Abstract
Renal dendritic cells (DC) and macrophages (Mac) represent a constitutive, extensive and contiguous network of innate immune cells that provide sentinel and immune intelligence function. They induce and regulate inflammatory responses to freely-filtered antigenic material and protect the kidney from infection. Tissue–resident or infiltrating DC and Mac are key to the initiation and propagation of renal disease, as well as essential contributors to subsequent tissue regeneration regardless of its etiology and pathogenesis. Their identification, functional and phenotypic distinction, interplay and relationship with effector and regulatory adaptive immune cells is complex and incompletely understood. This review discusses both the common and distinct characteristics of these cells, as well as recent key advances in the field that have identified renal-specific functions of DC and Mac that enable these important, phagocytic, antigen-presenting, cells to mediate or mitigate intrinsic kidney disease. We also identify priority areas for further investigation and prospects for translational and therapeutic application of acquired knowledge.
Keywords: dendritic cells, macrophages, kidney disease
Introduction
The manifestations of kidney disease are protean and include acute kidney injury (AKI) due to ischemia-reperfusion or direct tubular cytotoxicity, autoimmune glomerulonephritis, and rejection of kidney transplants affecting glomerular, interstitial and vascular compartments. Increasing molecular evidence now demonstrates the pivotal role of non-parenchymal cells, predominantly dendritic cells (DC) and macrophages (Mac), in determining both renal tissue injury and subsequent reparative responses following diverse insults.
Advances in technology and the understanding of innate and adaptive immunopathological cellular responses have facilitated DC and Mac identification and assessment of their function in non-lymphoid solid organs such as the kidney. DC are long-established as vital, systemic sentinels capable of responding to endogenous and exogenous ‘danger’ signals that activate cells to initiate and propagate immune responses to inciting antigen (Ag) 1–3. Mac have also been defined as a distinct, but related population of Ag-presenting cells (albeit less potent than DC) whose primary function is tissue homeostasis and phagocytic clearance. Both cell types reside within the renal interstitium and possess the capacity to drive and regulate both protective and deleterious renal pathology.
In this review we examine the archetypal components of the non-parenchymal compartment in the kidney. We consider recent advances in understanding the discrete yet overlapping roles of both DC and Mac - a difficult task due to the significant degree of functional and phenotypic similarity between these mononuclear phagocyte populations (Figure 1). We also review how innovations such as identification of distinct lineage markers and gene expression profiles, use of transgenic mice, and implementation of methods for cell ablation have facilitated the discrimination of subsets of each cell type that function in tissue homeostasis and immune tolerance, acute and chronic inflammation, and alloimmunity. We note however that significant limitations remain with many of these models, particularly the capacity to accurately target DC/Mac to modulate disease outcome, as well as their relevance to human disorders.
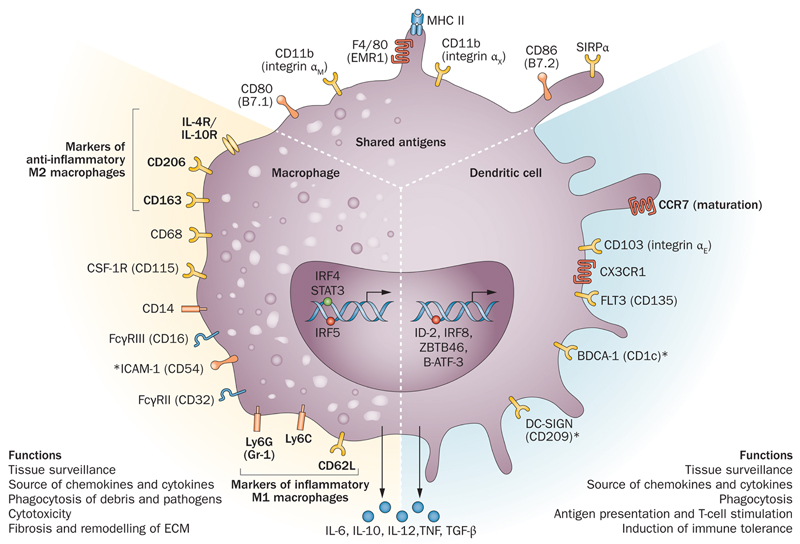
Figure 1. The heterogeneous but overlapping phenotype and function of renal DC and Mac.
DC are traditionally described as mediators of immune surveillance and antigen presentation and the primary determinants of antigenic fate through initiation of immune effector cell function or the development of tolerance. Mac also function as innate immune cells predominantly through phagocytosis, and production of toxic metabolites. However, the classical paradigm of DC versus Mac is increasingly indistinct within the kidney, as these cells exhibit overlapping surface markers, functional capabilities, and ontogenic pathways.
Abbreviations: AT3 – antithrombin 3; Batf3 – basic leucine zipper transcription factor ATF-like 3; csf – colony stimulating factor; DC-SIGN – dendritic cell-specific ICAM-3-grabbing non-integrin; flt3 – fms-like tyrosine kinase 3; ICAM – intercellular adhesion molecule; Id2 – inhibitor of DNA binding 2; IRF – interferon regulatory factor; SIRPα – signal regulatory protein alpha; STAT – signal transducer and activator of transcription; Zbtbf46 – zinc finger and BTB domain containing 46
* denotes human markers only
What defines a DC or Mac?
There is considerable flexibility, heterogeneity and complexity in the monocyte-myeloid developmental lineage 4, and both DC and Mac arise from progenitor cells in the bone marrow (BM) under the influence of key growth factors: colony-stimulating factor 1 (csf-1), fms-like related tyrosine kinase 3 ligand (Flt3L), and granulocyte-macrophage colony stimulating factor (GM-CSF) (Figure 2). Furthermore, monocytes recruited from the circulation may become tissue Mac or inflammatory DC during disease processes 5, 6.
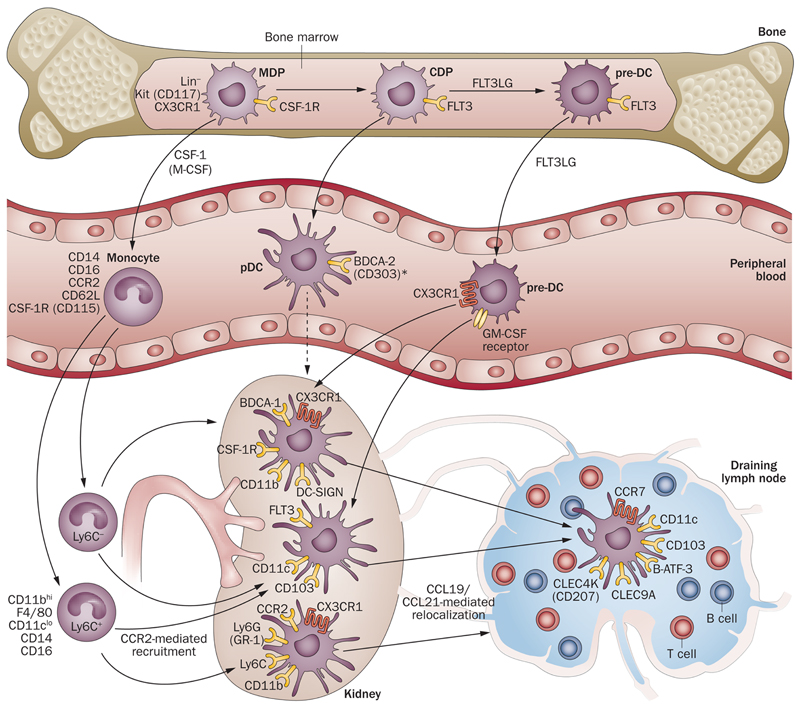
Figure 2. Ontogeny of renal-resident DC and Mac.
Bone marrow-resident monocyte-dendritic cell precursors (MDP) release monocytes to the peripheral circulation under homeostatic and inflammatory conditions. Cells also develop into common and pre-DC precursors (CDP and pre-DC) that migrate from blood to the renal interstitial compartment, with regular turnover. Pre-DC under the influence of different growth factors, become distinct, tissue-based subsets (broadly characterized as CX3CR1+ or CD103+) capable of exodus to the draining lymph node for antigen presentation to T cells. Pre-DC also give rise to plasmacytoid DC (pDC), although their presence in murine kidney is disputed.
An important issue has been how best to define DC versus Mac (Figure 1) and this area remains controversial (see Box 1) 7–10. Currently there is no definitive cellular demarcation and studies have tended to investigate either DC or Mac with segregation based on traditional views of identification. This research (predominantly in mouse models) has been based on the supposition that Mac or DC could be accurately identified by their expression level of the ‘macrophage’ markers F4/80 and CD11b or the ‘DC marker’ CD11c, but these markers are not exclusive 11. BM-derived Mac may express CD11c in vitro, whilst a DC may express F4/80 and CD11b, suggesting that these markers do not identify an unique cell population. The incomplete restriction of such cell surface markers complicates the interpretation of experimental data based upon this method of cell identification. This should be borne in mind when interpreting studies involving conditional cell ablation, or using transgenic mice expressing the human diphtheria toxin receptor (DTR) under the control of the CD11b 12 or CD11c 13 promoter (vide infra and Table 2) since cells other than DC or Mac may be affected following DT administration. These caveats are important to acknowledge, as there is likely to be overlap of function between Mac and DC, depending upon biological context. It is also desirable to determine the expression levels of multiple cell surface markers if possible (e.g. F4/80, CD11b, CD11c, CD103, major histocompatibility complex [MHC] class II), as both the pattern and level of expression of these cell surface markers may be informative. The analysis of transcription factors typically expressed by DC or Mac has also been utilized to characterize cells14. Whilst DC and Mac may have overlapping functions such as Ag uptake and presentation, there are several core functions that may be considered separately.
Box 1. DC versus Mac – why the confusion?
-
the markers are not exclusive
-
–
CD11c, F4/80, CD11b, MHC class II are all co-expressed
-
–
CD11c expression is induced with inflammation (in macrophages, neutrophils as well as DC)
-
–
-
similar growth factors promote their development
-
–
mononuclear cells from progenitors requires M-CSF, GM-CSF and Flt3L
-
–
cell phenotype in vitro demonstrate plasticity depending on the growth factors used
-
–
CSF1R (a traditional macrophage marker) is expressed by all classical DC; CSF1 and CSF1R mutations cause significant DC population depletion
-
–
injection of CSF1 into mice causes expansion of CD11c+ cells, including macrophages, conventional DC, and plasmacytoid DC
-
–
GM-CSF promotes DC development in vitro; GM-CSF produces alternative macrophage with ‘mature DC-type’ cytokine profile
-
–
-
functions are similar
-
–
both are highly phagocytic
-
–
antigen presenting capacity is typically attributed to DC; macrophages can suppress T cell activation in an antigen-specific manner
-
–
Table 2.
