Abstract
Introduction
Argentina and Uruguay have a high prevalence of smoking during pregnancy. However, and despite national recommendations, pregnant women are not routinely receiving cessation counseling during antenatal care (ANC). We evaluated a multifaceted strategy designed to increase the frequency of pregnant women who received a brief smoking cessation counseling based on the 5As (Ask, Advise, Assess, Assist, and Arrange).
Methods
We randomly assigned (1:1) 20 ANC clusters in Buenos Aires, Argentina and Montevideo, Uruguay to receive a multifaceted intervention to implement brief smoking cessation counseling into routine ANC, or to receive no intervention. The primary outcome was the frequency of women who recalled receiving the 5As during ANC at more than one visit. Frequency of women who smoked until the end of pregnancy, and attitudes and readiness of ANC providers towards providing counseling were secondary outcomes. Women’s outcomes were measured at baseline and at the end of the 14- to 18-month intervention, by administering questionnaires at the postpartum hospital stay. Self-reported cessation was verified with saliva cotinine. The trial took place between October 03, 2011 and November 29, 2013.
Results
The rate of women who recalled receiving the 5As increased from 14.0% to 33.6% in the intervention group (median rate change, 22.1%), and from 10.8% to 17.0% in the control group (median rate change, 4.6%; P = .001 for the difference in change between groups). The effect of the intervention was larger in Argentina than in Uruguay. The proportion of women who continued smoking during pregnancy was unchanged at follow-up in both groups and the relative difference between groups was not statistically significant (ratio of odds ratios 1.16, 95% CI: 0.98–1.37; P = .086). No significant changes were observed in knowledge, attitudes, and self-confidence of ANC providers.
Conclusions
The intervention showed a moderate effect in increasing the proportion of women who recalled receiving the 5As, with a third of women receiving counseling in more than one visit. However, the frequency of women who smoked until the end of the pregnancy was not significantly reduced by the intervention.
Implications
No implementation trials of smoking cessation interventions for pregnant women have been carried out in Latin American or in middle-income countries where health care systems or capacities may differ. We evaluated a multifaceted strategy designed to increase the frequency of pregnant women who receive brief smoking cessation counseling based on the 5As in Argentina and Uruguay. We found that the intervention showed a moderate effect in increasing the proportion of women receiving the 5As, with a third of women receiving counseling in more than one visit. However, the frequency of women who smoked until the end of the pregnancy was not significantly reduced by the intervention.
Introduction
Argentina and Uruguay are among the countries with the highest proportion of women who smoke during pregnancy, 10.3% and 18.3%, respectively.1 Smoking during pregnancy is associated with increased risks of stillbirth, preterm birth, low birth weight, restricted fetal growth, congenital anomalies such as cleft lip and palate, and sudden infant death syndrome.2,3 Maternal tobacco use is also likely to expose infants and children to secondhand smoke (SHS) and increase a child’s risk of becoming a smoker themselves.4 Because smoking poses serious risks to fetal and infant health, pregnant women are an important population for tobacco control efforts.
Brief cessation counseling interventions, based on the 5As (Ask, Advise, Assess, Assist, and Arrange), are effective for smoking cessation in a wide variety of settings and populations, and can be delivered by various provider types.5 In a meta-analysis of 27 studies, women receiving smoking cessation counseling were 44% more likely to not smoke during pregnancy compared to women receiving usual care. Moreover, women receiving counseling have shown a 10%–13% reduction in infants with low birth weight or born preterm, although these differences were not statistically significant.6 The World Health Organization’s Recommendations for the Prevention and Management of Tobacco Use and Secondhand Smoke Exposure in Pregnancy recommend that health care providers routinely offer advice and psychosocial interventions for tobacco cessation to all pregnant women who are either current tobacco users or recent tobacco quitters.2 National tobacco programs and guidelines in Argentina and Uruguay7,8 recommend a brief counseling strategy for all patients, including pregnant women, during antenatal care (ANC).
Despite national recommendations, women attending ANC at public maternity hospitals and clinics in Argentina and Uruguay are not routinely receiving brief cessation counseling. A survey conducted in 20 public maternity hospitals in Buenos Aires, Argentina, and Montevideo, Uruguay in 2010 showed that only 10% of smokers were receiving brief counseling during prenatal care.9 A survey conducted in Argentina in 2005, showed that only 22% of obstetrician/gynaecologists had received training in cessation counseling and 48.5% reported insufficient knowledge to provide cessation advice. Although 88.9% consistently advised women to stop smoking, three out of four providers believed it was acceptable for pregnant women to smoke up to six cigarettes per day.10 Studies reporting barriers for the uptake of interventions for prenatal smoking cessation include competing demands on time, uncertainty over the effectiveness of interventions, low staff confidence in counseling skills, and lack of service guidelines.11
A multifaceted intervention that combines implementation of clinical practice guidelines with quality-improvement strategies to improve professional practice may improve implementation of brief counseling into routine prenatal care. While evaluation of such strategies have been conducted in Australia,12,13 no implementation trials of smoking cessation interventions for pregnant women have been carried out in Latin American or in middle-income countries where health care systems or capacities may differ. We report the results of a multicenter, cluster randomized trial in 20 public maternity care settings in the Province of Buenos Aires, Argentina, and in Montevideo, Uruguay that evaluated a multifaceted strategy to increase the frequency of pregnant women who receive brief cessation counseling based on the 5As.
