence of PTX3 on the appearance of severe stenosis was independent on demographic factors, lipid status parameters and hsCRP. We had similar findings for hsCRP, which was also independent of demographic factors, lipid parameters and PTX3 as a predictor for coronary stenosis.
Circulating PTX3 has been shown to function as a fast responding biomarker in cardiovascular pathologies (8), and this reflects the degree of local inflammation in coronary atherosclerosis. Haybar et al. (30) showed that PTX3 in the coronary sinus and femoral artery predict the probability of CAD among intermediate-risk populations with stable angina independently of the established CAD risk factors. The MONICA-KORA case-cohort study (2006) showed that the increased concentration of hsCRP remained strong and was an independent predictor of CVD risk in men even after adjustment for traditional cardiovascular risk factors, lipid parameters and for another potent inflammatory marker, interleukin-6 (31). Our study may contribute to identifying the best combination of inflammatory markers for risk assessment of future cardiovascular events.
ROC analysis showed that hsCRP and PTX3 alone have moderate ability to separate CAD(1–3) from CAD(0) subjects. Thus, our findings were similar to those obtained for these two markers in the studies of Tong et al. (32) and Tamura et al. (33). ROC curve analysis showed that the AUC for determining these two parameters together was greater than 0.7.
Therefore, we performed tests to discriminate and calibrate different prediction models. Discriminative analysis of a model with lipid parameters and hsCRP and PTX3 showed that the AUC increased significantly. The predictive performance also improved. Considering the other new statistical metrics, proposed recently to quantify the degree of correct reclassification, the inclusion of hsCRP and PTX3 in a model with lipid parameters was associated with an NRI improvement of 13.5%, suggesting moderately effective reclassification. Thus, the combination of hsCRP and PTX3 provided a better tool for risk stratification than either factor alone and supports the theory that the scores for risk assessment should be supplemented with inflammatory parameters.
Until now, evidence on which emerging biomarker is more suitable for the diagnosis or prognosis of CAD has been inconsistent. Several authors have concluded that high hsCRP is associated with increased prevalence of myocardial infarction: de Ferranti et al. in their study suggested that hsCRP augments the predictive capacity of the Framingham Risk Score (FRS) (20), Tamon et al. (34) presented the relationship of hsCRP with FRS (34), Koenig et al. (35) showed that CRP enhances global coronary risk as assessed by FRS, especially in intermediate risk groups. In contrast, Cristell et al. established limitations of hsCRP as an individual prognostic marker and as an indicator of a generalized inflammatory pathogenic component of myocardial infarction with ST-elevation. The main weakness of this biomarker is its systemic inflammatory role. However, PTX3, being a marker of local inflammation, is independent of systemic inflammatory status and this might be a potential advantage of PTX3 in coronary disease prediction compared with hsCRP.
Our study showed that increased circulating levels of PTX3 and hsCRP represent inflammatory markers of advanced atherosclerosis in patients with CAD, and both markers might be quantitative indicators of disease extent. It would be best if we could have compared the values obtained in the patients with the corresponding values found in a group of stenosis-free subjects. However, as coronary angiography is an aggressive method, it is never performed without a good reason, and it is impossible to recruit such a group.
PTX3 is a promising independent diagnostic marker for identifying patients with CAD, and a useful indicator of disease progression. In all analyses PTX3 showed better performance than CRP. A combination of PTX3, hsCRP and lipid status parameters provides risk stratification of the development of coronary stenosis and better classification than their individual application. Finding the best combination of biomarkers for assessing coronary stenosis development is our future aim.
Limitations
Use of PTX3 as a marker to improve reclassification of patients with CAD is limited until it becomes widely available on immunochemistry platforms. However, the commercially available PTX3 assay used in our study is simple to perform and the results are available within a few hours. The cost per sample of PTX3 measurement is less compared to the prohibitive healthcare costs of the management of CAD complications. Additionally, hsCRP and PTX3 after inclusion in different models gave only useful accuracy for discrimination of patients with and without significant stenosis and cost-effectiveness studies are needed to evaluate their utility value. It can be assumed that PTX3 could be a successful marker for improving reclassification of patients with CAD, but the limitation of this study is the small group of patients included, and this opens up the possibility for further research on a larger group of patients.
Acknowledgements
The work was financially supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (Project No. 175035) and COST ACTION BM0904.