Rodent models of DC and Mac ablation
| Model | Mechanism | Utility | Specific Disadvantages |
|---|---|---|---|
| CD11c-DTR mouse196 | Simian diphtheria toxin receptor-enhanced green fluorescent protein (DTR-eGFP) fusion gene inserted after the CD11c promoter; | Short-term (48h) ablation of 85-90% conventional DC in vivo, with preservation of plasmacytoid compartment 197, to a lesser extent cytotoxic T lymphocytes196 and NK cells 198 are affected. B cells and Mac unaffected | Depletes all CD11c+ DC systemically, including F4/80+ cells79 Late (>72h) neutrophilia 199 Repeated systemic administration of DT is lethal |
| CD11b-DTR mouse 200 | DTR-eGFP fusion gene inserted after the CD11b promoter | CD11b+ cells predominantly affected; CD3+ T cells, B cells and neutrophils not affected | Depletes (>90%) all CD11b+ cells systemically at 24h (Gr1-CCR2-CX3CR1+ and Gr1+CCR2+CX3CR1-); counts still low at 48h69 |
| Liposomal clodronate67 | Liposomes phagocytosed by Mac, followed by intracellular clodronate accumulation, cell dies by apoptosis66 | Depletes phagocytic cells; total Mac (Gr1+ and Gr1-) decreased by >75% after 24h; Gr1+ cells rebound higher69; preserved CD11c+ population | All cells with significant phagocytic capacity potentially affected Clodronate leakage from dead/dying cells may affect inflammatory reactions65 |
DC and Mac – the basic immunological paradigm
Dendritic cells (DC)
DC represent a rare group of heterogeneous and potent BM-derived antigen presenting cells (APC) that play a critical role in immune surveillance and the instigation of immunity or tolerance. DC mediate a critical link between the innate and adaptive immunological armamentarium 1, 15, and control the generation of naïve and memory T cell responses16. DC arise from hematopoietic progenitor cells via developmental pathways that exhibit considerable plasticity17; their generation is governed predominantly by the hematopoietic growth factor Flt3L 18–20. They are present in the peripheral circulation (<0.1% of total circulating leukocytes), but also reside in virtually all tissues (especially near portals of entry), and display broad maturational and functional diversity 1, 15. They are highly proficient at both internalizing Ag and Ag processing21. Via a highly-orchestrated process, they subsequently present peptide fragments of processed foreign or self Ag bound to major histocompatibility complex (MHC) class I or II molecules to T cells bearing appropriate receptors 15. Once activated in the periphery, DC exhibit enhanced migratory capacity and convey processed Ag to secondary lymphoid organs for T cell stimulation that requires both MHC (signal 1) and co-stimulatory molecules (signal 2) provided by the DC22. DC activation, maturation and migration are triggered by a wide array of cell surface-expressed receptors for Toll-like receptor (TLR) ligands, C-type lectins, cytokines and chemokines involved in inflammatory responses (well-reviewed in 3, 18, 23).
Macrophages (Mac)
Mac also comprise a diverse group of innate immune regulatory cells that are particularly adept at phagocytosis of tissue debris and infectious material. They are rich in lysosomes, can also serve as APC, and play role in wound healing 24. Mac precursor populations arise from the BM with a similar lineage profile to DC, although their development, differentiation and proliferation are governed by csf-1 (also known as macrophage colony-stimulating factor [M-CSF]). Administration of csf-1 to mice post-natally increases kidney weight and Mac number 25. Mac arise during fetal life with a predominantly trophic role; developmentally their presence precedes the appearance of nephrons26, but they develop primary phagocytic function in adulthood27. Although the commonly accepted tenet is that Mac renewal within the interstitial compartment is derived from hematopoietic stem cells, recent evidence suggests contribution from embryonic progenitors28, 29 and in situ proliferation6, 30.
Several phenotypes that may be adopted by Mac led to the ‘M1/M2 macrophage paradigm’ in which M1 Mac (classically activated) are regarded as pro-inflammatory whilst M2 Mac (alternatively activated) are regarded as promoters of wound healing and fibrosis 31. These phenotypes have been defined by in vitro experiments and although undoubtedly simplistic, the M1/M2 concept emphasizes the fact that Mac, like DC, have inherent plasticity, so that the phenotype of individual cells and populations may change and evolve over time in vivo 32.
Renal DC and Mac in homeostasis
(i). Identification with the kidney
A range of mononuclear cells have been identified within the renal interstitum. CD3+, CD4+, CD8+, CD19+, and CD3−NK1.1+ lymphocytes have been isolated from unmanipulated rodent kidneys 33, as have regulatory CD4+CD25+34 and γδ T cells 33, 35, in addition to DC and Mac. Although mouse DC were first described in the seminal papers of Steinman and Cohn 40 years ago36–38, their localization, and phenotypic and functional characterization was confined initially to DC within secondary lymphoid tissue. Although DC (and their multiple subtypes) have now been described within virtually all lymphoid and non-lymphoid tissues, formal identification of renal DC, particularly CD11c+ DC, has been disputed. The renal DC population was thought initially to localize to the glomerular compartment and thus mediate the pathogenesis of glomerulonephritides. This assumption was supported by isolation of rodent glomerular cells phenotypically distinct from mesangial cells, that expressed MHC class II (Ia) and exhibited T cell allostimulatory capacity in primary mixed leukocyte cultures 39–42. Concurrent rodent and human studies confirmed the presence of MHC class II+ cells within the cortical interstitium 43, 44. Electron microscopic examination of rodent peritubular interstitium enabled differentiation of fibroblasts from immune cells with features suggestive of DC (high expression of MHC class II) or Mac (abundant primary and secondary lysosomes)45. Immunohistochemical identification of CD11c+ DC requires minimization of protein denaturation by avoiding high temperatures46, and has facilitated the detection and localization of DC proximate to peritubular capillaries47.
(ii). Phenotypic characterization
In mice, CD11c+ renal DC are heterogeneous, expressing MHC class II, CD11b and F4/80; although they exhibit allogeneic T cell stimulatory capacity, this is lower than that of splenic CD11c+ DC47. Extensive phenotypic characterization of conventional CD11c+ DC (cDC) has revealed an immature state (low CD80 and CD86, and negligible CD40 expression) and substantial phagocytic capacity, in addition to the absence of non-conventional plasmacytoid (p)DC markers (CD8α or B220),. CD11c+MHC II+ renal DC segregate into two distinct subsets: CD103 (integrin αEβ7)+ (CD11bloCX3CR1-F4/80-SIRP-α-) and CD11b+ (CD103-CX3CR1+F4/80+SIRP-α+), both of which divide in the steady-state48. CD103+ renal DC arise primarily from BM-derived precursor (pre)-cDC (see Figure 2), express higher inhibitor of DNA protein 2 (Id2) and interferon regulatory factor (IRF) 8, as well as Flt3. Both the Flt3 receptor and its ligand (Flt3L) are an absolute requirement for the development of this DC subset48. CD11b+ DC express csf-1 receptor (csf-1R) in keeping with a developmental bias towards this growth factor, but are also dependent upon Flt3L for complete reconstitution48.Mac, conventionally defined as CD11b+, express greater levels of the csf-1R (CD115) whencompared to DC 49. The csf1R-enhanced green fluorescent protein transgenic-reporter mouse (the so-called ‘MacGreen’ mouse, vide infra) has been used to track Mac appearance post-natally, with cells present prior to nephrogenesis in close apposition to developing renal tubules and increasing with csf-1 treatment 26. Tissue-resident Mac derive from BM-resident monocyte precursors 50, which may also be characterized as CX3CR1+CD117+Lin-; additional recruitment of Ly6C+ cells can occur under the influence of CCR2 (Figure 2)51. Tissue Mac derived from infiltrating monocytes may undergo differentiation into 2 broad categories depending on context: classically (M1) or alternatively-activated (M2) cells. M1 Mac may be induced by encountering danger-associated molecular pathogens (DAMPs) or pro-inflammatory cytokines, and produce IL-12 and IL-23 (as do DC) to promote CD4+ T helper (Th)cell polarization. . M2 Mac may develop from deactivated M1 Mac or arise de novo, and are immunoregulatory, and produce anti-inflammatory IL-10 as well as wingless-type MMTV integration site family member 7B (Wnt7B)52.
Studies in CX3CR1GFP/+ 53 and MacGreen 54 mice (Table 1) attest to the network of DC and Mac within the kidney, but despite improvements in renal DC characterization, absolute numbers following their isolation remain relatively low when compared to secondary lymphoid organs47. Systemic administration of Flt3L to mice55 and non-human primates56 has enabled expansion of both renal cDC and pDC in vivo. Ex vivo, freshly-isolated mobilized DC retain an immature phenotype and promote CD4+CD25+ IL-10-producing regulatory T cells (Treg) 55. Low chemokine receptor (1, 2, 5 and 7) transcript levels by these DC reflect a failure to migrate in vitro in response to chemokines (CCL3, 5 and 20), although their migratory capacity to the lymphoid tissue-homing chemokines CCL19/20 can be augmented ex vivo by exposure to bacterial lipopolysaccharide (LPS)57.
Table 1.
DC and Mac reporter mice
| Mouse model | Model basis | Utility | Cells identified | Disadvantages |
|---|---|---|---|---|
| CX3CR1GFP/+ 195 | Coding exon on one allele of CX3CR1 replaced by open reading frame for enhanced green fluorescent protein (eGFP) | Fate mapping of CX3CR1 DC that differentiate from BM-derived monocytes of the same lineage | CD11b+MHCII+ CX3CR1+CD11c+/-F4/80+/-CD103- ie CD11b+ type Mac (approximately 90% of total renal DC/Mac population) | Does not identify the remaining CD11b-MHCII+CX3CR1-CD11c+F4/80-CD103+ cell population (estimated 5% of renal-resident DC) |
| MacGreen49, 54 | EGFP gene driven by c-fms promoter (which encodes colony stimulating factor-1 receptor [CSF-1R]) combined with the first intron54 | Identification of Mac | BM: 50% eGFP+ cells are also F4/80+ and CSF+1R+ Peripheral blood: all eGFP+ cells express F4/80 and CD11b Kidney: peri-epithelial Mac-like cells correlate with F4/80 expression, predominantly medullary location61 |
Does not distinguish cell subset |
While no candidate precursor for renal-resident DC has been formally identified, they are thought to be derived from common DC precursors that arise from BM progenitors and subsequent blood-borne pre-DC precursors58 (Figure 2). Ly6C- ‘patrolling’ circulating monocytes may also contribute to renal resident DC, while Ly6C+ (Gr-1+) cells infiltrate the kidney under inflammatory conditions6, 59 in a manner requiring CX3CR1 (the fractalkine receptor) 60.
The complexity of resident renal DC/Mac populations has been elegantly reinforced in recent work demonstrating multiple discrete subsets in the kidney, distinguished by cell surface markers, cytokine production, and transcription factor and chemokine receptor expression 14. The populations were initially defined according to the expression level of CD11b and CD11c. Subsets 1 and 4 exhibit a DC phenotype, and an ability to robustly stimulate CD4+ T cell proliferation that includes induction of Foxp3+ Treg. However, these cells also phagocytose latex beads, and express the Mac marker CD68 in conjunction with developmental and reparative growth factors such as insulin-like growth factor 1, platelet derived growth factor and Wnt7B . In contrast, subsets 2 and 3 display a Mac phenotype with phagocytic capability but an absent or limited capacity to induce T cell proliferation. These cells also express growth factors and produce IL-10 following LPS stimulation. Thus, the concept of overlapping DC/Mac characteristics should be borne in mind in experimental studies or transgenic mice that utilize a single cell marker such as CD11b, CD11c, CX3CR1 or csf-1R as these are all variably expressed by DC and Mac in the normal kidney. A further limitation when assessing DC/Mac populations, is that their characterization is based predominantly on whole kidney, rather than compartmentalized digests. Different renal microenvironments may well influence the presence of distinct DC/Mac subtypes leading to erroneous conclusions regarding cell localization: for example glomerular Mac fail to express F4/80 but may be identified by CD6861, 62.
(iii). Mouse models that enhance DC and Mac identification, imaging and function: reporter mice (Table 1)
Transgenic reporter mice, such as CX3CR1GFP/+ mice that map DC derived from CX3CR1+ monocytes discharged from the BM, have confirmed the homeostatic presence of renal DC throughout the entire interstitial scaffold, encasing glomeruli and present in low numbers within mesangial matrix53. The ‘MacGreen’ mouse54 allows visualization of Mac surrounding glomeruli and encasing renal tubular epithelial cells (RTEC) within the medulla (http://www.macrophages.com/macrophage-images).
(iv). Models of Mac and DC ablation (Table 2)
Various methods have been used to conditionally ablate Mac and/or DC in vivo with the aim of dissecting their function in disease (Table 2). Early work utilized the administration of liposomal clodronate that was toxic to phagocytic cells, particularly Mac63–67. The systemic administration of liposomal clodronate profoundly ablates Mac populations in the kidney, as well as liver and spleen, such that its effect may be secondary to intra-renal or extra-renal effects. Despite these caveats, the administration of liposomal clodronate has been used in multiple mouse and rat models of renal disease to selectively deplete DC and Mac (Table 4). Selective ablation has been attempted using transgenic mice expressing the human DTR under the control of the CD11b 12 or CD11c 13 promoter to deplete Mac or DC respectively following diphtheria toxin (DT) administration. Although it is inevitable that cells other than DC or Mac may also be deleted (Table 2), use of CD11b-DTR and CD11c-DTR mice has been highly informative.