Methods
Trial Design and Participants
The study was a two-arm parallel cluster randomized trial with baseline and follow-up cross sectional measurements conducted in a total of 20 clusters, 10 in Buenos Aires, Argentina and 10 in Montevideo, Uruguay. Clusters were ANC clinics serving more than 250 unique pregnant women per year, that did not have a smoking cessation program based on the 5As for pregnant women, and had physicians, midwives or nurses as part of their ANC clinic staff. In Argentina, each cluster consisted of a maternity hospital and one to three associated ANC clinics at health centers. In Uruguay, each cluster consisted of one ANC clinic at a health center. Health providers worked as ANC attendants in the clinic or clinics belonging to one cluster only. Health centers were publicly funded by the ministries of health and free of charge. Women attending these centers came from the most deprived economic sectors in both countries. More study details are published elsewhere.9,14
In the intervention clusters, midwives and obstetrician/gynaecologists interested in participating as facilitators of the program were trained on how to implement the 5As during ANC. Control clusters only received a brief seminar to increase awareness of the importance of smoking cessation during pregnancy. For both countries at the time of the study, there were no pregnancy-specific cessation services outside of what was provided in ANC. Outcomes were measured during the postpartum hospital stay. Women were screened if they attended ANC at any of participating clinic; those who attended ANC at any of the participating clinics were invited to be enrolled. Participants were evaluated at a single time period. Data were collected during two 6-month periods: before randomization (baseline) and the last 6 months of the 18-month intervention (follow-up). The 24-month trial was conducted between October 03, 2011 and November 29, 2013.
Approval for the study was obtained from the ethics review boards of all participating local and partner institutions (listed in the Supplementary Appendix). Informed consent was read aloud to eligible women, and all eligible women provided written consent if they agreed to be interviewed, and if applicable, to provide a saliva sample. Responsible authorities from all the participating facilities signed a participation agreement. The protocol was in accordance with the Ottawa Statement.15 The authors confirm that all related trials for this intervention are registered (http://clinicaltrials.gov:NCT01852617).
Randomization and Masking
A covariate-constrained randomization procedure16 ensured that the intervention and control clusters were balanced with respect to the frequency of women recalling the 5As, the frequency of women who smoked during pregnancy, the relative number of providers per women attended at the clinics, the relative frequency of midwives or nurses who attended ANC over the total of providers, and country of location. An independent statistician performed the randomization using the above covariate information from the baseline data collection period. Each cluster was informed of the randomization allocation after baseline data collection and prior to implementation of the intervention. The nature of the trial precluded masking of randomization allocation.
Procedures
Intervention Description
The intervention lasted 14–18 months. Intervention strategies were chosen for their effectiveness in leading to a change in practice5,17 and were based on the diffusion of innovation theory.18 To tailor the intervention, formative research was conducted with ANC clinic directors and providers (physicians, midwives, nurses), in nonparticipating facilities similar to the study clinics and hospitals, and with pregnant smokers from participating clinics.19 The results of this formative research were discussed among the investigators to evaluate whether the intervention components addressed the identified barriers, and whether those components were feasible to be implemented and acceptable to the health providers.
A team of ANC providers from each of the intervention clusters was trained during a 2-day workshop on how to implement the 5As intervention during ANC. Expert trainers in smoking cessation programs conducted the workshop which included a general overview of smoking prevalence and trends, a review of the health consequences of smoking during pregnancy for the mother and the newborn, and a discussion about the role of the health care provider in preventing smoking among pregnant women. The 5As intervention, adapted to pregnant women, was explained in detail as the standard of care. After the theory was explained, all participants practiced communication and motivational interviewing skills in several role-play scenarios. During the second day of the workshop each cluster team developed a plan to implement the 5As program at their clinics. Two main implementation models were defined: (1) all ANC providers were trained to provide counseling to all women according to their smoking status; and (2) providers were trained to ask and provide brief advice to all patients, and to identify and refer smokers to a second team trained to provide specific counseling to smokers. Details on the implementation models at each intervention cluster are shown in Supplementary Appendix Table 1. It should be noted that both implementation models were selected by clinics within all clusters.
In the month after the workshop, the facilitators’ teams, with the support of the 5As trainers, replicated the training on the 5As to the rest of the providers, according to the chosen implementation model. Additional components of the strategy for all intervention clinics included printed pregnancy-specific self-help materials for the women and posters and reminders for waiting rooms and offices. Research staff visited the clusters on a monthly basis to monitor the implementation and assess completion of planned activities and to collect data on process measures. Some of these monitoring visits included an assessment of the quality of the counseling done by observation of prenatal care visits chosen at random and using a standardized checklist developed specifically for this purpose. This assessment was not intended to be representative and will not be reported quantitatively.
Outcomes
The primary outcome was the frequency of women who recalled receiving the 5As among those attending ANC at the participating clinics. The recall of 5As was defined positive if nonsmokers and spontaneous quitters received the first two components of the strategy (ask and advice), and later quitters and smokers received the five components (ask, advice, assess, assist, and arrange) at more than one prenatal care visit. We decided to consider the counseling as positive if it was recalled in more than one visit because it is routine in prenatal care in Argentina and Uruguay to assess smoking status condition and to advise smokers to quit in the first visit. We also report the outcome as 5As recall in at least one visit. The secondary outcomes were the frequency of women who smoked until the end of pregnancy, and the ANC providers’ attitudes and readiness to provide smoking cessation counseling. Women’s outcomes were measured at baseline and follow-up in a survey conducted by trained interviewers within the first 48 hours after delivery, during women’s postpartum hospital stay. Women were eligible if they attended ANC at one of the participating clinics. Women with mental or physical impairments that prevented them from being interviewed and women with a diagnosis of stillbirth at admission were ineligible to participate. All consecutively eligible women who signed an informed consent were included until reaching the sample size. The survey included: basic demographic data (extracted from the clinical record); knowledge and attitudes regarding tobacco; tobacco use behaviors; SHS exposure; and tobacco cessation counseling received during ANC. Additionally, tobacco status among women who self-reported smoking cessation during pregnancy was validated by cotinine analysis of saliva submitted within the first 12 hours postpartum. Data quality and validity was periodically checked against information abstracted from hospital records.