Abbreviations
- CRP
C-reactive protein
- CVD
cardiovascular disease
- hsCRP
high sensitivity C-reactive protein
- PTX3
pentraxin-3
- CAD
coronary artery disease
- HT
hypertension
- BMI
body mass index
- W/H
waist-to-hip ratio
- EDTA
ethylene diamine-tetraacetic acid
- TC
total cholesterol
- TG
triglycerides
- HDL-C-HDL
cholesterol
- LDL-C-LDL
cholesterol
- CI
confidence interval
- OR
odds ratio
- ROC
receiver operating characteristic
- AUC
the area under ROC curve
- FRS
Framingham Risk Score
Footnotes
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Knoflach M, Kiechl S, Mantovani A, Cuccovillo I, Botazzi B, Xu Q, et al. Pentraxin as a marker of advanced atherosclerosis. Results from Bruneck, ARMY and ARFY studies. PLoS ONE. 2012;7(2):e31474. doi: 10.1371/journal.pone.003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jylhava J, Haarala A, Kahonen M, Lehtimaki T, Jula A, Moilanen L, et al. Pentraxin 3 (PTX3) is associated with cardiovascular risk factors: the Health 2000 survey. Clin Exp Immunol. 2011;164:211–7. doi: 10.1111/j.1365-2249.2011.04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korita I, Bulo A, Langlois, Blaton V. Inflammation in patients with cardiovascular disease and metabolic syndrome. J Med Biochem. 2013;32:214–9. [Google Scholar]
- 4.Coskun A, Serteser M, Unsal I. Inhibition of cholesterol biosynthesis in hypercholesterolemia – is it the right choice. J Med Biochem. 2013;32:16–19. [Google Scholar]
- 5.Ridker PM, Rifai N, Rose l, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 6.Zanetti M, Bosutti A, Ferreira C, Vinci P, Biolo G, Fonda M, et al. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: evidence for association with atherogenic lipid profile. Clin Exp Med. 2009;9:243–8. doi: 10.1007/s10238-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 7.Bogavac-Stanojević N, Jelić-Ivanović Z, Spasojević-Kalimanovska V, Spasić S, Kalimanovska-Oštrić D. Lipid and inflammatory markers for the prediction of coronary artery disease: A multi-marker approach. Clin Biochem. 2007;40:1000–6. doi: 10.1016/j.clinbiochem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Soeki T, Niki T, Kusunose K, Bando S, Hirata Y, Tomita N, et al. Elevated concentrations of pentraxin 3 are associated with coronary plaque vulnerability. J Cardiol. 2011;58:151–7. doi: 10.1016/j.jjcc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto T, Qureshi AR, Heimburger O, Barany P, Carrearo K, Sjoberg B, et al. Inverse relationship between the inflammatory marker pentraxin-3, fat body mass, and abdominal obesity in end-stage renal disease. Clin J Am Soc Nephrol. 2011;6:2785–91. doi: 10.2215/CJN.02320311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson G. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–e14. doi: 10.1161/01.ATV.0000015595.95497.2F. [DOI] [PubMed] [Google Scholar]
- 11.Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 12.Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: A modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovas Med. 2010;20:35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–64. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 14.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 15.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Ferranti SD, Rifai N. C-reactive protein: a nontraditional serum marker of cardiovascular risk. Cardiovasc Path. 2007;16:14–21. doi: 10.1016/j.carpath.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by long pentraxin PTX3. Nat Immunol. 2010;11:328–35. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 22.Hollan I, Nebuloni M, Bottazzi B, Mikkelsen K, Førre OT, Almdahl SM, et al. Pentraxin 3, a novel cardiovascular biomarker, is expressed in aortic specimens of patients with coronary artery disease with and without rheumatoid arthritis. Cardiovasc Pathol. 2013 doi: 10.1016/j.carpath.2013.01.007.. [DOI] [PubMed] [Google Scholar]