Table 4.
Rodent models of renal injury and the effect of DC or Mac depletion
| Mouse model | Phenotype | DC/Mac effect | CD11c-DTR | CD11b-DTR | LipClo |
|---|---|---|---|---|---|
| AKI: | |||||
| UUO | Model of renal fibrosis | CD11b+Gr-1+ or F4/80+ DC interstitial infiltrate | No change in fibrosis79, 80 | Reduced fibrosis6 | Reduced fibrosis 77 |
| IRI | Coagulative necrosis of renal tubular epithelial cells | Neutrophilic, Mac and CD11c+ DC interstitial infiltrate | Exacerbated 68; protection 128 | Exacerbated 68; no change69 | Exacerbated 134; protection 68, 69, 71; decreased TNF production 145 |
| Cisplatin | Renally-excreted drug, concentrated in cortex; predominant damage is to S3 segment of the proximal tubule; coagulative necrosis of RTEC | Not described | At time of cisplatin treatment,- exacerbated injury 127 | Not studied | Not studied |
| Adriamycin | Glomerular capillary permeability, tubular atrophy via ROS | Adoptive transfer of LPS-treated pDC ameliorates disease208 | Not studied | Not studied | Not studied |
| GN: | |||||
| Nephrotoxic | Crescentic GN | DC capture Ag; | At day+4 or | Decreased | Decreased |
| nephritis (NTN) | (see Table 3) | present Ag to T cells | day+10, aggravates renal injury 92; depletion at day+7 reduces injury 90 | glomerular crescents and proteinuria; improved renal function96 | proteinuria 213; prevention of protection by CRP treatment 123 |
| GN (NOH mice) | Podocyte-based glomerular pathology (see Table 3) | CD11c+CD11b+Gr-1+ glomerular infiltrates | Decreased CD11c+CD11b+Gr-1+ glomerular infiltrates | Not studied | Not studied |
| Lupus NZB/W mice | Severe GN with polyI:C 209 | CD11b+GR1-F4/80+ glomerular infiltrates210 | Not studied | Not studied | Decreased accumulation of Mac and glomerular injury 209 |
| MRL.Fas(lpr) mice | See Table 3 | Mature CD11c+ DC within kidney 117; TLR9 expression on DC and Mac in affected glomeruli 211 | Decreased disease severity; autoAb formation (CD11c-DTR mice) 212 | Not studied | Not studied |
| Infection Pyelonephritis | E. coli-induced 70 | DC production of CXCL2 recruits neutrophils 70 | Decreased neutrophil recruitment | Not studied | Not studied |
Abbreviations: AKI – acute kidney injury; BM, bone marrow; CRP – C-reactive protein; GN – glomerulonephritis; IRF – interferon regulatory factor; IRI – ischemia reperfusion injury; LipClo, liposomal clodronate; LPS – lipopolysaccharide; ROS – reactive oxygen species; SIGIRR – single immunoglobulin and toll-interleukin-1 receptor; UUO – unilateral ureteric obstruction
Despite the apparent simplicity of cell depletion studies, there are multiple caveats to their interpretation. For example, what might be the possible explanations of an experiment in which depletion of CD11c+ cells reduced tissue injury? These would include i) the production of injurious factors by resident or infiltrating renal CD11c+ cells, ii) the production of protective factors by the residual surviving renal CD11c+ cells, other hematopoietic cells, or parenchymal cells (e.g. generated subsequent to interaction with the apoptotic corpses of ablated cells), iii) the promotion of systemic protective effects by renal or extra-renal CD11c+ cell depletion (e.g. skewing of immune responses), iv) the regeneration of CD11c+ cells following transient depletion might be protective, and any combination of these potential mechanisms.
Investigators have typically used a single cell ablation method, although the concurrent use of multiple methods can also be informative. Thus, administration of liposomal clodronate to CD11b-DTR mice was markedly protective against ischemia reperfusion injury (IRI), whilst administration of DT to CD11b-DTR mice had no effect on injury 68, 69. Intriguingly, the combined administration of both liposomal clodronate and DT to mice provides no protection i.e. the addition of DT removes the protective effect of the clodronate 69. These data suggest that the phenotype of the surviving residual cells in the kidney may be key to protection. These potential caveats and complexities should be borne in mind when cell ablation models are employed.
(v). Intrarenal DC and Mac function: cell recruitment, renal repair and fibrosis
Renal DC appear able to recruit other cells that perform immune functions. Thus, in a mouse model of Escherischia coli pyelonephritis, CD11c+ renal DC are the predominant producers of CXCL2 that recruits neutrophils to facilitate bacterial clearance70 (Figure 3), an effect that is impeded following conditional DC ablation (employing CD11c-DTR mice).
Figure 3. Renal DC function in health and disease.
DC provide homeostatic functions including tolerance to peripheral antigens typically cleared by the glomerulus, and anti-infectious immunosurveillance. Interaction with bacteria promotes generation of chemokines to attract effector cells, such as neutrophils. DC responsiveness and maturation state may be regulated by ongoing interaction with tubular epithelial cells. They also operate to exacerbate or mitigate a wide range of parenchymal disease and this role may be determined by either tissue-resident or influxing cells.
Abbreviations: AKI – acute kidney injury; IRI – ischemia reperfusion injury; NTN – nephrotoxic nephritis; SLE – systemic lupus erythematosus; UUO – unilateral ureteral obstruction
The progression of many experimental models of kidney injury (such as chronic allograft and diabetic nephropathy) can be limited by various maneuvers, but these do not typically lead to renal regeneration. Renal regeneration does occur, however, following moderate AKI induced by renal IRI and this has been used to explore the role of Mac in renal repair. Thus depletion of Mac using either liposomal clodronate 71 or DT treatment of CD11b-DTR mice during the reparative phase of AKI markedly retards restoration of tubular integrity and renal function. Recent work implicates Mac-derived Wnt7B and TLR4-induced IL-22 as key mediators of renal repair and tubular regeneration 52, 72.
Fibrosis is associated with progression of kidney disease to chronic renal impairment (well reviewed in 73, 74) . Mac may adopt a wound healing M2 phenotype during inflammation that promotes the production of extracellular matrix by myofibroblasts and scar formation (Figure 4). This is detrimental in the kidney, and may occur in conjunction with other pro-fibrotic processes such as epithelial cell cycle arrest75. Mac have been implicated in the development of renal fibrosis since administration of liposomal clodronate is protective in the rodent unilateral ureteric obstruction (UUO) model 76, 77. Interestingly, reduced fibrosis is evident in DT treated CD11b-DTR mice 6, 78, while DT treatment of CD11c-DTR mice does not reduce renal scarring 79, 80 despite the fact that renal DC exhibit increased maturation, activation and Ag-presenting capacity during UUO80. Studies involving the use of BM chimeric-CD11b-DTR mice in which either renal resident DC/Mac or infiltrating BM-derived cells are depleted have demonstrated that resident renal non-parenchymal cells proliferate in UUO but do not actively contribute to fibrosis 6. The cells with a pro-fibrotic transcriptional profile were Ly6Clo cells that had matured from kidney-infiltrating Ly6Chigh monocytes 6. Also, persistent pro-inflammatory Mac activation promotes tubular atrophy and fibrosis following AKI 81. Collectively, these data suggest that Mac rather than DC may drive renal scarring but the mechanism is unclear. Although transforming growth factor beta (TGFβ) 1 is believed to be a key pro-fibrotic cytokine, mice with Mac-specific deletion of TGFβ1 exhibited comparable levels of fibrosis after AKI or UUO suggesting that Mac-derived TGFβ1 is not critical 82. Mac-derived mediators such as the lectin galectin 3 78 may be important, but further work is needed to clarify their identity and role.
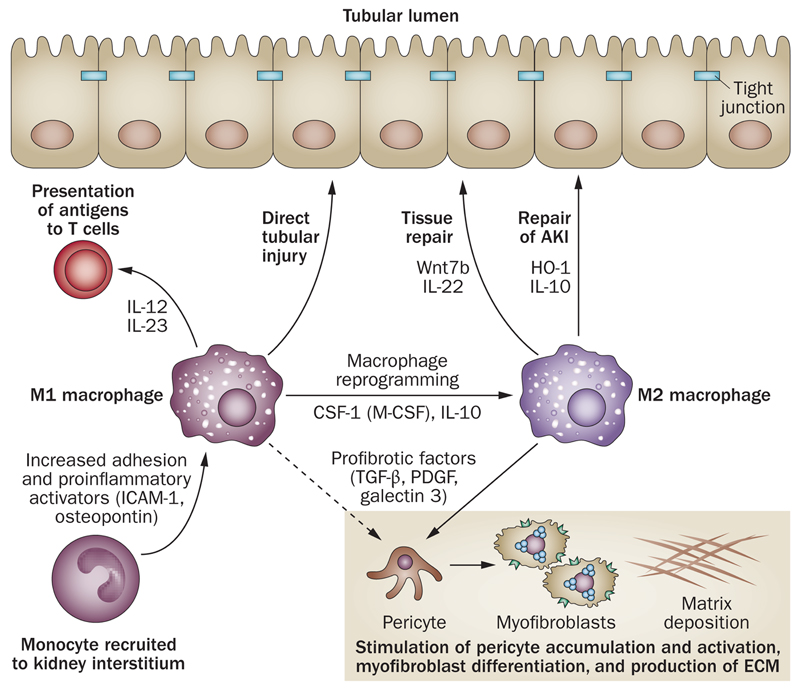
Figure 4. Mac in renal disease.
Tissue-resident Mac or infiltrating pro-inflammatory monocytes may become classically-activated (M1) to produce IL-12 and IL-23 and engage T cells for antigen presentation and activation or induce pro-fibrotic parenchymal change. M1 Mac may be reprogrammed to become alternatively-activated (M2) Mac by anti-inflammatory cytokines or apoptotic cell ingestion. M2 Mac may facilitate restoration of tubular cell integrity following injury . Mac may also express anti-inflammatory mediators such as HO-1 or IL-10 that act to limit tissue injury and promote resolution of inflammation, but may also drive pericyte and myofibroblast activation.
Abbreviations: AKI – acute kidney injury; HO-1 – heme oxygenase-1; ICAM-1 – intercellular adhesion molecule-1; M-CSF – macrophage colony-stimulating factor (also known as csf-1); OPN – osteopontin; PDGF – platelet-derived growth factor; Wnt7b – wingless-related MMTV integration site 7B.
(vi). Extrarenal DC function
Following activation, renal DC traffic to the draining renal lymph node where they present Ag. Several studies have shed light on the behavior of these DC when exposed to antigenic material. CD11c+ renal DC preferentially and rapidly endocytose small (40kDa) molecules, compared to splenic DC that endocytose larger (500kDa) Ag 83. DC in the draining renal lymph node also endocytose low molecular weight Ag that has been transported by cell-independent mechanisms. CD8+XCR1+Batf3+ (basic leucine zipper transcriptional factor ATF-like 3) renal lymph node-based DC are capable of Ag cross-presentation to subsequently delete cytotoxic T cells in a programmed death ligand 1 (PD-L1)-dependent manner84. This implies a necessary tolerogenic function for renal DC continually exposed to innocuous Ag. This view is further supported by the finding that proteasomal processing of albumin in vitro by BM-derived rat renal DC allows these cells to prime and subsequently activate CD8+ T cells85. Similar observations have been made in rats subjected to five-sixth nephrectomy to induce focal segmental glomerulosclerosis that demonstrated interstitial inflammation, including infiltrating CD11c+OX62+ DC85. Infiltrates were reduced in animals treated with the proteasome inhibitor bortezomib. In the same study, DC isolated from draining renal lymph nodes activated syngeneic CD8+ T cells in a manner that was time-dependent and reduced by bortezomib treatment.