ANC providers’ outcomes were measured at baseline and during the last month of the intervention period. The providers completed anonymous, self-administered questionnaires to describe their current practice, knowledge, attitudes and self-confidence regarding counseling for smoking cessation and SHS exposure. The questionnaires were collected in opaque envelopes and placed in sealed containers at each hospital.
Statistical Analysis
The statistical power was estimated for both primary and secondary outcomes, using Monte Carlo simulation20 with 3000 repetitions. The assumptions were: (1) the intervention and control group each contains seven clinics, with a minimum of 200 women at each time point, and three smaller clinics with 120 women at each time point; (2) an increase in the frequency of women recalling the 5As by the end of pregnancy from 10% to 20% in the control group and from 10% to 50% in the intervention group (ie, an intervention effect of 30%); (3) a decrease in the frequency of women who smoke at the end of the pregnancy from 18% to 17% in the control group and from 18% to 12% in the intervention group (ie, an intervention effect of 5%); and (4) an intra-cluster correlation coefficient between 2 outcomes from different women at different times (pretest/post-test) = intra-cluster correlation coefficient between two different women at the same time of 0.05. The value of the latter intra-cluster correlation coefficient was observed in a previous study carried out in Argentina and Uruguay.21
The sample size of twenty clusters (14 clusters of 200 women and six clusters of 120 women at each time point, respectively) would provide statistical power at the 5% level of significance (two-sided) of 100% for the primary outcome and 89% for the secondary outcome of frequency of women who were smokers at end of pregnancy.
Analyses were performed according to the intention-to-treat principle, and no cluster was excluded from the analysis after allocation. To test the primary hypothesis that the intervention would increase the frequency of women recalling the 5As during pregnancy in the intervention clinics compared to the control clinics, the clusters were the unit of analysis. We were interested in observing how the absolute difference of the percentage of women recalling the 5As differs between the control and intervention group. We computed the outcome rate for each ANC clinic at baseline and the follow-up periods, and then we calculated the outcome rate change as the absolute difference between the follow-up and baseline rates for the intervention and control groups. The median of these differences for the intervention and control groups was determined as the median rate change. Finally, the absolute difference between the median rate change in the intervention clusters and the median rate change in the control clusters was calculated as the intervention effect, and tested with the use of an exact Wilcoxon rank test. The confidence interval (CI) and P value were estimated using a permutation method implemented in the “wilcox.test” function in the R Project for Statistical Computing.22
For the second hypothesis that the intervention would decrease the frequency of women who smoke by the end of pregnancy, we used the woman as the unit of analysis because we wanted to study the effect of the intervention on the individual. For that analysis, we fit a model in which the variables included were the intervention (categorized as control group and intervention group), the time (baseline and follow-up measures) and the “intervention by time” interaction. To test the effect of the intervention, we focused on the significance of the interaction. We used a generalized estimation equation (GEE) with a logit link to estimate the model and reported the effect size as an odds ratio (OR) and 95% CI. To test if the intervention changed providers’ outcomes, we used the same analytical approach used for the primary hypothesis. Those outcomes variables measured using the Likert scale were dichotomized in higher than 6 and 6 or less. A Z-score to percentile rank transformation was also used to compare the results.
To assess differences in the magnitude of the intervention effects on the rate of women recalling the 5As in Argentina and Uruguay, we used a median (50th quantile) regression analysis23 on the outcome rate change for each ANC. Independent variables were intervention (categorized as control group and intervention group), country, and the “intervention by country” interaction. These analyses involved calculating the intervention effect for each country and the difference of intervention effects between countries. Logistic regression analyses using generalized estimation equation and median regression analyses were completed using SAS version 9.3 (SAS Institute Inc, Cary, NC).24
Results
Characteristics of ANC Clinics, ANC Providers, and Women at Baseline
Twenty clusters completed baseline data collection, ten were randomized to the intervention group and ten to the control group (Figure 1). All 20 clusters completed the trial. The characteristics of the clusters and ANC providers were generally similar in the two groups (Table 1).
Figure 1.
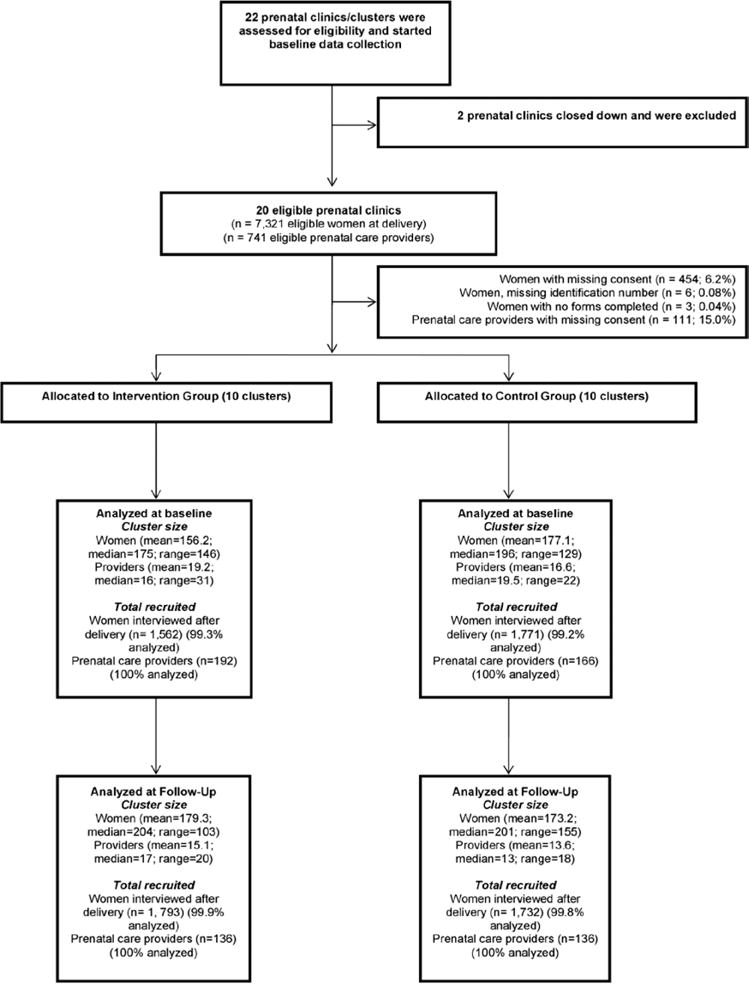
Trial diagram.