- 23.Memon L, Spasojević-Kalimanovska V, Bogavac-Stanojević N, Kalimanovska-Oštrić D, Jelić-Ivanović Z, Spasić S, et al. Association of C-reactive protein with the presence and extent of angiographically verified coronary artery disease. Tohoku J Exp Med. 2006;209:197–206. doi: 10.1620/tjem.209.197. [DOI] [PubMed] [Google Scholar]
- 24.Arthurs ZM, Andersen C, Starnes BW. A prospective evaluation of C-reactive protein in the progression of carotid artery stenosis. J Vasc Surg. 2008;47:744–751. doi: 10.1016/j.jvs.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 25.Kadoglou NP, Gerasimidis T, Golemati S, Kapelouzou A, Karayannacos PE, Liapis CD, et al. The relationship between serum levels of vascular calcification inhibitors and carotid plaque vulnerability. J Vasc Surg. 2008;47:55–62. doi: 10.1016/j.jvs.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 26.Sadat U, Weerakkody RA, Bowden DJ, Young VE, Graves MJ, Li ZY, et al. Utility of high resolution MR imaging to assess carotid plaque morphology: A comparison of acute symptomatic, recently symptomatic and asymptomatic patients with carotid artery disease. Atherosclerosis. 2009;207:434–9. doi: 10.1016/j.atherosclerosis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Hollan I, Bottazzi B, Cuccovillo I, Førre OT, Mikkelsen K, Saatvedt K, et al. Increased levels of serum pentraxin 3, a novel cardiovascular biomarker, in patients with inflammatory rheumatic disease. Arthritis Care Res. 2010;62:378–85. doi: 10.1002/acr.20094. [DOI] [PubMed] [Google Scholar]
- 28.Inoue K, Kodama T, Daida H. Pentraxin 3: A novel biomarker for inflammatory cardiovascular disease. Int J Vasc Med. 2012 doi: 10.1155/2012/657025. http://dx.doi.org/10.1155/2012/657025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa T, Kawano Y, Imamura T, Kawakita K, Sagara M, Matsuo T, et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity. 2010;18:1871–4. doi: 10.1038/oby.2009.507. [DOI] [PubMed] [Google Scholar]
- 30.Haybar H, Assareh A, Ghotbi Y, Torabizadeh M, Bozorgmanesh M. Incremental diagnostic value of circulating pentraxin in patients with intermediate risk of coronary artery disease. Heart. 2013 doi: 10.1136/heartjnl-2012-303560. [DOI] [PubMed] [Google Scholar]
- 31.Koenig W, Khuseyinova N, Baumert J, Thorand B, Loewel H, Chambless L, et al. Increased concentrations of C-reactive protein and IL-6 but not IL-18 are independently associated with incident coronary events in middle-aged men and women. Results from MONICA/KORA Augsburg case-cohort study, 1984–2002. Arterioscl Thromb Vasc Biol. 2006;26:2745–51. doi: 10.1161/01.ATV.0000248096.62495.73. [DOI] [PubMed] [Google Scholar]
- 32.Tong M, Carrero JJ, Qureshi AR, Anderstam B, Heimburger O, Barany P, et al. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2007;2:889–97. doi: 10.2215/CJN.00870207. [DOI] [PubMed] [Google Scholar]
- 33.Tamura Y, Ono T, Kuwana M, Inoue K, Takei M, Yamamoto T, et al. Human pentraxin 3 (PTX3) as a novel biomarker for the diagnosis of pulmonary arterial hypertension. PloS ONE. 2012;7(9):e45834. doi: 10.1371/journal.pone.0045834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamon R, Kou Y, Yoshida Y, Ogawa Y, Imaki M. Study on the evaluation of serum hsCRP as predictor of the coronary artery disease by using Framingham Risk Score (FRS) J An Bio-Sci. 2011;34:324–30. [Google Scholar]
- 35.Koenig W, Löwel H, Baumert J, Meisinger C. C-Reactive Protein modulates risk prediction based on the Framingham Score: Implications for future risk assessment: Results from a large cohort study in Southern Germany. Circulation. 2004;109:1349–53. doi: 10.1161/01.CIR.0000120707.98922.E3. Baumert and Christa Meisinger. [DOI] [PubMed] [Google Scholar]
- 36.Cristell N, Cianflone D, Durante A, Ammirati E, Vanuzzo D, Banfi M, et al. High-sensitivity C-reactive protein is within normal levels at the very onset of first ST-segment elevation acute myocardial infarction in 41% of cases. J Am Coll Cardiol. 2011;58:2654–61. doi: 10.1016/j.jacc.2011.08.055. [DOI] [PubMed] [Google Scholar]