Systemic administration of LPS to mice initiates DC efflux from the kidney to the draining renal lymph node over 24 to 48 hours86, 87. Renal subcapsular placement of ovalbumin (OVA) and subsequent LPS administration results in DO11.10 (OVA-specific) CD4+ T cell division in the ipsilateral renal lymph node with Ag presentation mediated by CD11c+ renal DC. A similar migratory response has been seen following IRI. This work is mechanistically relevant to ex vivo analysis of Flt3L-mobilized renal DC that upregulate CCR7 in response to LPS57, a necessary requirement for DC migration to draining lymph nodes via CCL19 and 21.
Renal DC and Mac in intrisinc renal disease models (Tables 3&4)
Table 3.
Experimental models of murine glomerular disease with known DC- and Mac-related pathogeneses
| Model | Mechanism | DC/Mac function |
|---|---|---|
| Nephrotoxic nephritis (NTN)89 | Glomerular injury- induced by the injection of mouse/rat renal cortex into a heterologous species (eg sheep, rabbits), then the Abs introduced into rodents | Ag/Ab complexes endocytosed by MHC II+ DC and Ag presented to CD4+ T cells. Mac are the main effector cells201. Aggravation of damage after early depletion of CD11c+ DC92. |
| Glomerulonephritis – (NOH mice 104) | Podocyte injury - the promoter for podocyte-specific human nephrin fused to cDNA encoding the transmembrane domain of the transferrin receptor, ovalbumin (OVA), and hen egg lysozyme (HEL) | Injection of activated OT-II (OVA-specific) Th cells, intra-renal stimulation. Periglomerular infiltrate, Mac-like: CD11c+, CD86+, MHC II+, CD11bhi/int, Gr1+ |
| Lupus models: | ||
| BWF1 mice 202 | F1 hybrid strain created from mating New Zealand black and white mice205, 206 | Mononuclear cell infiltration in kidney. Attraction of autoreactive T and B cells to kidney and expansion in draining lymph node114. CD11b+CD11c+ intrarenal DC/Mac are the source of B lymphocyte chemokine106. |
| B6.TC mice203 | Mice homozygous for the NZM2410 lupus susceptibility QTL (Sle1, Sle2 and Sle3) | DC from these mice demonstrate higher CD40 expression 207 DC have higher T cell allostimulatory capacity111 |
| NZM2328 mice 204 | Derived by selective inbreeding of progeny of a cross between NZB and NZW mice | Intraglomerular CD11c+ DC, F4/80+ Mac are confined to interstitial regions |
Multiple rodent models exist as mimics of clinical renal disease to provide mechanistic insights into glomerular and tubular pathophysiology (well reviewed in 88). We discuss a number of standard immunological and cytotoxin-based examples of renal disease where DC and/or Mac have been investigated extensively.
Glomerulonephritides
Use of ovine (or rabbit or goat) antiserum produced following vaccination with mouse renal cortex and then injected into mice, generates rapid and progressive glomerular damage (nephrotoxic nephritis - NTN). This mimics the crescentic glomerulonephritis (GN) seen in human autoimmune disease89. The glomerular-based Ag-Ab complex that is generated is captured by both DC and Mac and presented subsequently to T cells. The cells presenting Ag within the kidney during the initial injury phase of NTN are typically CD11c+CD11b+ with a maturing phenotype90. Renal DC also induce CD4+ T cell proliferation ex vivo and promote concurrent secretion of IFNγ and IL-10. Tubulointerstitial DC release IL 1β to promote NLRP3 inflammasome-caspase-1 pathway activation91. Ly6C- renal DC produce most of the pro-inflammatory tumor necrosis factor-α and IL12-23p4090.
Renal DC may also provide a protective role in NTN as their deletion (in CD11c-DTR mice) at days 4 or 10 after disease induction aggravates the extent of injury92. The mechanism of DC-mediated renal protection in NTN centers on IL-10 production and regulation of Th1 responses 93, 94. Initial evidence suggested that this effect might be mediated via enhanced expression of inducible costimulator (ICOS)-L specifically on renal DC, that could promote IL-10 secretion following its ligation by T cells 95. In contrast, DC depletion at day 7 reduces the presence of effector DC and T cells90, implying a biphasic role for DC in this disease process. Mac ablation (using CD11b-DTR mice) attenuates glomerular disease, tubular injury and the presence of effector CD4+ T cells 96. Other methods that block either Mac recruitment or activation factors, such as CCL2 are also protective in GN 97, 98.
Rat mesangioproliferative GN (anti-Thy 1.1 GN) is produced by injection of mouse monoclonal antibody to Thy1.1 and results in mesangial-based Mac infiltration99. Anti-glomerular basement membrane (GBM) disease also demonstrates ingress (or localization of adoptively transferred) monocyte-derived Mac that proliferate within glomeruli and correlate with histopathological severity100, 101, a phenomenon that can be reversed by inhibiting csf-1102 or treatment with CD80/86 monoclonal antibody103.
In a mouse model of podocyte immunopathology in which Ag are expressed in glomerular podocytes (so-called NOH mice) 104, glomerular infiltrates demonstrate increased CD11c+CD11bint/hi DC, and CD11c-CD11b+ Mac, as well as pro-inflammatory CD11c+Gr1+ DC or CD11cloGR1+ Mac. In this model, DC produce IL-12 and upregulate CD86 and CD40, consistent with their activation; in addition, the DC component, and consequently the periglomerular infiltrates are eliminated in DT-treated NOHxCD11c-DTR mice. Presentation of Ag by DC to CD4+ T cells is intrarenal only and facilitates CD8+ cytotoxic T lymphocyte accumulation.
Lupus Nephritis
Systemic lupus erythematosus (SLE) is a common autoimmune disease with a clinical predilection for catastrophic renal involvement. In animal models, the majority of studies have centered on the role of systemic DC in disease pathogenesis. However, the role of kidney-resident DC and Mac in disease initiation and progression have been increasingly described (Table 3).
NZB/WF1 mice develop lupus nephritis spontaneously and histologically this is characterized by extensive mononuclear cell infiltration. The presence of CD11b+F4/80hiCD80+CD86+ Mac has been shown to correlate with disease severity 105, and intrarenal CD11b+CD11c+ cDC express B lymphocyte chemokine (BLC, CXCL13) with the ability to recruit autoAb-producing B cells 106. MRL-Fas(lpr) mice demonstrate age-dependent lupus nephritis that correlates with renal expression of C1q (a subcomponent of complement activation via the classical pathway) that is generated locally by renal CD11c+ DC107. The pro-inflammatory cytokine IL-12, produced intrarenally by both DC and Mac, promotes the accumulation of IFNγ-secreting T cells to foster progression of nephritis. Not surprisingly, MRL-Fas(lpr)IL-12-/- mice demonstrate reduced renal damage and lower mononuclear cell infiltrates108. In contrast, overexpression of CCL2 promotes monocyte recruitment, mitigated by blockade of the chemokine or its receptor (CCR2)109, 110. B6.TC mice also develop GN with concomitant autoAbs. DC from these mice have higher T cell allostimulatory capacity and block the suppressive function of CD4+CD25+ Treg via overproduction of IL-6111, in addition to facilitating B cell autoAb production and proliferation112. NZM2328 mice also display a proliferative, gender-dependent GN113. Renal histology is characterized by intraglomerular CD11c+ DC that co-localize with T cells114, while F4/80+ Mac are confined to interstitial regions, suggesting that intrarenal DC mediate the presentation of (auto)Ag to promote disease progression.
Targeting Dc and Mac regulates experimental renal disease
(i). GN
Administration of cyclophosphamide and/or co-stimulation blockade (CTLA4-Ig or anti-CD154 mAb) to NZB/WF1 mice decreases CD11c+ renal DC infiltrates and induces lupus remission 115. Moreover, amelioration of disease in MRL-Fas(lpr) mice has been demonstrated using a p38 mitogen-activated protein kinase (MAPK) inhibitor 116 that decreases renal CD11c+ DC, including those producing high-mobility group box 1 (HMGB-1) displaying a mature phenotype 117. Creation of NZB mice lacking IRF8 (and thus lacking pDC) or mice carrying a mutation of the peptide-histone transporter solute carrier family 15, member 4 (SLC154, that retain pDC incapable of producing type I IFN) demonstrate amelioration of lupus pathology, implicating pDC in lupus pathogenesis 118. In perinuclear anti-neutrophil cytoplasmic Ab (pANCA) positive SCG/Kj mice119, a novel triazolopyrimidine derivative (NK026680) that inhibits DC function suppresses multiple disease manifestations and Ab development120.
Manipulation of the humoral immune response may also mitigate disease in GN. Ligation of Fc receptors (particularly FcγRIIb) by immunoglobulin provides an inhibitory signal to prevent TLR-mediated DC maturation and attenuate lupus nephritis following adoptive transfer of immature DC121. MRL-lpr mice lacking FcγRIIb also display greater B cell activity and DC-derived IL-12122. Administration of C-reactive protein reverses renal manifestations of NTN in a Mac-dependent manner123. Immune complexes also suppress Mac inflammatory responses via FcγRIIb124 which is protective in a GN model125. Binding of tissue-bound Ab or immune complexes may also activate Mac to produce pro-inflammatory mediators via activation of various signal transduction pathways including spleen tyrosine kinase (Syk). The Syk inhibitor fostamatinib markedly ameliorates NTN in rats and limits Mac activation following treatment with aggregated IgG126.
(ii). AKI
Both IRI and cisplatin induce tubular epithelial cell coagulative necrosis. CD11bloF4/80hi DC and CD11bhiF4/80lo Mac (both differentiated from Ly6Chi infiltrating cells) can be identified early (3 hours) following IRI 60, peaking at 24 hours and become predominantly Mac in phenotype. Ablation of DC exacerbates cisplatin-induced AKI127, although DC-mediated protection has been disputed in models of IRI 68, 128. Mac ablation may provide renal protection in an IRI model 129–131, that is reversed by adoptive transfer of the mouse Mac cell line RAW 264.7 cells71, 130. Mac also display distinct phenotypes depending upon time post-injury, with M1 predominance initially (1-3 days following reperfusion) and M2 skewing during repair (with critical Wnt7b52 and csf-1132 production) and phenotype switching following adoptive transfer 133. The significance of each Mac subset is reflected in loss-of-function experiments demonstrating that depletion of M1 cells reduces injury, while reduction in M2 cells delays tissue restoration in IRI-induced AKI133, 134.
In a variation of the DTR models described above, mice selectively expressing the DTR gene in proximal tubular epithelial cells (where a human heparin-binding epidermal growth factor construct is linked to the promoter region of gamma glutamyl transferase 1 specific to proximal tubule cells) develop AKI in response to DT132. Administration of DT leads to renal infiltration with CD11b+F4/80+CD11c+CX3CR1+CD86+ DC/Mac. Prior administration of liposomal clodronate worsens renal injury, increases mortality and blunts the rapidity of renal repair, as does the use of DT in mice concomitantly expressing DTR in both CD11c+ DC and proximal tubules, demonstrating the necessity of DC/Mac for tubular recovery.
Ablation of F4/80+CD11c+ DC/Mac 24 hours after renal IRI using liposomal clodronate aggravates injury and retards regeneration by promoting pro-inflammatory cytokines and dampening intrinsic IL-10 release by these cells134. In the same model, injury is partly alleviated by adoptive transfer of CD11c+ DC. The protective effects of IL-10 generated by renal DC have been seen in other models of AKI, including cisplatin nephrotoxicity. Renal DC express higher IL-10 levels following cisplatin treatment (with concomitant increases in IL-10R1 expression). Conversely, IL-10KO mice display enhanced cisplatin-mediated injury, as do chimeric mice with DC that lack the capacity to produce IL-10135. This finding is also in keeping with increased expression of ICOS-L on renal DC in cisplatin-treated mice127.