Table 1.
Characteristics of Clusters, ANC Providers, and Women at Baseline and Follow-upa
| Characteristics | Baseline periodb
|
Follow-up periodc
|
||
|---|---|---|---|---|
| Intervention group (N = 10) | Control group (N = 10) | Intervention group (N = 10) | Control group (N = 10) | |
| Clusters | ||||
| Ratio of providers/women attending prenatal care | 0.053 ± 0.027 | 0.051 ± 0.030 | ||
| Percentage of nurses or midwives/providers | 50.6 ± 11.5 | 50.8 ± 18.5 | ||
| Prenatal care providers | ||||
| Age (y) | 44.0 ± 3.1 | 42.8 ± 2.5 | 44.2 ± 4.0 | 40.7 ± 5.0 |
| Female (%) | 88.3 ± 7.4 | 74.9 ± 12.7 | 85.0 ± 9.0 | 85.2 ± 9.8 |
| Number of years since graduation | 16.8 ± 2.5 | 16.6 ± 3.2 | 17.1 ± 4.0 | 14.3 ± 4.4 |
| Profession (%) | ||||
| Physician | 49.5 ± 15.3 | 53.7 ± 17.9 | 50.4 ± 21.8 | 50.3 ± 28.1 |
| Midwife | 29.9 ± 17.3 | 23.1 ± 23.0 | 38.3 ± 21.2 | 27.0 ± 23.9 |
| Nurses | 17.3 ± 15.7 | 21.9 ± 14.6 | 10.8 ± 13.8 | 20.5 ± 24.3 |
| Other | 3.2 ± 7.8 | 1.4 ± 2.3 | 0.5 ± 1.7 | 2.2 ± 5.4 |
| Women’s and newborns’ characteristics | ||||
| Maternal age <20 years (%) | 20.6 ± 4.4 | 20.8 ± 5.4 | 24.5 ± 5.1 | 22.3 ± 8.6 |
| Primiparous women (%) | 35.5 ± 7.1 | 36.9 ± 7.1 | 42.4 ± 14.7 | 41.7 ± 11.5 |
| Number of prenatal care visits | 7.9 ± 1.3 | 8.0 ± 1.5 | 7.8 ± 1.5 | 7.6 ± 1.5 |
| Recall 5As during prenatal cared (%) | 4.1 ± 4.1 | 3.7 ± 4.6 | ||
| Continue smoking during pregnancye (%) | 21.9 ± 11.1 | 21.8 ± 10.0 | ||
| Nonsmokers | 69.1 ± 8.1 | 69.0 ± 9.7 | ||
| Smoke-free home rule (%) | 68.0 ± 7.6 | 64.5 ± 9.1 | ||
| Partner/household member smokes (%) | 45.9 ± 6.4 | 42.1 ± 4.0 | ||
| Infant’s birth weight <2500 g (%) | 6.7 ± 2.3 | 7.5 ± 2.1 | ||
ANC = antenatal care.
The mean (SD) of clusters’ rates (in percent) is reported. For age and years since graduation of the prenatal care providers and for the number of prenatal care visits, the mean ± SD of cluster’s means is reported. No significant difference between groups at baseline or follow-up.
There were 192 prenatal care providers and 1562 mothers in the intervention clusters, and 166 prenatal care providers and 1771 women in the control clusters.
There were 136 prenatal care providers and 1793 mothers in the intervention clusters, and 136 prenatal care providers and 1732 women in the control clusters.
For randomization purposes only, recall of the 5As (Ask, Advise, Assess, Assist, and Arrange) was defined as nonsmokers and quitters reported receiving the first 2As at all visits and smokers reported recalling the 5As at all visits. This variable definition differed from the definition of the 5As for the trial outcome measure.
Continued smokers were those who reported smoking every day or some days throughout pregnancy or within the last week prior to delivery.
Baseline data were collected for 1562 women from the intervention group and for 1771 women from the control group. The groups were similar with respect to maternal and newborn characteristics, number of ANC visits, rates of women recalling the 5As during ANC, and smoking status and secondhand exposure during pregnancy (Table 1). Baseline data were missing for less than 1% of women, with the exception of biochemically-confirmed smoking status which was missing in 46 (2.9%) and 61 (3.4%) women who self-reported smoking cessation in the intervention and control groups, respectively. All women who reported quitting and had missing biochemical confirmation at baseline and follow-up were considered smokers for the purpose of this analysis.
The questionnaires to ANC providers were administered at baseline to 226 and 170 providers in the intervention and control groups, respectively. The mean response rate was 90.2% for providers in the intervention and 96.5% for the control group.
Intervention Process Measures
Overall, the compliance with the intervention was high. Among the intervention clusters, 94% of the clinic providers received training in the 5As that included 49.5% physicians, 41.6% midwives and 8.9% nurses. Most of them were female (93.6%) and trained providers were available during the intervention period in almost all ANC shifts (median rate 92%; range 60%–100%). The printed materials and reminders were distributed throughout the intervention period (median rate 99%; range 80%–100%), and monitoring reports were conducted by clinic staff (median rate 87%; range 17%–100%). The quality of the counseling assessed in a nonrepresentative sample of prenatal care visits showed a heterogeneous quality of the 5As components, including cases in which the steps of the 5As were not implemented as required during the intervention training. No intervention activities were conducted in control clusters, and there were no changes in policies regarding smoking cessation during the intervention period.
Outcome Measures
During the follow-up period, data were collected from 1793 women in the intervention group and from 1732 women in the control group (Figure 1). Maternal, newborn, and providers’ characteristics were similar to the baseline period (Table 1). Data were missing for less than 0.3% of the women for self-reported smoking status and 4.4% and 4.6%, for the biochemical confirmation of smoking status in women who self-reported smoking cessation, which was missing in the intervention group and in the control group. These missing values were due to woman who did not consent saliva extraction, women with insufficient saliva sample, or women with no sample.
The rate of women recalling the 5As increased from 14.0% to 33.6% in the intervention group (median rate change, 22.1%), and from 10.8% to 17.0% in the control group (median rate change, 4.6%). The size of the intervention effect, measured as the differences between the rate changes in the intervention and control groups, was 17.4% (95% CI: 8.5–26.8; P = .001; Table 2). The absolute changes in rates at each individual cluster are shown in Supplementary Appendix Figure 1. There was a statistically significant increase in each of the individual components of the 5As; the difference in rate changes ranged between 14.9% and 29.2% for “Ask,” “Advice,” “Assess,” and “Assist.” For the “Arrange” step, the difference in size of the effect was 2.7%. The rate of women recalling the 5As at least in one visit changed from 41.2% to 47.5% in the intervention group (median rate change, 12.5%), and from 29.2% to 31.2% in the control group (median rate change, −0.1%). The intervention effect on recalling the 5As in at least one visit was a 12.6% (95% CI: −8.0–30.5; P = .212) absolute increase, although not statistically significant.
Table 2.
Effect of the Intervention on the Rates of Implementation of the 5As During Antenatal Care
| Intervention group (10 clusters)
|
Control group (10 clusters)
|
Intervention effect
|
||||||
|---|---|---|---|---|---|---|---|---|
| Median baseline rate (N = 1551) | Median follow-up period rate (N = 1791) | Median rate changea | Median baseline rate (N = 1757) | Median follow-up period rate (N = 1729) | Median rate changea | Absolute difference in median rate change (95% CI)b | Pc | |
| Recalled 5As during antenatal care according to smoking statusd | 14.0 | 33.6 | 22.1 | 10.8 | 17.0 | 4.6 | 17.4 (8.5; 26.8) | .001 |
| Ask about tobacco use (at more than one visit) | 43.1 | 73.2 | 33.1 | 43.5 | 48.9 | 3.9 | 29.2 (17.5; 38.0) | <.005 |
| Advice about tobacco use (at more than one visit) | 24.9 | 55.4 | 29.0 | 22.6 | 28.6 | 2.6 | 26.4 (13.9; 40.2) | <.005 |
| Assess late quitters and continuous smokers are ready to quit (at more than one visit)e | 8.3 | 28.9 | 20.2 | 9.6 | 14.7 | 5.3 | 14.9 (2.5; 32.7) | .015 |
| Assist late quitters and continuous smokers in the quitting process (at more than one visit)e | 6.2 | 31.7 | 25.0 | 6.3 | 12.7 | 3.5 | 21.5 (10.6; 31.8) | <.005 |
| Arrange follow-up with late quitters and smokers (at more than one visit)e | 0.0 | 2.7 | 2.7 | 0.7 | 0.0 | 0.0 | 2.7 (0.0; 17.2) | .026 |
The median rate change is the median of the differences between the follow-up and baseline rate for each cluster.
The absolute difference in median rate change is the difference between the median rate change in the intervention clusters and the median rate change in the control clusters. Small discrepancies in the differences are due to rounding. The confidence interval (CI) was estimated using a permutation method.
P values were calculated using the Wilcoxon Test.
Recall of 5As (Ask, Advise, Assess, Assist, and Arrange) was defined “Yes” if nonsmokers and spontaneous quitters received the first 2As at more than one visit and if later quitters and smokers received the 5As at more than one visit.
Only women who quit smoking and those who continued smoking during pregnancy were considered for these steps of the 5As. There were 387 and 428 women in the intervention and control clusters at baseline and 476 and 407 women in the intervention and control clusters during follow-up period.
The size of the effects on the rate of women recalling the 5As in Argentina and Uruguay were statistically different (median regression, P = .02). In Argentina, there was a 29.7% relative increase in the percentage of women recalling the 5As (95% CI: 12.6–46.8; P = .002), while the effect in Uruguay was 8.5% (95% CI: 3.1–13.9; P = .004). Similarly, the effect on each individual component increased in both countries, with the increase for each component lower in Uruguay than in Argentina (Supplementary Appendix Tables 2 and 3).
The intervention group showed a statistically significant absolute increase on the rates of asking and advising on SHS at home and at work, compared to the control group (data not shown). Overall, asking about exposure at work and at home increased 24.0% (95% CI: 10.7–40.1) and 32.8% (95% CI: 21.1–45.0), respectively. As with the 5As, the effect was larger in Argentina than in Uruguay: asking about exposure at work increased 24.8% (95% CI: −0.3–49.9) in Argentina and 13.5% (95% CI: −4.2–31.3) in Uruguay and asking about exposure at home increased 43.3% (95% CI: 10.8–75.7) in Argentina and 25.6% in Uruguay (95%CI: 16.5–34.6). Overall, advising on SHS consequences on women’s and babies’ health increased 30.1% (95% CI: 14.7–41.3) and 30.0% (95% CI: 15.5–44.2), respectively. Again, the effect was larger in Argentina than in Uruguay: advising on SHS consequences on women’s health increased 38.8% (95% CI: 16.4–61.3) in Argentina and 16.0% (95% CI: 4.7–27.4) in Uruguay and advising on SHS consequences on baby’s health increased 43.5% (95% CI: 19.4–49.7) in Argentina and 21.3% in Uruguay (95% CI: 7.15–35.6).