CD11c+ DC may also provide protection against AKI by modulating the effects of additional cell populations. Thus the protective role of Treg (both CD4+CD25+FoxP3+ and CD4+CD25+IL-10+)136 induced by CD11c+ DC following renal IRI has been well-recognized 137–140. Moreover, infusion of mesenchymal stem cells abrogates renal IRI (reviewed in 141), via an effect that is partly mediated by CD11c+ DC142. The immature phenotype of tissue-resident DC and intrarenal FoxP3+ expression are lost in DT-treated CD11c-DTR mice administered mesenchymal stem cells, and partially restored by the adoptive transfer of CD11c+ DC although not if they lack IL-10. There is increasing evidence that the cytokine milieu associated with sterile inflammation established in IRI is modulated by renal DC/Mac to drive pathologic and reparative processes. RTEC express csf-1 to drive Mac proliferation in situ132, 143. TLR-activated RTEC limit classical Mac activation in vitro with results of Ab inhibition experiments suggesting a role for IL-10144. Furthermore, a series of in vitro and in vivo experiments demonstrated that RTEC can induce non-programmed, quiescent Mac or programmed pro-inflammatory M1 Mac to adopt a M2 phenotype that limits acute renal inflammation and promotes repair133. F4/80+CD11c+ renal DC/Mac numbers remain static, and F4/80- DC/Mac with a mature phenotype increase in IRI kidneys. TNF-α, as well as IL-6 and CCL2 are produced in greater quantities by renal DC isolated from ischemic kidneys, and in vivo depletion of DC diminishes total TNF-α secretion within the renal CD45+ cell compartment 145.
(iii). Unilateral ureteric outlet obstruction (UUO)
UUO is a well-characterized model in which early inflammation is followed by renal fibrosis. CD11c+ DC exhibit phenotypic maturation and enhanced Ag presentation to and activation of T cells following UUO, with accompanying interstitial infiltration by CD11b+Gr-1+ or F4/80+ DC (depending upon the cell surface marker used)79, 80, 146. DC depletion in CD11c-DTR mice at various times following induction of UUO does not alter subsequent development of renal fibrosis79, 80 although this feature is ameliorated by Mac depletion in CD11b-DTR mice6 or by administration of liposomal clodronate77. Additional data confirm decreased IFNγ and IL-17 in T cells following DC depletion146. In addition, 3 distinct populations of CD11b+Ly6Chi/int/lo Mac have been identified that derive from BM monocytes or tissue-resident Mac 6. This is in keeping with data demonstrating that CCL2 or CCR2 blockade (and thus recruitment of inflammatory-type monocytes/Mac) impedes Mac accumulation147, and that attenuation of Mac matrix metalloproteinase 9 production decreases fibrosis148.
Cell surface receptors that play a role in mediating AKI are often expressed concomitantly on renal parenchymal and interstitial cells. Single Ig IL-1-related receptor (SIGIRR), also known as Toll-IL-1 receptor 8 (TIR8), modulates TLR signaling responses, particularly APC function in response to LPS challenge and ischemic renal damage149, 150. Thus, SIGIRR-deficient mice display susceptibility to tissue damage, including renal IRI, with increased production of IL-6, CXCL2 and CCL2. This response is abrogated in WT mice transplanted with SIGIRR-/- BM and subjected to renal IRI, as well as in SIGIRR-/- mice given liposomal clodronate149. Transplantation of SIGIRR-/- renal allografts in a fully–MHC-mismatched mouse model has also been associated with expansion and maturation of CD11b+CD11c+ resident DC/Mac that prime T cells and impede the development of CD4+CD25+FoxP3+ Treg 151. Renal IRI-mediated induction of IRF 4 is localized to CD45+CD11c+ DC/Mac, whereas IRF4-/- mice have increased renal damage that is abrogated by liposomal clodronate152.
Transplantation
The involvement of DC and Mac in renal transplantation has been studied 153, but there is relatively little information concerning the effects of standard immunosuppressive agents on renal DC or Mac number and function. Both donor Mac and DC are transferred within the allograft at the time of transplantation, and then host DC/Mac are recruited into the transplant154, 155. Mac proliferate intensely within kidney allografts in the absence of immunosuppression, and this is promoted by csf-1 156. Early animal studies revealed the lack of MHC class II induction in kidney allografts when recipients received cyclosporine compared to untreated transplants157. Cyclosporine also increases DC number and maturation status in rats158, 159. Warm ischemia alone leads to loss of the CD11c+CD11b+CD103- DC subset, whereas cold ischemia results in additional loss of the CD103+ subset from syngeneic grafts and their replacement by host CD11b+CD11c+CD103+TNFα+ DC160.
The ability of DC to subvert the alloimmune response following transplantation has been documented extensively in the literature (well reviewed in 18). Given their typical immature phenotype 55 renal DC represent an ideal potential source of regulatory DC, however their use is limited by inherent difficulties in isolating adequate numbers of cells. Mobilization of mouse renal DC using Flt3L increases their absolute numbers for subsequent infusion prior to cardiac transplantation, prolonging allograft survival in the absence of immunosuppression55.
Mac are also significant contributors to both acute and chronic allograft injury in animal models. In loss-of-function studies, administration of liposomal clodronate mitigates functional and tissue injury in a rat renal transplant model without affecting lymphocyte numbers or activation status 161. Similarly, DT treatment of CD11b-DTR mice protects the renal allograft from acute injury and rejection 162. Use of a pharmacological antagonist of the csf-1/csf-1R pathway reduces tubulointerstitial injury163, Mac infiltration, acute cellular rejection and T cell activation164. Interestingly, the treatment had no effect on humoral rejection that occurred later in the model. Persistent Mac infiltration also characterizes the development of chronic allograft nephropathy in rodents 165, that is ameliorated by Mac-specific blockade166, 167.
Renal DC and Mac in human health and disease
DC and Mac in normal human kidneys have been studied less extensively than those in rodents. Currently, most studies demonstrate DC within renal parenchyma using immunohistochemistry and their putative function is based on animal models.Both cDC (BDCA1+[CD1c]+ DC-SIGN+[CD209]CD68+ and BDCA1+DC-SIGN-CD68-) and pDC (BDCA2+DC-SIGN-) have been demonstrated within the normal renal interstitium and are rarely present within glomeruli168.
Examination of human renal allograft biopsies during acute cellular rejection reveals an increased number of DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN)+ DC, particularly intraglomerular cells168. BDCA-1+, BDCA-2 (CD303)+, and DC-LAMP (DC-lysosomal associated membrane protein 3, CD208)+ cells have also been identified with in acutely rejecting renal allografts168. CCR1+DC-SIGN+ DC are also evident following transplantation169. A recent publication demonstrated increased CD141hiCLEC9A+ and CD1c+CD1a-DCSIGN- mDC170, as well as pDC in human allograft biopsies with histological evidence of fibrosis. The mDC phenotype suggested that they were derived from the peripheral circulation, as well as being the predominant source of TGFβ.
Mac (CD68+ cells ) have also been identified in renal allografts with histological evidence of Ab-mediated171, 172, acute cellular 173 and chronic rejection174. Impairment of estimated glomerular filtration rate correlated with Mac transcript sets in patients biopsied for cause175, and histological changes of both interstitial fibrosis/tubular atrophy and inflammation were associated with a Mac signature in microarray analyses176, 177. It is unclear whether these data represent a subset of acute rejection or a reparative phase to injury. Glomerular178, 179 or interstitial180, 181 Mac infiltration is a poor prognostic sign and marker of unfavorable graft outcomes.
Mac infiltration has been shown to be associated with an adverse outcome in native kidney disease. CD68+ Mac infiltration is an independent risk factor for progression to end-stage renal disease in patients with membranous nephropathy182. Biopsies from patients with a diverse range of chronic kidney disease, demonstrate that Mac infiltration correlate with both chronic damage and serum creatinine183, and these cells co-localize to areas of capillary rarefaction, implicating a role for Mac in microvasculature injury184.
Early studies detected CD1b+ DC within active areas of interstitial inflammation and glomerular crescent formation185. Observations regarding DC-based infiltration have also been made in kidneys with IgA nephropathy168. In patients with lupus nephritis, increased numbers of BDCA-1, -3 (CD141; thymomodulin) and -4 (CD304; neuropilin-1) positive cells (putative cDC), infrequent DC-LAMP (CD208)+ mature DC, and BDCA-2+ChemR23 (chemokine receptor-like 1)+ pDC are seen 186. BDCA-1+ myeloid DC also express C1q, with concordant animal model data suggesting that intrinsic renal DC contribute to local C1q synthesis107. However, there may be compartmental separation of mononuclear cell subtypes, with CD68+CD209+ tubulointerstitial cell accumulation but CD68+CD209- intraglomerular cells, as well as correlation between the number of DC and degree of renal injury187. Mature (DC-LAMP+) DC are not present in healthy kidneys but are evident at low numbers in diseased tissue, including lupus nephritis 168, 188. Cross-talk between T cells and DC is well-recognized in mouse models and there is evidence from human kidney samples that a similar interrelationship initiates an (in)appropriate immunological response. Biopsies of patients with anti-neutrophil cytoplasmic Ab (ANCA)-associated vasculitis demonstrate close proximity between CD3+ T cells and DC-SIGN+ DC189. Tertiary lymphoid structures that develop within the renal parenchyma following chronic Ag stimulation have also demonstrated clusters of follicular (CD21+) DC expressing the B lymphocyte-specific chemokine BCA-1 (CXCL13) 190, and neolymphangiogenesis with admixed CD4+ and CD8+ T cells, CD20+ B cells, S-100+ cDC and pDC191. These observations suggest intrarenal Ag presentation by DC to T and B cells to promote a chronic, immune-mediated inflammatory response.
The presence of pDC within diseased kidney has been demonstrated in mouse models (vide supra), but their role in human disease has been disputed. The contribution of pDC to renal pathology has been most demonstrated consistently in lupus nephritis, where secretion of IL-18 by resident glomerular cells is a potent chemoattractant for IL-18R-expressing DC 192, 193. BDCA-2+ChemR23+ pDC are present in severe lupus nephritis. pDC are also evident in increased numbers following delayed graft function (compared to calcineurin inhibitor toxicity where there is a preponderance of myeloid DC)194.
Conclusions
Renal DC and Mac are phenotypically and functionally heterogeneous cells that regulate tissue responses to renal injury and disease. The considerable overlap between DC and Mac represents a continuum of phenotype, as well as plasticity of cells of the myeloid-monocytic lineage both in vivo and in vitro. Our knowledge of renal DC and Mac function lags behind that described for other organs. This limitation has been improved by advances in cell isolation, identification, in vivo propagation, and use of innovative genetically-modified mouse models. The central role of renal DC and Mac in homeostasis, as well as their capacity to modulate physiological function to drive immune or non-immune disorders, is increasingly recognized. These cells provide a reservoir for ongoing immunosurveillance within the renal tubulointerstitium extending to the draining lymph node, in addition to affording an immune privileged site within the glomerulus.
Extensive small animal work has provided an important basis for increased phenotypic and functional understanding, however a consensus regarding precise cell identification and extrapolation to human kidney disease is lacking providing avenues for future research (see Box 2). However, after several decades of research the question of whether renal DC and Mac constitute sufficiently specific targets for therapeutic intervention to potentially ameliorate kidney disease progression remains.
Box 2. Renal DC and Mac – challenges for the future.
-
consensus regarding differences/similarities between DC and macrophages for appropriate identification and classification
-
–
glomerular versus tubulointerstitial
-
–
tissue-resident versus recruited
-
–
immunogenic versus tolerogenic
-
–
fibrotic versus reparative
-
–
correlation of terminology between murine and human cells
exploration of RTEC-DC-macrophage crosstalk
expansion of understanding of DC/macrophages in non-immunological and chronic disease processes such as diabetic kidney disease
development of models with kidney-specific DC/macrophage ablation
emphasis on translational work and applicability of findings to clinical disease
Key points.