The proportion of women who continued smoking during pregnancy was not reduced between baseline and follow-up either in the intervention or control groups (Table 3). Moreover, a slight statistically significant increase from baseline to follow-up in the proportion that continued smoking was observed in the intervention group (OR 1.14, 95% CI: 1.01–1.29), while no change occurred in the control group (OR 0.99, 95% CI: 0.88–1.11). The relative difference between baseline and follow-up changes in intervention and control groups was not statistically significant (ROR 1.16, 95% CI: 0.98–1.37). The quit rates among smokers at first ANC visit decreased in both groups at follow-up. However, the reduction was larger in the control group but the 95% CI included 1.0 (ROR 1.29, 95% CI: 0.84–1.97). In stratified analyses (data not shown), these trends were similar in both countries. We conducted a sensitivity analysis excluding women without saliva samples instead of treating them as smokers. The results did not change. For description purposes, Supplementary Appendix Table 4 reports the rates of women according their smoking status at baseline and follow-up by country, in intervention and control groups.
Table 3.
Effect of Intervention on Smoking During Pregnancy
| Intervention group (10 clusters)
|
Control group (10 clusters)
|
Intervention effect
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline rate n/N (%) | Follow-up period rate n/N (%) | ORa | Pb | Baseline rate n/N (%) | Follow-up period rate n/N (%) | ORa | Pb | RORc (95% CI) | Pd | |
| Continuous smoking | 330/1551 (21.3) | 424/1791 (23.7) | 1.14 (1.01–1.29) | .031 | 368/1757 (20.9) | 374/1729 (21.6) | 0.99 (0.88–1.11) | .814 | 1.16 (0.98–1.37) | .082 |
| Quit smoking during pregnancye | 57/387 (14.7) | 52/476 (10.9) | 0.74 (0.55–1.00) | .051 | 60/428 (14.0) | 33/407 (8.1) | 0.58 (0.43–0.78) | <.001 | 1.29 (0.84–1.97) | .239 |
CI = confidence interval.
Odds ratio (OR) comparing follow-up to baseline period calculated using Generalized Estimation Equation (GEE).
Significance of the OR of the time period.
Relative odds ratio (ROR) is the ratio of the odds ratio for the intervention group to the odds ratio for the control group.
Significance of the ROR. An ROR estimates statistically different from one implies a significant intervention effect.
The outcome “Quit smoking during pregnancy” was calculated considering as denominators the women who quit smoking during pregnancy plus the women who continued smoking during pregnancy.
At follow-up, a questionnaire was administered to 169 and 168 ANC providers in the intervention and control groups, respectively. The mean response rates were 85.2% for the intervention group and 86.8% for the control group. Evidence-based knowledge, positive attitudes, and self-confidence of providers were, in general, higher than 50% in both intervention and control groups at both baseline and follow-up. No significant changes were observed in the items explored between intervention and control groups, with the exception of the provision to women of smoking cessation self-help materials as a barrier with an increase of 50.6% (95% CI: 31.4–60.0) and decrease in the perception of the lack of materials as a barrier at −46.4% (95% CI: −59.3 to −14.8). Similar results were found when we used the Z-score to percentile rank transformation or scores higher than 6 to analyze the Likert-scales outcomes (data not shown). The results are shown in Supplementary Appendix Tables 5 and 6.
Discussion
Summary of Findings
In this cluster RCT, we assessed the effects of a multifaceted intervention to deliver the 5As intervention during ANC in public clinics in Argentina and Uruguay. The intervention resulted in an overall increase of 17 percentage points in the frequency of women recalling the 5As (30% in Argentina and 9% in Uruguay). However and despite this increase, less than one third of the women attending the intervention clinics recalled the 5As in more than one visit. The intervention also increased the frequency of women recalling advice regarding avoiding SHS. The intervention did not significantly affect the rate of women who continued smoking during pregnancy, or the rate of smokers quitting during pregnancy. Additionally, a slight increase in the rate of women who continued smoking and a decrease in the quitting rates among smokers were observed at follow-up in both groups. Finally, overall, the intervention did not have a significant effect on the knowledge, attitudes, and self-confidence of the ANC providers towards providing the 5As, which were highly positive in both intervention and control groups at baseline.
Strengths and Limitations
The study had several strengths. We used a rigorous experimental design and achieved similar groups by using a covariate-constrained randomization approach. Careful training of interviewers and monitoring of data acquisition resulted in minimal missing data. The outcome data collection in women during the postpartum stay, conducted by data collectors well separated from the intervention teams, prevented observer bias of the women’s outcomes. The selected intervention strategies were documented as effective in changing behavior and were tailored according to formative research. Finally, the multifaceted strategy was implemented with a pragmatic approach to integrate the intervention within the existing health systems, suggesting that the reported effects might be similar in a programme using these components.
Nonetheless, the study has a few limitations. Interviewing women during the postpartum stay and not during pregnancy could have affected recall of the ANC process, increasing outcome misclassification. We cannot rule out the possibility of social desirability bias in the responses of women interviewed in the intervention group. However, the clear separation of the outcome assessment at the postpartum stay conducted in different facilities and by interviewers independent of the ANC clinics makes this unlikely. Also, missing biochemical validation was a limitation, although we used a conservative approach considering all those who had missing values as smokers.
Interpretation
The intervention showed a moderate effect in increasing the proportion of women recalling the 5As, but, at follow-up, only a third of women in the intervention group recalled counseling in more than one visit. The high compliance with the intervention implementation at the antenatal clinic level among the intervention group suggests that the factors preventing a higher coverage of the 5As might be more likely related to variation in the behavior of the individual providers, as we found 40% of observations providers did not implement the 5As as required. Alternatively, the providers’ high positive attitudes and self-confidence towards smoking cessation counseling during pregnancy suggest that the barriers might be more related to some characteristics of the 5As. Despite the evidence of effectiveness of brief cessation counseling, our findings are consistent with implementation challenges found in clinical practice.6,25 Clinical practice guidelines that change the existing routines, demand training and new skills, and require substantial organizational changes have been associated with lower guideline implementation.26–28 The larger effect shown on the frequency of women recalling SHS advice with around 60% of women recalling advice at follow-up compared to baseline, suggest that the main barriers to full intervention implementation might be in the advice to smokers. Additionally, 50% of the providers in the intervention group at follow-up mentioned lack of time as a barrier to providing counseling (Supplementary Appendix Table 6).
Why the intervention showed a significantly differential effect in Argentina than in Uruguay is unclear and was unexpected. Compliance with the intervention components was similar in both countries, as well as the effect on rates of providers’ readiness. However, the overall quality of the intervention is unknown, as it was not rigorously evaluated and could have varied by country. These findings suggest that other areas should be further explored as potential factors of the differences. However, because of the pragmatic design, we did not systematically collect detailed process data on the intervention implementation that might be useful to propose alternative explanations.
The intervention did not affect either the rate of women who continued smoking or who quit smoking during pregnancy. The modest 17 percentage point increase in the frequency of women receiving the 5As between the intervention and control group, and the overall low percentage of women who received the 5As in more than one visit are the most likely explanations. The quality of the counseling might have been modest and could have contributed as well.
The slight increase in the rate of women who continued smoking and the decrease in the rate of quitters during pregnancy is a matter of concern for public health in both countries. This trend is not consistent with other reports that show a decline is smoking prevalence among women of reproductive age, including Argentina and Uruguay.29
In Context
To our knowledge, two published trials conducted in Australia evaluated comparable interventions to disseminate smoking cessation programs at antenatal clinics.12,13 Cooke and colleagues randomly allocated clinics to two groups, which received the program of brief intervention for smoking cessation either by simple or intensive dissemination and trained midwives who provided support and training for the program. The study showed no differences in the adoption of the smoking cessation program between the two groups.12 Lowe and colleagues performed a cluster RCT comparing hospitals conducting a behaviorally-based intervention, which included training workshops and reminders, compared to hospitals receiving printed materials. At 1-year follow-up, 68% of the intervention hospitals were providing antenatal smoking cessation to smoking pregnant women, compared with only 14% in the control hospitals.13 Both trials evaluated the intervention effect with managers and providers, a method highly susceptible to information bias, and did not measure the outcomes in women. Thus, our results are difficult to be compared.
Implications for Practice and Policy
In summary, these results show that by deploying similar interventions it can be expected a modest to moderate increase (depending on the country) in the provision of brief counseling for smoking cessation using the 5As strategy (measured by patient recall), but not to the majority of women and with uncertain quality. Strategies to increase the fidelity of delivery of the intervention are needed if policy makers are to expect reductions in the proportion of smokers among pregnant women.
Implications for Research
Can we improve this kind of pragmatic multifaceted intervention to make it more effective to increase coverage and quality of the brief counseling for smoking cessation to pregnant women? Exploring any differential effects according to whether only a special team of providers or all providers counsel women will be important to orient future designs. Additionally, exploring the reasons for the differential effects between countries will be important to identify other hidden barriers and facilitators. Finally, the trends of smoking during pregnancy, and among low-income groups, should be closely monitored in these countries to assess whether our observation was isolated or was part of a more generalized problem.
Supplementary Material
Acknowledgments
We wish to acknowledge the field team: Argentina Site: Hospital Materno-Infantil Dr Carlos Gianantonio: D. Fernández (coordinator), J. Carril; M. Rubino, M. Martinez (data managers); Hospital Zonal General de Agudos Héroes de Malvinas: J. Antón (coordinator), B. Clark, M. Rebotaro (data managers); Hospital General de Agudos Dr Carlos Bocalandro: E. Macagno (coordinator), N. Echarri, M. Muñoz (data manager); Hospital Zonal General de Agudos Magdalena V. de Martínez: M. Ferrary (coordinator), C. Buttner (data manager); Instituto de Maternidad Santa Rosa: V. Nicolaci (coordinator) M. Colmenero, M. Debis, K. Ratel, V. Soraire, G. Alarcon (data manager); Hospital Zonal General de Agudos San Roque: M.T. Moreno (coordinator), S. Fernandez, G. Velazquez, M. Agüero (data manager); Hospital Zonal General de Agudos Dr Narciso López: M.R. Sabbadín (coordinator) S. Tavella, M R Miño (data managers); Hospital Zonal General de Agudos Evita Pueblo: S. Souza (coordinator), S. Perconti (data manager); Hospital Municipal Ostaciana B. de Lavignolle: C. Muzzio (coordinator), S. Rodriguez (data manager); Hospital Zonal General de Agudos Lucio Meléndez: L. Frías (coordinator), B. Von Kaven, B. Aballay (data manager). Uruguay Site: Administración de los Servicios de Salud del Estado: O. Graña and E. Gómez (coordinator, data manager), Griselda Bittar (coordinator, data manager). Banco de Previsión Social: A. Raggio (coordinator, data manager), Claudio Albarracín (data manager). Ana Claudia Maisonev (data manager). We wish to acknowledge Eduardo Bergel, PhD, who assisted with the randomization. FA had the original idea. MB, AA, MC, PM, LG, and PMB designed the initial protocol. All authors contributed to the final protocol. MB, PM, AA, AM, MC, AB, LL, and FA oversaw the study execution. AC, LG, and RAS provided statistical and analytic expertise. MB, PM, AA and FA drafted the manuscript and all authors provided critical comments to it, and approved the final version to be published.
Funding
The study was supported through CDC Cooperative Agreement 5U48DP001948-04 (SIP09-18) to Tulane University. The Centers for Disease Control and Prevention (CDC) had input into the study design and data interpretation and reviewed and approved the report. The findings and conclusions are those of the authors and do not necessarily represent the official position of CDC. LG, RAS, AC, and FA had access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Footnotes
Supplementary Material
Supplementary Appendix Figure 1 and Tables 1–6 and can be found online at http://www.ntr.oxfordjournals.org
Declaration of Interests
None declared.