-
-
Dendritic cells and macrophages are distinct cell types but also demonstrate similarities in terms of ontogeny, phenotype and function
-
-
Both cells are present within the renal interstitium and are critical to homeostatic regulation of the kidney environment
-
-
Dendritic cells and macrophages increase in number following renal injury
-
-
The manifestation of glomerular or tubular kidney disease, as well as the outcome in pre-clinical models is determined by the type of dendritic cell or macrophage involved
-
-
A number of methods are available to eliminate dendritic cells or macrophages, but the effects are not renal-specific
Acknowledgments
Source of support: This work was supported by the National Institutes of Health (NIH) grants R01 HL-108954 and HL-112914 (to JSI), R01AI67541 and U01AI51698 (AWT); University of Pittsburgh O’Brien Kidney Pilot Award (JSI); Cunningham Trust (JH), the Mrs AE Hogg Charitable Trust (JH), the Kidney Research UK and UK Medical Research Council (JH); American Heart Association Postdoctoral Award (13POST14520003) and American Society of Transplantation/Pfizer Basic Science Fellowship (NMR); Wellcome Trust Intermediate fellowship (DAF). This work was also supported by the Institute for Transfusion Medicine, the Hemophilia Center of Western Pennsylvania, and the Vascular Medicine Institute (JSI).
Biography
Angus W. Thomson is a Distinguished Professor of Surgery and Immunology at the Starzl Transplantation Institute, University of Pittsburgh School of Medicine. He trained in basic and applied immunology at the University of Birmingham, UK, received his PhD from the University of Aberdeen, UK and conducted postdoctoral research as an MRC Travelling Fellow at the Kolling Institute of Medical Research, University of Sydney, Australia. Dr. Thomson’s principal research interests concern mechanisms underlying tolerance induction, the immunobiology and tolerogenicity of dendritic leukocytes, and cell therapy in transplantation. Dr. Thomson has received numerous NIH grants, and is Program Director of the NIAID-funded University of Pittsburgh institutional training grant in transplantation biology.
Jeremy Hughes graduated in medicine from the Universities of Cambridge and London and focused upon glomerular inflammation for his PhD studies as a MRC Clinical Training Fellow at the Royal Postgraduate Medical School in London. After a University lectureship in Nottingham, he undertook a 3 year Wellcome Advanced Fellowship in Seattle in Professor Richard Johnson’s laboratory. He became a Wellcome Senior Fellow in Clinical Science at the University of Edinburgh in 2000 and is now Professor of Experimental Nephrology. He has broad research interests, including macrophage biology, hemeoxygenase-1, acute kidney injury and renal fibrosis.
David Ferenbach is a Wellcome Trust Intermediate Clinical Fellow in the Bonventre Laboratory at Harvard Medical School, Boston, MA. He trained in Nephrology in Glasgow and Edinburgh, where he obtained his PhD examining the role of phagocyte biology in the pathogenesis of acute kidney injury. He is currently examining the mechanisms underlying the susceptibility to AKI seen in the ageing kidney.
Natasha Rogers is currently a postdoctoral fellow at the Vascular Medicine and Starzl Transplant Institutes at the University of Pittsburgh, Pennsylvania, USA. She received her MD and PhD from the University of Adelaide, Australia. Following an internal medicine residency she specialized in Nephrology. She has a current research interest in transplantation, acute kidney injury and dendritic cell biology.
Jeffrey S. Isenberg is an Associate Professor of Medicine and a principal investigator of the Vascular Medicine Institute, University of Pittsburgh School of Medicine, Pennsylvania, USA. He received his B.A. from the University of Pennsylvania and his medical training at Tulane University School of Medicine, Louisiana (M.D., M.P.H.). Following general surgery residency he specialized in reconstructive surgery, hand surgery and microsurgery training at Yale University, Connecticut and the University of Southern California. He completed postdoctoral training in the Laboratory of Pathology in the Center for Cancer Research, National Cancer Institute, National Institutes of Health in Bethesda with Dr. David D. Roberts. His research interests include understanding the mechanism that control tissue perfusion and survival, blood flow and cardiovascular responses. He has published widely on ischemia reperfusion injury. These studies have led to new work in whole organ transplantation.
Footnotes
Conflict of Interest: JSI is chair of the Scientific Advisory Boards of Vasculox (St Louis, MO) and Radiation Control Technologies (Rockville, MD). AWT is co-inventor of a US patent for the generation of tolerogenic dendritic cells to promote transplant tolerance.
A search for original, peer-reviewed articles was performed in MEDLINE and PubMed in November 2013 and again in February 2014. The search terms used were “kidney”, “dendritic cells”, “macrophages”, “glomerulonephritis”, “acute kidney injury”, “lupus”, “fibrosis” and “transplantation”, alone and in combination. All articles identified were English language, full-text papers. The reference lists of identified articles were searched for further relevant papers.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Geissmann F. The origin of dendritic cells. Nat Immunol. 2007;8:558–60. doi: 10.1038/ni0607-558. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 4.Nelson PJ, et al. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012;23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 6.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–43. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 7.Miller JC, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–99. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hume DA. Plenary perspective: the complexity of constitutive and inducible gene expression in mononuclear phagocytes. J Leukoc Biol. 2012;92:433–44. doi: 10.1189/jlb.0312166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–35. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 10.Hume DA, Mabbott N, Raza S, Freeman TC. Can DCs be distinguished from macrophages by molecular signatures? Nat Immunol. 2013;14:187–9. doi: 10.1038/ni.2516. [DOI] [PubMed] [Google Scholar]
- 11.Ferenbach D, Hughes J. Macrophages and dendritic cells: what is the difference? Kidney Int. 2008;74:5–7. doi: 10.1038/ki.2008.189. [DOI] [PubMed] [Google Scholar]
- 12.Cailhier JF, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–42. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 13.Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami T, et al. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J Immunol. 2013;191:3358–72. doi: 10.4049/jimmunol.1300342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 16.Diebold SS. Determination of T-cell fate by dendritic cells. Immunol Cell Biol. 2008;86:389–97. doi: 10.1038/icb.2008.26. [DOI] [PubMed] [Google Scholar]
- 17.Naik SH. Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol. 2008;86:439–52. doi: 10.1038/icb.2008.28. [DOI] [PubMed] [Google Scholar]
- 18.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 20.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 21.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 22.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 24.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alikhan MA, et al. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol. 2011;179:1243–56. doi: 10.1016/j.ajpath.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rae F, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308:232–46. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Shepard JL, Zon LI. Developmental derivation of embryonic and adult macrophages. Curr Opin Hematol. 2000;7:3–8. doi: 10.1097/00062752-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 29.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2014;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rees AJ. Monocyte and macrophage biology: an overview. Semin Nephrol. 2010;30:216–33. doi: 10.1016/j.semnephrol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascon DB, et al. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol. 2006;177:3380–7. doi: 10.4049/jimmunol.177.5.3380. [DOI] [PubMed] [Google Scholar]
- 34.Paust HJ, et al. Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int. 2011;80:154–64. doi: 10.1038/ki.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, et al. Depletion of gammadelta T cells exacerbates murine adriamycin nephropathy. J Am Soc Nephrol. 2007;18:1180–9. doi: 10.1681/ASN.2006060622. [DOI] [PubMed] [Google Scholar]
- 36.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–97. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974;139:1431–45. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiner GF, Kiely JM, Cotran RS, Unanue ER. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest. 1981;68:920–31. doi: 10.1172/JCI110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiner GF, Cotran RS, Unanue ER. Modulation of Ia and leukocyte common antigen expression in rat glomeruli during the course of glomerulonephritis and aminonucleoside nephrosis. Lab Invest. 1984;51:524–33. [PubMed] [Google Scholar]
- 41.Schreiner GF, Cotran RS. Localization of an Ia-bearing glomerular cell in the mesangium. J Cell Biol. 1982;94:483–8. doi: 10.1083/jcb.94.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gieseler R, et al. Enrichment and characterization of dendritic cells from rat renal mesangium. Scand J Immunol. 1997;46:587–96. doi: 10.1046/j.1365-3083.1997.d01-175.x. [DOI] [PubMed] [Google Scholar]
- 43.Hart DN, Fabre JW. Major histocompatibility complex antigens in rat kidney, ureter, and bladder. Localization with monoclonal antibodies and demonstration of Ia-positive dendritic cells. Transplantation. 1981;31:318–25. doi: 10.1097/00007890-198105010-00003. [DOI] [PubMed] [Google Scholar]
- 44.Hart DN, et al. Localization of HLA-ABC and DR antigens in human kidney. Transplantation. 1981;31:428–33. doi: 10.1097/00007890-198106000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Kaissling B, Hegyi I, Loffing J, Le Hir M. Morphology of interstitial cells in the healthy kidney. Anat Embryol (Berl) 1996;193:303–18. doi: 10.1007/BF00186688. [DOI] [PubMed] [Google Scholar]
- 46.Pajak B, et al. Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualisation of dendritic cells in peripheral organs. J Clin Pathol. 2000;53:518–24. doi: 10.1136/jcp.53.7.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruger T, et al. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol. 2004;15:613–21. doi: 10.1097/01.asn.0000114553.36258.91. [DOI] [PubMed] [Google Scholar]
- 48.Ginhoux F, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald KP, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–63. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 50.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 51.Sunderkotter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 52.Lin SL, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–9. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soos TJ, et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–6. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 54.Sasmono RT, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–63. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 55.Coates PT, et al. In vivo-mobilized kidney dendritic cells are functionally immature, subvert alloreactive T-cell responses, and prolong organ allograft survival. Transplantation. 2004;77:1080–9. doi: 10.1097/01.tp.0000122183.60680.c9. [DOI] [PubMed] [Google Scholar]
- 56.Morelli AE, et al. Growth factor-induced mobilization of dendritic cells in kidney and liver of rhesus macaques: implications for transplantation. Transplantation. 2007;83:656–62. doi: 10.1097/01.tp.0000255320.00061.e9. [DOI] [PubMed] [Google Scholar]
- 57.Coates PT, et al. CCR and CC chemokine expression in relation to Flt3 ligand-induced renal dendritic cell mobilization. Kidney Int. 2004;66:1907–17. doi: 10.1111/j.1523-1755.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- 58.Schraml BU, et al. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell. 154:843–58. doi: 10.1016/j.cell.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–37. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hume DA, Gordon S. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J Exp Med. 1983;157:1704–9. doi: 10.1084/jem.157.5.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masaki T, Chow F, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Heterogeneity of antigen expression explains controversy over glomerular macrophage accumulation in mouse glomerulonephritis. Nephrol Dial Transplant. 2003;18:178–81. doi: 10.1093/ndt/18.1.178. [DOI] [PubMed] [Google Scholar]
- 63.van Rooijen N. Liposomes as an in vivo tool to study and manipulate macrophage function. Res Immunol. 1992;143:177–8. doi: 10.1016/s0923-2494(92)80160-m. [DOI] [PubMed] [Google Scholar]
- 64.van Rooijen N. Liposome-mediated elimination of macrophages. Res Immunol. 1992;143:215–9. doi: 10.1016/s0923-2494(92)80169-l. [DOI] [PubMed] [Google Scholar]
- 65.van Rooijen N, Sanders A. Elimination, blocking, and activation of macrophages: three of a kind? J Leukoc Biol. 1997;62:702–9. doi: 10.1002/jlb.62.6.702. [DOI] [PubMed] [Google Scholar]