References
- 1.Bloch M, Althabe F, Onyamboko M, et al. Tobacco use and secondhand smoke exposure during pregnancy: an investigative survey of women in 9 developing nations. Am J Public Health. 2008;98(10):1833–1840. doi: 10.2105/AJPH.2007.117887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Recommendations for the Prevention and Management of Tobacco Use and Second-hand Smoke Exposure in Pregnancy. Geneva, Switzerland: World Health Organization; 2013. www.who.int/tobacco/publications/pregnancy/guidelinestobaccosmokeexposure/en/. Accessed January 26, 2015. [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 4.World Health Organization. International Consultation on Environmental Tobacco Smoke (ETS) and Child Health. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 5.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 6.Chamberlain C, O’Mara-Eves A, Oliver S, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev. 2013;10:CD001055. doi: 10.1002/14651858.CD001055.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministerio de Salud y Ambiente de la Nación. Guía Nacional de Tratamiento de la adicción al tabaco. Buenos Aires, Argentina: Ministerio de Salud, Presidencia de la Nacion; 2011. www.msal.gov.ar/tabaco/index.php/informacion-para-profesionales/cesacion-tabaquica. Accessed February 18, 2014. [Google Scholar]
- 8.Ministerio de Salud Pública. Guía Nacional para el Abordaje del Tabaquismo; Uruguay: 2009. http://cieturuguay.org/?p=1195. Accessed January 16, 2014. [Google Scholar]
- 9.Althabe F, Aleman A, Mazzoni A, et al. Tobacco cessation intervention for pregnant women in Argentina and Uruguay: study protocol. Reprod Health. 2013;10(1):44. doi: 10.1186/1742-4755-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mejia R, Buil Martinez V, Gregorich S, Perez Estable E. Physician counselling of pregnant women about active and second hand smoking in Argentina. Acta Obstet Gynecol Scand. 2010;89(4):490–495. doi: 10.3109/00016341003739567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxter S, Everson-Hock E, Messina J, Guillaume L, Burrows J, Goyder E. Factors relating to the uptake of interventions for smoking cessation among pregnant women: a systematic review and qualitative synthesis. Nicotine Tob Res. 2010;12(7):685–694. doi: 10.1093/ntr/ntq072. [DOI] [PubMed] [Google Scholar]
- 12.Cooke M, Mattick RP, Campbell E. The dissemination of a smoking cessation program to 23 antenatal clinics: the predictors of initial program adoption by managers. Aust N Z J Public Health. 1999;23(1):99–103. doi: 10.1111/j.1467-842x.1999.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 13.Lowe JB, Balanda PK, Stanton WR, Del Mar C, O’Connor V. Dissemination of an efficacious antenatal smoking cessation program in public hospitals in Australia: a randomized controlled trial. Health Educ Behav. 2002;29(5):608–619. doi: 10.1177/109019802237028. [DOI] [PubMed] [Google Scholar]
- 14.Berrueta M, Morello P, Alemán A, et al. Smoking patterns and receipt of cessation services among pregnant women in Argentina and Uruguay [published online ahead of print June 27, 2015] Nicotine Tob Res. doi: 10.1093/ntr/ntv145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weijer C, Grimshaw JM, Eccles MP, et al. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012;9(11):e1001346. doi: 10.1371/journal.pmed.1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivers NM, Halperin IJ, Barnsley J, et al. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials. 2012;13:120. doi: 10.1186/1745-6215-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melvin C, Dolan-Mullen P, Windsor R, Pennington Whiteside H, Jr, Goldenberg R. Recommended cessation counselling for pregnant women who smoke: a review of the evidence. Tob Control. 2000;9(suppl III):iii80–iii84. doi: 10.1136/tc.9.suppl_3.iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers EM. Diffusion of Innovations. 3rd. New York, NY: The Free Press; 1983. [Google Scholar]
- 19.Colomar M, Tong V, Morello P, et al. Barriers and promoters of an evidenced-based smoking cessation counselling during prenatal care in Argentina and Uruguay. Matern Child Health J. 2015;19(7):1481–1489. doi: 10.1007/s10995-014-1652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manly BFJ. Randomization and Monte Carlo Methods in Biology. London, United Kingdom: Chapman & Hall; 1991. [Google Scholar]
- 21.Althabe F, Colomar M, Gibbons L, Belizan JM, Buekens P. Smoking during pregnancy in Argentina and Uruguay. Medicina (B Aires) 2008;68(1):48–54. [PubMed] [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; www.r-project.org. Accessed August 18, 2014. [Google Scholar]
- 23.Koenker R, Bassett G. Regression quantiles. Econometrica. 1978;46(1):33–50. [Google Scholar]
- 24.SAS Institute Inc. SAS: Version 9.3. Cary, NC: SAS Institute Inc; 2010. [Google Scholar]
- 25.Stead LF, Buitrago D, Preciado N, et al. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;5:CD000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foy R, MacLennan G, Grimshaw J, Penney G, Campbell M, Grol R. Attributes of clinical recommendations that influence change in practice following audit and feedback. J Clin Epidemiol. 2002;55(7):717–722. doi: 10.1016/s0895-4356(02)00403-1. [DOI] [PubMed] [Google Scholar]
- 27.Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317(7162):858–861. doi: 10.1136/bmj.317.7162.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgers JS, Grol RP, Zaat JO, Spies TH, van der Bij AK, Mokkink HG. Characteristics of effective clinical guidelines for general practice. Br J Gen Pract. 2003;53(486):15–19. [PMC free article] [PubMed] [Google Scholar]
- 29.Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385(9972):966–976. doi: 10.1016/S0140-6736(15)60264-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