- 66.van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–9. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 67.van Rooijen N, van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 1984;238:355–8. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]
- 68.Lu L, et al. Depletion of macrophages and dendritic cells in ischemic acute kidney injury. Am J Nephrol. 2012;35:181–90. doi: 10.1159/000335582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferenbach DA, et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–33. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 70.Tittel AP, et al. Kidney dendritic cells induce innate immunity against bacterial pyelonephritis. J Am Soc Nephrol. 2011;22:1435–41. doi: 10.1681/ASN.2010101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vinuesa E, et al. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol. 2008;214:104–13. doi: 10.1002/path.2259. [DOI] [PubMed] [Google Scholar]
- 72.Kulkarni OP, et al. Toll-Like Receptor 4-Induced IL-22 Accelerates Kidney Regeneration. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–25. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 74.Kaissling B, Lehir M, Kriz W. Renal epithelial injury and fibrosis. Biochim Biophys Acta. 2013;1832:931–9. doi: 10.1016/j.bbadis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–43. doi: 10.1038/nm.2144. 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sung SA, Jo SK, Cho WY, Won NH, Kim HK. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron Exp Nephrol. 2007;105:e1–9. doi: 10.1159/000096859. [DOI] [PubMed] [Google Scholar]
- 77.Kitamoto K, et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci. 2009;111:285–92. doi: 10.1254/jphs.09227fp. [DOI] [PubMed] [Google Scholar]
- 78.Henderson NC, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–98. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Machida Y, et al. Renal fibrosis in murine obstructive nephropathy is attenuated by depletion of monocyte lineage, not dendritic cells. J Pharmacol Sci. 2010;114:464–73. doi: 10.1254/jphs.10246fp. [DOI] [PubMed] [Google Scholar]
- 80.Snelgrove SL, et al. Renal dendritic cells adopt a pro-inflammatory phenotype in obstructive uropathy to activate T cells but do not directly contribute to fibrosis. Am J Pathol. 2012;180:91–103. doi: 10.1016/j.ajpath.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 81.Lech M, et al. Macrophage Phenotype Controls Long-Term AKI Outcomes--Kidney Regeneration versus Atrophy. J Am Soc Nephrol. 2014;25:292–304. doi: 10.1681/ASN.2013020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huen SC, Moeckel GW, Cantley LG. Macrophage-specific deletion of transforming growth factor-beta1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am J Physiol Renal Physiol. 2013;305:F477–84. doi: 10.1152/ajprenal.00624.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lukacs-Kornek V, et al. The kidney-renal lymph node-system contributes to cross-tolerance against innocuous circulating antigen. J Immunol. 2008;180:706–15. doi: 10.4049/jimmunol.180.2.706. [DOI] [PubMed] [Google Scholar]
- 84.Gottschalk C, et al. Batf3-dependent dendritic cells in the renal lymph node induce tolerance against circulating antigens. J Am Soc Nephrol. 2013;24:543–9. doi: 10.1681/ASN.2012101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macconi D, et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol. 2009;20:123–30. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong X, et al. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 2005;68:1096–108. doi: 10.1111/j.1523-1755.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 87.Roake JA, et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–47. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurts C, Heymann F, Lukacs-Kornek V, Boor P, Floege J. Role of T cells and dendritic cells in glomerular immunopathology. Semin Immunopathol. 2007;29:317–35. doi: 10.1007/s00281-007-0096-x. [DOI] [PubMed] [Google Scholar]
- 89.Assmann KJ, Tangelder MM, Lange WP, Schrijver G, Koene RA. Anti-GBM nephritis in the mouse: severe proteinuria in the heterologous phase. Virchows Arch A Pathol Anat Histopathol. 1985;406:285–99. doi: 10.1007/BF00704298. [DOI] [PubMed] [Google Scholar]
- 90.Hochheiser K, et al. Kidney Dendritic Cells Become Pathogenic during Crescentic Glomerulonephritis with Proteinuria. J Am Soc Nephrol. 2011;22:306–16. doi: 10.1681/ASN.2010050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lichtnekert J, et al. Anti-GBM glomerulonephritis involves IL-1 but is independent of NLRP3/ASC inflammasome-mediated activation of caspase-1. PLoS One. 2011;6:e26778. doi: 10.1371/journal.pone.0026778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scholz J, et al. Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J Am Soc Nephrol. 2008;19:527–37. doi: 10.1681/ASN.2007060684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kitching AR, Tipping PG, Huang XR, Mutch DA, Holdsworth SR. Interleukin-4 and interleukin-10 attenuate established crescentic glomerulonephritis in mice. Kidney Int. 1997;52:52–9. doi: 10.1038/ki.1997.303. [DOI] [PubMed] [Google Scholar]
- 94.Kitching AR, Tipping PG, Timoshanko JR, Holdsworth SR. Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int. 2000;57:518–25. doi: 10.1046/j.1523-1755.2000.00872.x. [DOI] [PubMed] [Google Scholar]
- 95.Witsch EJ, et al. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur J Immunol. 2002;32:2680–6. doi: 10.1002/1521-4141(200209)32:9<2680::AID-IMMU2680>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 96.Duffield JS, et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–19. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lloyd CM, Dorf ME, Proudfoot A, Salant DJ, Gutierrez-Ramos JC. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukoc Biol. 1997;62:676–80. doi: 10.1002/jlb.62.5.676. [DOI] [PubMed] [Google Scholar]
- 98.Wada T, et al. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1) FASEB J. 1996;10:1418–25. [PubMed] [Google Scholar]
- 99.Yamamoto T, Wilson CB. Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int. 1987;32:514–25. doi: 10.1038/ki.1987.240. [DOI] [PubMed] [Google Scholar]
- 100.Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 101.Lan HY, Nikolic-Paterson DJ, Mu W, Atkins RC. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int. 1995;48:753–60. doi: 10.1038/ki.1995.347. [DOI] [PubMed] [Google Scholar]
- 102.Han Y, Ma FY, Tesch GH, Manthey CL, Nikolic-Paterson DJ. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab Invest. 2011;91:978–91. doi: 10.1038/labinvest.2011.61. [DOI] [PubMed] [Google Scholar]
- 103.Odobasic D, et al. Glomerular expression of CD80 and CD86 is required for leukocyte accumulation and injury in crescentic glomerulonephritis. J Am Soc Nephrol. 2005;16:2012–22. doi: 10.1681/ASN.2004060437. [DOI] [PubMed] [Google Scholar]
- 104.Heymann F, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119:1286–97. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schiffer L, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–47. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishikawa S, et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castellano G, et al. Infiltrating dendritic cells contribute to local synthesis of C1q in murine and human lupus nephritis. Mol Immunol. 2010;47:2129–37. doi: 10.1016/j.molimm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 108.Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–25. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 109.Kulkarni O, et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol. 2007;18:2350–8. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- 110.Perez de Lema G, et al. Chemokine receptor Ccr2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J Am Soc Nephrol. 2005;16:3592–601. doi: 10.1681/ASN.2005040426. [DOI] [PubMed] [Google Scholar]
- 111.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–9. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 112.Wan S, Zhou Z, Duan B, Morel L. Direct B cell stimulation by dendritic cells in a mouse model of lupus. Arthritis Rheum. 2008;58:1741–50. doi: 10.1002/art.23515. [DOI] [PubMed] [Google Scholar]
- 113.Bagavant H, Thompson C, Ohno K, Setiady Y, Tung KS. Differential effect of neonatal thymectomy on systemic and organ-specific autoimmune disease. Int Immunol. 2002;14:1397–406. doi: 10.1093/intimm/dxf105. [DOI] [PubMed] [Google Scholar]
- 114.Bagavant H, Deshmukh US, Wang H, Ly T, Fu SM. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J Immunol. 2006;177:8258–65. doi: 10.4049/jimmunol.177.11.8258. [DOI] [PubMed] [Google Scholar]
- 115.Schiffer L, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489–97. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 116.Iwata Y, et al. p38 Mitogen-activated protein kinase contributes to autoimmune renal injury in MRL-Fas lpr mice. J Am Soc Nephrol. 2003;14:57–67. doi: 10.1097/01.asn.0000037402.83851.5f. [DOI] [PubMed] [Google Scholar]
- 117.Iwata Y, et al. Dendritic cells contribute to autoimmune kidney injury in MRL-Faslpr mice. J Rheumatol. 2009;36:306–14. doi: 10.3899/jrheum.080318. [DOI] [PubMed] [Google Scholar]
- 118.Baccala R, et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc Natl Acad Sci U S A. 2012;110:2940–5. doi: 10.1073/pnas.1222798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kinjoh K, Kyogoku M, Good RA. Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc Natl Acad Sci U S A. 1993;90:3413–7. doi: 10.1073/pnas.90.8.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saiga K, et al. NK026680, a novel suppressant of dendritic cell function, prevents the development of rapidly progressive glomerulonephritis and perinuclear antineutrophil cytoplasmic antibody in SCG/Kj mice. Arthritis Rheum. 2006;54:3707–15. doi: 10.1002/art.22187. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Y, et al. Immune complex enhances tolerogenecity of immature dendritic cells via FcgammaRIIb and promotes FcgammaRIIb-overexpressing dendritic cells to attenuate lupus. Eur J Immunol. 2011;41:1154–64. doi: 10.1002/eji.201040767. [DOI] [PubMed] [Google Scholar]
- 122.McGaha TL, Karlsson MC, Ravetch JV. FcgammaRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J Immunol. 2008;180:5670–9. doi: 10.4049/jimmunol.180.8.5670. [DOI] [PubMed] [Google Scholar]
- 123.Rodriguez W, et al. C-reactive protein-mediated suppression of nephrotoxic nephritis: role of macrophages, complement, and Fcgamma receptors. J Immunol. 2007;178:530–8. doi: 10.4049/jimmunol.178.1.530. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, et al. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through Fc gamma RIIb-dependent PGE2 production. J Immunol. 2009;182:554–62. doi: 10.4049/jimmunol.182.1.554. [DOI] [PubMed] [Google Scholar]
- 125.Muhlfeld AS, et al. Deletion of the fcgamma receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis. Am J Pathol. 2003;163:1127–36. doi: 10.1016/s0002-9440(10)63472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith J, et al. A spleen tyrosine kinase inhibitor reduces the severity of established glomerulonephritis. J Am Soc Nephrol. 2010;21:231–6. doi: 10.1681/ASN.2009030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tadagavadi RK, Reeves WB. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol. 2009;21:53–63. doi: 10.1681/ASN.2009040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30:268–77. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21:1231–9. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 130.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–31. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 131.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2008;23:842–52. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 132.Zhang MZ, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–32. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee S, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–26. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim MG, et al. Depletion of kidney CD11c+ F4/80+ cells impairs the recovery process in ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010;25:2908–21. doi: 10.1093/ndt/gfq183. [DOI] [PubMed] [Google Scholar]
- 135.Tadagavadi RK, Reeves WB. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J Immunol. 2010;185:4904–11. doi: 10.4049/jimmunol.1000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77:771–80. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kinsey GR, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–53. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gandolfo MT, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–29. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 139.Kim MG, et al. IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J Am Soc Nephrol. 2013;24:1529–36. doi: 10.1681/ASN.2012080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cho WY, et al. The role of Tregs and CD11c(+) macrophages/dendritic cells in ischemic preconditioning of the kidney. Kidney Int. 2010;78:981–92. doi: 10.1038/ki.2010.266. [DOI] [PubMed] [Google Scholar]
- 141.Cantaluppi V, et al. Rationale of mesenchymal stem cell therapy in kidney injury. Am J Kidney Dis. 2013;61:300–9. doi: 10.1053/j.ajkd.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 142.Kim MG, et al. CD11c(+) Cells Partially Mediate the Renoprotective Effect Induced by Bone Marrow-Derived Mesenchymal Stem Cells. PLoS One. 2013;8:e72544. doi: 10.1371/journal.pone.0072544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Menke J, et al. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest. 2009;119:2330–42. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y, Tay YC, Harris DC. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int. 2004;66:655–62. doi: 10.1111/j.1523-1755.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 145.Dong X, et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–28. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 146.Dong X, Bachman LA, Miller MN, Nath KA, Griffin MD. Dendritic cells facilitate accumulation of IL-17 T cells in the kidney following acute renal obstruction. Kidney Int. 2008;74:1294–309. doi: 10.1038/ki.2008.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kitagawa K, et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–46. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tan TK, et al. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am J Pathol. 2010;176:1256–70. doi: 10.2353/ajpath.2010.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lech M, et al. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol. 2009;183:4109–18. doi: 10.4049/jimmunol.0900118. [DOI] [PubMed] [Google Scholar]
- 150.Garlanda C, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–6. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noris M, et al. The Toll-IL-1R member Tir8/SIGIRR negatively regulates adaptive immunity against kidney grafts. J Immunol. 2009;183:4249–60. doi: 10.4049/jimmunol.0803549. [DOI] [PubMed] [Google Scholar]
- 152.Lassen S, et al. Ischemia reperfusion induces IFN regulatory factor 4 in renal dendritic cells, which suppresses postischemic inflammation and prevents acute renal failure. J Immunol. 2009;185:1976–83. doi: 10.4049/jimmunol.0904207. [DOI] [PubMed] [Google Scholar]
- 153.Chadban SJ, Wu H, Hughes J. Macrophages and kidney transplantation. Semin Nephrol. 2010;30:278–89. doi: 10.1016/j.semnephrol.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 154.Penfield JG, et al. Transplant surgery injury recruits recipient MHC class II-positive leukocytes into the kidney. Kidney Int. 1999;56:1759–69. doi: 10.1046/j.1523-1755.1999.00741.x. [DOI] [PubMed] [Google Scholar]
- 155.Penfield JG, et al. Syngeneic renal transplantation increases the number of renal dendritic cells in the rat. Transpl Immunol. 1999;7:197–200. doi: 10.1016/s0966-3274(99)80002-1. [DOI] [PubMed] [Google Scholar]
- 156.Le Meur Y, Jose MD, Mu W, Atkins RC, Chadban SJ. Macrophage colony-stimulating factor expression and macrophage accumulation in renal allograft rejection. Transplantation. 2002;73:1318–24. doi: 10.1097/00007890-200204270-00022. [DOI] [PubMed] [Google Scholar]
- 157.Milton AD, Spencer SC, Fabre JW. The effects of cyclosporine on the induction of donor class I and class II MHC antigens in heart and kidney allografts in the rat. Transplantation. 1986;42:337–47. doi: 10.1097/00007890-198610000-00002. [DOI] [PubMed] [Google Scholar]
- 158.Ahn KO, et al. Influence of angiotensin II on expression of toll-like receptor 2 and maturation of dendritic cells in chronic cyclosporine nephropathy. Transplantation. 2007;83:938–47. doi: 10.1097/01.tp.0000258589.39006.94. [DOI] [PubMed] [Google Scholar]
- 159.Lim SW, et al. Cyclosporine-induced renal injury induces toll-like receptor and maturation of dendritic cells. Transplantation. 2005;80:691–9. doi: 10.1097/01.tp.0000173594.69089.a0. [DOI] [PubMed] [Google Scholar]
- 160.Ozaki KS, et al. The loss of renal dendritic cells and activation of host adaptive immunity are long-term effects of ischemia/reperfusion injury following syngeneic kidney transplantation. Kidney Int. 2012;81:1015–25. doi: 10.1038/ki.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76:1015–22. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 162.Qi F, et al. Depletion of cells of monocyte lineage prevents loss of renal microvasculature in murine kidney transplantation. Transplantation. 2008;86:1267–74. doi: 10.1097/TP.0b013e318188d433. [DOI] [PubMed] [Google Scholar]
- 163.Jose MD, Le Meur Y, Atkins RC, Chadban SJ. Blockade of macrophage colony-stimulating factor reduces macrophage proliferation and accumulation in renal allograft rejection. Am J Transplant. 2003;3:294–300. doi: 10.1034/j.1600-6143.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 164.Ma FY, Woodman N, Mulley WR, Kanellis J, Nikolic-Paterson DJ. Macrophages contribute to cellular but not humoral mechanisms of acute rejection in rat renal allografts. Transplantation. 2013;96:949–57. doi: 10.1097/TP.0b013e3182a4befa. [DOI] [PubMed] [Google Scholar]
- 165.Shimizu A, Yamada K, Sachs DH, Colvin RB. Mechanisms of chronic renal allograft rejection. II. Progressive allograft glomerulopathy in miniature swine. Lab Invest. 2002;82:673–86. doi: 10.1097/01.lab.0000017370.74529.89. [DOI] [PubMed] [Google Scholar]
- 166.Azuma H, Nadeau KC, Ishibashi M, Tilney NL. Prevention of functional, structural, and molecular changes of chronic rejection of rat renal allografts by a specific macrophage inhibitor. Transplantation. 1995;60:1577–82. doi: 10.1097/00007890-199560120-00034. [DOI] [PubMed] [Google Scholar]
- 167.Yang J, et al. Targeting of macrophage activity by adenovirus-mediated intragraft overexpression of TNFRp55-Ig, IL-12p40, and vIL-10 ameliorates adenovirus-mediated chronic graft injury, whereas stimulation of macrophages by overexpression of IFN-gamma accelerates chronic graft injury in a rat renal allograft model. J Am Soc Nephrol. 2003;14:214–25. doi: 10.1097/01.asn.0000037703.73850.72. [DOI] [PubMed] [Google Scholar]
- 168.Woltman AM, et al. Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int. 2007;71:1001–8. doi: 10.1038/sj.ki.5002187. [DOI] [PubMed] [Google Scholar]
- 169.Mayer V, et al. Expression of the chemokine receptor CCR1 in human renal allografts. Nephrol Dial Transplant. 2007;22:1720–9. doi: 10.1093/ndt/gfm007. [DOI] [PubMed] [Google Scholar]
- 170.Kassianos AJ, et al. Increased tubulointerstitial recruitment of human CD141(hi) CLEC9A(+) and CD1c(+) myeloid dendritic cell subsets in renal fibrosis and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F1391–401. doi: 10.1152/ajprenal.00318.2013. [DOI] [PubMed] [Google Scholar]
- 171.Fahim T, et al. The cellular lesion of humoral rejection: predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant. 2007;7:385–93. doi: 10.1111/j.1600-6143.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 172.Sund S, Reisaeter AV, Scott H, Mollnes TE, Hovig T. Glomerular monocyte/macrophage influx correlates strongly with complement activation in 1-week protocol kidney allograft biopsies. Clin Nephrol. 2004;62:121–30. doi: 10.5414/cnp62121. [DOI] [PubMed] [Google Scholar]
- 173.Grimm PC, et al. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999;10:1582–9. doi: 10.1681/ASN.V1071582. [DOI] [PubMed] [Google Scholar]
- 174.Pilmore HL, Painter DM, Bishop GA, McCaughan GW, Eris JM. Early up-regulation of macrophages and myofibroblasts: a new marker for development of chronic renal allograft rejection. Transplantation. 2000;69:2658–62. doi: 10.1097/00007890-200006270-00028. [DOI] [PubMed] [Google Scholar]
- 175.Bunnag S, et al. Molecular correlates of renal function in kidney transplant biopsies. J Am Soc Nephrol. 2009;20:1149–60. doi: 10.1681/ASN.2008080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Scian MJ, et al. Gene expression changes are associated with loss of kidney graft function and interstitial fibrosis and tubular atrophy: diagnosis versus prediction. Transplantation. 2011;91:657–65. doi: 10.1097/TP.0b013e3182094a5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mengel M, et al. The molecular phenotype of 6-week protocol biopsies from human renal allografts: reflections of prior injury but not future course. Am J Transplant. 2011;11:708–18. doi: 10.1111/j.1600-6143.2010.03339.x. [DOI] [PubMed] [Google Scholar]
- 178.Papadimitriou JC, et al. Glomerular inflammation in renal allografts biopsies after the first year: cell types and relationship with antibody-mediated rejection and graft outcome. Transplantation. 2010;90:1478–85. doi: 10.1097/TP.0b013e3181ff87f5. [DOI] [PubMed] [Google Scholar]
- 179.Ozdemir BH, Demirhan B, Gungen Y. The presence and prognostic importance of glomerular macrophage infiltration in renal allografts. Nephron. 2002;90:442–6. doi: 10.1159/000054732. [DOI] [PubMed] [Google Scholar]
- 180.Hoffmann U, et al. Impact of chemokine receptor CX3CR1 in human renal allograft rejection. Transpl Immunol. 2010;23:204–8. doi: 10.1016/j.trim.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 181.Copin MC, et al. Diagnostic and predictive value of an immunohistochemical profile in asymptomatic acute rejection of renal allografts. Transpl Immunol. 1995;3:229–39. doi: 10.1016/0966-3274(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 182.Yoshimoto K, et al. CD68 and MCP-1/CCR2 expression of initial biopsies reflect the outcomes of membranous nephropathy. Nephron Clin Pract. 2004;98:c25–34. doi: 10.1159/000079924. [DOI] [PubMed] [Google Scholar]
- 183.Eardley KS, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–97. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 184.Eardley KS, et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 185.Cuzic S, Ritz E, Waldherr R. Dendritic cells in glomerulonephritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:357–63. doi: 10.1007/BF02899704. [DOI] [PubMed] [Google Scholar]
- 186.De Palma G, et al. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 2011;79:1228–35. doi: 10.1038/ki.2011.32. [DOI] [PubMed] [Google Scholar]
- 187.Segerer S, et al. Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int. 2008;74:37–46. doi: 10.1038/ki.2008.99. [DOI] [PubMed] [Google Scholar]
- 188.Noessner E, Lindenmeyer M, Nelson PJ, Segerer S. Dendritic cells in human renal inflammation--Part II. Nephron Exp Nephrol. 2011;119:e91–8. doi: 10.1159/000332032. [DOI] [PubMed] [Google Scholar]
- 189.Wilde B, et al. Dendritic cells in renal biopsies of patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2009;24:2151–6. doi: 10.1093/ndt/gfp019. [DOI] [PubMed] [Google Scholar]
- 190.Steinmetz OM, et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 2008;74:448–57. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]
- 191.Kerjaschki D, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 192.Tucci M, Ciavarella S, Strippoli S, Dammacco F, Silvestris F. Oversecretion of cytokines and chemokines in lupus nephritis is regulated by intraparenchymal dendritic cells: a review. Ann N Y Acad Sci. 2009;1173:449–57. doi: 10.1111/j.1749-6632.2009.04805.x. [DOI] [PubMed] [Google Scholar]
- 193.Tucci M, et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58:251–62. doi: 10.1002/art.23186. [DOI] [PubMed] [Google Scholar]
- 194.Loverre A, et al. Ischemia-reperfusion injury-induced abnormal dendritic cell traffic in the transplanted kidney with delayed graft function. Kidney Int. 2007;72:994–1003. doi: 10.1038/sj.ki.5002468. [DOI] [PubMed] [Google Scholar]
- 195.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sapoznikov A, et al. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med. 2007;204:1923–33. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–7. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 199.Tittel AP, et al. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods. 2012;9:385–90. doi: 10.1038/nmeth.1905. [DOI] [PubMed] [Google Scholar]
- 200.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–63. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- 202.Shirai T, Hirose S, Okada T, Nishimura H. In: Immunological Disorders in Mice. Rihova EBaVV, V V., editors. CRC Press Inc; Boca Raton, Florida: 1991. pp. 95–136. [Google Scholar]
- 203.Morel L, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97:6670–5. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest. 1993;68:419–26. [PubMed] [Google Scholar]
- 205.Dubois EL, Horowitz RE, Demopoulos HB, Teplitz R. NZB/NZW mice as a model of systemic lupus erythematosus. JAMA. 1966;195:285–9. [PubMed] [Google Scholar]
- 206.Rigby RJ, et al. New loci from New Zealand Black and New Zealand White mice on chromosomes 4 and 12 contribute to lupus-like disease in the context of BALB/c. J Immunol. 2004;172:4609–17. doi: 10.4049/jimmunol.172.7.4609. [DOI] [PubMed] [Google Scholar]
- 207.Colonna L, et al. Abnormal costimulatory phenotype and function of dendritic cells before and after the onset of severe murine lupus. Arthritis Res Ther. 2006;8:R49. doi: 10.1186/ar1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zheng D, et al. Lipopolysaccharide-pretreated plasmacytoid dendritic cells ameliorate experimental chronic kidney disease. Kidney Int. 2012;81:892–902. doi: 10.1038/ki.2011.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Triantafyllopoulou A, et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci U S A. 2010;107:3012–7. doi: 10.1073/pnas.0914902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bethunaickan R, et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol. 2011;186:4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Anders HJ, et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J. 2004;18:534–6. doi: 10.1096/fj.03-0646fje. [DOI] [PubMed] [Google Scholar]
- 212.Teichmann LL, et al. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–78. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Huang XR, et al. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol. 1997;109:134–42. doi: 10.1046/j.1365-2249.1997.4091307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]